Study of the Volatile Constituents in Radix Flemingiae Macrophyllae and a Substitute by Gas Chromatography-Mass Spectrometry and Chemometric Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Resolution of Overlapped Peaks with SFA
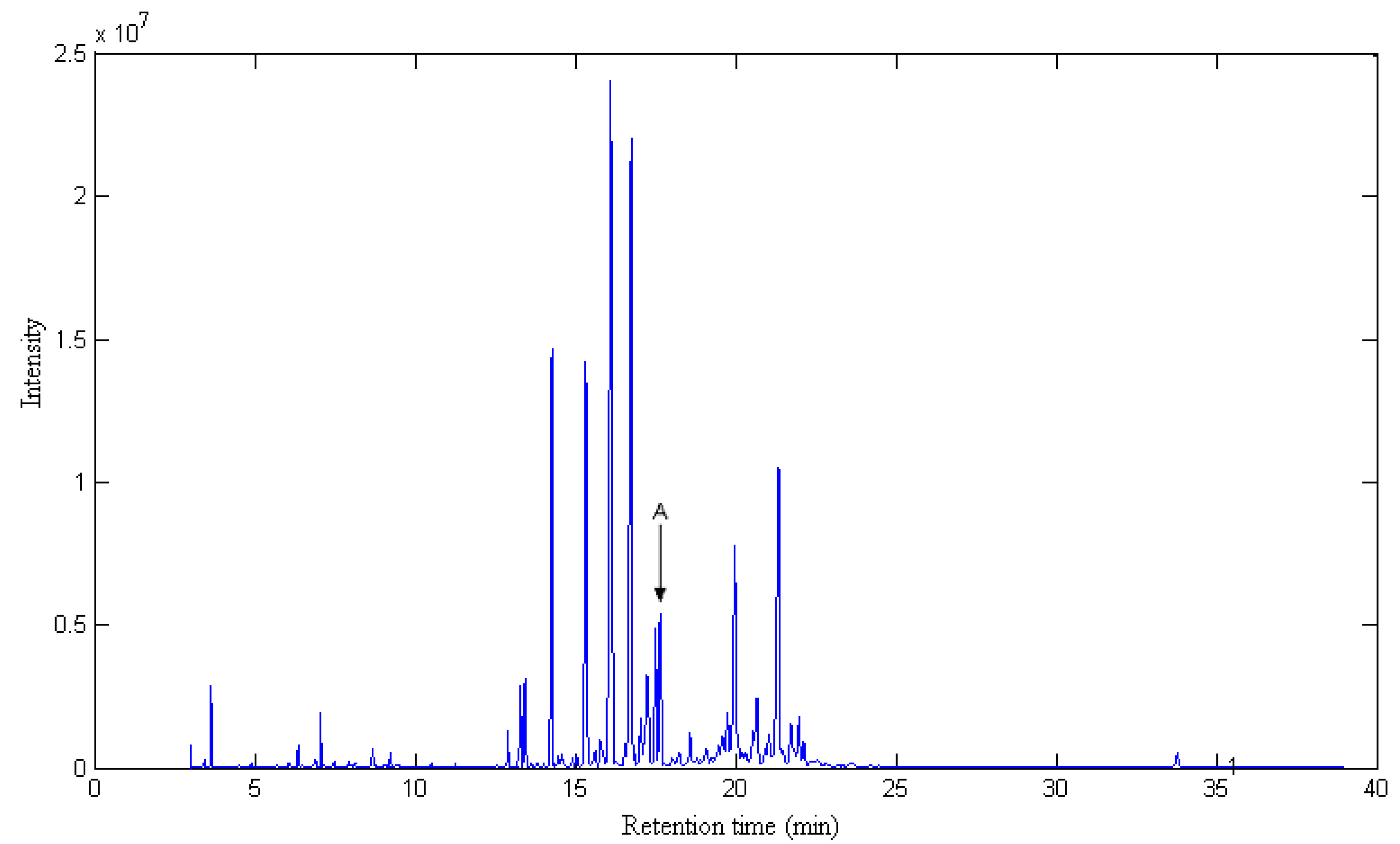
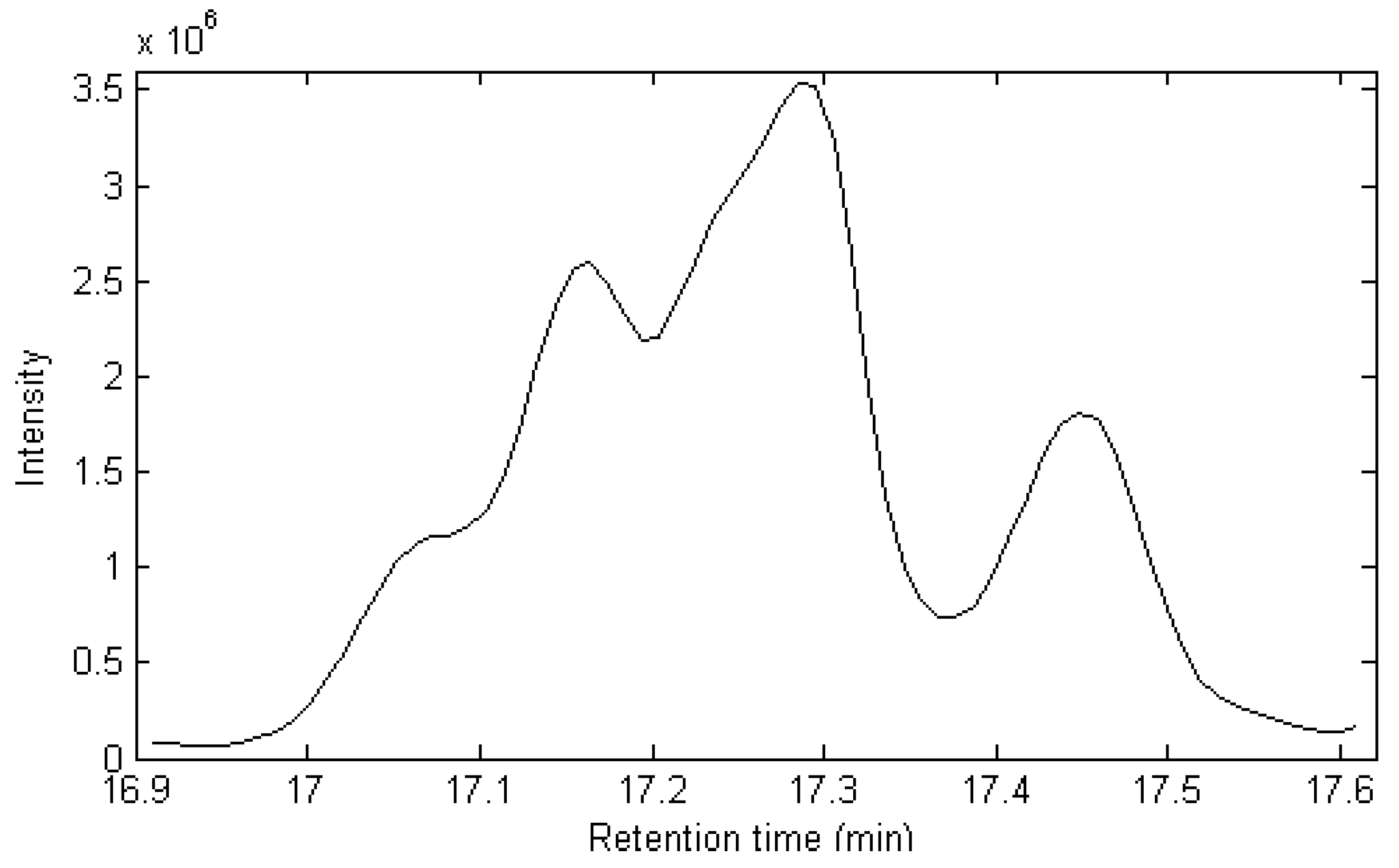
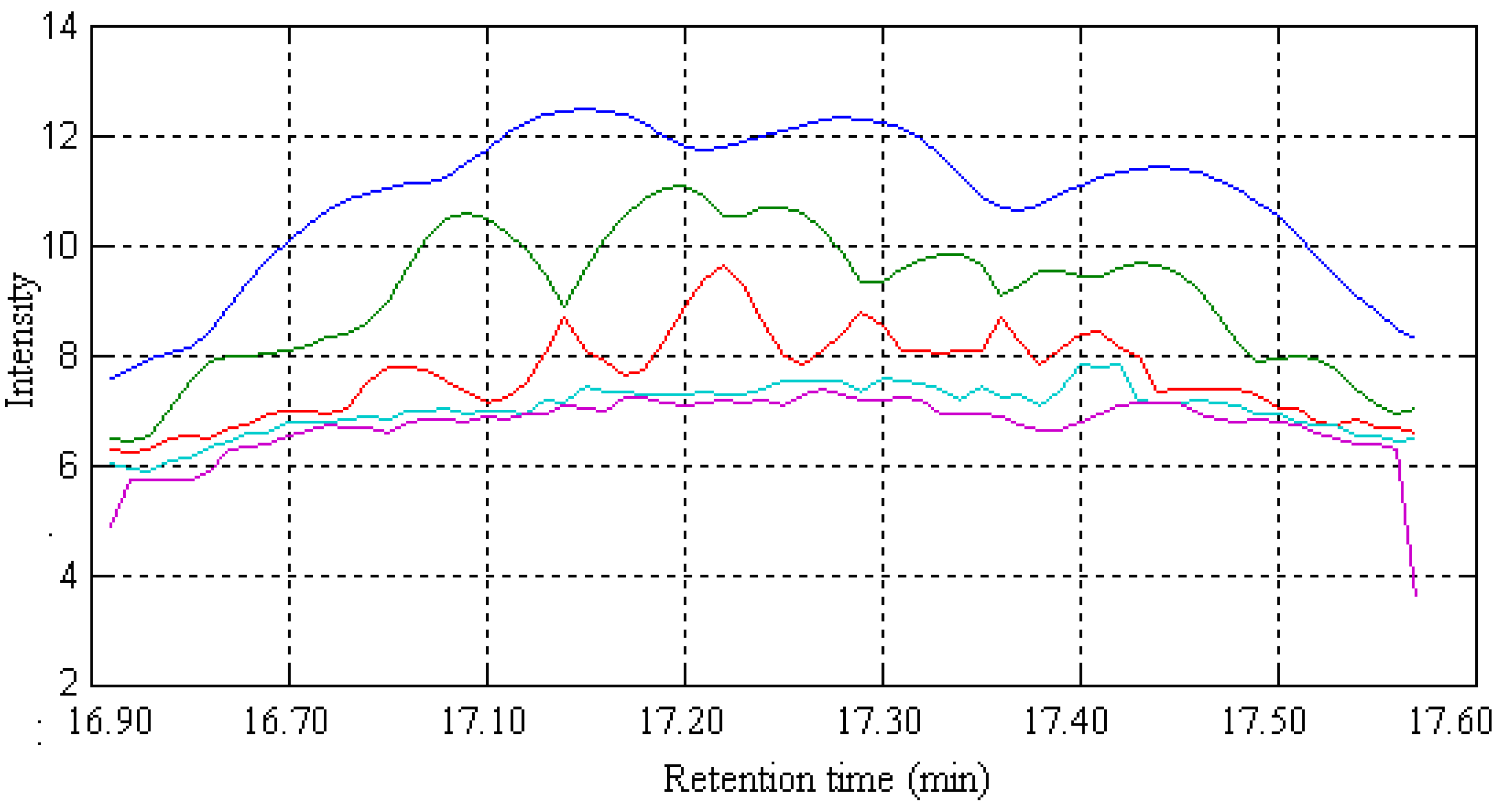
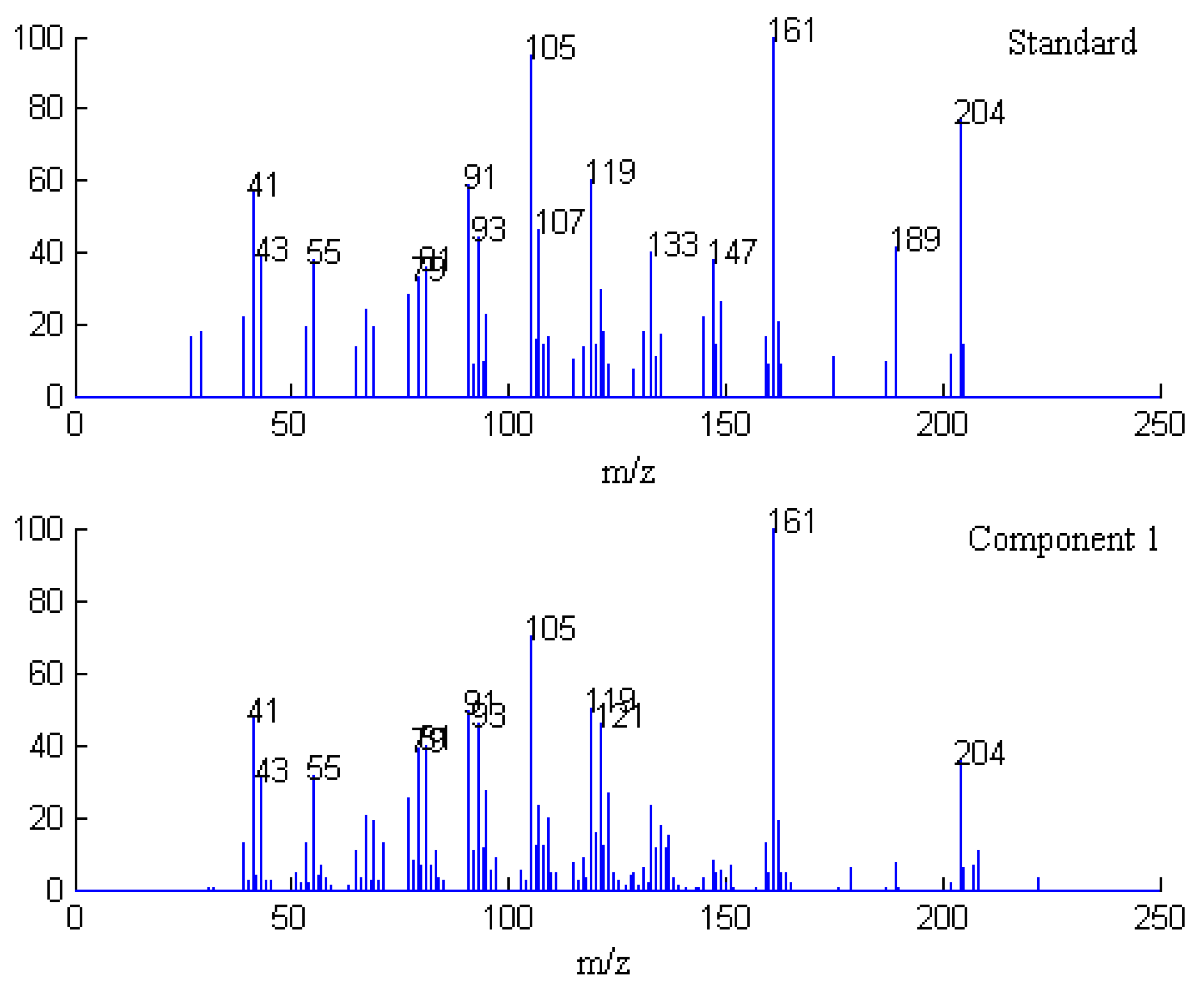
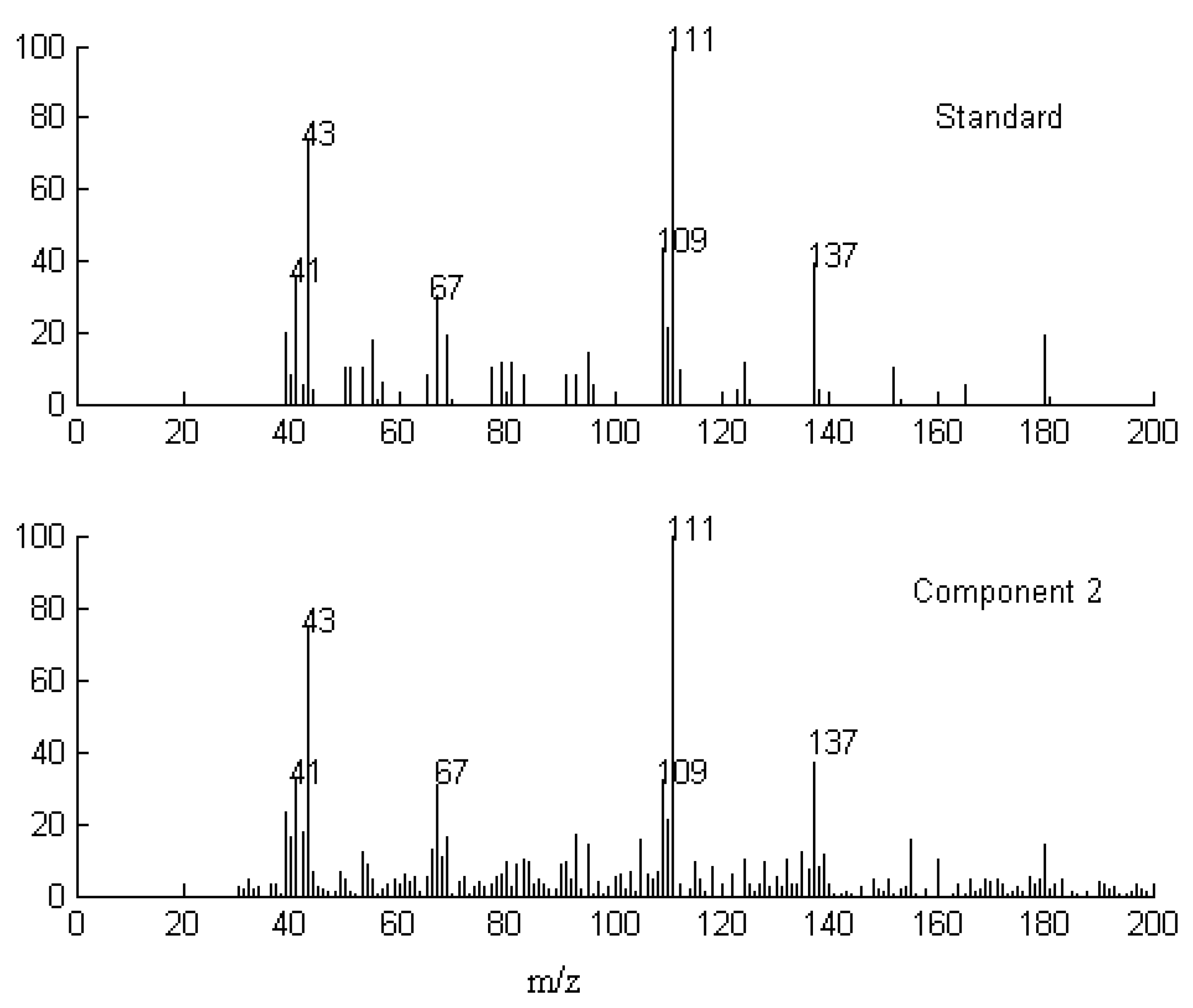
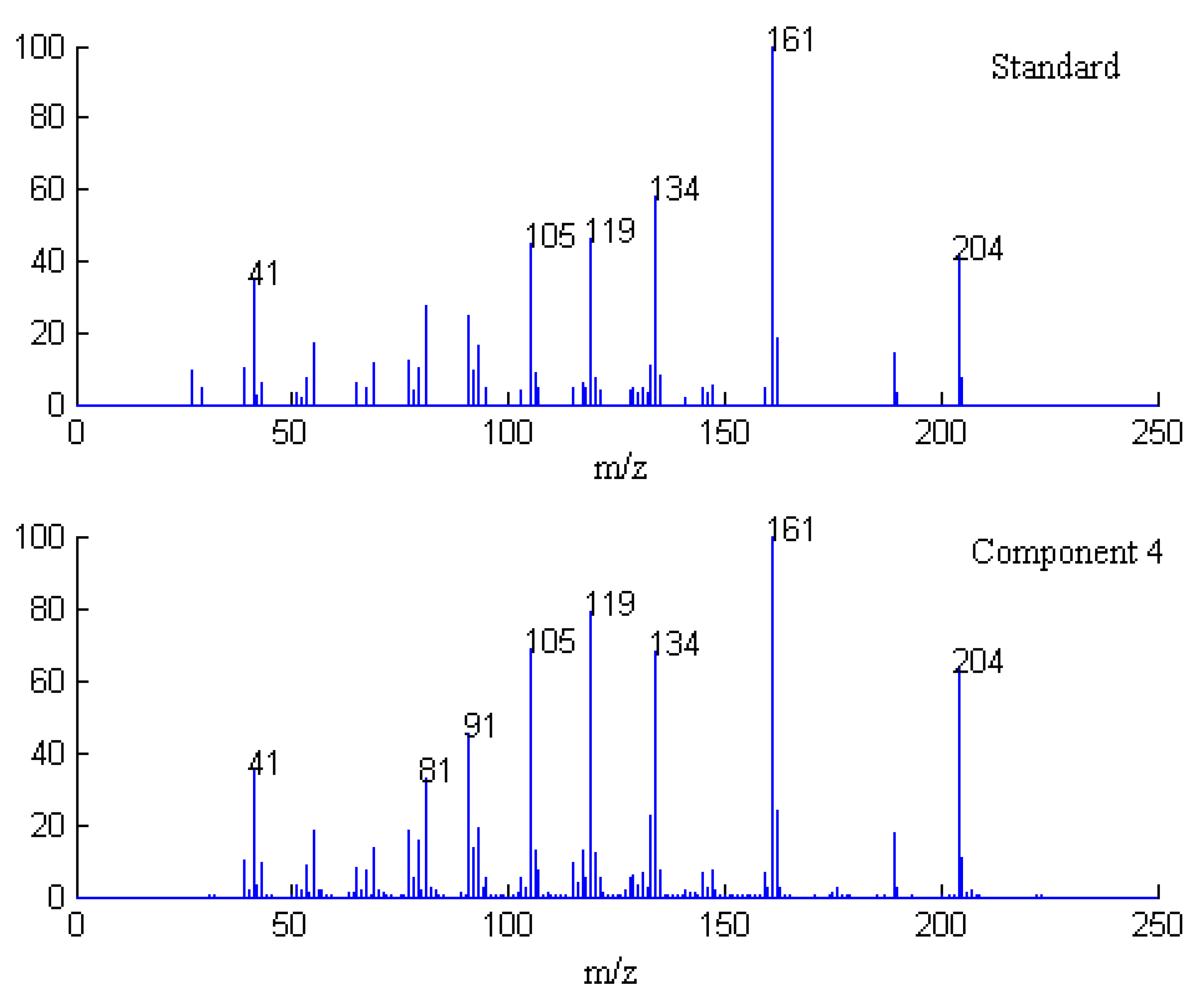
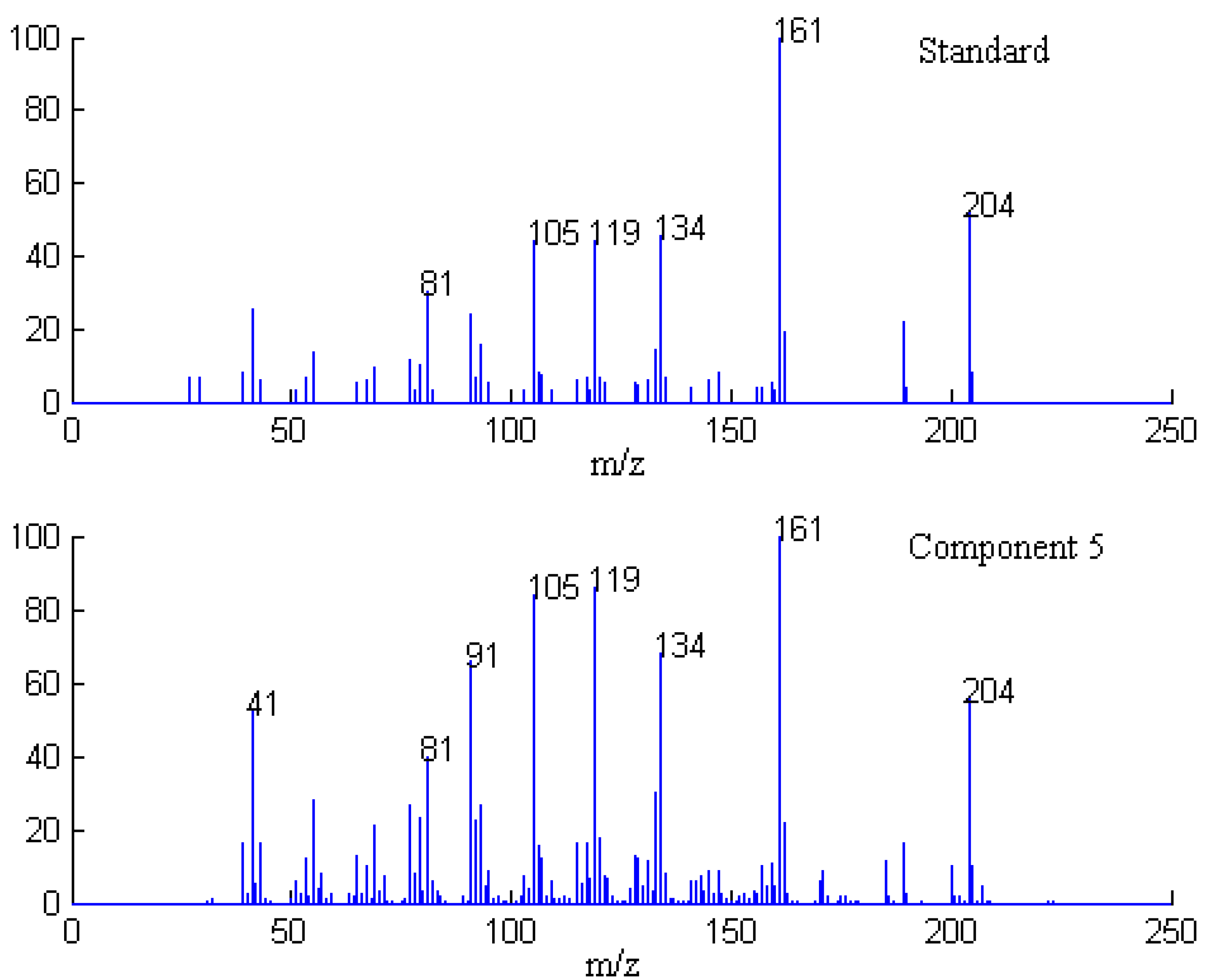
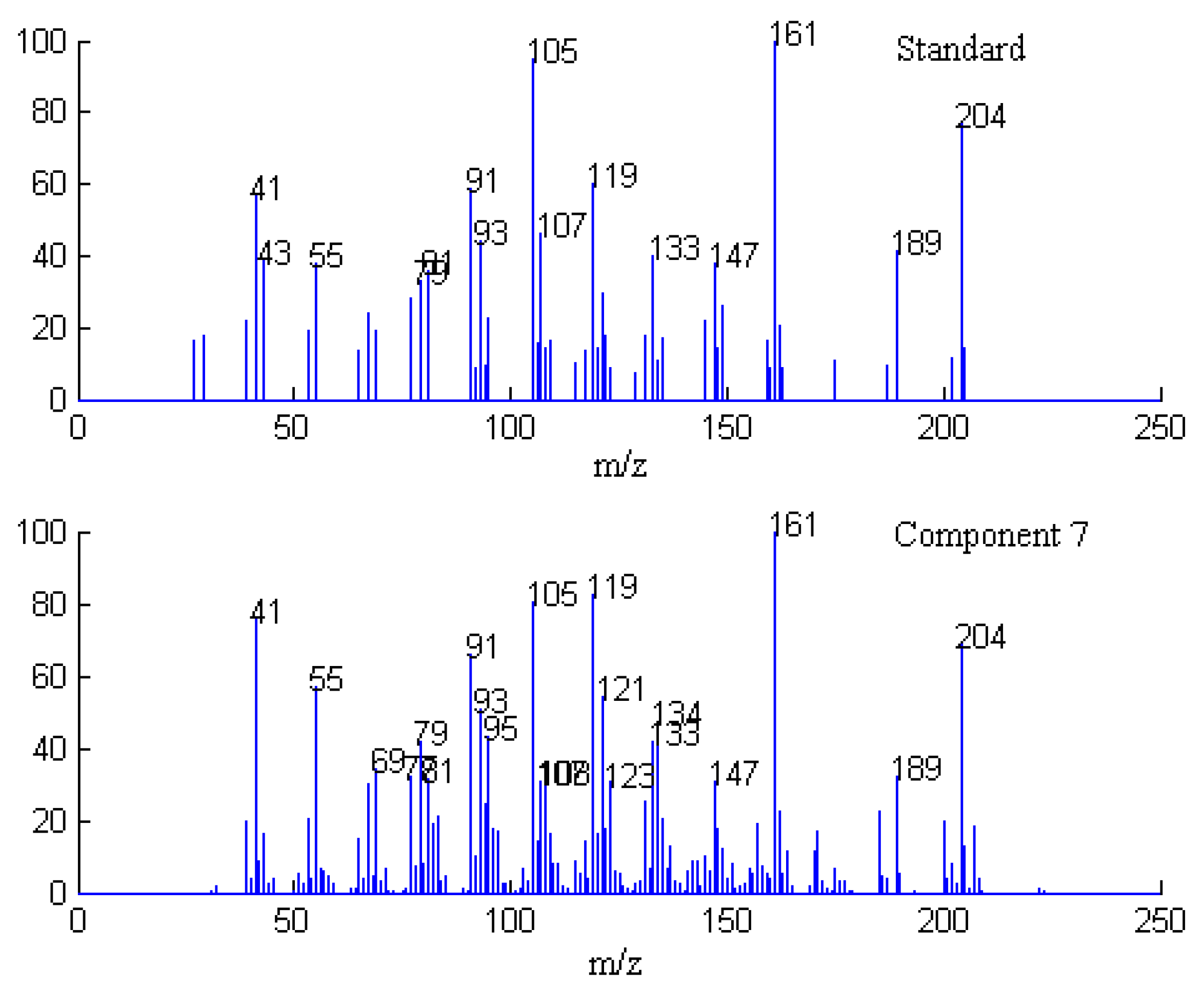
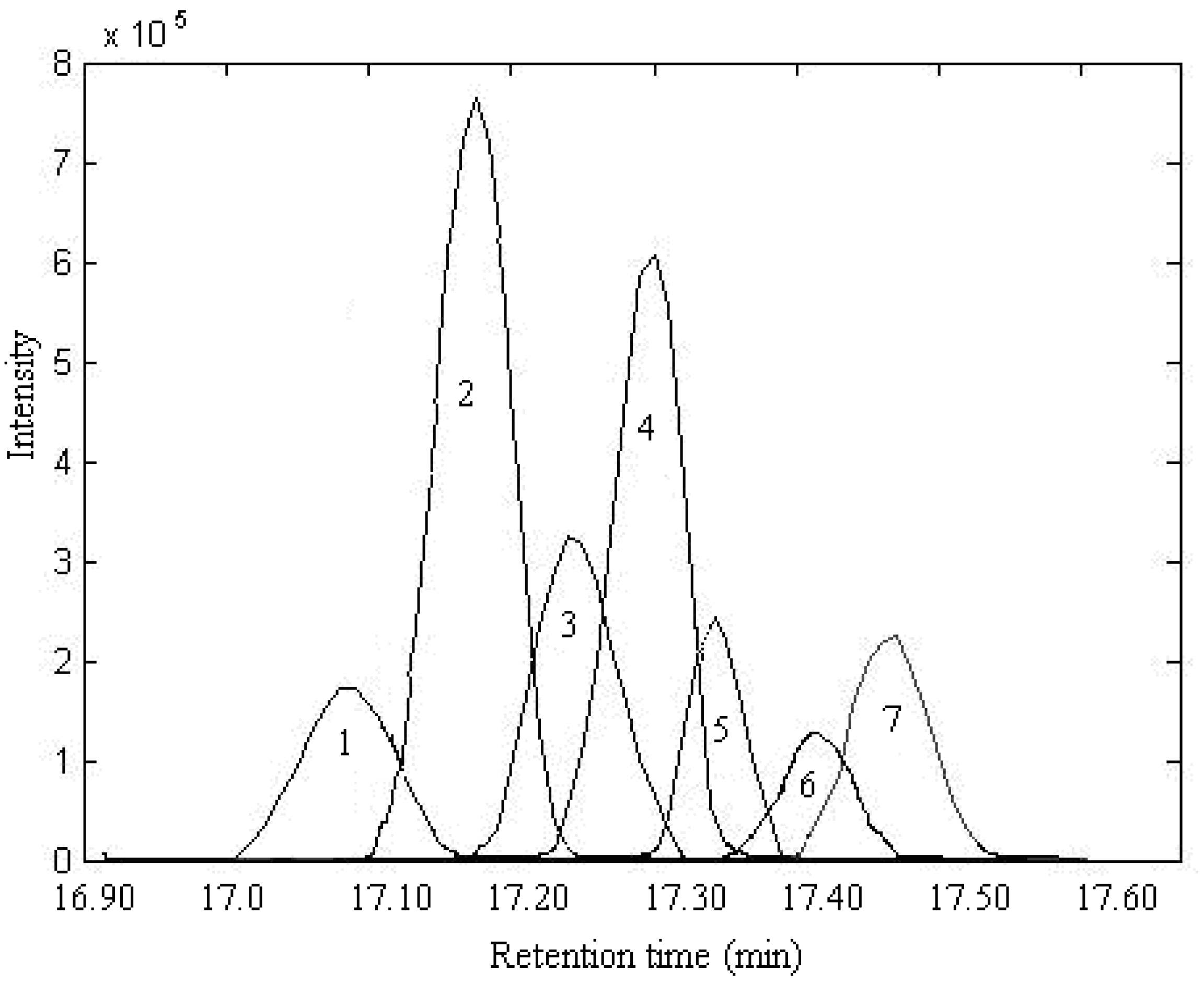
| Series No. | Retention time (min) | Compound name | Molecularformula | Relative content (%) | |
|---|---|---|---|---|---|
| X1 a | X2 b | ||||
| 1 | 3.439 | - | Prenal | C5H8O | 0.07 |
| 2 | 3.653 | 3.658 | Hexanal | C6H12O | 0.35 |
| 3 | 6.368 | 6.372 | 2-Amylfuran | C9H14O | 0.03 |
| 4 | 7.065 | - | D-Limonene | C9H20 | 0.27 |
| 5 | 7.979 | 7.984 | Linalool | C10H18O | 0.98 |
| 6 | 8.682 | 8.691 | L-Camphor | C10H16O | 1.91 |
| 7 | 9.104 | - | l-2-Bornanol | C10H18O | 0.06 |
| 8 | 9.267 | 9.275 | p-Menth-1-en-4-ol | C10H18O | 0.66 |
| 9 | 9.419 | 9.428 | p-Menth-1-en-8-ol | C10H18O | 0.87 |
| 10 | 10.529 | 10.536 | Nonanoic acid | C9H18O2 | 0.46 |
| 11 | 10.671 | - | Perillyl aldehyde | C10H14O | 0.04 |
| 12 | 10.914 | 10.925 | Anethole | C10H12O | 1.62 |
| 13 | 11.065 | 11.081 | Bornyl acetate | C12H20O2 | 0.72 |
| 14 | 11.294 | 11.308 | Tricyclo[3.2.1.02,7]oct-3-ene, 2,3,4,5-tetramethyl- | C12H 18 | 0.57 |
| 15 | 11.504 | - | Furan, 2,5-dibutyl- | C12H20O | 0.03 |
| 16 | 12.465 | 12.478 | Decanoic acid | C10H20O2 | 0.06 |
| 17 | 12.720 | 12.731 | α-Cubebene | C15H24 | 0.56 |
| 18 | 12.859 | 12.875 | α-Longipinene | C15H24 | 1.47 |
| 19 | 13.325 | 13.331 | Ylangene | C15H24 | 1.69 |
| 20 | 13.476 | 13.493 | Longicyclene | C15H24 | 2.84 |
| 21 | 13.771 | - | Copaene | C15H24 | 0.64 |
| 22 | 14.305 | 14.312 | Longofolene | C15H24 | 3.21 |
| 23 | 14.437 | - | Di-epi-α-cedrene | C15H26 | 0.08 |
| 24 | 14530 | 14.542 | β-Caryophyllene | C15H24 | 0.82 |
| 25 | 14.575 | 14.583 | β-Cedrene | C15H24 | 5.92 |
| 26 | 14.744 | 14.762 | Germacrene D | C15H24 | 0.65 |
| 27 | 15.057 | 15.071 | (Z)-β-Farnesene | C15H24 | 0.61 |
| 28 | 15.108 | - | Himachala-2,4-diene | C15H24 | 0.07 |
| 29 | 15.385 | 15.435 | α-Himachalene | C15H24 | 4.13 |
| 30 | 15.572 | 15.590 | α-Caryophyllene | C15H24 | 0.44 |
| 31 | 15.593 | - | Acoradiene | C15H24 | 0.33 |
| 32 | 15.673 | 15.685 | Dihydrocurcumene | C15H24 | 0.03 |
| 33 | 15.858 | 15.869 | Eremophilene | C15H24 | 1.30 |
| 34 | 16.006 | 16.021 | (+)-Cycloisosativene | C15H24 | 0.05 |
| 35 | 16.108 | 16.117 | Humulen-(v1) | C15H24 | 0.92 |
| 36 | 16.210 | 16.215 | Longifolene-(V4) | C15H24 | 6.82 |
| 37 | 16.321 | 16.327 | α-Guaiene | C15H24 | 0.49 |
| 38 | 16.419 | - | Patchoulene | C15H24 | 0.88 |
| 39 | 16.721 | 16.730 | β-Himachalene | C15H24 | 5.26 |
| 40 | 17.077 | 17.086 | β-Guaiene | C15H24 | 0.72 |
| 41 | 17.162 | 17.184 | Dihydroactinidiolide | C11H16O2 | 2.14 |
| 42 | 17.275 | 17.292 | δ-Cadinene | C15H24 | 1.53 |
| 43 | 17.321 | 17.334 | β-Cadinece | C15H24 | 0.56 |
| 44 | 17.510 | - | Valencene | C15H24 | 0.67 |
| 45 | 18.710 | 18.722 | Caryophyllenyl alcohol | C15H26O | 0.31 |
| 46 | 19.076 | 19.085 | Caryophyllene oxide | C15H24O | 0.85 |
| 47 | 19.142 | 19.155 | Drimenol | C15H26O | 1.27 |
| 48 | 19.470 | - | 3-Isobutyl-4,5-dimethyl-3H-isobenzofuran-1-one | C14H18O2 | 0.06 |
| 49 | 19.732 | 19.740 | Cedrol | C15H26O | 0.83 |
| 50 | 19.918 | 19.932 | Bulnesol | C15H26O | 4.17 |
| 51 | 20.235 | - | Mansonone C | C15H26O2 | 0.07 |
| 52 | 20.342 | 20.360 | Epiglobulol | C15H26O | 0.78 |
| 53 | 20.621 | 20.647 | Cubenol | C15H26O | 1.74 |
| 54 | 20.967 | 20.982 | ι-Cadinol | C15H26O | 0.56 |
| 55 | 21.151 | 21.165 | δ-Cadinol | C15H26O | 1.82 |
| 56 | 21.494 | 21.522 | Torreyol | C15H26O | 0.38 |
| 57 | 21.662 | 21.684 | β-Selinenol | C15H26O | 7.12 |
| 58 | 21.867 | 21.887 | α-Eudesmol | C15H26O | 5.16 |
| 59 | 22.061 | 22.077 | Cadalene | C15H18 | 2.22 |
| 60 | 23.167 | 23.185 | Hedycaryol | C15H26O | 0.51 |
| 61 | 23.286 | 23.310 | Peruviol | C15H26O | 1.26 |
| 62 | 26.172 | 26.190 | Farnesol isomer a | C15H26O | 4.87 |
| 63 | 26.362 | 26.384 | cis-Farnesal | C15H24O | 0.46 |
| 64 | 26.471 | - | Tetradecanoic acid | C14H28O2 | 0.24 |
| 65 | 33.571 | 33.590 | Hexadecanoic acid | C16H32O2 | 0.58 |
2.2. Quantitative Analysis
2.3. Identification of Common Components
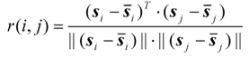
 denotes the mean of the spectral vector, and
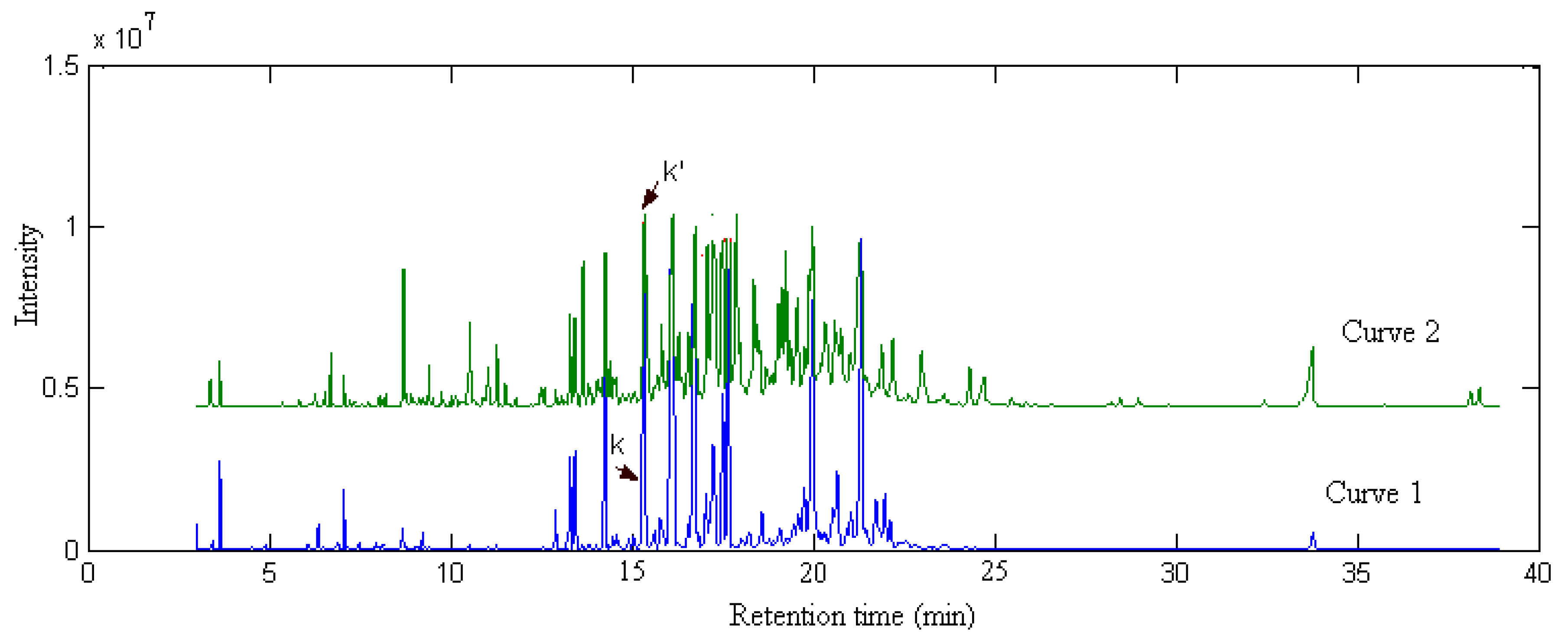
denotes the mean of the spectral vector, and  is Frobenius norm. The values r(i, j) of spectral correlation coefficient are in the range −1 ≤ r ≤ 1, and the larger the value of r is the more correlative are the components between the ith and the jth peak. When r equals to 1, these two components are identical. On account of errors and interference from noise and background, etc., in actual systems, the maximum of r is not equal to 1 but close to 1. In order to check if the component existing in X1 really also exists in X2, we may first extract si (the component to be investigated) from X1 and then calculate its corresponding correlation coefficient with every row vector, say xjT (j = 1, …, m), in X2 as shown in Equation (2)?:
is Frobenius norm. The values r(i, j) of spectral correlation coefficient are in the range −1 ≤ r ≤ 1, and the larger the value of r is the more correlative are the components between the ith and the jth peak. When r equals to 1, these two components are identical. On account of errors and interference from noise and background, etc., in actual systems, the maximum of r is not equal to 1 but close to 1. In order to check if the component existing in X1 really also exists in X2, we may first extract si (the component to be investigated) from X1 and then calculate its corresponding correlation coefficient with every row vector, say xjT (j = 1, …, m), in X2 as shown in Equation (2)?: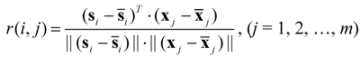
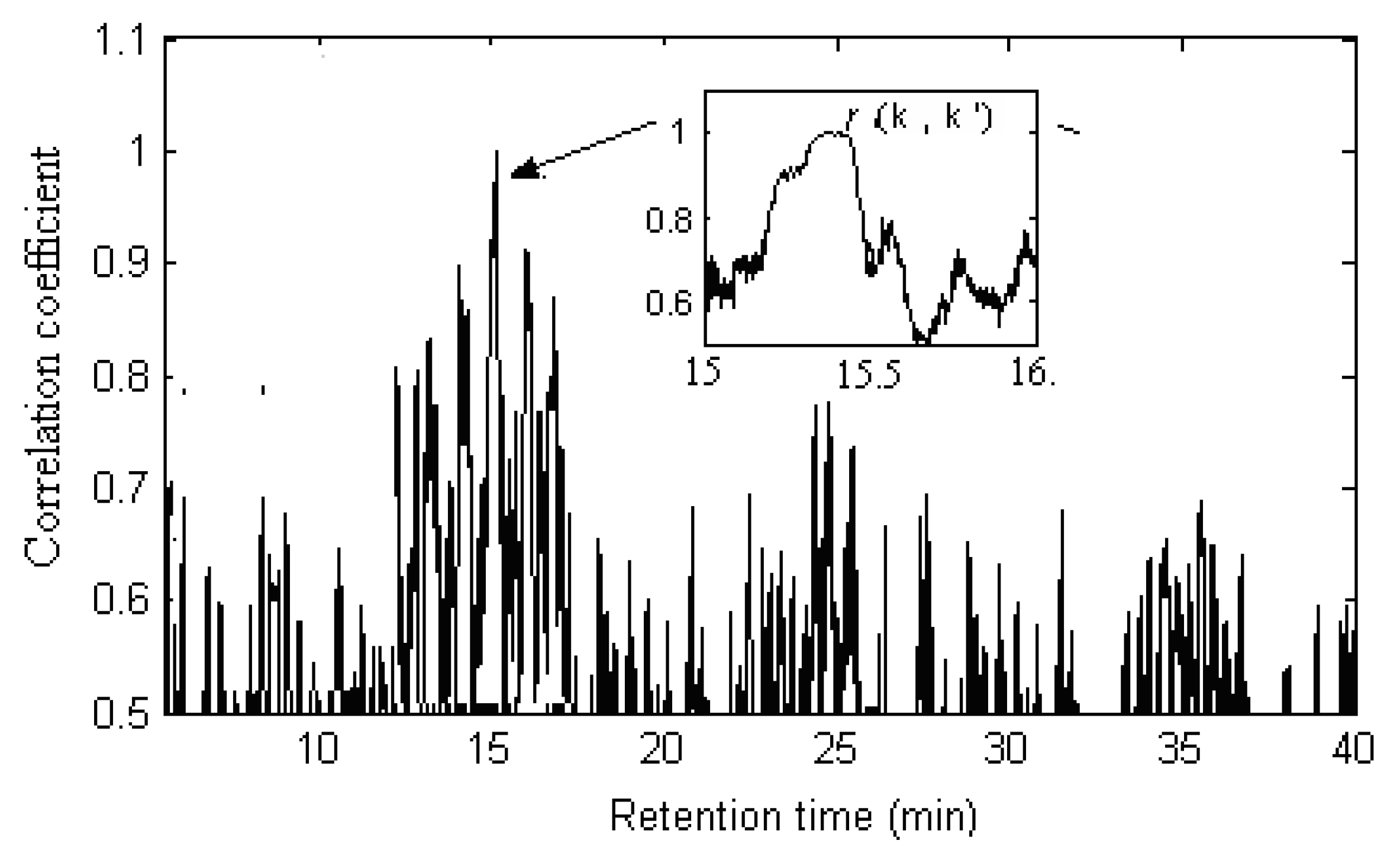
2.4. Comparison of Samples
| Series No. | Retention time (min) | Compound name | Molecule structure |
|---|---|---|---|
| 1 | 6.914 | m-Cymene | C10H16 |
| 2 | 9.128 | Octanoic acid | C8H16O2 |
| 3 | 10.719 | 4-Hydroxy-3-methylacetophenone | C9H10O2 |
| 4 | 11.526 | Di-epi-α-cedrene | C10H18O |
| 5 | 14.430 | α-Cedrene | C15H32 |
| 6 | 15.112 | 1 H-Benzocycloheptene, 2,4a,5,6,7,8,9,9a-octahydro-3,5,5-trimethyl-9-methylene- | C15H24 |
| 7 | 15.672 | Dihydrocurcumene | C15H24 |
| 8 | 16.541 | α-Muurolene | C15H24 |
| 9 | 17.512 | Eudesma-3,7(11)-diene | C15H24 |
| 10 | 19.513 | Caryophyllenyl alcohol | C15H26O |
| 11 | 20.247 | 1-Cyclohexen-1-ol, 2,6-dimethyl-, acetate | C10H16O2 |
| 12 | 26.490 | (E)-10-Pentadecenol | C15H30O |
| 13 | 34.172 | cis-7-Tetradecen-1-ol | C14H28O |
3. Experimental
3.1. Instrumentation and Materials
3.2. Extraction of the Essential Oil
3.3. Gas Chromatography-Mass Spectrometry
3.4. Data Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the extracts from Radix Flemingiae Macrophyllae and Radix Flemingiae Latifolia Benth are available from the authors.
References
- Chen, Q.; Li, X.Y.; Cai, Y.; Zhu, Y.L.; Chen, S.Q. Pharmacognostical identification of Flemingia fruticulosa. West China J. Pharm. Sci. 2012, 27, 83–85. [Google Scholar]
- Zeng, C.L.; Zhong, Z.X.; Lu, W.J.; Wang, J.; Li, S.B.; Li, X.J. Study on the Pharmacological Action of Flemingia macrophylla(Willd.) Prain. Guiding J. Tradit. Chin. Med. Pharm. 2011, 17, 79–81. [Google Scholar]
- Shiao, Y.J.; Wang, C.N.; Wang, W.Y. Neuroprotective flavonoids from Flemingia macrophylla. Planta Med. 2005, 71, 835–840. [Google Scholar] [CrossRef]
- Li, D.Y.; Fu, Y.B.; Hua, H.M.; Li, Z.L. Chemical constituents from roots of Flemingia macrophylla. Chi. Tradit. Herbal Drugs 2012, 43, 1259–1262. [Google Scholar]
- Li, L.; Liu, Z.H.; Qin, M.J. Simultaneous Determination of Seven Flavonoids in the Flemingia macrophylla by HPLC. Chin. Wild Plant Resour. 2011, 30, 54–58. [Google Scholar]
- Ahn, E.M.; Nakamura, N.; Akao, T. Estrogenic and Antiestrogenic Activities of the Roots of Moghania philippinensis and Their Constituents. Biol. Pharm. Bull. 2004, 27, 548–553. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.L.; Han, G.H. Chemical constituents of the roots of Moghania philippinensis. Chin. J. Med. Chem. 2009, 19, 364–371. [Google Scholar]
- Wang, X.Q.; Yang, S.D.; Yang, Z.Y. Study on the chemical constituents of volatile components from Radix Flemingiae Macrophyllae. J. Yunnan Univ. Tradit. Chin. Med. 2008, 31, 12–15. [Google Scholar]
- Liu, J.H.; Gao, L.X.; Gao, Y.Q. Study on the chemical constituents of volatile oil from Radix Flemingiae Macrophyllae. Chin. Tradit. Pat. Med. 2003, 25, 485–487. [Google Scholar]
- Wei, D.; Edmund, R. Malinowski.Investigation of copper (II)- Ethylenediaminetetraacetate complexation by window factor analysis of ultraviolet spectra. J. Chemom. 1993, 7, 89–98. [Google Scholar]
- Shen, H.L.; Manne, R.; Xu, Q.S.; Chen, D.Z.; Liang, YZ. Local resolution ofhyphenated chromatographic data. Chemom. Intell. Lab. Syst. 1999, 45, 323–330. [Google Scholar] [CrossRef]
- Xu, C.J.; Jiang, J.H.; Liang, Y.Z. Evolving window orthogonal projections method for two-way Data Resolution. Analyst 1999, 124, 1471–1476. [Google Scholar] [CrossRef]
- Sanchez, F.C.; Rutan, S.C.; Gil Garcia, N.D. Resolution of multicomponent overlapped peaks by the orthogonal projection approach, evolving factor analysis and window factor analysis. Chemom. Intell. Lab. Syst. 1997, 36, 153–164. [Google Scholar] [CrossRef]
- Manne, R.; Shen, H.L.; Liang, Y.Z. Subwindow factor analysis. Chemom. Intell. Lab. Syst. 1999, 45, 171–176. [Google Scholar] [CrossRef]
- Li, B.Y.; Hu, Y.; Liang, Y.Z.; Huang, L.F.; Xu, C.J.; Xie, P.S. Spectral correlative chromatography and its application to analysis of chromatographic fingerprints of herbal medicines. J. Sep. Sci. 2003, 27, 581–588. [Google Scholar]
- Liang, Y.Z. White, Grey and Black Multicomponent Systems and Their Chemometric Algorithms; Hunan Publishing House of Science and Technology: Changsha, China, 1996. [Google Scholar]
- Guo, F.Q.; Liang, Y.Z.; Xu, C.J.; Huang, L.F. Determination of the volatile chemical constituents of Notoptergium Incium by gas chromatography- mass spectrometry and iterative or non-iterative chemometrics resolution methods. J. Chromatogr. A 1016, 99–110. [Google Scholar]
- Wang, Y.M.; Yi, L.Z.; Liang, Y.Z.; Li, H.D.; Yuan, D.L.; Gao, H.Y.; Zeng, M.M. Comparative analysis of essential oil components in Pericarpium Citri Reticulatae Viride and Pericarpium Citri Reticulatae by GC-MS combined with chemometric resolution method. J. Pharm. Biomed. Anal. 2008, 46, 66–74. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee, Chinese Pharmacopoeia; Publishing House of People’s Health: Beijing, China, 2000.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, S.-Y.; Xie, Y.; Feng, X.-L.; Huang, L.-F. Study of the Volatile Constituents in Radix Flemingiae Macrophyllae and a Substitute by Gas Chromatography-Mass Spectrometry and Chemometric Methods. Molecules 2012, 17, 14111-14125. https://doi.org/10.3390/molecules171214111
Cheng S-Y, Xie Y, Feng X-L, Huang L-F. Study of the Volatile Constituents in Radix Flemingiae Macrophyllae and a Substitute by Gas Chromatography-Mass Spectrometry and Chemometric Methods. Molecules. 2012; 17(12):14111-14125. https://doi.org/10.3390/molecules171214111
Chicago/Turabian StyleCheng, Shen-Yu, Yan Xie, Xiao-Liang Feng, and Lan-Fang Huang. 2012. "Study of the Volatile Constituents in Radix Flemingiae Macrophyllae and a Substitute by Gas Chromatography-Mass Spectrometry and Chemometric Methods" Molecules 17, no. 12: 14111-14125. https://doi.org/10.3390/molecules171214111
APA StyleCheng, S.-Y., Xie, Y., Feng, X.-L., & Huang, L.-F. (2012). Study of the Volatile Constituents in Radix Flemingiae Macrophyllae and a Substitute by Gas Chromatography-Mass Spectrometry and Chemometric Methods. Molecules, 17(12), 14111-14125. https://doi.org/10.3390/molecules171214111




