Synthesis and Characterization of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole: A High Nitrogen Energetic Compound with Good Oxygen Balance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectral Studies of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole (4)
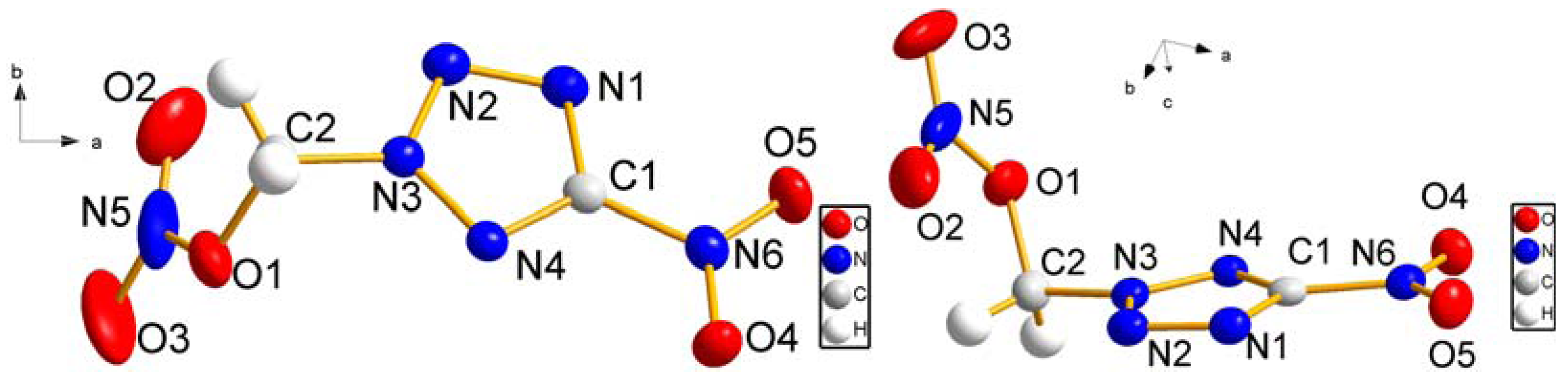
2.2. Crystal Structure Determination
| Empirical formula | C2H2N6O5 |
|---|---|
| Formula weight | 190.10 g·mol−1 |
| Temperature | 148(2) K |
| Wavelength | 0.71073 Ǻ |
| Crystal system | Orthorhombic |
| Space group | Pna2(1) |
| Unit cell dimensions | a = 2.1209(8) nm α = 90° |
| b = 0.52805(19) nm β = 90° | |
| c = 0.6246(2) nm γ = 90° | |
| Volume | 699.5(4) nm 3 |
| Z | 4 |
| Density calculated | 1.805 g·cm−3 |
| Absorption coefficient | 0.174 mm−1 |
| F(000) | 384 |
| Crystal size | 0.54 × 0.52 × 0.08 mm3 |
| Theta range for data collection | 3.262° to 29.103° |
| Limiting indices | −25 ≤ h ≤ 29, −7 ≤ k ≤ 7, −8 ≤ l ≤ 7 |
| Reflections collected / unique | 5552 / 1749 [R(int) = 0.0263] |
| Completeness to theta = 29.13 | 99.8% |
| Refinement method | Full-matrix least-squares on F2 |
| Goodness-of-fit on F2 | 0.998 |
| Final R indices (I > 2sigma(I)) | R1 = 0.0296, wR2 = 0.0594 |
| R indices (all data) | R1 = 0.0333 wR2 = 0.0609 |
| Largest diff. peak and hole | 0.205 and −0.133 e.A−3 |
| CCDC No. | 868752 |
| Atoms | Parameter | Atoms | Parameter |
|---|---|---|---|
| Distances | |||
| O(1)–C(2) | 1.407(19) | N(1)–C(1) | 1.338(18) |
| O(1)–N(5) | 1.448(2) | N(2)–N(3) | 1.333(17) |
| O(2)–N(5) | 1.198(2) | N(3)–N(4) | 1.324(16) |
| O(3)–N(5) | 1.193(2) | N(3)–C(2) | 1.460(18) |
| O(4)–N(6) | 1.226(16) | N(4)–C(1) | 1.316(17) |
| O(5)–N(6) | 1.222(16) | N(6)–C(1) | 1.446(19) |
| N(1)–N(2) | 1.310(17) | ||
| Angles | |||
| C(2)–O(1)–N(5) | 113.2(14) | O(2)–N(5)–O(1) | 117.6(15) |
| N(2)–N(1)–C(1) | 104.7(12) | O(5)–N(6)–O(4) | 125.6(12) |
| N(1)–N(2)–N(3) | 105.9(11) | O(5)–N(6)–C(1) | 117.0(12) |
| N(4)–N(3)–N(2) | 114.6(11) | O(4)–N(6)–C(1) | 117.4(11) |
| N(4)–N(3)–C(2) | 123.4(12) | N(4)–C(1)–N(1) | 115.4(13) |
| N (2)–N(3)–C(2) | 122.0(12) | N(4)–C(1)–N(6) | 122.8(13) |
| C(1)–N(4)–N(3) | 99.5(12) | N(1)–C(1)–N(6) | 121.8(12) |
| O(3)–N(5)–O(2) | 132.0(2) | O(1)–C(2)–N(3) | 110.6(12) |
| O(3)–N(5)–O(1) | 110.4(19) | ||
| Torsion | |||
| C(1)–N(1)–N(2)–N(3) | 0.29(14) | N(2)–N(1)–C(1)–N(4) | −0.40(17) |
| N(1)–N(2)–N(3)–N(4) | −0.12(16) | N(2)–N(1)–C(1)–N(6) | −178.11(12) |
| N(1)–N(2)–N(3)–C(2) | 176.60(12) | O(5)–N(6)–C(1)–N(4) | −179.64(13) |
| N(2)–N(3)–N(4)–C(1) | −0.10(15) | O(4)–N(6)–C(1)–N(4) | 1.10(2) |
| C(2)–N(3)–N(4)–C(1) | −176.78(13) | O(5)–N(6)–C(1)–N(1) | −2.10(2) |
| C(2)–O(1)–N(5)–O(3) | 177.24(15) | O(4)–N(6)–C(1)–N(1) | 178.60(13) |
| C(2)–O(1)–N(5)–O(2) | −2.20(2) | N(5)–O(1)–C(2)–N(3) | −85.49(15) |
| N(3)–N(4)–C(1)–N(1) | 0.31(16) | N(4)–N(3)–C(2)–O(1) | −68.73(18) |
| N(3)–N(4)–C(1)–N(6) | 177.99(12) | N(2)–N(3)–C(2)–O(1) | 114.84(15) |

2.3. Differential Scanning Calorimety (DSC)
2.4. Quantum chemistry Calculation
| Compound | HOF(kJ·mol−1) | ρ(g·cm−3) | D(km·s−1) | P(GPa) | Q(%, CO2) |
|---|---|---|---|---|---|
| 4 | +228.07 | 1.801 | 9.26 | 37.92 | 0 |
| TNT | −52.22 | 1.650 | 7.02 | 20.70 | −73.9 |
| RDX | −192 | 1.816 | 8.75 | 34.7 | −21.6 |
| HMX | −250 | 1.90 | 9.09 | 39.0 | −21.6 |
2.5. Impact Sensitivity Test (H50)
3. Experimental
3.1. General Methods
3.2. Synthesis of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole (4)
3.3. X-Ray Data Collection and Structure Refinement
3.4. Calculation Details
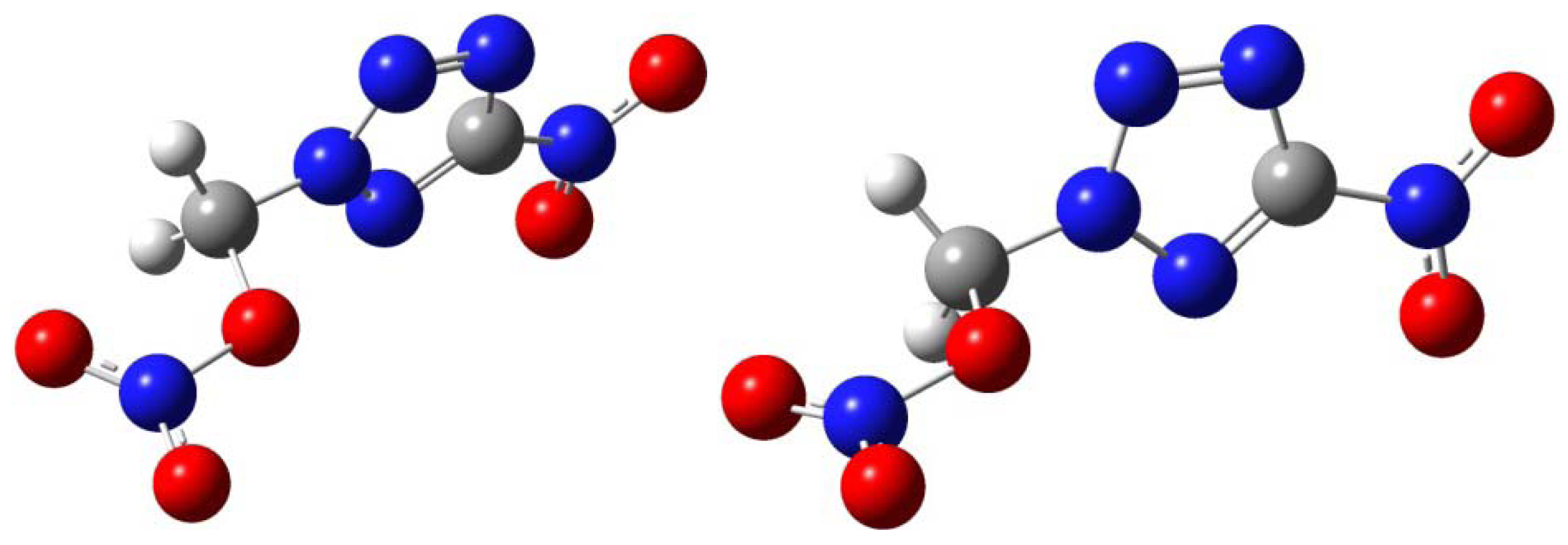
| X | Y | Z | |
|---|---|---|---|
| C | 0.183381 | −0.020822 | 0.059801 |
| N | −0.153487 | −0.039073 | 1.448934 |
| O | 0.540248 | 0.596306 | 2.232762 |
| O | −1.162338 | −0.708725 | 1.711098 |
| C | 1.733866 | 1.089352 | −2.791983 |
| H | 1.823865 | 0.462761 | −3.687781 |
| H | 2.716947 | 1.381967 | −2.404333 |
| O | 0.929433 | 2.262760 | −3.076652 |
| N | 1.487473 | 3.081719 | −4.109374 |
| O | 2.529516 | 2.704601 | −4.596656 |
| O | 0.820637 | 4.047085 | −4.341554 |
| N | −0.474628 | −0.813272 | −0.866547 |
| N | −0.004918 | −0.478340 | −2.132613 |
| N | 1.131773 | 0.679581 | −0.487432 |
| N | 1.036714 | 0.356866 | −1.785979 |
4. Conclusions
Acknowledgments
Supporting Information Available
References and Notes
- Hammerl, A.; Hiskey, M.A.; Holl, G.; Klapötke, T.M.; Polborn, K.; Stierstorfer, J.; Weigand, J.J. Azidoformamidimium and guanidinium 5,5'-azotetrazolate salts. Chem. Mater. 2005, 17, 3784–3793. [Google Scholar] [CrossRef]
- Li, Y.C. Study on Synthesis of Nitrogen-Rich Energetic Compounds (in Chinese). PhD Thesis, Beijing Institute of Technology, Beijing, China, 2009. [Google Scholar]
- Klapötke, T.M.; Krumm, B.; Martin, F.A.; Stierstorfer, J. New azidotetrazoles: Structurally interesting and extremely sensitive. Chem. Asian J. 2012, 7, 214–224. [Google Scholar]
- Harel, T.; Rozen, S. The tetrazole 3-N-oxide synthesis. J. Org. Chem. 2010, 75, 3141–3143. [Google Scholar]
- Klapötke, T.M.; Sabaté, C.M.; Rasp, M. Synthesis and properties of 5-nitrotetrazole derivatives as new energetic materials. J. Mater. Chem. 2009, 19, 2240–2252. [Google Scholar]
- Gao, H.X.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Bottaro, J.C.; Petrie, M.A; Penwell, P.E.; Dodge, A.L.; Malhotra, R. Nano/HEDM Technology: Late Stage Exploratory Effort; Report No. A466714; SRI International: Menlo Park, CA, USA, 2003. [Google Scholar]
- Klapötke, T.M.; Mayer, P.; Sabaté, C.M.; Welch, J.M.; Wiegand, N. Simple, nitrogen-rich, energetic salts of 5-nitrotetrazole. Inorg. Chem. 2008, 47, 6014–6027. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M.; Stierstorfer, J. Neutral 5-nitrotetrazoles: Easy initiation with low pollution. New J. Chem. 2009, 33, 136–147. [Google Scholar] [CrossRef]
- Koldobskii, G.I.; Soldatenko, D.S.; Gerasimova, E.S.; Khokhryakova, N.R.; Shcherbinin, M.B.; Lebedev, V.P.; Ostrovskii, V.A. Tetrazoles: XXXVI. Synthesis, structure, and properties of 5-nitrotetrazole. Russ. J. Org. Chem. 1997, 33, 1771–1783. [Google Scholar]
- Semenov, V.V.; Shevelev, S.A. Reactivity of the low-nucleophilic N-dinitromethyl carbanion center in polynitromethylazoles. Mendeleev Comm. 2010, 20, 332–334. [Google Scholar] [CrossRef]
- Semenov, V.V.; Shevelev, S.A.; Bruskin, A.B.; Kanishchev, M.I.; Baryshnikov, A.T. Mechanism of nitration of nitrogen-containing heterocyclic N-acetonyl derivatives. General approach to the synthesis of N-dinitromethylazoles. Russ. Chem. Bull. 2009, 58, 2077–2096. [Google Scholar] [CrossRef]
- Vasiliev, A.D.; Astachov, A.M.; Golubtsova, O.A.; Pekhotin, K.V.; Rogozin, M.V.; Kruglyakova, L.A.; Stepanov, R.S. 5-Nitro-2-nitromethyl-2H-1,2,3,4-tetrazole. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, C57, 1101–1102. [Google Scholar]
- Li, G.Q.; Li, Y.C.; Ma, Q.L.; Sun, C.H.; Pang, S.P. Research progress in synthesis of nitro-rich zole-ring compounds by cycloaddition reaction (in Chinese). Chin. J. Org. Chem. 2010, 30, 1431–1440. [Google Scholar]
- Sun, C.H.; Li, Y.C.; Li, Y.Y.; Li, G.Q.; Pang, S.P. Study on synthesis and reactivity of 5-nitro-2H-tetrazole and crystal structure of some products (in Chinese). Chin. J. Org. Chem. 2010, 30, 424–430. [Google Scholar]
- von Herz, E. C-Nitrotetrazole Compounds. US Patent 2066954, 1937. [Google Scholar]
- Li, Y.C.; Qi, C.; Sun, C.H.; Pang, S.P.; Zhao, X.Q. Synthesis and quantum chemical study on 2,4,6,8,10,12-hexaazaisowurtzitane (in Chinese). Chin. J. Energ. Mater. 2010, 18, 121–127. [Google Scholar]
- Lin, Q.H.; Li, Y.C.; Li, Y.Y.; Wang, Z.; Liu, W.; Qi, C.; Pang, S.P. Energetic salts based on 1-amino-1,2,3-triazole and 3-methyl-1-amino-1,2,3-triazole. J. Mater. Chem. 2012, 22, 666–674. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision F. 02; Gaussian, Inc.: Wallingford, CT, USA, 2006. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar]
- Hariharan, P.C.; Pople, J.A. Influence of polarization functions on molecular-orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; John Wiley and Sons: New York, NY, USA, 1985. [Google Scholar]
- Li, Y.C.; Qi, C.; Li, S.H.; Zhang, H.J.; Sun, C.H.; Yu, Y.Z.; Pang, S.P. 1,1'-Azobis-1,2,3-trizole: A high-nitrogen compound with stable N8 structure and photochromism. J. Am. Chem. Soc. 2010, 132, 12172–12173. [Google Scholar]
- Branham, M.L.; Singh, P.; Bisetty, K.; Sabela, M.; Govender, T. Preparation, spectrochemical, and computational analysis of L-carnosine (2-[(3-aminopropanoyl) amino]-3-(1H-imidazol-5-yl) propanoic acid) and its ruthenium (II) coordination complexes in aqueous solution. Molecules 2011, 16, 10269–10291. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Liu, W.; Pang, S. Synthesis and Characterization of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole: A High Nitrogen Energetic Compound with Good Oxygen Balance. Molecules 2012, 17, 5040-5049. https://doi.org/10.3390/molecules17055040
Li Y, Liu W, Pang S. Synthesis and Characterization of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole: A High Nitrogen Energetic Compound with Good Oxygen Balance. Molecules. 2012; 17(5):5040-5049. https://doi.org/10.3390/molecules17055040
Chicago/Turabian StyleLi, Yuchuan, Wei Liu, and Siping Pang. 2012. "Synthesis and Characterization of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole: A High Nitrogen Energetic Compound with Good Oxygen Balance" Molecules 17, no. 5: 5040-5049. https://doi.org/10.3390/molecules17055040
APA StyleLi, Y., Liu, W., & Pang, S. (2012). Synthesis and Characterization of 5-Nitro-2-nitratomethyl-1,2,3,4-tetrazole: A High Nitrogen Energetic Compound with Good Oxygen Balance. Molecules, 17(5), 5040-5049. https://doi.org/10.3390/molecules17055040




