Chemical Composition, Antimicrobial and Antitumor Activities of the Essential Oils and Crude Extracts of Euphorbia macrorrhiza
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
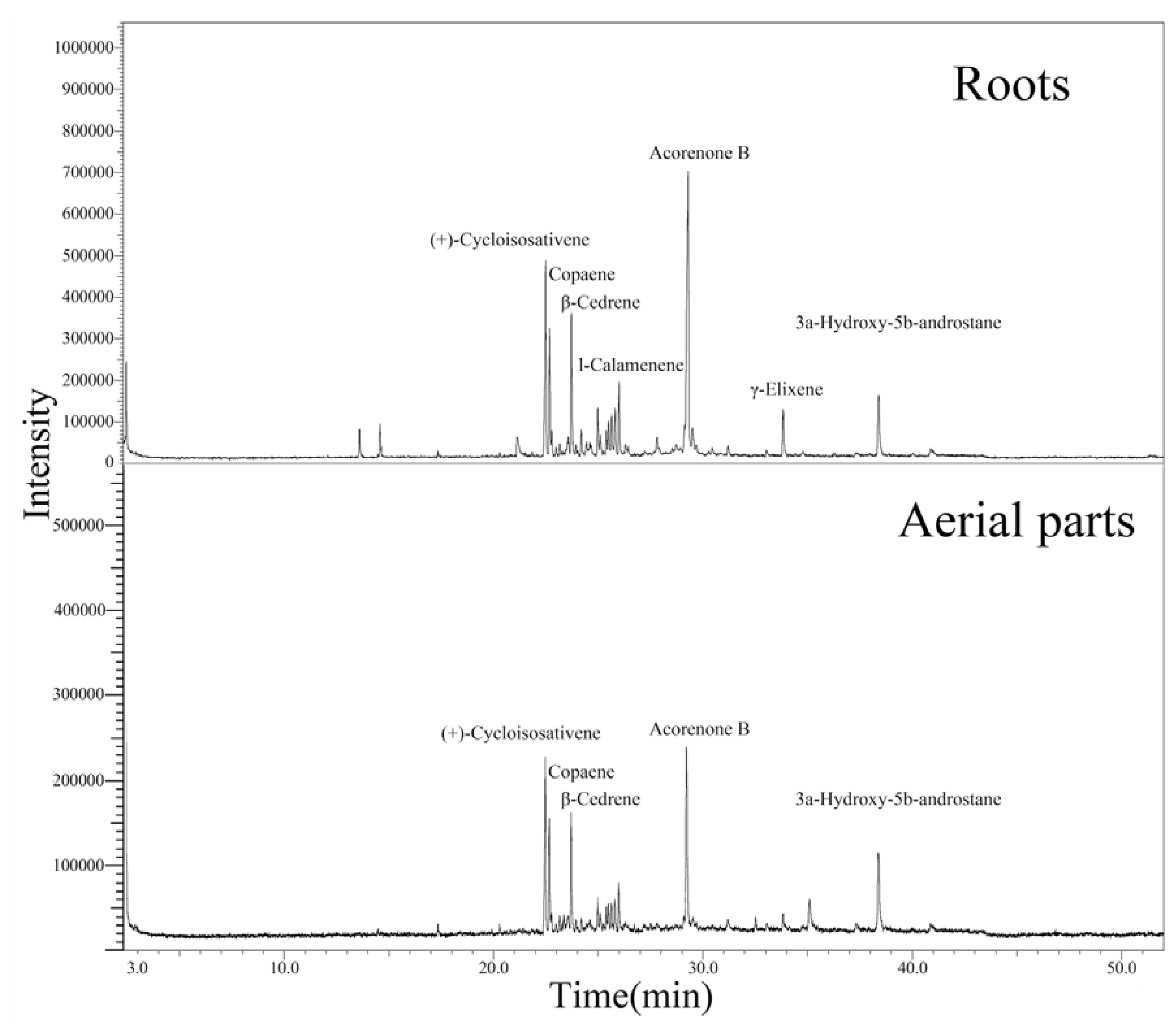
| No. | Compounds | RI | Peak area (%) | |
|---|---|---|---|---|
| aerial parts | roots | |||
| 1 | Thymene | 1025 | - | 1.49 |
| 2 | 3,7-Dimethyldecane | 1056 | - | 0.14 |
| 3 | γ-Terpinene | 1060 | - | 1.59 |
| 4 | Cyclohexyl(dimethoxy)methylsilane | 1160 | - | 0.36 |
| 5 | Nonane,5-(2-methylpropyl) | 1276 | 0.70 | - |
| 6 | Thymecamphor | 1310 | - | 2.70 |
| 7 | (+)-Cycloisosativene | 1369 | 14.94 | 12.40 |
| 8 | Copaene | 1377 | 7.37 | 6.29 |
| 9 | α-Cedrene | 1382 | 1.29 | 1.36 |
| 10 | Octadecyl chloride | 1399 | - | 0.73 |
| 11 | Valencene | 1407 | 0.93 | - |
| 12 | Zingiberene | 1416 | 1.46 | - |
| 13 | β-Cedrene | 1423 | 8.40 | 7.98 |
| 14 | Widdrene | 1433 | 0.89 | 0.47 |
| 15 | trans-Caryophyllene | 1444 | - | 1.54 |
| 16 | α-Guaiene | 1462 | - | 0.41 |
| 17 | Bicyclosesquiphellandrene | 1464 | - | 0.26 |
| 18 | β-Chamigrene | 1478 | 2.41 | 2.68 |
| 19 | Curcumene | 1483 | 1.58 | 1.30 |
| 20 | Pentadecane | 1496 | 1.58 | 1.38 |
| 21 | (−)-α-Muurolene | 1501 | 1.98 | 1.54 |
| 22 | Cuparene | 1507 | 1.98 | 1.83 |
| 23 | γ-Cadinene | 1515 | 2.89 | 3.03 |
| 24 | l-Calamenene | 1524 | 4.13 | 4.65 |
| 25 | a-Cadinene | 1538 | - | 0.68 |
| 26 | Calacorene | 1545 | - | 0.50 |
| 27 | Humulane-1,6-dien-3-ol | 1609 | - | 0.94 |
| 28 | Tricyclo[4.4.0.0(2,7)]dec-3-ene-3- | 1653 | - | 0.59 |
| methanol, 1-methyl-8-(1-methylethyl) - | ||||
| 29 | (Z)-3-Heptadecene | 1674 | - | 1.96 |
| 30 | Acorenone B | 1681 | 16.72 | 25.8 |
| 31 | 1,3,7,7-Tetramethyl-2-oxa-bicyclo(4.4.0)-dec-5-en-4-one | 1691 | - | 1.66 |
| 32 | Naphthalene, 1,2,3,4-tetrahydro- 1-methyl-8-(1-methylethyl)- | 1776 | - | 0.87 |
| 33 | Hexahydrofarnesyl acetone | 1841 | 1.35 | - |
| 34 | γ-Elixene | 1905 | 1.81 | 3.67 |
| 35 | Palmitinic acid | 1971 | 5.68 | - |
| 36 | 3a-Hydroxy-5b-androstane | 2146 | 10.62 | 5.52 |
| Total | 88.71 | 97.80 | ||
2.2. Antimicrobial and Antitumor Activities
| Concentration (μg/mL) | Inhibition ratio (%) | IC50 (μg/mL) | |
|---|---|---|---|
| Aerial parts | |||
| EO | 250 | 96.12 | 78.32 |
| HAF | 250 | 83.57 | - |
| CHF | 250 | 68.22 | - |
| EAF | 250 | 4.61 | - |
| BAF | 250 | - | - |
| RMF | 250 | - | - |
| Roots | |||
| EO | 250 | 96.32 | 11.86 |
| HAF | 250 | 72.52 | - |
| CHF | 250 | 82.52 | - |
| EAF | 250 | 2.32 | - |
| BAF | 250 | - | - |
| RMF | 250 | - | - |
| Staphyloccocus aureus | Escherichia coli | Candidaalbicans | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MFC | |
| Aerial parts | ||||||
| EO | 5.6 | 22.0 | >20.0 | >20.0 | >20.0 | >20.0 |
| HAF | 1000 | 2000 | >2000 | >2000 | >2000 | >2000 |
| CHF | 500 | 1000 | >2000 | >2000 | >2000 | >2000 |
| EAF | 1000 | 1000 | >2000 | >2000 | >2000 | >2000 |
| BAF | 2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| RMF | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Roots | ||||||
| EO | 2.8 | 5.6 | >20.0 | >20.0 | >20.0 | >20.0 |
| HAF | 1000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| CHF | 500 | 2000 | >2000 | >2000 | >2000 | >2000 |
| EAF | 500 | 1000 | >2000 | >2000 | >2000 | >2000 |
| BAF | 2000 | 2000 | >2000 | >2000 | >2000 | >2000 |
| RMF | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Ampicillin | 0.25 | 2.5 | >20.0 | >20.0 | n.t. | n.t. |
| Amphotericin B | n.t. | n.t. | n.t. | n.t. | 0.25 | 0.25 |
3. Experimental
3.1. Plant Materials
3.2. Extraction of Essential Oils
3.3. Preparation of Crude Extracts
3.4. GC-MS/MS Analysis
3.5. Antitumor Activity
3.6. Antimicrobial Activity
4. Conclusions
Acknowledgments
References and Notes
- Sahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Webester, G. Annals of the Missouri Botanical Garden: Systematic of the Euphorbiaceae; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; Volume 81, pp. 33–144. [Google Scholar]
- Singla, A.K.; Pathak, K. Phytoconstituents of Euphorbia species. Fitoterapia 1990, 61, 483–516. [Google Scholar]
- Mazoir, N.; Benharref, A.; Bailén, M.; Reina, M.; González-Coloma, A. Bioactive triterpene derivatives from latex of two Euphorbia species. Phytochemistry 2008, 69, 1328–1338. [Google Scholar]
- Fernandez-Arche, A.; Saenz, M.T.; Arroyo, M.; de la Puerta, R.; Garcia, M.D. Topical anti-inflammatory effect of tirucallol, a triterpene isolated from Euphorbia lactea latex. Phytomedicine 2010, 17, 146–148. [Google Scholar] [CrossRef]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Kassem, S.M.I.; Doe, M.; Baba, M.; Nakatani, M. Diterpenoids from Euphorbia paralias. Phytochemistry 2001, 58, 1135–1139. [Google Scholar]
- Ferreira, A.M.V.D.; Carvalho, L.H.M.; Carvalho, M.J.M.; Sequeira, M.M.; Silva, A.M.S. Jatrophane and lathyrane diterpenoids from Euphorbia hyberna L. Phytochemistry 2002, 61, 373–377. [Google Scholar]
- Haba, H.; Lavaud, C.; Harkat, H.; Alabdul Magid, A.; Marcourt, L.; Benkhaled, M. Diterpenoids and triterpenoids from Euphorbia guyoniana. Phytochemistry 2007, 68, 1255–1260. [Google Scholar]
- Ayatollahi, A.M.; Ghanadian, M.; Afsharypuor, S.; Choudhary, M.I.; Kobarfard, F.; Rahmati, M. Two new lathyrane type diterpenoids from Euphorbia aellenii. Fitoterapia 2010, 81, 891–893. [Google Scholar] [CrossRef]
- Bagci, E.; Kocak, A. Essential oil composition of two endemic Tanacetum (T. nitens (Boiss.& Noe) Grierson and T. argenteum (Lam.) Willd. subsp. argenteum) (Asteraceae) taxa, growing wild in Turkey. Ind. Crop. Prod. 2010, 31, 542–545. [Google Scholar] [CrossRef]
- Huang, Y.; Aisa, H.A. Jatrophane diterpenoids from fructus Euphorbia sororia. Phytochem. Lett. 2010, 3, 176–180. [Google Scholar] [CrossRef]
- Song, Z.-Q.; Mu, S.-Z.; Di, Y.-T.; Hao, X.-J. A new jatrophane diterpenoid from Euphorbia peplus. Chin. J. Nat. Med. 2010, 8, 81–83. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Chianese, G.; Zolfaghari, B.; Sajjadi, S.-E.; Appendino, G.; Taglialatela-Scafati, O. Macrocyclic diterpenoids from the Iranian plant Euphorbia bungei Boiss. Fitoterapia 2011, 82, 317–322. [Google Scholar] [CrossRef]
- Valente, C.; Pedro, M.; Duarte, A.; Nascimento, M.S.J.; Abreu, P.M.; Ferreira, M.-J.U. Bioactive diterpenoids, a new jatrophane and two ent-abietanes, and other constituents from Euphorbia pubescens. J. Nat. Prod. 2004, 67, 902–904. [Google Scholar] [CrossRef]
- Hohmann, J.; Rédei, D.; Forgo, P.; Molnár, J.; Dombi, G.; Zorig, T. Jatrophane diterpenoids from Euphorbia mongolica as modulators of the multidrug resistance of L5128 mouse lymphoma cells. J. Nat. Prod. 2003, 66, 976–979. [Google Scholar] [CrossRef]
- Sutthivaiyakit, S.; Thapsut, M.; Prachayasittikul, V. Constituents and bioactivity of the tubers of Euphorbia sessiliflora. Phytochemistry 2000, 53, 947–950. [Google Scholar]
- Natarajan, D.; Britto, S.J.; Srinivasan, K.; Nagamurugan, N.; Mohanasundari, C.; Perumal, G. Anti-bacterial activity of Euphorbia fusiformis—A rare medicinal herb. J. Ethnopharmacol. 2005, 102, 123–126. [Google Scholar] [CrossRef]
- Sudhakar, M.; Rao, C.V.; Rao, P.M.; Raju, D.B.; Venkateswarlu, Y. Antimicrobial activity of Caesalpinia pulcherrima, Euphorbia hirta and Asystasia gangeticum. Fitoterapia 2006, 77, 378–380. [Google Scholar] [CrossRef]
- Gören, N.; Jakupovic, J.; Topal, Ş. Sesquiterpene lactones with antibacterial activity from Tanacetum argyrophyllum var. argyrophyllum. Phytochemistry 1990, 29, 1467–1469. [Google Scholar]
- Claeson, P.; Rådström, P.; Sköld, O.; Nilsson, Å.; Höglund, S. Bactericidal effect of the sesquiterpene T-cadinol on Staphylococcus aureus. Phytother. Res. 1992, 6, 94–98. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, J.; Dou, J.; Xu, J.; Aisa, H.A. Chemical Composition, Antimicrobial and Antitumor Activities of the Essential Oils and Crude Extracts of Euphorbia macrorrhiza. Molecules 2012, 17, 5030-5039. https://doi.org/10.3390/molecules17055030
Lin J, Dou J, Xu J, Aisa HA. Chemical Composition, Antimicrobial and Antitumor Activities of the Essential Oils and Crude Extracts of Euphorbia macrorrhiza. Molecules. 2012; 17(5):5030-5039. https://doi.org/10.3390/molecules17055030
Chicago/Turabian StyleLin, Jianbo, Jun Dou, Jiangling Xu, and Haji Akber Aisa. 2012. "Chemical Composition, Antimicrobial and Antitumor Activities of the Essential Oils and Crude Extracts of Euphorbia macrorrhiza" Molecules 17, no. 5: 5030-5039. https://doi.org/10.3390/molecules17055030
APA StyleLin, J., Dou, J., Xu, J., & Aisa, H. A. (2012). Chemical Composition, Antimicrobial and Antitumor Activities of the Essential Oils and Crude Extracts of Euphorbia macrorrhiza. Molecules, 17(5), 5030-5039. https://doi.org/10.3390/molecules17055030







