Biological Activities of a-Pinene and β-Pinene Enantiomers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Minimal Inhibitory Concentration (MIC) of (+)-α-Pinene and (+)-β-Pinene Standards
| Microorganisms | MIC * (µg/mL) | |||||
|---|---|---|---|---|---|---|
| (+)-α-pinene | (−)-α-pinene | (+)-β-pinene | (−)-β-pinene | AMB | CIP | |
| C. albicans | 3,125 | na | 187 | na | 0.125 | - |
| C. neoformans | 117 | na | 234 | na | 0.125 | - |
| R. oryzae | 390 | na | 780 | na | 0.48 | - |
| MRSA | 4,150 | na | 6,250 | na | - | 0.5 |
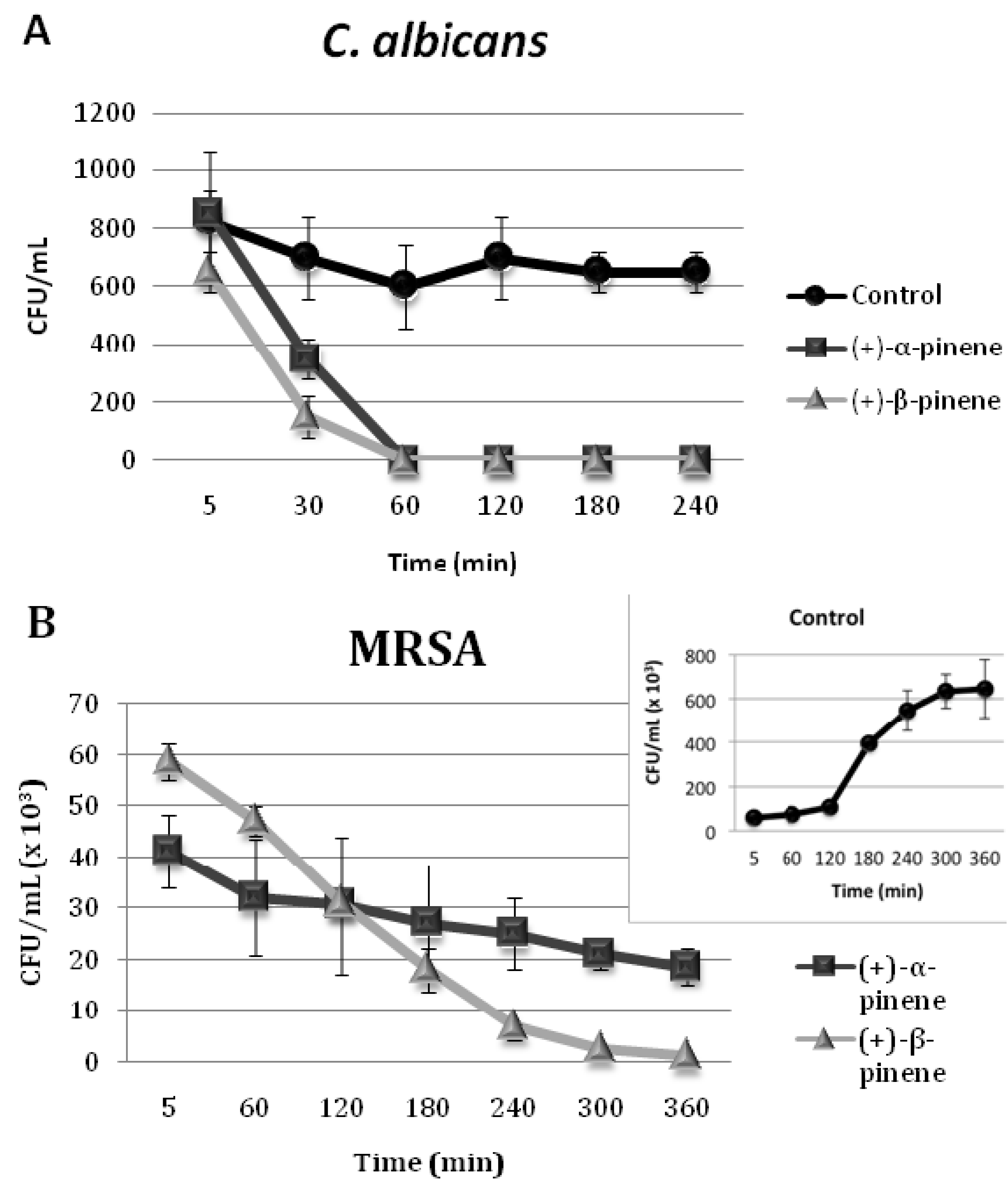
2.2. Time-Kill Curves
2.3. Synergistic Activity of (+)-α-Pinene and (+)-β-Pinene Standards with Commercial Antimicrobials against Microorganisms
| M.o. | (+)-α-pinene and AMB | (+)-α-Pinene and CIP | (+)-β-Pinene and AMB | (+)-β-Pinene and CIP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC in combination | FIC index | MIC in combination | FIC index | MIC in combination | FIC index | MIC in combination | FIC index | |||||
| α-pinene | AMB | α-pinene | CIP | β-pinene | AMB | β-pinene | CIP | |||||
| MRSA | - | - | - | 1037 | 0.003 | 0.256 (S) | - | - | - | 662 | 0.06 | 0.226 (S) |
| C. albicans | 390 | 0.06 | 0.62 (I) | - | - | - | 187 | 0.11 | 1.93 (I) | - | - | - |
| C. neoformans | 78.12 | 0.053 | 1.10 (I) | - | - | - | 78.12 | 0.11 | 1.29 (I) | - | - | - |
| R. oryzae | 390 | 0.026 | 1.05 (I) | - | - | - | 390 | 0.11 | 0.72 (I) | - | - | - |
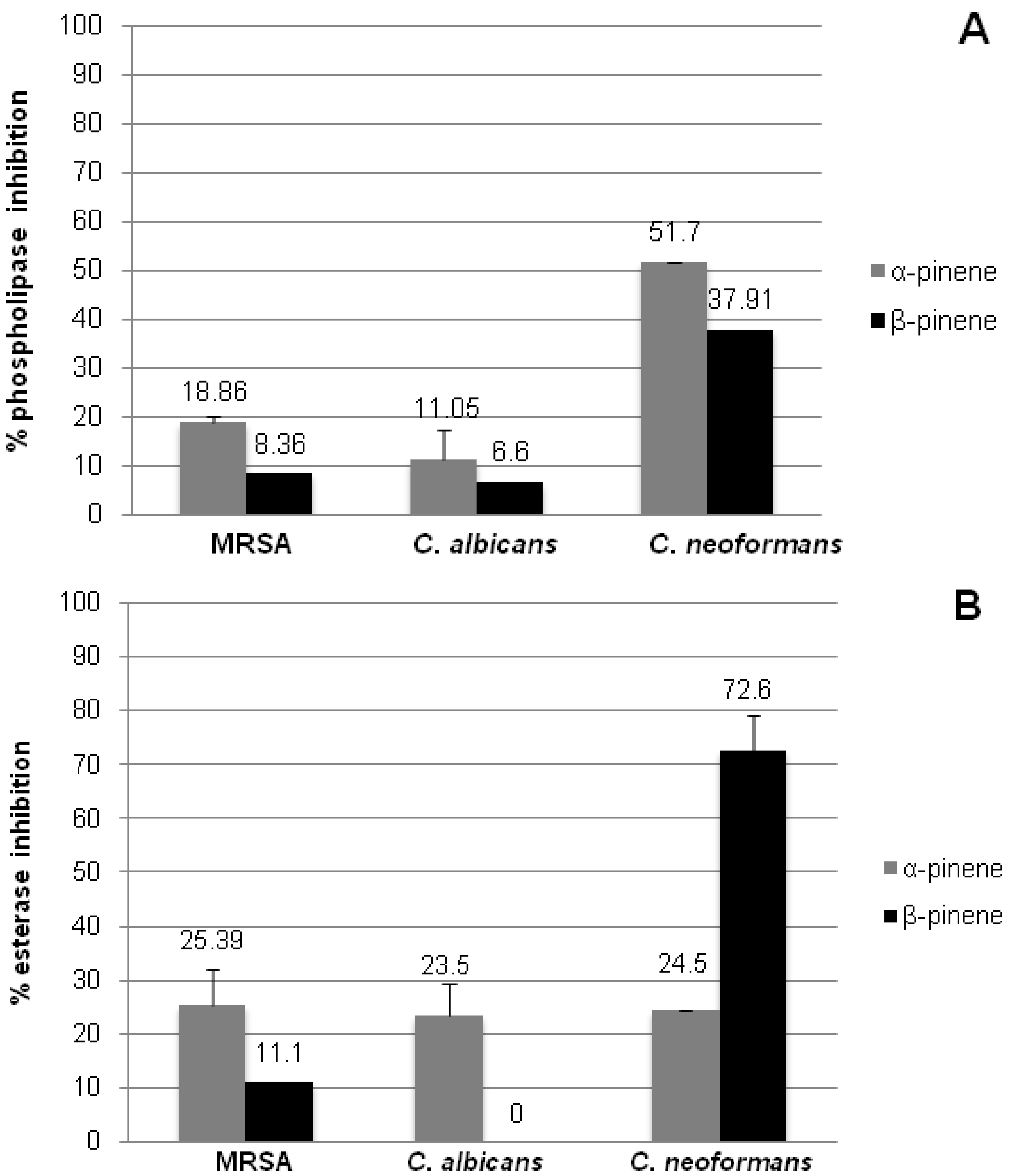
2.4. Inhibition of Microbial Phospholipase and Esterase Activities
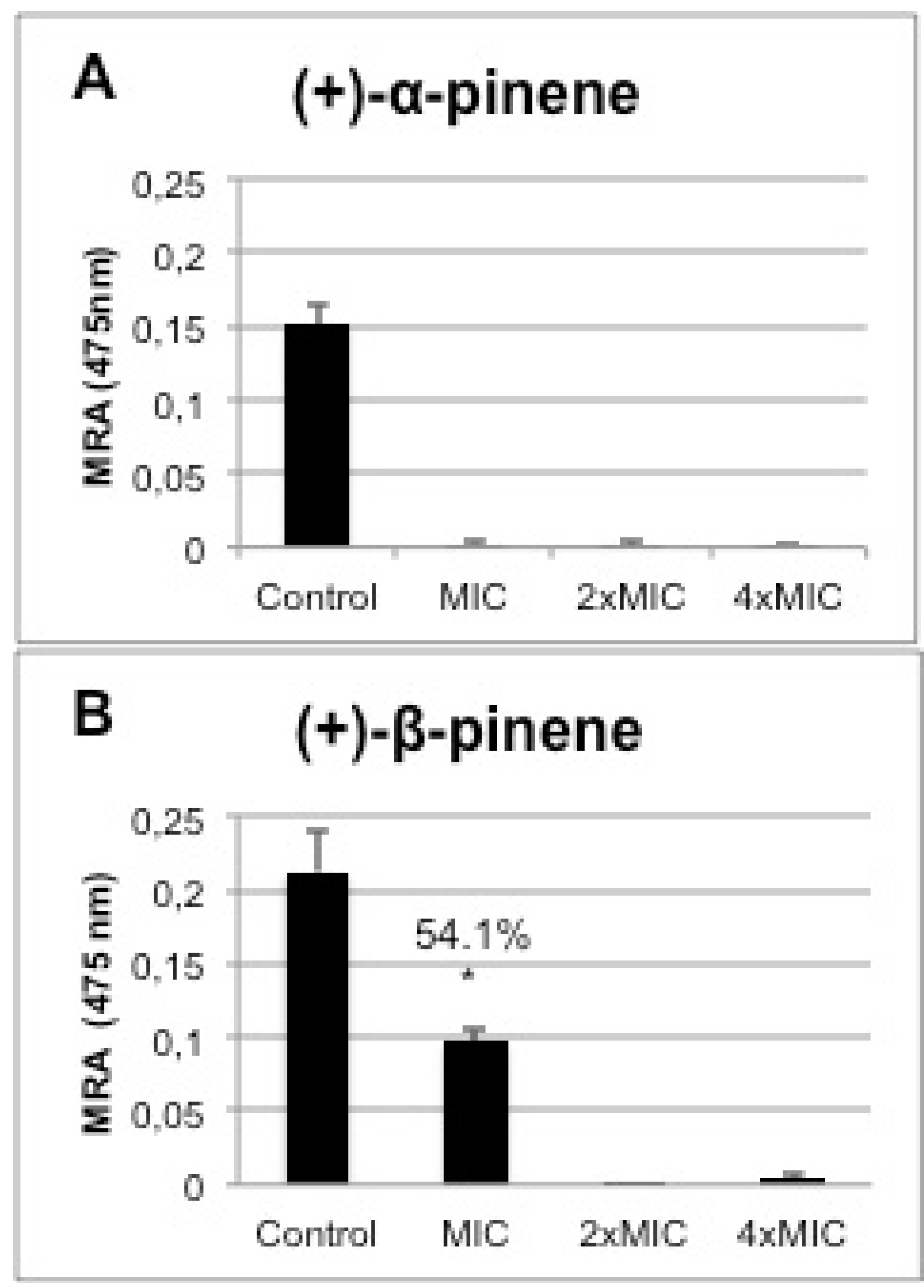
2.5. In vitro Biofilm Susceptibility Assay
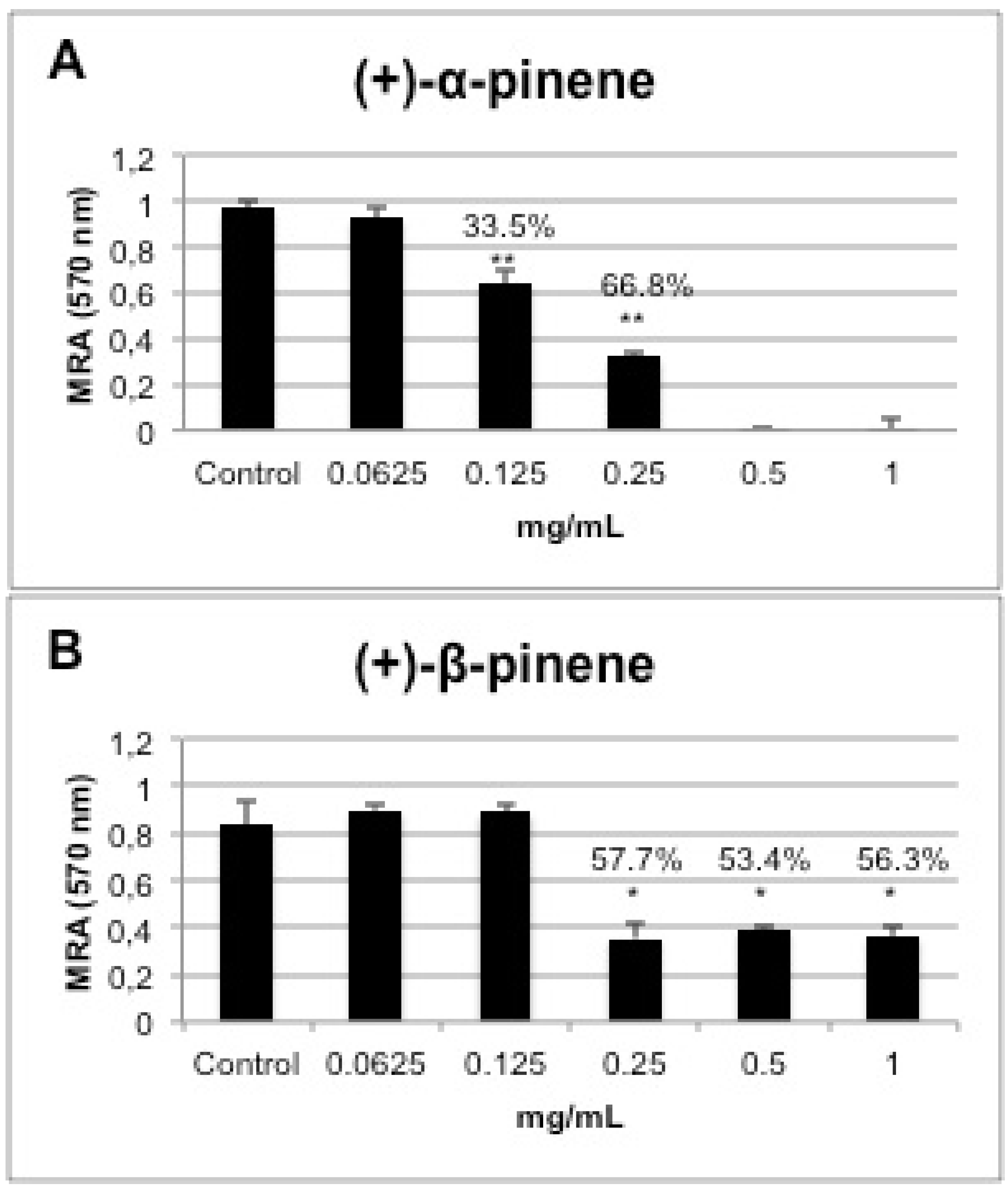
2.6. Cytotoxicity of the Positive Enantiomers of Pinene
3. Experimental
3.1. Chemicals and Microorganisms
3.2. Minimal Inhibitory Concentration (MIC) of Pinene Standards against Microorganisms
3.3. Time-Kill Curves
3.4. Synergistic Activity of Pinenes and Antimicrobial Drugs against Microorganisms
3.5. Inhibition of Microbial Phospholipase and Esterase
3.6. Inhibition of Biofilm Formation
3.7. Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
- Samples Availability: Not available.
References and Notes
- Koudou, J.; Abena, A.A.; Ngaissona, P.; Bessière, J.M. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia 2005, 76, 700–703. [Google Scholar] [CrossRef]
- Loizzoa, M.R.; Saabb, A.; Tundisa, R.; Stattia, G.A.; Lamprontic, I.; Menichinia, F.; Gambarid, R.; Cinatle, J.; Doer, H.W. Phytochemical analysis and in vitro evaluation of the biological activity against herpes simplex virus type 1 (HSV-1) of Cedrus libani A. Rich. Phytomedicine 2008, 15, 79–83. [Google Scholar] [CrossRef]
- M’Barek, L.A.; Ait Mouse, H.; Jaâfari, A.; Aboufatima, R.; Benharref, A.; Kamal, M.; Bénard, J.; El Abbadi, N.; Bensalah, M.; Gamouh, A.; et al. Cytotoxic effect of essential oil of thyme (Thymus broussonettii) on the IGR-OV1 tumor cells resistant to chemotherapy. Braz. J. Med. Biol. Res. 2007, 40, 1537–1544. [Google Scholar] [CrossRef]
- Zarai, Z.; Kadri, A.; Chobba, I.B.; Mansour, R.B.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 10, 161. [Google Scholar] [CrossRef]
- Alviano, D.S.; Alviano, C.S. Plant extracts: Search for new alternatives to treat microbial diseases. Curr. Pharm. Biotechnol. 2009, 10, 106–121. [Google Scholar] [CrossRef]
- Paiva, A.P.O. Fenómeno da quiralidade: Base da Esterioquímica. Quimica 2006, 56, 56–61. [Google Scholar]
- Özek, T.; Tabanca, N.; Demirci, F.; Wedge, D.E.; Baser, K.H.C. Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod. 2010, 4, 180–192. [Google Scholar]
- Martins, C.V.B.; de Resende, M.A.; da Silva, D.L.; Magalhães, T.F.F.; Modolo, L.V.; Pilli, R.A.; de Fátima, A. In vitro studies of anticandidal activity of goniothalamin enantiomers. J. Appl. Microbiol. 2009, 107, 1279–1286. [Google Scholar] [CrossRef]
- Solomons, T.W.G.; Fryhle, C.B. Química Organica, 9th ed; LTC: Rio de Janeiro, RJ, Brazil, 2009; pp. 188–190. [Google Scholar]
- Tabanca, N.; Demirci, B.; Crockett, S.L.; Baser, K.H.C.; Wedge, D.E. Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus essential oils. J. Agric. Food Chem. 2007, 55, 8430–8435. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, N.; Zu, Y.; Fu, Y. Comparative anti-infectious bronchitis virus (IBV) activity of (−)-pinene: Effect on nucleocapsid (N) protein. Molecules 2011, 16, 1044–1054. [Google Scholar] [CrossRef]
- Albuquerque, M.R.J.R.; Costa, S.M.O.; Bandeira, P.N.; Santiago, G.M.P.; Andrade-Neto, M.; Silveira, E.R.; Pessoa, O.D.L. Nematicidal and larvicidal activities of the essential oils from aerial parts of Pectis oligocephala and Pectis apodocephala Baker. Ann. Braz. Acad. Sci. 2007, 79, 209–213. [Google Scholar]
- Aligiannis, N.; Kalpoutzakis, E.; Chinou, I.B.; Mitakou, S. Composition and antibacterial activity of the essential oils of five taxa of Sideritis from Greece. J. Agric. Food Chem. 2001, 49, 811–815. [Google Scholar] [CrossRef]
- Couladis, M.; Chinou, B.; Tzakou, O.; Petrakis, P.V. Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. apollinis (Boiss. & Heldr.). Phytother. Res. 2003, 17, 152–154. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.O.; Souza, E.L.; Diniz, M.F.F.M.; Trajano, V.N.; Medeiros, I.A. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar]
- Angioni, A.; Barra, A.; Russo, A.T.; Coroneo, A.; Dessiä, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Zore, G.B; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of Biofilm Resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Mowat, E.; Butcher, J.; Lang, S.; Williams, C.; Ramage, G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J. Med. Microbiol. 2007, 56, 1205–1212. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI), Methods for Dilution Antimicrobial Susceptibility Tests. Approved Standards, M27-A3, M38-A2, M11-A6 e M7-A4, 4th ed; CLSI: Wayne, PA, USA, 2008.
- Price, M.F.; Wilkinson, I.D.; Gentry, L. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 15, 179–185. [Google Scholar]
- Schoofs, A.; Odds, F.C.; Colebunders, R.; Leven, M.; Goussens, H. Use of specialized isolation media for recognition and identification of Candida dubliniensis isolates from HIV infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 296–300. [Google Scholar] [CrossRef]
- Krom, B.P.; Cohen, J.B.; Feser, G.E.M.; Cihlar, R.L. Optimized candidal biofilm microtiter assay. J. Microbiol. Methods 2007, 6, 421–423. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silva, A.C.R.d.; Lopes, P.M.; Azevedo, M.M.B.d.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305-6316. https://doi.org/10.3390/molecules17066305
Silva ACRd, Lopes PM, Azevedo MMBd, Costa DCM, Alviano CS, Alviano DS. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules. 2012; 17(6):6305-6316. https://doi.org/10.3390/molecules17066305
Chicago/Turabian StyleSilva, Ana Cristina Rivas da, Paula Monteiro Lopes, Mariana Maria Barros de Azevedo, Danielle Cristina Machado Costa, Celuta Sales Alviano, and Daniela Sales Alviano. 2012. "Biological Activities of a-Pinene and β-Pinene Enantiomers" Molecules 17, no. 6: 6305-6316. https://doi.org/10.3390/molecules17066305
APA StyleSilva, A. C. R. d., Lopes, P. M., Azevedo, M. M. B. d., Costa, D. C. M., Alviano, C. S., & Alviano, D. S. (2012). Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules, 17(6), 6305-6316. https://doi.org/10.3390/molecules17066305




