Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam Analogues
Abstract
:1. Introduction
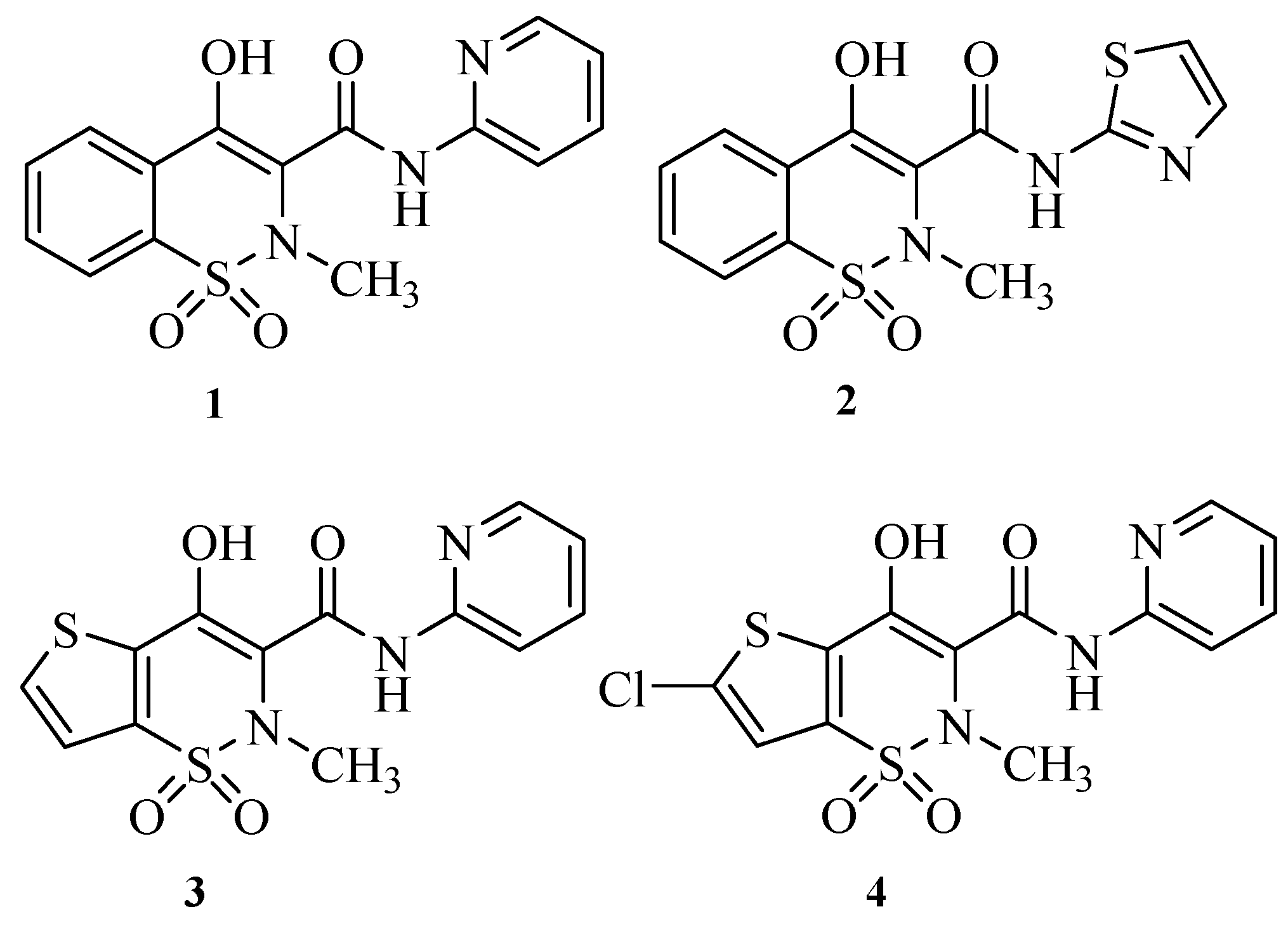
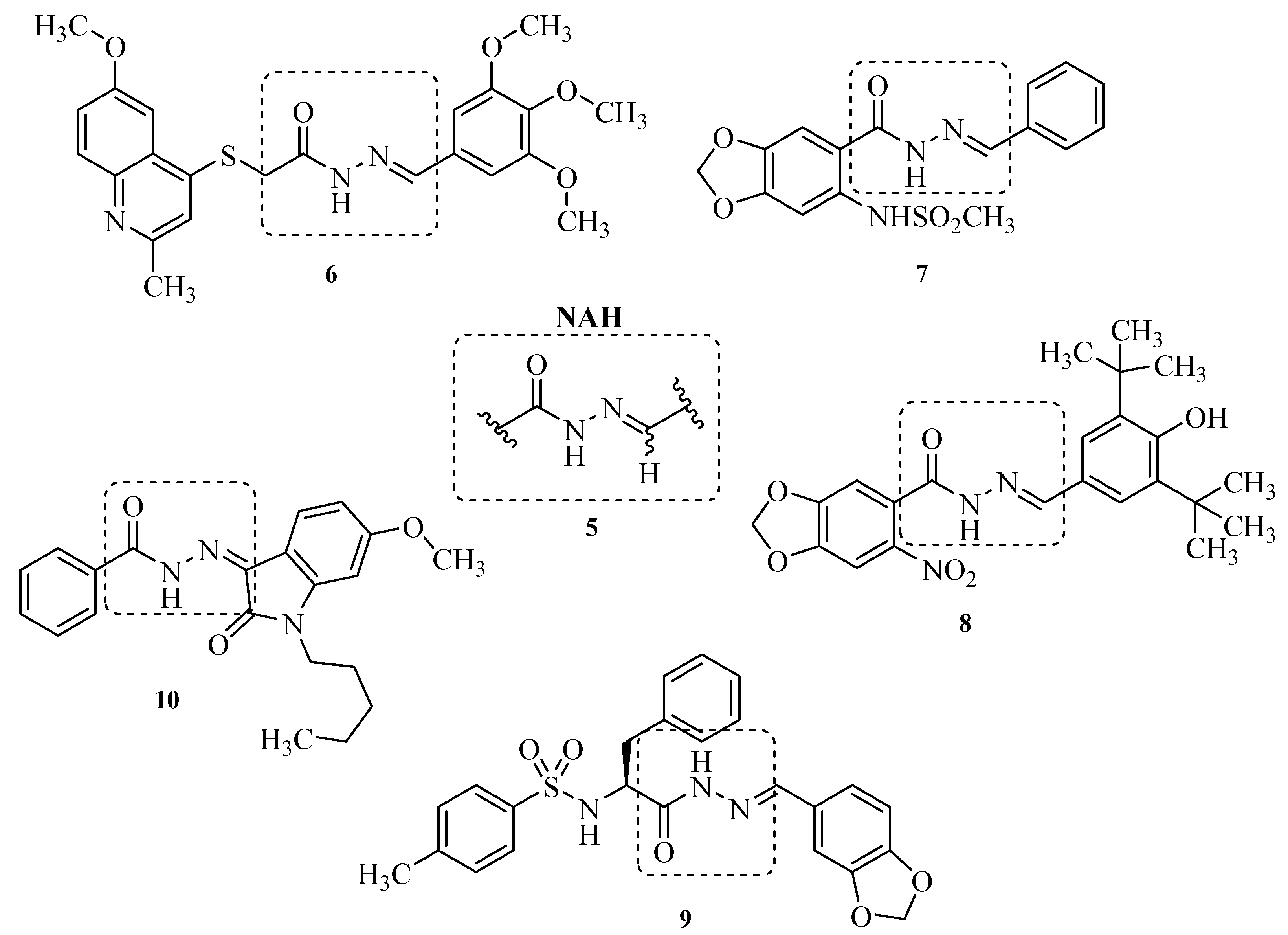

2. Results and Discussion
2.1. Chemistry
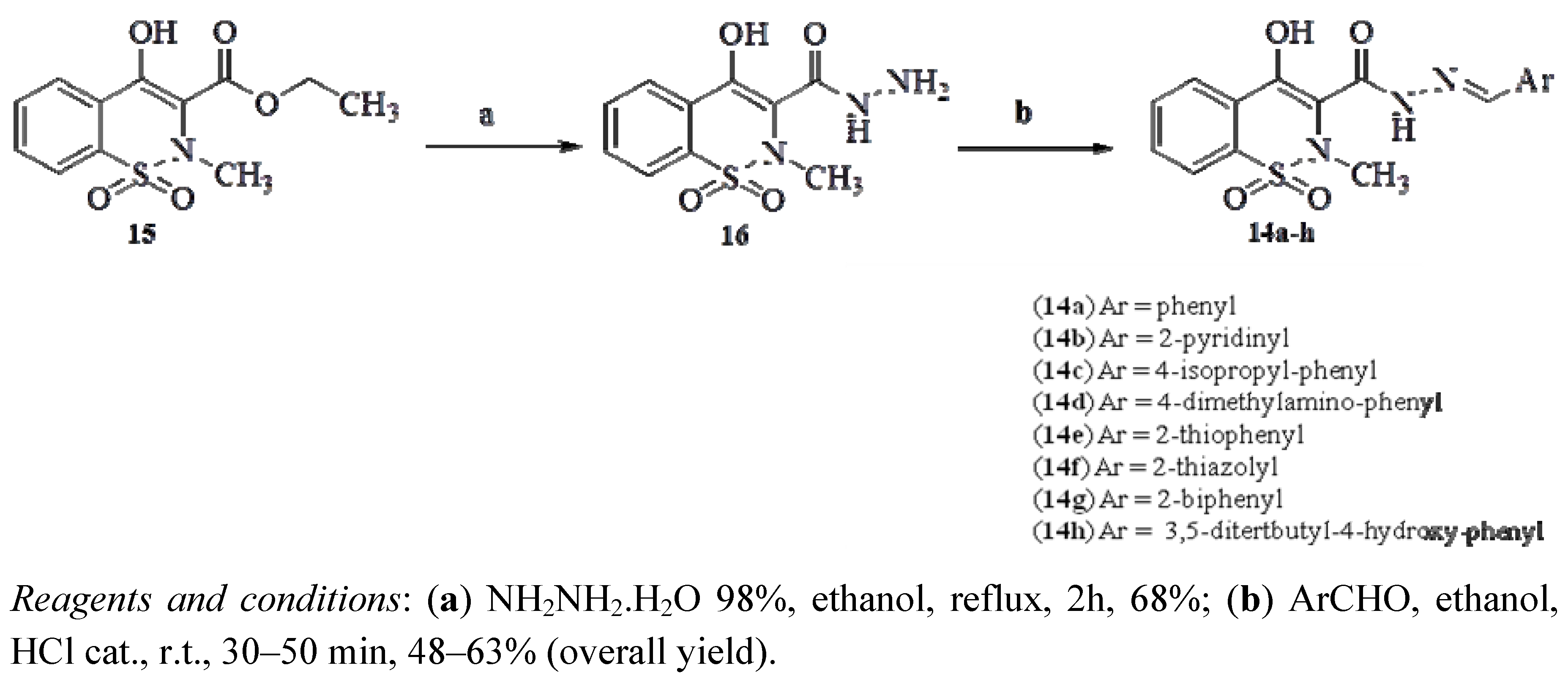
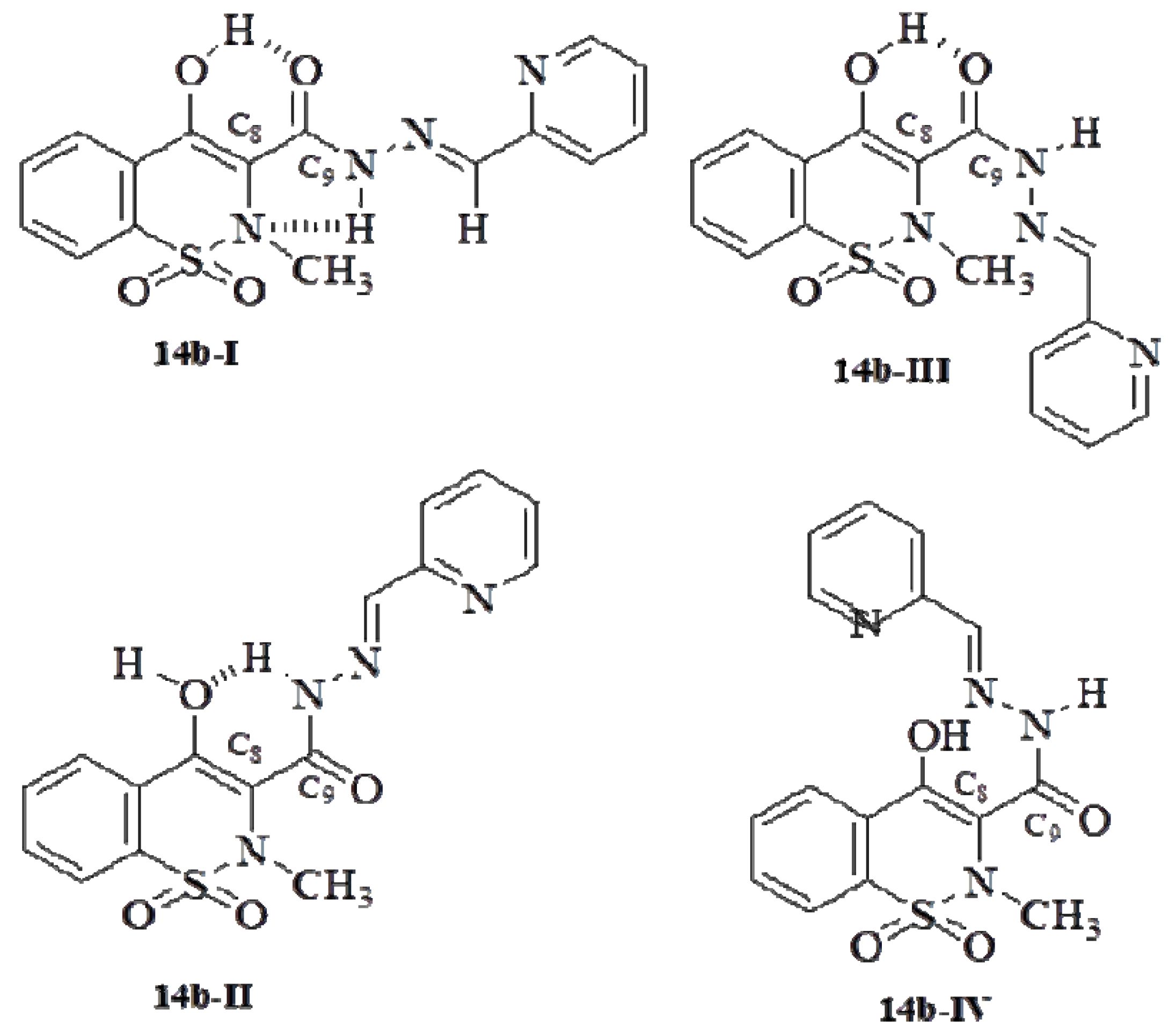
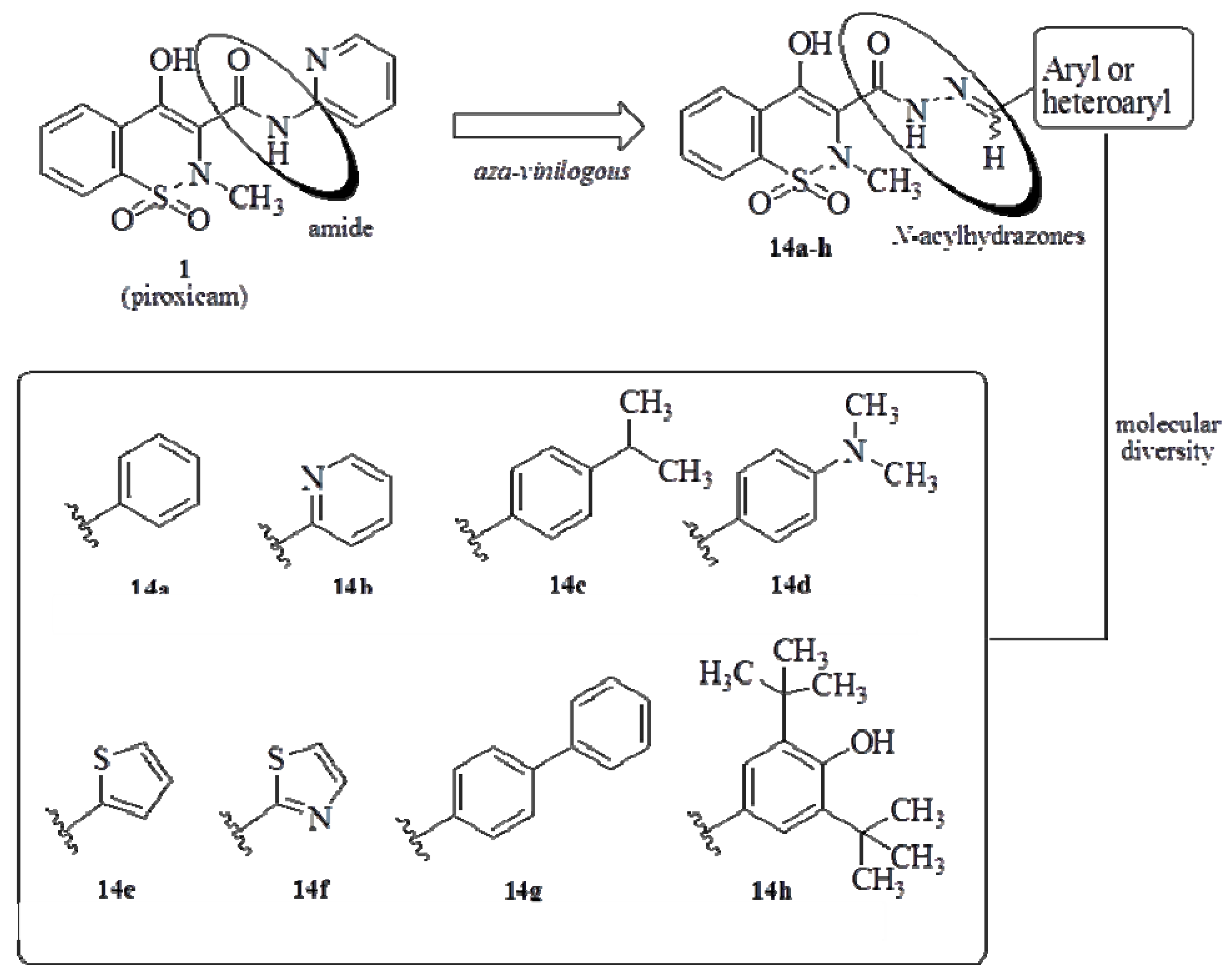
2.2. Pharmacology
| Compound | Writhing Number a | % of Inhibition |
|---|---|---|
| Control | 39.4 ± 4.9 | - |
| Piroxicam | 1.8 ± 0.6 ** | 95.4% |
| 14a (LASSBio-1606) | 9.4 ± 1.6 * | 76.1% |
| 14b (LASSBio-1617) | 16.3 ± 2.5 * | 58.6% |
| 14c (LASSBio-1605) | 9.8 ± 5.1 * | 75.1% |
| 14d (LASSBio-1607) | 16.6 ± 2.9 ** | 57.9% |
| 14e (LASSBio-1604) | 3.8 ± 1.7 ** | 90.4% |
| 14f (LASSBio-1637) | 14.7 ± 2.6 ** | 62.7% |
| 14g (LASSBio-1638) | 6.3 ± 1.5 ** | 84.0% |
| 14h (LASSBio-1639) | 6.8 ± 2.3 ** | 82.7% |
| Compound | ID50 (µmol/kg) | Maximum efficacy |
|---|---|---|
| piroxicam | 0.40 (0.0013–115.6) | 95.4% |
| 14b (LASSBio-1617) | 115.6 (0.17–808.30) | 86.00% |
| 14e (LASSBio-1604) | 28.57 (6.29–49.61) | 90.30% |
| 14f (LASSBio-1637) | 17.19 (0.189–156.1) | 84.77% |
| 14g (LASSBio-1638) | 14.07 (1.06–187.3) | 80.20% |
| 14h (LASSBio-1639) | 2.87 (0.06–130.5) | 88.83% |
| Compound | Phase 1 | Phase 2 | % of inhibition Phase 2 |
|---|---|---|---|
| Control | 50.9 ± 5.8 | 194.0 ± 10.5 | - |
| Piroxicam | 50.0 ± 5.8 | 89.3 ± 22.8 ** | 53.9% |
| 14a (LASSBio-1606) | 49.6 ± 11.2 | 172.5 ± 25.9 | 11.1% |
| 14b (LASSBio-1617) | 21.3 ± 5.2 * | 119.6 ± 23.9 * | 38.4% |
| 14c (LASSBio-1605) | 36.1 ± 6.2 | 176.3 ± 21.9 | 9.1% |
| 14d (LASSBio-1607) | 38.8 ± 8.8 | 161.4 ± 27.9 | 16.8% |
| 14e (LASSBio-1604) | 49.6 ± 10.7 | 131.0 ± 13.1 ** | 32.5% |
| 14f (LASSBio-1637) | 50.3 ± 10.6 | 77.5 ± 16.7 * | 60.1% |
| 14g (LASSBio-1638) | 56.6 ± 10.5 | 88.8 ± 18.5 ** | 54.2% |
| 14h (LASSBio-1639) | 54.8 ± 5.7 | 140.7 ± 13.9 * | 27.5% |
| Compound | Cell Number X 106/mL | % of inhibition |
|---|---|---|
| Control | 38.0 ± 1.0 | - |
| Piroxicam | 16.2 ± 1.1 ** | 57.4% |
| 14a (LASSBio-1606) | 21.4 ± 2.4 * | 43.7% |
| 14b (LASSBio-1617) | 9.9 ± 0.7 ** | 73.9% |
| 14c (LASSBio-1605) | 16.5 ± 3.7 ** | 56.6% |
| 14d (LASSBio-1607) | 10.6 ± 0.4 ** | 72.1% |
| 14e (LASSBio-1604) | 23.9 ± 1.7 ** | 37.1% |
| 14f (LASSBio-1637) | 8.1 ± 1.8 ** | 78.7% |
| 14g (LASSBio-1638) | 7.1 ± 0.6 ** | 81.3% |
| 14h (LASSBio-1639) | 6.6 ± 1.3 ** | 82.6% |
| Compound | Cell Number X 106/mL | % of inhibition |
|---|---|---|
| Control | 20.9 ± 1.6 ** | - |
| Piroxicam | 15.5 ± 0.3 ** | 25.8% |
| 14a (LASSBio-1606) | 8.9 ± 0.5 ** | 57.4% |
| 14b (LASSBio-1617) | 16.2 ± 0.7 ** | 22.5% |
| 14c (LASSBio-1605) | 7.9 ± 0.8 ** | 62.2% |
| 14d (LASSBio-1607) | 12.0 ± 1.5 ** | 42.6% |
| 14e (LASSBio-1604) | 5.4 ± 0.6 ** | 74.2% |
| 14f (LASSBio-1637) | 5.6 ± 0.5 ** | 73.2% |
| 14g (LASSBio-1638) | 4.7 ± 0.7 ** | 77.5% |
| 14h (LASSBio-1639) | 3.8 ± 0.6 ** | 81.8% |
3. Experimental
3.1. General
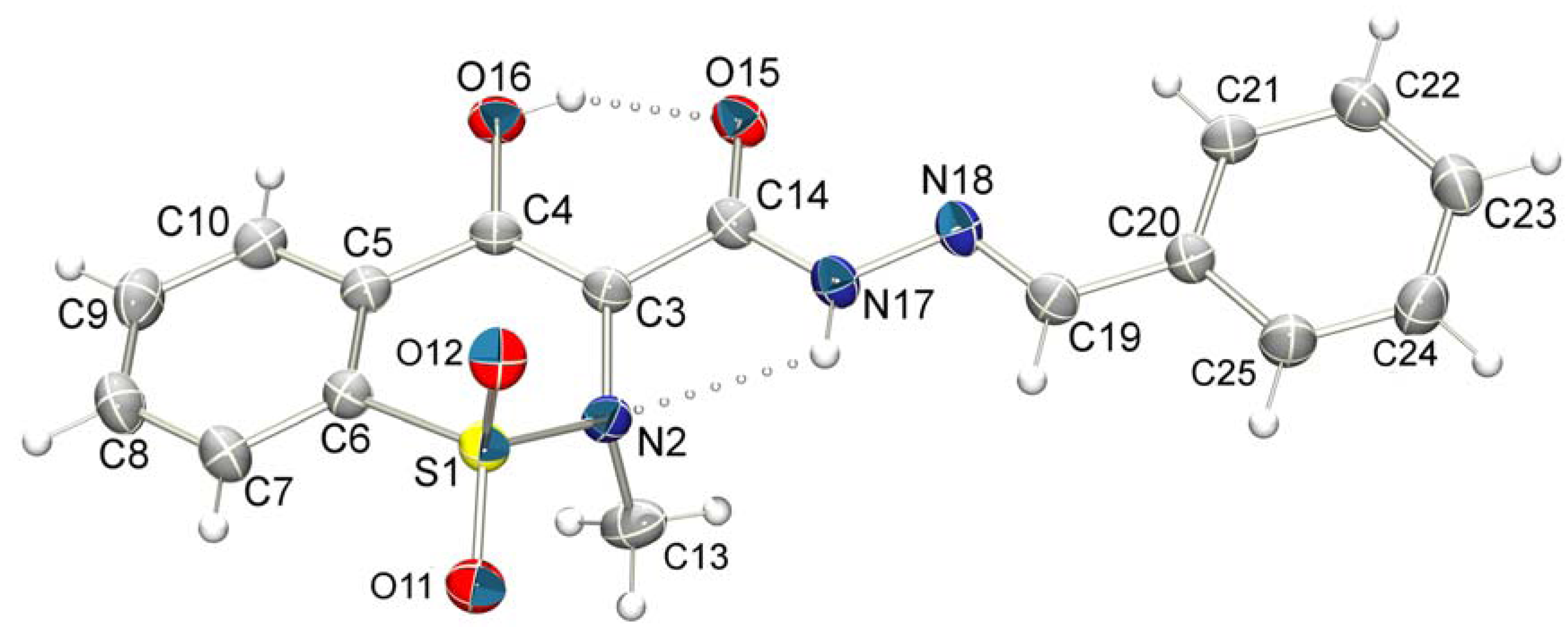
3.2. X-ray Crystallography
| D—H...A | D—H (Å) | H...A (Å) | D...A (Å) | D—H...A (°) | Symmetry operation |
|---|---|---|---|---|---|
| N17-H17...N2 | 0.86 | 2.33 | 2.723(3) | 108.1 | |
| O16-H16...O15 | 0.82 | 1.81 | 2.541(3) | 147.4 | |
| N17-H17...O12 i | 0.86 | 2.39 | 3.156(3) | 149.3 | (i) 1/2-x, 1/2-y, -z |
| C10-H10...O12 ii | 0.93 | 2.69 | 3.268(4) | 121.3 | (ii) 1-x, -y, -z |
| C13-H13B...O15 iii | 0.96 | 2.55 | 3.466(4) | 158.7 | (iii) 1-x, 1-y, -z |
| C22-H22...O11 iv | 0.93 | 2.48 | 3.373(4) | 162.3 | (iv) x, 1-y, -1/2+z |
3.3. Pharmacological Evaluation
3.3.1. Animals
3.3.2. Reagents
3.3.3. Acetic Acid-induced Writhing Test
3.3.4. Formalin-induced Nociception
3.3.5. Hot-plate Test
3.3.6. Zymosan-induced Peritonitis
3.3.7. Carrageenan-induced Peritonitis
3.3.8. Evaluation of Human COX-1/COX-2 Inhibition
3.3.9. Test Evaluation of the Inhibition of LOX
3.3.10. Statistical Analysis
4. Conclusions
Supplementary Material
Acknowledgements
- Sample Availability: Samples of the compounds 14a–h are available from the authors.
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar]
- Henson, P.M. Dampening inflammation. Nat. Immunol. 2005, 6, 1179–1181. [Google Scholar] [CrossRef]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Dis. 2009, 8, 480–499. [Google Scholar]
- Lombardino, J.G.; Wiseman, E.H.J. Sudoxicam and Related N-Hetherocyclic Carboxamides of 4-Hidroxy-2H-1,2- benzothiaziene 1,1- Dioxide. Potent Nonsteroidal Antiinflammatory Agents. J. Med. Chem. 1972, 15, 848–849. [Google Scholar]
- Whitehouse, M.W. Drugs to treat inflammation: A historical introduction. Curr. Med. Chem. 2005, 12, 2931–2942. [Google Scholar] [CrossRef]
- Engelhardt, G.; Homma, D.; Schlegel, K.; Ultzmann, R.; Schnitzler, C. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, A new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm. Res. 1995, 44, 423–433. [Google Scholar] [CrossRef]
- Bradshaw, D.; Cashin, C.H.; Kennedy, A.D.; Roberts, N.A. Pharmacological and biochemical activities of Tenoxicam (Ro 12–0068), A new non-steroidal anti-inflammatory drug. Agents Actions 1984, 15, 569–577. [Google Scholar] [CrossRef]
- Balfour, J.A.; Fitton, L.B. Barradell, Lornoxicam: A review of its pharmacology and therapeutic potential in the management of painful and inflammatory conditions. Drugs 1996, 51, 639–657. [Google Scholar] [CrossRef]
- Hobbs, Y.D.C.; Twomey, T.M.J. Piroxicam pharmacokinetics in man: Aspirin and antacid interaction studies. Clin. Pharmacol. 1979, 19, 270–281. [Google Scholar]
- Richy, F.; Scarpignato, C.; Lanas, A.; Reginster, J.-Y. Efficacy and safety of piroxicam revisited. A global meta-analysis of randomized clinical trials. Pharmacol. Res. 2009, 60, 254–263. [Google Scholar]
- Dannhardt, G.; Kiefer, W. Cyclooxygenase inhibitors—Current status and future prospects. Eur. J. Med. Chem. 2001, 36, 109–126. [Google Scholar] [CrossRef]
- Brideau, C.; Kargman, S.; Liu, S.; Dallob, A.L. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflamm. Res. 1996, 45, 68–74. [Google Scholar] [CrossRef]
- Duarte, C.D.; Barreiro, E.J.; Fraga, C.A.M. Privileged Structures: A Useful Concept for the Rational Design of New Lead Drug Candidates. Mini-Rev. Med. Chem. 2007, 7, 1108–1119. [Google Scholar]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; Dipardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Cage, J.L.; Onrust, R.; Johnston, D.; Osnowski, A.; MacDonald, W.; Mitchell, L.; Ürogdi, L.; Rohde, A.; Harbol, K.; Gragerov, S.; et al. N-Acylhydrazones as inhibitors of PDE10A. Bioorg. Med. Chem. 2011, 21, 4155–4159. [Google Scholar] [CrossRef]
- Tributino, J.M.L.; Duarte, C.D.; Corrêa, R.S.; Doriguetto, A.C.; Ellena, J.; Romeiro, N.C.; Castro, N.G.; Miranda, A.L.P.; Barreiro, E.J. Novel 6-methanesulfonamide-3,4-methylenedioxyphenyl-N-acylhydrazones: Orally effective anti-inflammatory drug candidates. Bioorg. Med. Chem. 2009, 17, 1125–1131. [Google Scholar]
- Duarte, C.D.; Tributino, J.L.M.; Lacerda, D.I.; Martins, M.V.; Alexandre Moreira, M.S.; Dutra, F.; Bechara, E.J.H.; De Paula, F.S.; Goulart, M.O.F.; Ferreira, J.; et al. Synthesis, Pharmacological evaluation and electrochemical studies of novel 6-nitro-3,4-methylenedioxyphenyl-N-acylhydrazone derivatives: Discovery of LASSBio-881, A new ligand of cannabinoid receptors. Bioorg. Med. Chem. 2007, 15, 2421–2433. [Google Scholar]
- Tian, B.; He, M.; Tang, S.; Hewlett, I.; Tan, Z.; Li, J.; Jin, Y.; Yang, M. Synthesis and antiviral activities of novel acylhydrazone derivatives targeting HIV-1 capsid protein. Bioorg. Med. Chem Lett. 2009, 19, 2162–2167. [Google Scholar]
- Diaz, P.; Phatak, S.S.; Xu, J.; Astruc-Diaz, F.; Cavasotto, C.N.; Naguib, M.J. 6-methoxy-VV-alkyl isatin acylhydrazone derivatives as a novel series of potent selective cannabinoid receptor 2 inverse agonists: Design, Synthesis, and binding mode prediction. Med. Chem. 2009, 52, 433–444. [Google Scholar] [CrossRef]
- Fraga, C.A.M.; Barreiro, E.J. Medicinal chemistry of N-acylhydrazones: New lead-compounds of analgesic, Antiinflammatory and antithrombotic drugs. Curr. Med. Chem. 2006, 13, 167–198. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Fraga, C.A.M.; Miranda, A.L.P.; Rodrigues, C.R. A química medicinal de N-acilidrazonas: novos compostos protótipos de fármacos analgésicos, antiinflamatórios e anti-trombóticos. Quim. Nova 2002, 25, 129–148. [Google Scholar] [CrossRef]
- Mahy, J.P.; Gaspard, S.; Mansuy, D. Phenylhydrazones as new good substrates for the dioxygenase and peroxydase reactions of prostaglandin synthase: Formation of Iron (III)-sigma-phenyl complexes. Biochemistry 1993, 32, 4014–4021. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Choudary, J.A.; Elsegood, M.R.J.; Siddiqui, H.L.; Khan, K.M. A facile synthesis of novel biologically active 4-hydroxy-N'-(benzylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 2009, 44, 1311–1316. [Google Scholar] [CrossRef]
- Ahmad, M.; Zia-ur-Rehman, M.; Siddiqui, H.L.; Ullah, M.F.; Parvez, M. Microwave assisted synthesis and structure-activity relationship of 4-hydroxy-N'-[1-phenylethylidene]-2H/2-methyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides as anti-microbial agents. Eur. J. Med. Chem. 2011, 46, 2368–2377. [Google Scholar] [CrossRef]
- Ahmad, M.; Rizvi, S.U.F.; Siddiqui, H.L.; Ahmad, S.; Parvez, M.; Suliman, R. Antioxidant and antimicrobial studies of novel N0-(substituted-2-chloroquinolin-3-yl)methylidene-4-hydroxy-2H-1,2-benzothiazine-3-carbohydrazides 1,1-dioxides. Med. Chem. Res. 2012, 21, 2340–2348. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Wiseman, E.H.; Chianini, J. Potent Antiinflammatory N-Hetherocyclic 3-Carboxamides of 4-Hydroxi-2-methyl-2H-1,2-benzothiazine 1,1-dioxide. J. Med. Chem. 1973, 16, 493–496. [Google Scholar] [CrossRef]
- Wang, J.; Limburg, D.; Carter, J.; Mbalaviele, G.; Gierse, J.; Vazquez, M. Selective inducible microsomal prostaglandin E2 synthase-1 (mPGES-1) inhibitors derived from an oxicam template. Bioorg. Med. Chem. Lett. 2010, 20, 1604–1609. [Google Scholar] [CrossRef]
- de Leval, X.; Julémon, F.; Delarge, J.; Pirotte, B.; Dogné, J.-M. New Trends in Dual 5-LOX/COX Inhibition. Curr. Med. Chem. 2002, 9, 941–962. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Wiseman, E.H.; McLamore, W.M. Synthesis and Antiinflammatory Activity of some 3-carboxamides of 2-alkyl-4-hydroxy-2H-1,2-benzothiazine-1,1-dioxide. J. Med. Chem. 1971, 14, 1171–1175. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; Silva, K.C.P.; Leda, P.H.O.; Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Kümmerle, A.E.; Raimundo, J.M.; Leal, C.M.; da Silva, G.S.; Balliano, T.L.; Pereira, M.A.; de Simone, C.A.; Sudo, R.T.; Zapata-Sudo, G.; Fraga, C.A.M.; et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur. J. Med. Chem. 2009, 44, 4004–4009. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Ling, Y.; Huang, J.; Cui, J.; Wang, J.R.; Yang, X. New class of potent antitumor acylhydrazone derivatives containing furan. Eur. J. Med. Chem. 2010, 45, 5576–5584. [Google Scholar] [CrossRef]
- Cunha, A.C.; Figueiredo, J.M.; Tributino, J.L.; Miranda, A.L.; Castro, H.C.; Zingali, R.B.; Fraga, C.A.; de Souza, M.C.; Ferreira, V.F.; Barreiro, E.J. Antiplatelet properties of novel N-substituted-phenyl-1,2,3-triazole-4-acylhydrazone derivatives. Bioorg. Med. Chem. 2003, 11, 2051–2059. [Google Scholar]
- Silva, G.A.; Costa, L.M.M.; Brito, F.C.F.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. New class of potent antinociceptive and antiplatelet 10H-phenothiazine-1-acylhydrazone derivatives. Bioorg. Med. Chem. 2004, 12, 3149–3158. [Google Scholar] [CrossRef]
- Syakaev, V.V.; Podyachev, S.N.; Buzykin, B.I.; Latypov, S.K.; Habicher, W.D.; Konovalov, A.I. NMR study of conformation and isomerization of aryl- and heteroarylaldehyde 4-tert-butylphenoxyacetylhydrazones. J. Mol. Struct. 2006, 788, 55–62. [Google Scholar] [CrossRef]
- Silva, Y.K.C.; Augusto, C.V.; Barbosa, M.L.C.; Melo, G.M.A.; Queiroz, A.C.; Dias, T.L.M.F.; Bispo-Júnior, W.; Barreiro, E.J.; Lima, L.M.; Alexandre-Moreira, M.S. Synthesis and pharmacological evaluation of pyrazine N-acylhydrazone derivatives designed as novel analgesic and anti-inflammatory drug candidates. Bioorg. Med. Chem. 2010, 18, 5007–5015. [Google Scholar]
- Palla, G.; Predieri, G.; Domiano, P.; Vignali, C.; Turner, W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron 1986, 42, 3649–3654. [Google Scholar] [CrossRef]
- Farrugia, L.J. Ortep-3 for Windows—A Version of ORTEP-III with a Graphical User. J. Appl. Cryst. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Collier, H.O.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its supression by analgesic drugs in the mouse. Br. J. Pharmacol. 1968, 32, 295–310. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: dissociation between inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified formalin test; characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: an evaluation of the method. Pain 1992, 51, 15–17. [Google Scholar]
- Woolfe, G.; MacDonald, A.D. Evaluation of analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenylamines and dithienylbutylamines. J. Pharmacol. Exp. Ther 1953, 107, 385–393. [Google Scholar]
- Kuraishi, Y.; Harada, Y.; Aratani, S.; Satoh, M.; Takagi, H. Involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: The differences in mechanical and thermal algesic tests. Brain Res. 1983, 273, 245–252. [Google Scholar] [CrossRef]
- Le Bars, D.M.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Doherty, N.S.; Poubelle, P.; Borgeat, P.; Beaver, T.H.; Westrich, G.L.; Schrader, N.L. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins 1985, 30, 769–789. [Google Scholar] [CrossRef]
- Leite, D.F.P.; Echevarria-Lima, J.; Ferreira, S.C.; Calixto, J.B.; Rumjanek, V.M.J. ABCC transporter inhibition reduces zymosan-induced peritonitis. Leuk. Biol. 2007, 82, 630–637. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Alcaraz, M.J. Antiinflammatory activity and inhibition of arachidonic acidmetabolism by flavonoids. Inflamm. Res. 1991, 32, 283–288. [Google Scholar]
- Glaser, K.; Sung, M.-L.; O’Neill, K.; Belfast, M.; Hartman, D.; Carlson, R.; Kreft, A.; Kubrak, D.; Hsiao, C.-L.; Weichman, B. Etodolac selectively inhibits human prostaglandin G/H synthase 2 (PGHS-2) versus human PGHS-1. Eur. J. Pharmacol. 1995, 281, 107–111. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX Suite for Single Crystal Small Molecule Crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Miranda, A.S.; Júnior, W.B.; Da Silva, Y.K.C.; Alexandre-Moreira, M.S.; Castro, R.D.P.; Sabino, J.R.; Lião, L.M.; Lima, L.M.; Barreiro, E.J. Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam Analogues. Molecules 2012, 17, 14126-14145. https://doi.org/10.3390/molecules171214126
De Miranda AS, Júnior WB, Da Silva YKC, Alexandre-Moreira MS, Castro RDP, Sabino JR, Lião LM, Lima LM, Barreiro EJ. Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam Analogues. Molecules. 2012; 17(12):14126-14145. https://doi.org/10.3390/molecules171214126
Chicago/Turabian StyleDe Miranda, Amanda Silva, Walfrido Bispo Júnior, Yolanda Karla Cupertino Da Silva, Magna Suzana Alexandre-Moreira, Rosane De Paula Castro, José Ricardo Sabino, Luciano Morais Lião, Lídia Moreira Lima, and Eliezer J. Barreiro. 2012. "Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam Analogues" Molecules 17, no. 12: 14126-14145. https://doi.org/10.3390/molecules171214126
APA StyleDe Miranda, A. S., Júnior, W. B., Da Silva, Y. K. C., Alexandre-Moreira, M. S., Castro, R. D. P., Sabino, J. R., Lião, L. M., Lima, L. M., & Barreiro, E. J. (2012). Design, Synthesis, Antinociceptive and Anti-Inflammatory Activities of Novel Piroxicam Analogues. Molecules, 17(12), 14126-14145. https://doi.org/10.3390/molecules171214126




