An ent-Kaurane-Type Diterpene in Croton antisyphiliticus Mart.
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Determination of Antimicrobial Activity by Bio-Autographic Assay and Minimum Inhibitory Concentration (MIC)
3.5. Microbial Strains
4. Conclusions
Supplementary Materials
Acknowledgements
References
- Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A.; Carter, S. World Checklist and Bibliography of Euphorbiaceae (and Pandaceae); Kew Royal Botanic Gardens: Kew, Richmond, UK, 2000; Volume 2, p. 415. [Google Scholar]
- Berry, P.E.; Hipp, A.L.; Wurdack, K.J.; van Ee, B.W.; Riina, R. Molecular phylogenetics of the giant genus Croton and tribe Crotoneae (Euphorbiaceae sensu stricto) using ITS and trnL-trnF sequence data. Am. J. Bot. 2005, 92, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Carneiro, V.A.; Santos, H.S.; Arruda, F.V. Casbane diterpene as a promising natural antimicrobial agent against biofilm-associated infections. Molecules 2011, 16, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Sittlie, A.; Bugyei, K. Acute toxicity studies of Croton membranaceus root extract. J. Ethonopharmacol. 2011, 134, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Suárez, A.L.; Oropeza, M.; Vásquez, L. Chemical composition of the essential oil of Croton Gossypiifolius from Venezuela. Nat. Prod. Commun. 2011, 6, 97–99. [Google Scholar] [PubMed]
- Pereira, U.; Garcia-Le Gal, C.; Lê Gal, G. Effects of sangre de drago in an in vitro model of cutaneous neurogenic inflammation. Exp. Dermatol. 2010, 19, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Sá, N.C.; Cavalcante, T.T.A.; Araújo, A.X.; Santos, H.S.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Cunha, R.M.S.; Cavada, B.S.; Teixeira, E.H. Antimicrobial and antibiofilm action of casbane diterpene from Croton nepetaefolius against oral bacteria. Arch. Oral Biol. 2012, 57, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Vila Verde, G.M.; Paula, J.R.; Caneiro, D.M. Levantamento etnobotânico das plantas medicinais do cerrado utilizadas pela população de Mossâmedes (GO). Rev. Bras. Farmacogn. 2003, 13, 64–66. [Google Scholar] [CrossRef]
- Rodrigues, V.E.G.; Carvalho, D.A. Levantamento etnobotânico de plantas medicinais no domínio do cerrado na região do alto Rio Grande-Minas Gerais. Ciênc. Agrotec. 2001, 25, 102–123. [Google Scholar]
- Nader, T.T.; Coppede, J.S.; Amaral, L.A.; Facchin, A.L.; Pereira, A.M.S.; Ferreira, L.M. Avaliação in vitro da eficácia de extratos de plantas medicinais do cerrado frente Staphylococcus aureus isolado de diferentes fontes de propriedades leiteiras. Arq. Inst. Biol. 2010, 77, 429–433. [Google Scholar]
- Velandia, J.R.; Carvalho, M.G.; Braz-Filho, R. Ácido ent-16α,17-dihydroxikauran-19-oic acid isolated from Ouratea semiserrata and the stereochemistry defiances of the chiral carbons C-4 and C-16. Quím. Nova 1998, 21, 397–404. [Google Scholar] [CrossRef]
- Norin, T. Chiral chemodiversity and its role for biological activity. Some observations from studies on insect/insect and insect/plant relationships. Pure Appl. Chem. 1996, 68, 2043–2049. [Google Scholar] [CrossRef]
- Davino, S.C.; Giesbrecht, A.M.; Roque, N.F. Antimicrobial activity of kaurenoic acid derivatives substituted on carbon-15. Braz. J. Med. Biol. Res. 1989, 22, 1127–1129. [Google Scholar] [PubMed]
- Drewes, S.E.; Mudau, K.E.; Vuuren, S.F.; Viljoen, A.M. Antimicrobial monomeric and dimeric diterpenes from the leaves of Helichrysum tenax var tenax. Phytochemistry 2006, 67, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Xu, Y.; Shimizu, K. Antibacterial activity of ent-kaurene diterpenoids from Rabdosia rosthornii. Phytother. Res. 2004, 18, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native tingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Wilkens, M.; Alarcon, C.; Urzua, A.; Mendoza, L. Characterization of the bactericidal activity of the natural diterpene kaurenoic acid. Planta Med. 2002, 68, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Iglesia, R.; Mendoza, L.; Gonzalez, B.; Wilkens, M. Soil bacteria are differentially affected by the resin of the medicinal plant Pseudognaphalium vira vira and its main component kaurenoic acid. Microb. Ecol. 2006, 52, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schmelza, E.A.; Kaplana, F.; Huffakera, A.; Dafoea, N.J.; Vaughana, M.M.; Nib, X.; Roccac, J.R.; Alborna, H.T.; Teal, P.E. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Soc. USA 2011, 108, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 6th ed.; CLSI: Wayne, PA, USA, 2003. [Google Scholar]
- Clinical and Laboratory Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 7th ed.; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
Sample Availability: Sample of the compound ent-kaur-16-en-18-oic acid is available from the authors. |
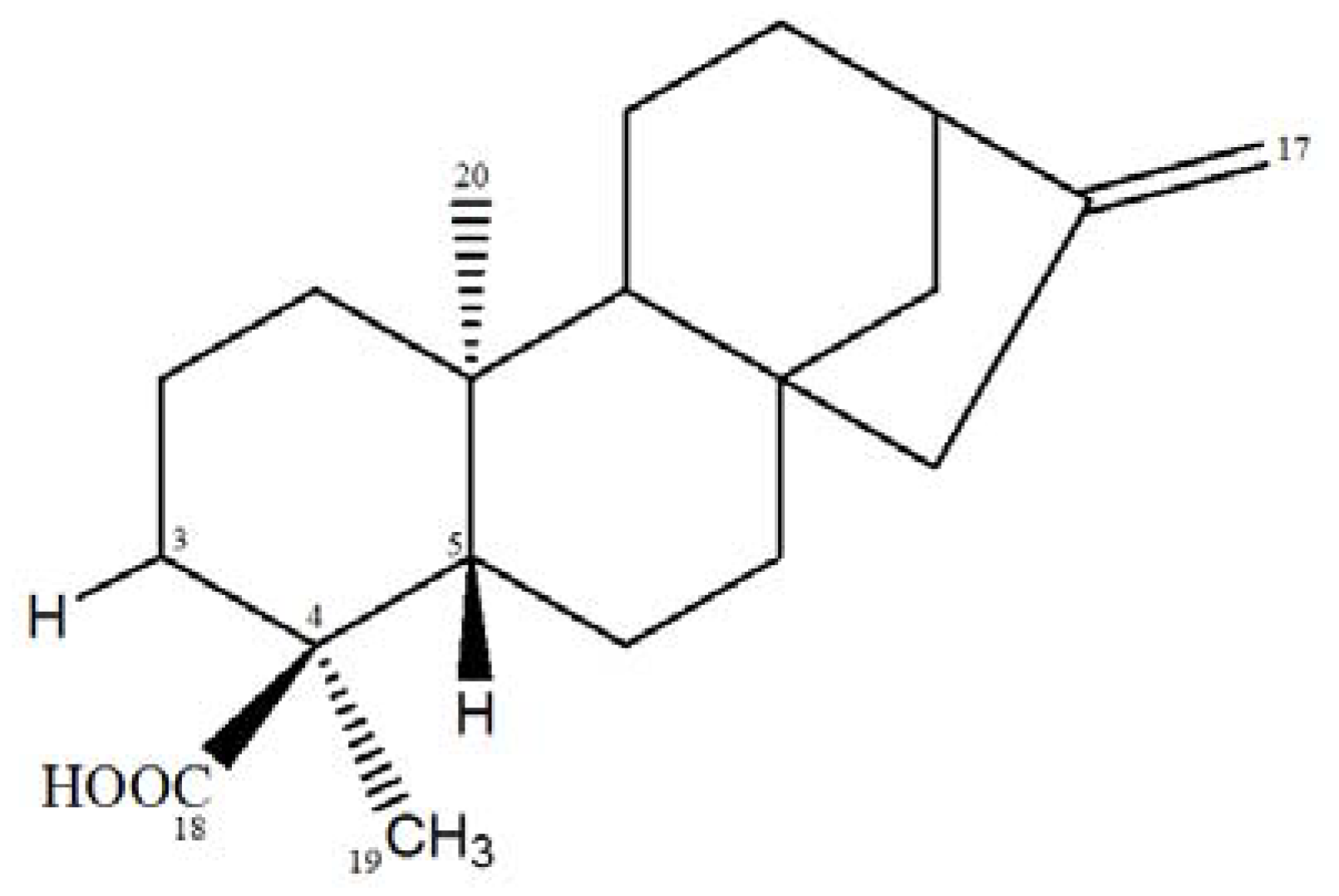
| C/H | δC | δH | 2JCH | 3JCH |
|---|---|---|---|---|
| C | ||||
| 4 | 47.9 | - | H3; H19 | H2; H6 |
| 8 | 44.7 | - | H7; H9; H14; H15 | H6; H11 |
| 10 | 39.0 | - | H1; H5; H9; H20 | H2; H6 |
| 16 | 156.1 | - | H15; H17 | H12 |
| 18 | 185.4 | - | - | H5; H19 |
| CH | ||||
| 5 | 50.6 | 1.64 a | H6 | H7; H19; H20 |
| 9 | 56.4 | 1.17 (br s) | H11 | H5; H7; H12; H14; H15; H20 |
| 13 | 44.3 | 2.65(s) | H12; H14 | H15; H17 |
| CH2 | ||||
| 1 | 40.2 | 1.10 a; 1.95 a | H2 | H3; H5; H9; H20 |
| 2 | 18.3 | 1.54 a | H1 | - |
| 3 | 37.3 | 1.54 a; 1.71 a | H2 | H1; H5; H19 |
| 6 | 23.6 | 1.16 (br s); 1.48 a | H5; H7 | - |
| 7 | 41.0 | 1.44 a; 1.59 a | H6 | H5; H9; H14 |
| 11 | 18.1 | 1.61 a | H9; H12 | - |
| 12 | 33.6 | 1.46 a; 1.59 a | H11 | H9; H14 |
| 14 | 39.9 | 0.86 a; 1.82 a | - | H7; H9; H12; H15 |
| 15 | 49.4 | 2.05 a | - | H7; H9: H14; H17 |
| 17 | 103.4 | 4.74 (s), 4.80 (s) | - | - |
| CH3 | ||||
| 19 | 18.2 | 1.06 (s) | - | H5 |
| 20 | 16.5 | 1.17 (br s) | - | H1; H5; H9 |
| Samples | Escherichia coli ATCC 25999 (mg/mL) | Escherichia coli from clinical isolates (mg/mL) | Staphylococcus aureus ATCC6538 (mg/mL) | Staphylococcus aureus from clinical isolates (mg/mL) |
| Crude extract CHCl3 | 2 | 2 | 2.5 | 2 |
| C1 | 2 | 2 | 2 | 2 |
| C2 | 2 | 2 | 2 | 2 |
| C3 | 2 | 2 | 2 | 2 |
| C4 | 2 | 2 | 1 | 2 |
| C5 | 2 | 2 | 2 | 2 |
| C6 | 2 | 2 | 2 | 2 |
| C7 | 2 | 2 | 2 | 2 |
| C8 | 2 | 2 | 2 | 2 |
| C9 | 2 | 2 | 2 | 2 |
| C10 | 2 | 2 | 2 | 2 |
| C11 | 2 | 2 | 2 | 2 |
| ent-kaur-16-en-18-oic acid * | 2 | 2 | 0.250 | 2 |
| kaurenoic acid | 1 | 2 | 0.125 | 2 |
| Gentamicin sulphate ** | 0.007 | 0.125 | 0.007 | 0.015 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, S.; Taleb-Contini, S.; Coppede, J.; Pereira, P.; Bertoni, B.; França, S.; Pereira, A.M. An ent-Kaurane-Type Diterpene in Croton antisyphiliticus Mart. Molecules 2012, 17, 8851-8858. https://doi.org/10.3390/molecules17088851
Pereira S, Taleb-Contini S, Coppede J, Pereira P, Bertoni B, França S, Pereira AM. An ent-Kaurane-Type Diterpene in Croton antisyphiliticus Mart. Molecules. 2012; 17(8):8851-8858. https://doi.org/10.3390/molecules17088851
Chicago/Turabian StylePereira, Sarazete, Silvia Taleb-Contini, Juliana Coppede, Paulo Pereira, Bianca Bertoni, Suzelei França, and Ana Maria Pereira. 2012. "An ent-Kaurane-Type Diterpene in Croton antisyphiliticus Mart." Molecules 17, no. 8: 8851-8858. https://doi.org/10.3390/molecules17088851
APA StylePereira, S., Taleb-Contini, S., Coppede, J., Pereira, P., Bertoni, B., França, S., & Pereira, A. M. (2012). An ent-Kaurane-Type Diterpene in Croton antisyphiliticus Mart. Molecules, 17(8), 8851-8858. https://doi.org/10.3390/molecules17088851




