GHGKHKNK Octapeptide (P-5m) Inhibits Metastasis of HCCLM3 Cell Lines via Regulation of MMP-2 Expression in in Vitro and in Vivo Studies
Abstract
:1. Introduction
2. Results and Discussion
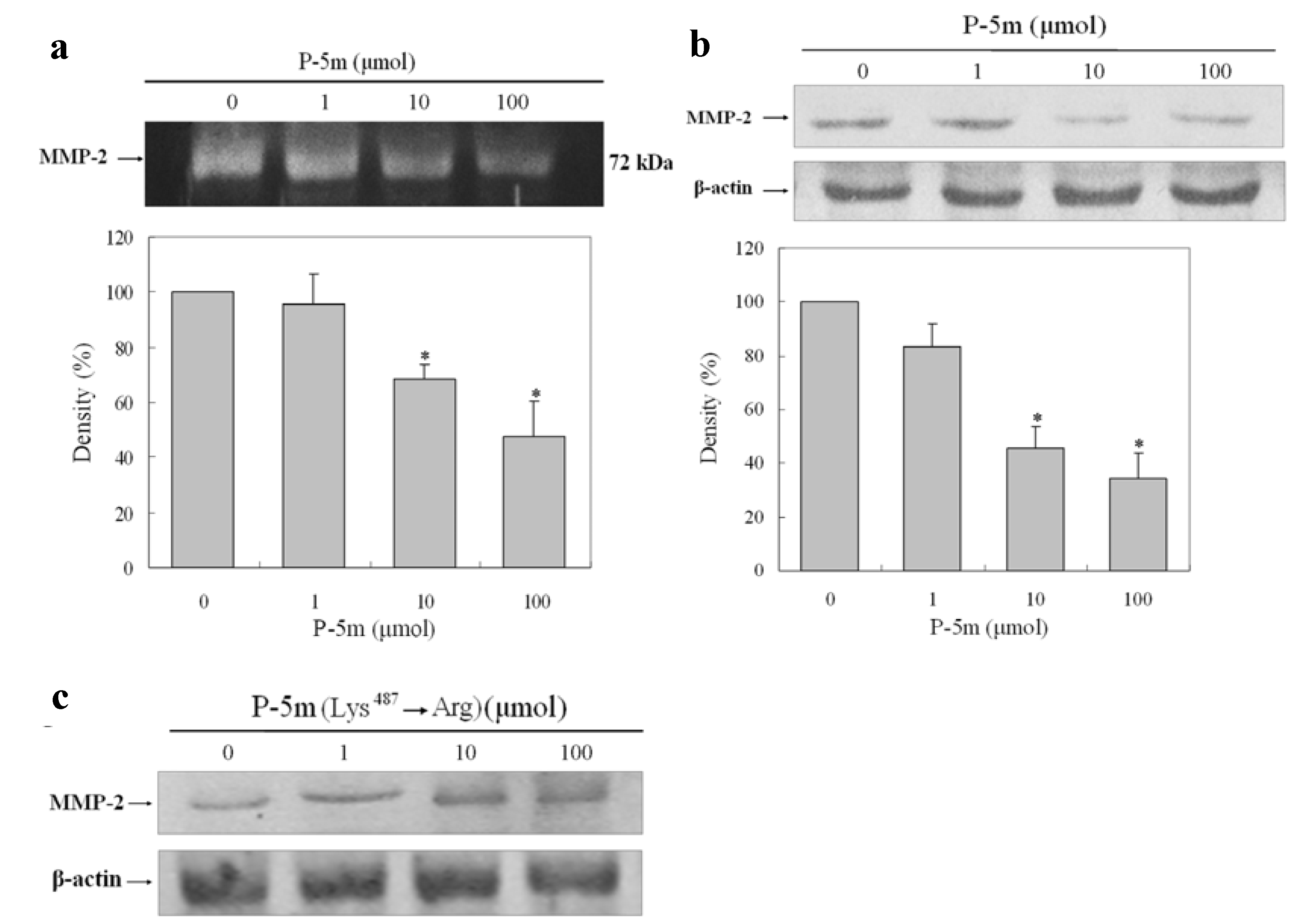
2.1. Effect of P-5m on the Activation of MMP-2 in HCCLM3 Cells
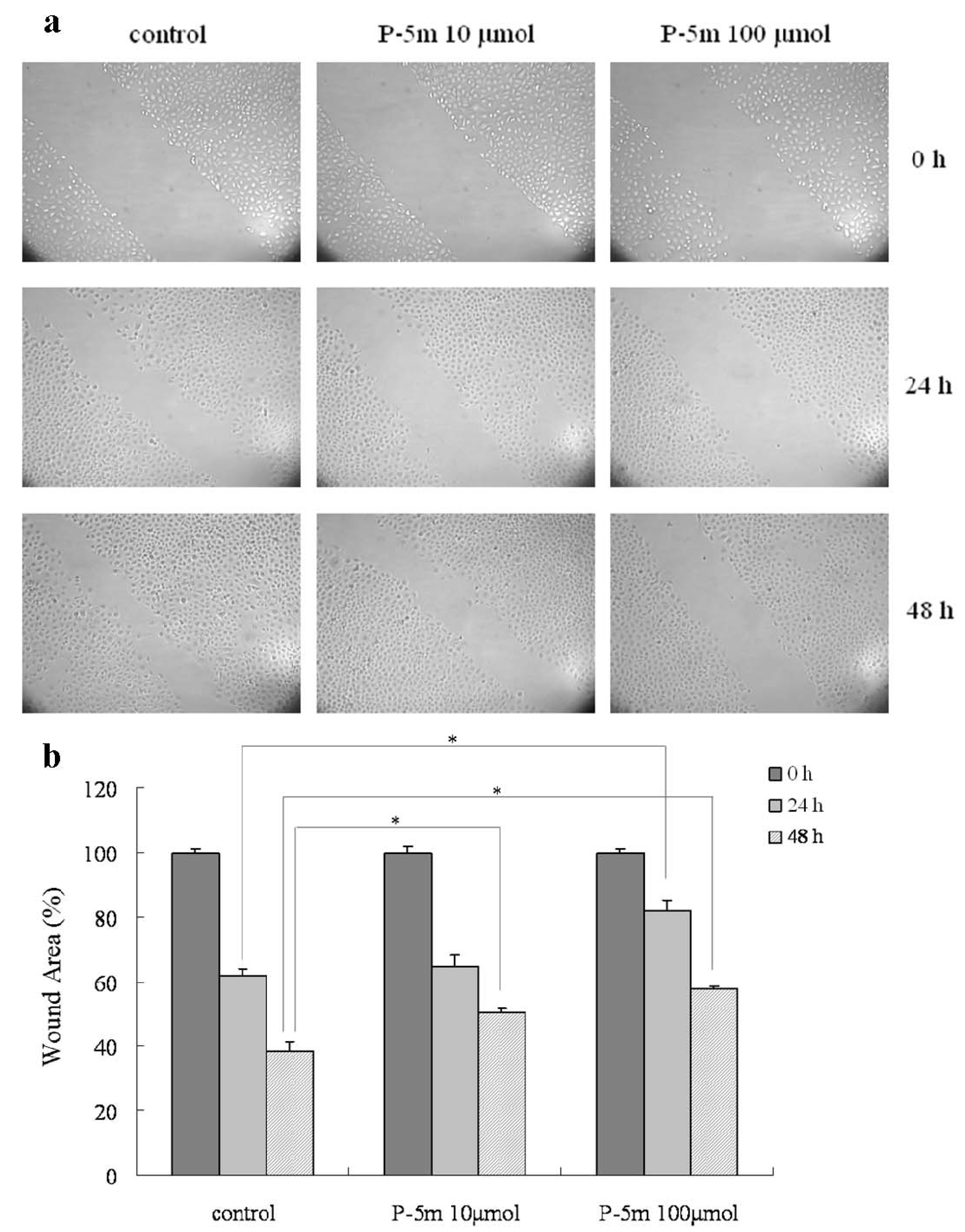
2.2. P-5m Inhibits the Migration and Invasion of HCCLM3 Cells

2.3. P-5m Suppresses Pulmonary Metastasis of Hepatocellular Carcinoma in Vivo
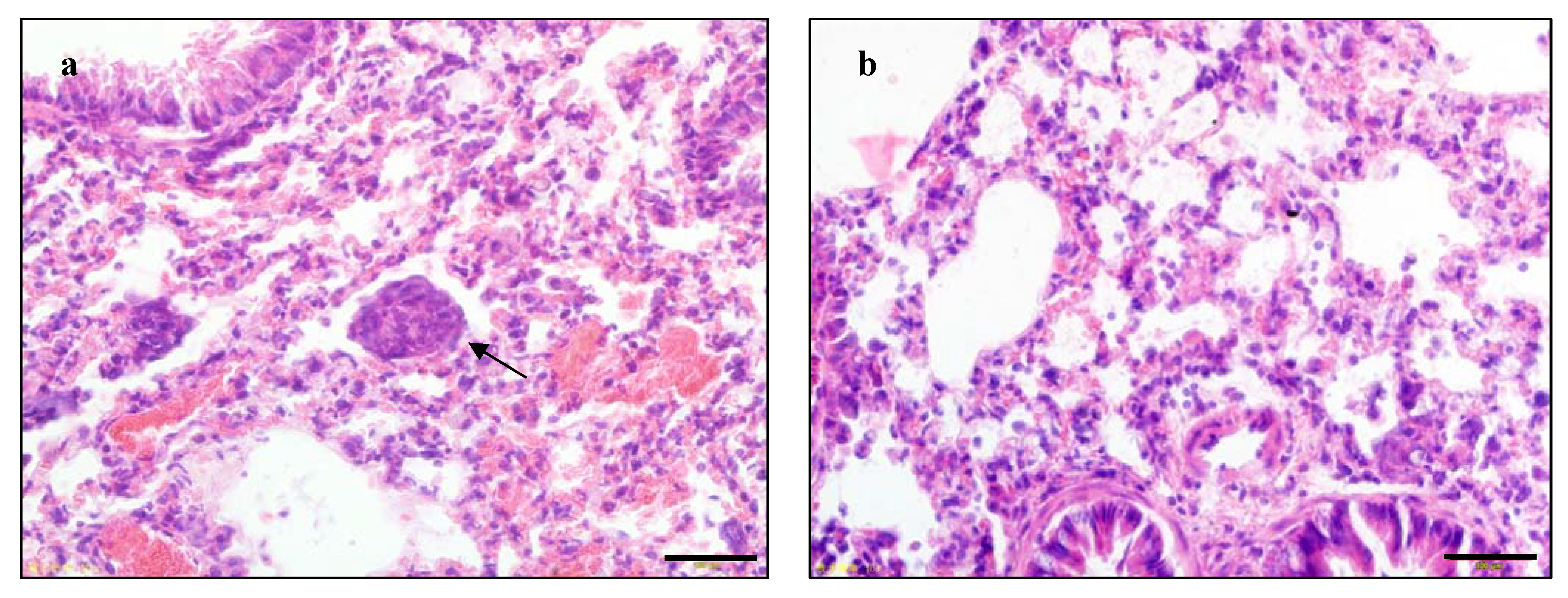
| Treatment group | Mice-bearing pulmonary metastasis/total mice | Percentage |
|---|---|---|
| Control | 6/8 | 75% |
| P-5m 50μg/kg | 2/8 | 25% |
| P-5m 500μg/kg | 4/8 | 50% |
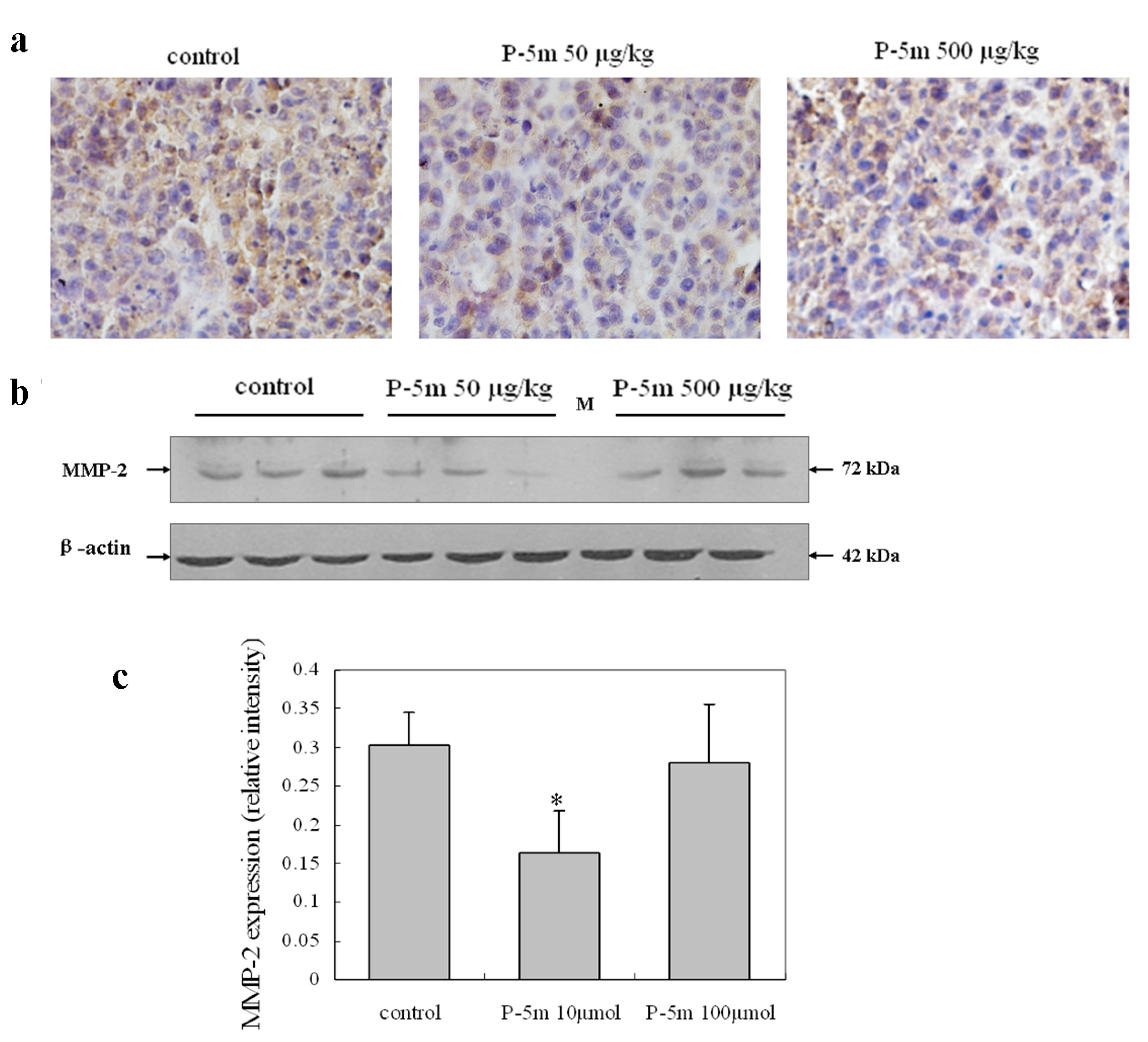
2.4. P-5m Inhibits MMP-2 Expression in Orthotopic Hepatoma Animal Model
2.5. Regulation of Tumor-Related Genes in Vivo by Treatment with P-5m
| Name of gene | Symbol | GenBank ID | Primer | Regulation fold | |
|---|---|---|---|---|---|
| Proteinases involved in the degradation of ECM | |||||
| Matrix metallopeptidase 2 | MMP-2 | NM_001127891.1 | 5'-ATTTGGCGGACTGTGACG-3' | 0.67 | |
| 5'-AGCCTTCTCCTCCTGTGGG-3' | |||||
| TIMP metallopeptidase inhibitor 1 | TIMP-1 | NM_003254.2 | 5'-CCGCAGCGAGGAGTTTCT-3' | 3.17 | |
| 5'-ACAGCCAACAGTGTAGGTCTT-3' | |||||
| TIMP metallopeptidase inhibitor 2 | TIMP-2 | NM_003255.4 | 5'-CTGGACGTTGGAGGAAAGAA-3' | 3.84 | |
| 5'-CTTCTTCTGGGTGGTGCTCA-3' | |||||
| Adhesion molecules | |||||
| CD44 molecule (Indian blood group) | CD44 | NM_000610 | 5'-ATCTTGGCATCCCTCTTGG-3' | 1.42 | |
| 5'-GTCCACTTGGCTTTCTGTCC-3' | |||||
| Cadherin 1, type 1, E-cadherin (epithelial) | CDH-1 | NM_004360.3 | GAATCCAAAGCCTCAGGTCA-3' | 2.11 | |
| 5'-TCAAAATGATAGATTCTTGGGTT-3' | |||||
| Vascular cell adhesion molecule 1 | VCAM-1 | NM_001078.3 | 5'-TGCCGAGCTAAATTACACATTG-3' | 0.91 | |
| 5'-ACAGAGCCACCTTCTTGCAG-3' | |||||
| Metastasis-related markers | |||||
| Non-metastatic cells 1, protein (NM23A) expressed in (NME1) | NM23 | NM_198175 | 5'-GGATTCCGCCTTGTTGGT-3' | 2.17 | |
| 5'-GGCCCTGAGTGCATGTATTT-3' | |||||
| Metastasis associated 1 | MTA-1 | NM_004689.3 | 5'-AGACCCTGCTGGCAGATAAAG-3' | 0.73 | |
| 5'-GCTTGTCTGTGAGTGGGTTGT-3' | |||||
| Metastasis suppressor 1 | MTSS-1 | NM_014751.4 | 5'-GAAGAACGTGGCCGATTC-3' | 3.74 | |
| 5'-GAGGGCAGTTTGTGAGGGTC-3' | |||||
| Apoptosis-related factors | |||||
| Cysteine protease CPP32 isoform alpha | CASP-3 | NM_004346 | 5'-CTGGTTTTCGGTGGGTGT-3' | 3.44 | |
| 5'-CAGTGTTCTCCATGGATACCTTT-3' | |||||
| B-cell CLL/lymphoma 2 | BCL-2 | NM_000633.2 | 5'-TGTCCCTTTGACCTTGTTTCT-3' | 1.27 | |
| 5'-TCATTTGCCATCTGGATTTT-3' | |||||
| Cell cycle regulators | |||||
| Cyclin-dependent kinase 4 | CDK-4 | NM_000075 | 5'-ACCTGTGGACATGTGGAGTG-3' | 0.94 | |
| 5'-ACATCTCGAGGCCAGTCATC-3' | |||||
| Cyclin D1 | CCND-1 | NM_053056 | 5'-TGTCCTACTACCGCCTCACAC-3' | 1.25 | |
| 5'-TTGGGGTCCATGTTCTGC-3' | |||||
| Cyclin-dependent kinase inhibitor 1A (p21) | CDKN-1A | NM_000389 | 5'-GGCAGACCAGCATGACAGAT-3' | 1.13 | |
| 5'-AGATGTAGAGCGGGCCTTTG-3' | |||||
| Cyclin-dependent kinase inhibitor 2A ( p16) | CDKN2A | NM_000077.4 | 5'-CATTCATGTGGGCATTTCTTG-3' | 1.94 | |
| 5'-TTTGGTTCTGCCATTTGCTA-3' | |||||
| Immune-regulating factors | |||||
| Tumor necrosis factor alpha | TNF-α | NM_000594 | 5'-CAGCCTCTTCTCCTTCCTGAT-3' | 2.12 | |
| 5'-GCCAGAGGGCTGATTAGAGA-3' | |||||
| Interleukin 6 | IL-6 | NM_000600 | 5'-GATGAGTACAAAAGTCCTGATCC-3' | 13.6 | |
| 5'-CTGCAGCCACTGGTTCTGT-3' | |||||
| Nitric oxide synthase 2, inducible | NOS-2A | NM_000625 | 5'-TGCCAAGCTGAAATTGAATG-3' | 1.33 | |
| 5'-CCACCCTGTCCTTCTTCG-3' | |||||
| Macrophage stimulating 1 receptor | MST-1R | NM_002447.2 | 5'-CTACATCAACTCCCACATCACC-3' | 2.19 | |
| 5'-ATTCAGGAAGGCGGCACA-3' | |||||
| Interferon gamma | IFNG | NM_000619.2 | 5'-CCAACGCAAAGCAATACATG-3' | 2.86 | |
| 5'-GCAGGACAACCATTACTGGG-3' | |||||
| Major histocompatibility complex, class I, E | HLA-E | NM_005516.5 | 5'-GCGCGGCTACTACAATCAGA-3' | 2.44 | |
| 5'-GGGTGAGATAATCCTTGCCG-3' | |||||
| Others | |||||
| Vascular endothelial growth factor A | VEGFA | NM_001025366 | 5'-GGCAGAATCATCACGAAGTG-3' | 1.45 | |
| 5'-CGATCTCATCAGGGTACTCCT-3' | |||||
| Transforming growth factor, beta 1 | TGF-β1 | NM_000660 | 5'-GCACGTGGAGCTGTACCA-3' | 3.43 | |
| 5'-AAGATAACCACTCTGGCGAGTC-3' | |||||
| V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 | ERBB-2 | NM_001005862.1 | 5'-TGGAGACCCGCTGAACAATA-3' | 0.95 | |
| 5'-GGTTCCGCTGGATCAAGAC-3' | |||||
| House-keeping gene | |||||
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | NM_002046 | 5'-GTGGACCTGACCTGCCGTCT-3' | - | |
| 5'-GGAGGAGTGGGTGTCGCTGT-3' | |||||
3. Experimental
3.1. Peptide Synthesis
3.2. Cell Lines and Animals
3.3. Gelatin Zymography
3.4. Wound Healing Assay
3.5. Invasion Assay
3.6. Orthotopic Tumor Model
3.7. Histology and Immunohistochemistry
3.8. Western Blotting
3.9. Quantitative Real-Time PCR
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
- Samples Availability: Contact the authors.
References and Notes
- Venook, A.P. Hepatocellular carcinoma. Curr. Treat. Options Oncol. 2000, 1, 407–415. [Google Scholar] [CrossRef]
- Huang, C.S.; Liao, J.W.; Hu, M.L. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 538–543. [Google Scholar]
- Winnard, P.T.; Pathak, A.P.; Dhara, S.; Cho, S.Y.; Raman, V.; Pomper, M.G. Molecular imaging of metastatic potential. J. Nucl. Med. 2008, 49, 96S–112S. [Google Scholar]
- Giannelli, G.; Bergamini, C.; Marinosci, F.; Fransvea, E.; Quaranta, M.; Lupo, L.; Schiraldi, O.; Antonaci, S. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int. J. Cancer 2002, 97, 425–431. [Google Scholar]
- Bjorklund, M.; Koivunen, E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta 1755, 37–69. [Google Scholar]
- Khan, S.T.; Pixley, R.A.; Liu, Y.; Bakdash, N.; Gordon, B.; Agelan, A.; Huang, Y.; Achary, M.P.; Colman, R.W. Inhibition of metastasis of syngeneic murine melanoma in vivo and vasculogenesis in vitro by monoclonal antibody C11C1 targeted to domain 5 of high molecular weight kininogen. Cancer Immunol. Immunother. 2010, 59, 1885–1893. [Google Scholar]
- Lv, G.; Tian, D.; An, L.; Zhang, C. Inbitory effect of peptide compound GHGKHKNK on invasion and metastasis of mouse melanoma cell line B16-F10. J. Jilin Univ. (Med. Ed.) 2008, 34, 825–828. [Google Scholar]
- Sainz, I.M.; Pixley, R.A.; Colman, R.W. Fifty years of research on the plasma kallikrein-kinin system: From protein structure and function to cell biology and in-vivo pathophysiology. Thromb. Haemost. 2007, 98, 77–83. [Google Scholar]
- Clapp, C.; Thebault, S.; Jeziorski, M.C.; Martinez de La Escalera, G. Peptide hormone regulation of angiogenesis. Physiol. Rev. 2009, 89, 1177–1215. [Google Scholar]
- Kawasaki, M.; Maeda, T.; Hanasawa, K.; Ohkubo, I.; Tani, T. Effect of His-Gly-Lys motif derived from domain 5 of high molecular weight kininogen on suppression of cancer metastasis both in vitro and in vivo. J. Biol. Chem. 2003, 278, 49301–49307. [Google Scholar]
- Kamiyama, F.; Maeda, T.; Yamane, T.; Li, Y.H.; Ogukubo, O.; Otsuka, T.; Ueyama, H.; Takahashi, S.; Ohkubo, I.; Matsui, N. Inhibition of vitronectin-mediated haptotaxis and haptoinvasion of MG-63 cells by domain 5 (D5(H)) of human high-molecular-weight kininogen and identification of a minimal amino acid sequence. Biochem. Biophys. Res. Commun. 2001, 288, 975–980. [Google Scholar] [CrossRef]
- Cao, D.J.; Guo, Y.L.; Colman, R.W. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ. Res. 2004, 94, 1227–1234. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, M.Y.; Lee, J.W.; Kim, S.C.; Cho, J.H. Enhancement of the cancer targeting specificity of buforin IIb by fusion with an anionic peptide via a matrix metalloproteinases-cleavable linker. Peptides 2011, 32, 895–899. [Google Scholar] [CrossRef]
- Lo, A.; Lin, C.T.; Wu, H.C. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol. Cancer Ther. 2008, 7, 579–589. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Lau, E.Y.; Luo, J.; Yao, N.; Shi, C.; Meza, L.; Tseng, H.; Maeda, Y.; Kumaresan, P.; et al. The use of one-bead one-compound combinatorial library technology to discover high-affinity alphavbeta3 integrin and cancer targeting arginine-glycine-aspartic acid ligands with a built-in handle. Mol. Cancer Ther. 2010, 9, 2714–2723. [Google Scholar]
- Yao, Z.; Lu, R.; Jia, J.; Zhao, P.; Yang, J.; Zheng, M.; Lu, J.; Jin, M.; Yang, H.; Gao, W. The effect of tripeptide tyroserleutide (YSL) on animal models of hepatocarcinoma. Peptides 2006, 27, 1167–1172. [Google Scholar]
- Rodriguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta 1803, 39–54. [Google Scholar]
- Wang, W.; Wu, F.; Fang, F.; Tao, Y.; Yang, L. Inhibition of invasion and metastasis of hepatocellular carcinoma cells via targeting RhoC in vitro and in vivo. Clin. Cancer Res. 2008, 14, 6804–6812. [Google Scholar]
- Morel, G. Internalization and nuclear localization of peptide hormones. Biochem. Pharmacol. 1994, 47, 63–76. [Google Scholar]
- Finch, A.R.; Caunt, C.J.; Armstrong, S.P.; McArdle, C.A. Agonist-induced internalization and downregulation of gonadotropin-releasing hormone receptors. Am. J. Physiol. Cell Physiol. 2009, 297, C591–C600. [Google Scholar] [CrossRef]
- Persad, R.; Liu, C.; Wu, T.T.; Houlihan, P.S.; Hamilton, S.R.; Diehl, A.M.; Rashid, A. Overexpression of caspase-3 in hepatocellular carcinomas. Mod. Pathol. 2004, 17, 861–867. [Google Scholar]
- Xie, F.; Ye, L.; Ta, M.; Zhang, L.; Jiang, W.G. MTSS1: A multifunctional protein and its role in cancer invasion and metastasis. Front. Biosci. (Schol. Ed.) 2011, 3, 621–631. [Google Scholar]
- Yang, J.D.; Nakamura, I.; Roberts, L.R. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin. Cancer Biol. 2011, 21, 35–43. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kiick, K.L. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules 2005, 6, 1921–1930. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H.; Yamada, S.; Windsor, J.; Pollard, A.H.; Lyons, G.; Stetler-Stevenson, W.; Birkedal-Hansen, B. Unit 10.8 Matrix metalloproteinases. Curr. Protoc. Cell Biol. 2008. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, J.Y.; Choi, J.H.; Kim, H.G.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem. Toxicol. 2006, 44, 1890–1896. [Google Scholar] [CrossRef]
- Lee, T.K.; Man, K.; Ho, J.W.; Wang, X.H.; Poon, R.T.; Xu, Y.; Ng, K.T.; Chu, A.C.; Sun, C.K.; Ng, I.O.; et al. FTY720: A promising agent for treatment of metastatic hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 8458–8466. [Google Scholar] [CrossRef]
- Martinez, C.; Vicente, V.; Yanez, M.J.; Garcia, J.M.; Canteras, M.; Alcaraz, M. Experimental model of pulmonary metastasis treatment with IFNα. Cancer Lett. 2005, 225, 75–83. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Han, X.; Yan, D.-M.; Zhao, X.-F.; Hiroshi, M.; Ding, W.-G.; Li, P.; Jiang, S.; Du, B.-R.; Du, P.-G.; Zhu, X. GHGKHKNK Octapeptide (P-5m) Inhibits Metastasis of HCCLM3 Cell Lines via Regulation of MMP-2 Expression in in Vitro and in Vivo Studies. Molecules 2012, 17, 1357-1372. https://doi.org/10.3390/molecules17021357
Han X, Yan D-M, Zhao X-F, Hiroshi M, Ding W-G, Li P, Jiang S, Du B-R, Du P-G, Zhu X. GHGKHKNK Octapeptide (P-5m) Inhibits Metastasis of HCCLM3 Cell Lines via Regulation of MMP-2 Expression in in Vitro and in Vivo Studies. Molecules. 2012; 17(2):1357-1372. https://doi.org/10.3390/molecules17021357
Chicago/Turabian StyleHan, Xiao, Dong-Mei Yan, Xiang-Feng Zhao, Matsuura Hiroshi, Wei-Guang Ding, Peng Li, Shuang Jiang, Bai-Rong Du, Pei-Ge Du, and Xun Zhu. 2012. "GHGKHKNK Octapeptide (P-5m) Inhibits Metastasis of HCCLM3 Cell Lines via Regulation of MMP-2 Expression in in Vitro and in Vivo Studies" Molecules 17, no. 2: 1357-1372. https://doi.org/10.3390/molecules17021357
APA StyleHan, X., Yan, D.-M., Zhao, X.-F., Hiroshi, M., Ding, W.-G., Li, P., Jiang, S., Du, B.-R., Du, P.-G., & Zhu, X. (2012). GHGKHKNK Octapeptide (P-5m) Inhibits Metastasis of HCCLM3 Cell Lines via Regulation of MMP-2 Expression in in Vitro and in Vivo Studies. Molecules, 17(2), 1357-1372. https://doi.org/10.3390/molecules17021357




