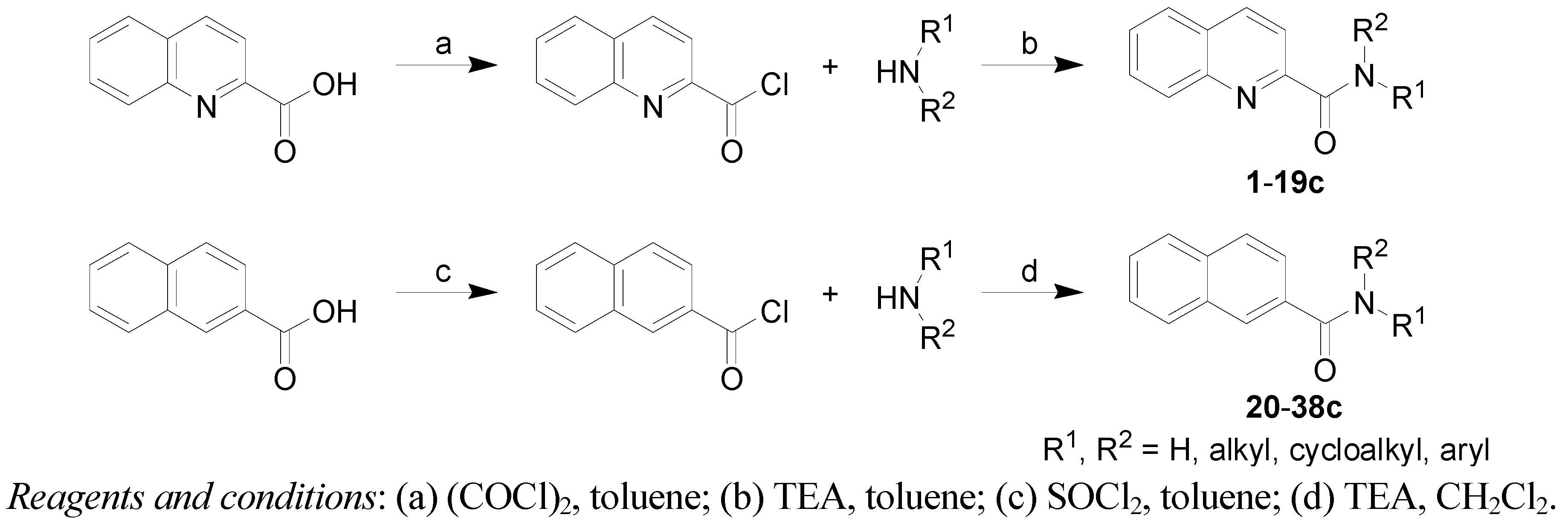
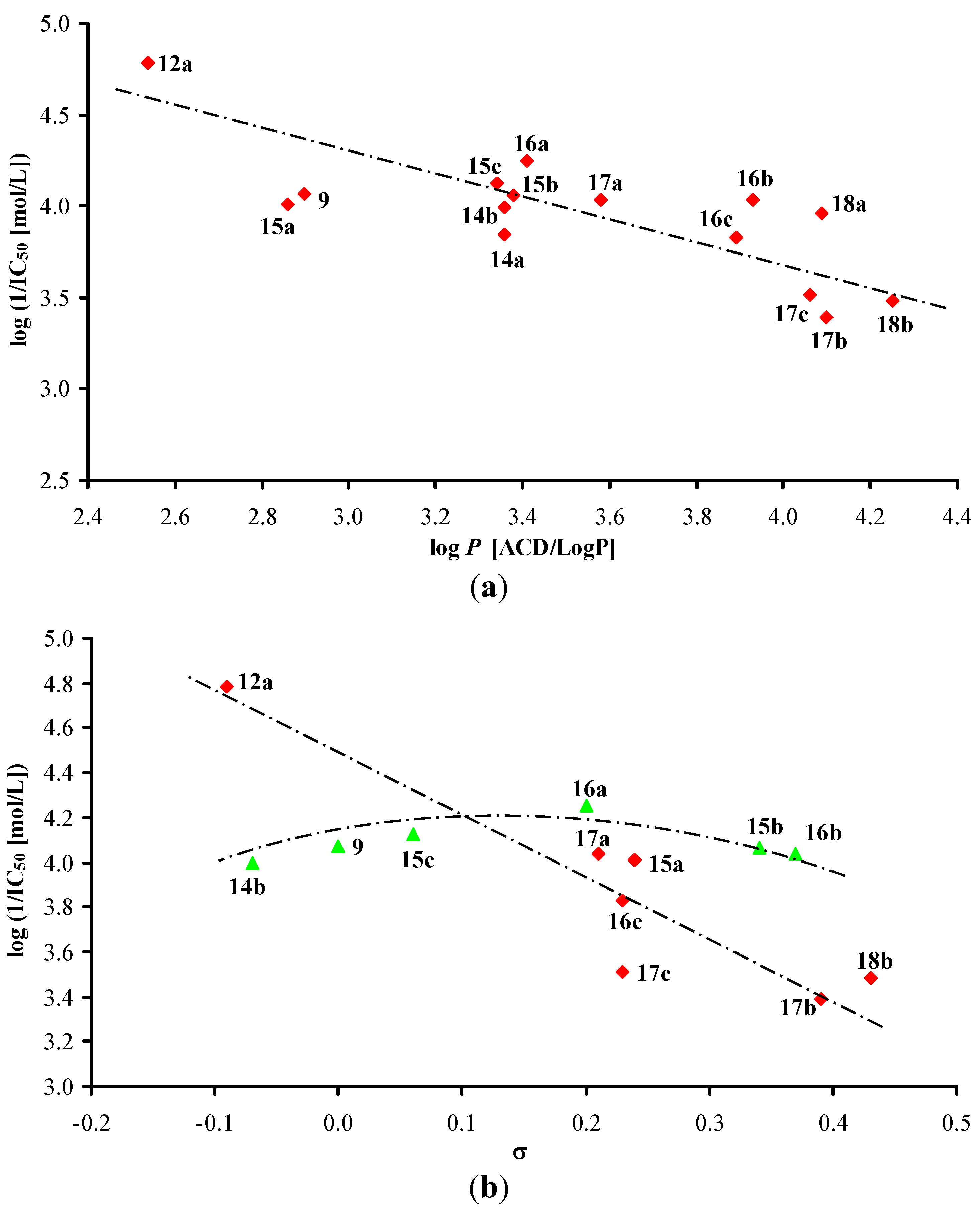
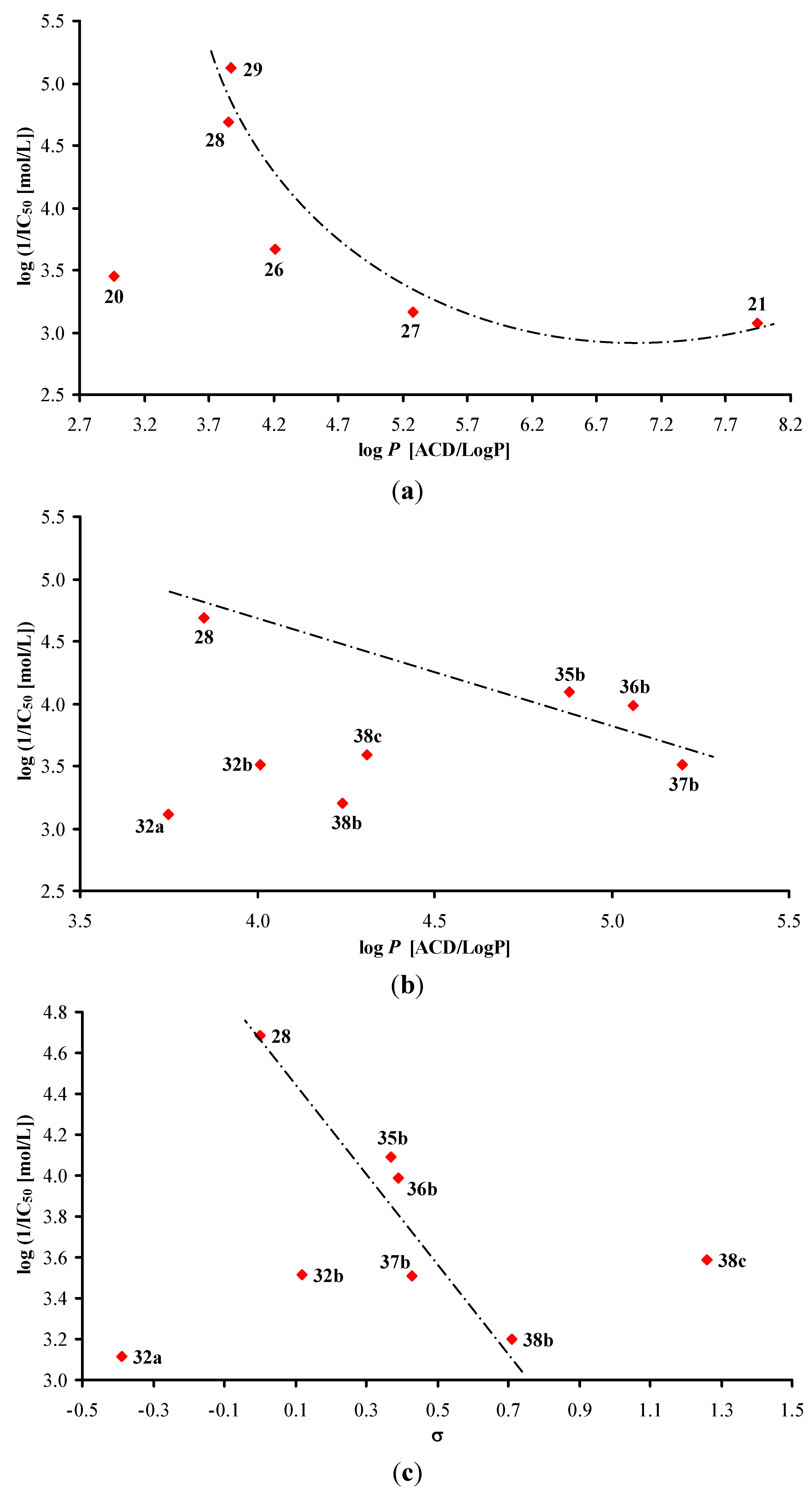
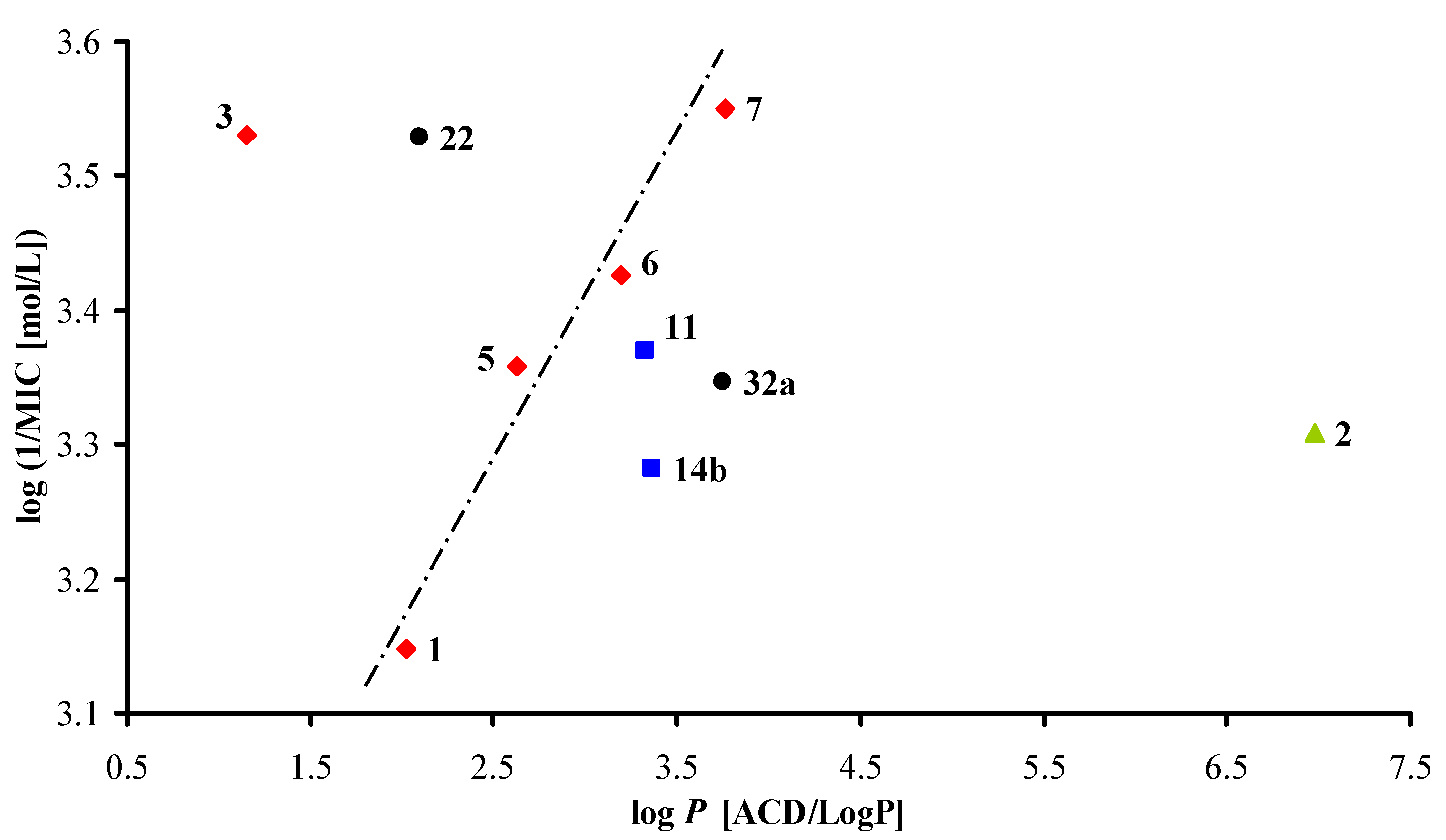
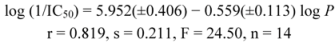3.2.1. General Procedure for Synthesis of Carboxamide Derivatives 1–19c
2-Quinaldic acid (1 g, 5.8 mmol) was suspended in dry toluene (15 mL) at room temperature and oxalyl chloride (1 mL, 1.61 g, 12.7 mmol, 2.2 eq.) was added dropwise. The reaction mixture was stirred for 30 min at the same temperature and then DMF (2 drops) was added. The mixture was stirred for 24 h and then evaporated to dryness. The residue was washed with petroleum ether and used directly in the next step. Into the solution of 2-quinaldic acid chloride in dry toluene (15 mL), triethylamine (4.5 mL, 2.92 g, 32.5 mmol) and corresponding substituted aniline (5.8 mmol) were added dropwise. The mixture was stirred at room temperature for 24 h after which the solvent was removed under reduced pressure. The residue was extracted with CHCl
3. Combined organic layers were washed with water and saturated aqueous solution of NaHCO
3 and dried over anhydrous MgSO
4. The solvent was evaporated to dryness under reduced pressure. The crude product was recrystallized from isopropanol or EtOAc. The studied compounds
1–
19c are presented in
Table 1.
N-Isopropylquinoline-2-carboxamide (1). Yield 40%; Mp. 75–76 °C; IR (Zn/Se ATR, cm−1): 3,339m, 3,052w, 2,925w, 2,852w, 1,676s, 1,589m, 1,556m, 1,525s, 1,501m, 1,434w, 1,418w, 1,205w, 1,119m, 903w, 841w, 770m, 747m, 686w, 666w; 1H-NMR (DMSO-d6), δ: 8.60 (d, J = 8.0 Hz, 1H), 8.54 (d, J = 8.5 Hz, 1H), 8.16 (d, J = 8.5 Hz, 2H), 8.05 (d, J = 8.3 Hz, 1H), 7.80–7.89 (m, 1H), 7.64–7.74 (m, 1H), 4.03–4.28 (m, 1H), 1.23 (d, J = 6.8 Hz, 6H); 13C-NMR (DMSO-d6), δ: 163.02, 150.32, 145.96, 137.83, 130.45, 129.18, 128.76, 128.07, 127.98, 118.65, 40.94, 22.27; HR-MS: for C13H15N2O [M+H]+ calculated 215.1179 m/z, found 215.1139 m/z.
N-Dodecylquinoline-2-carboxamide (2). Yield 24%; Mp. 45–46 °C; IR (Zn/Se ATR, cm−1): 3,411s, 3,052w, 2,983w, 2,901w, 1,671s, 1,589w, 1,561m, 1,521m, 1,492m, 1,450w, 1,423s, 1,201w, 1,131w, 1,107w, 1,074w, 1,054w, 907w, 841m, 768w, 735m, 696m; 1H-NMR (DMSO-d6), δ: 8.88 (t, J = 6.0 Hz, 1H), 8.53 (d, J = 8.3 Hz, 1H), 8.15 (d, J = 8.5 Hz, 1H), 8.11 (d, J = 8.5 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 7.81–7.86 (m, 1H), 7.65–7.71 (m, 1H), 3.31–3.38 (m, 2H), 1.54 (quin, J = 6.9 Hz, 2H), 1.10–1.31 (m, 18H), 0.78 (t, J = 6.9 Hz, 3H); 13C-NMR (DMSO-d6), δ: 163.78, 150.29, 145.99, 137.72, 130.37, 129.13, 128.76, 128.04, 127.89, 118.60, 38.96, 31.30, 29.24, 29.06, 29.03, 29.02, 29.00, 28.81, 28.73, 26.49, 22.09, 13.89; HR-MS: for C22H33N2O [M+H]+ calculated 341.2587 m/z, found 341.2599 m/z.
2-(Pyrrolidin-1-ylcarbonyl)quinoline (
3) [
39]. Yield 41%; Mp. 71–72 °C; IR (Zn/Se ATR, cm
−1): 3,291
m, 3,064
w, 2,919
s, 2,844
w, 1,644
s, 1,561
w, 1,532
s, 1,498
m, 1,467
m, 1,438
w, 1,422
m, 1,344
w, 1,324
w, 1,201
w, 1,168
m, 1,070
m, 1,009
w, 903
m, 842
m, 774
s, 735
m, 678
m;
1H-NMR (DMSO-
d6), δ: 8.46 (d,
J = 8.5 Hz, 1H), 8.03 (t,
J = 9.2 Hz, 2H), 7.77–7.83 (m, 2H), 7.66 (ddd,
J = 8.0 Hz,
J = 6.9 Hz,
J = 1.1 Hz, 1H), 3.67 (t,
J = 6.4 Hz, 2H), 3.55 (t,
J = 6.5 Hz, 2H), 1.78–1.89 (m, 4H);
13C-NMR (DMSO-
d6), δ: 165.56, 154.22, 145.87, 137.07, 130.14, 129.15, 127.90, 127.75, 127.64, 120.41, 48.46, 46.39, 26.05, 23.59; HR-MS: for C
14H
15N
2O [M+H]
+ calculated 227.1179
m/z, found 227.1203
m/z.
2-(Piperidin-1-ylcarbonyl)quinoline (
4) [
40]. Yield 59%; Mp. 83–84 °C; IR (Zn/Se ATR, cm
−1): 3,327
s, 3,023
w, 2,934
w, 1,655
s, 1,561
w, 1,522
m, 1,495
m, 1,454
m, 1,426
s, 1,360
m, 1,331
w, 1,230
w, 1,209
w, 1,156
m, 1,074
w, 837
m, 772
w, 735
m, 701
m;
1H-NMR (DMSO-
d6), δ: 8.48 (d,
J = 8.3 Hz, 1H), 7.96–8.07 (m, 2H), 7.81 (ddd,
J = 8.5 Hz,
J = 6.8 Hz,
J = 1.5 Hz, 1H), 7.57–7.71 (m, 2H), 3.61–3.69 (m, 2H), 3.22–3.35 (m, 2H), 1.38–1.73 (m, 6H);
13C-NMR (DMSO-
d6), δ: 166.63, 154.67, 146.14, 137.34, 130.26, 128.99, 128.00, 127.46, 127.38, 120.04, 47.45, 42.22, 25.97, 25.31, 23.97; HR-MS: for C
15H
17N
2O [M+H]
+ calculated 241.1335
m/z, found 241.1384
m/z.
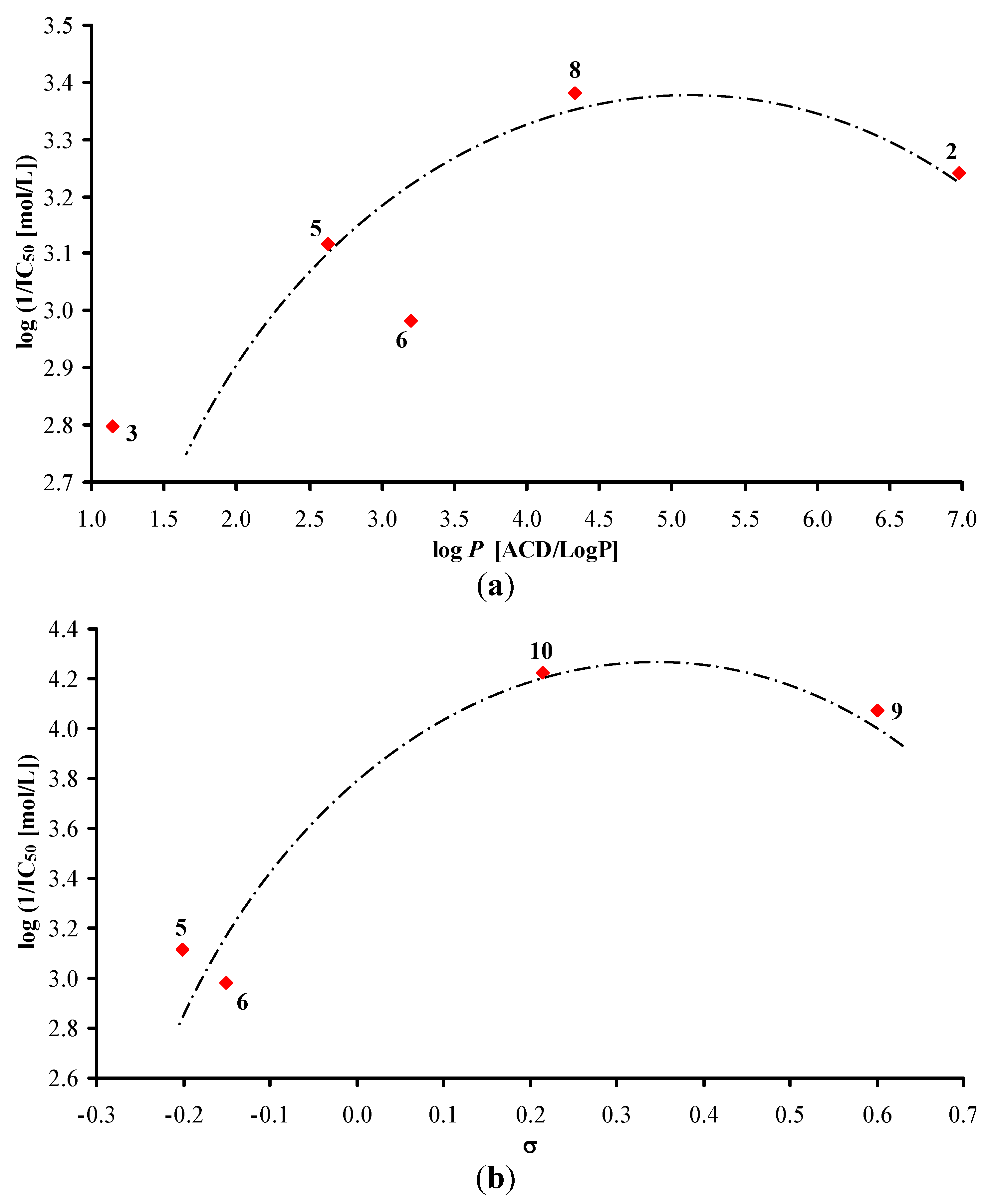
N-Cyclopentylquinoline-2-carboxamide (5). Yield 36%; Mp. 63–64 °C; IR (Zn/Se ATR, cm−1): 3,387m, 3,305m, 2,933m, 2,848m, 1,655s, 1,614w, 1,561w, 1,524s, 1,497s, 1,446m, 1,373w, 1,205w, 1,160m, 1,136m, 1,074w, 849m, 796w, 779s, 735w; 1H-NMR (DMSO-d6), δ: 8.68 (d, J = 8.0 Hz, 1H), 8.54 (d, J = 8.5 Hz, 1H), 8.15 (dd, J = 8.4 Hz, J = 2.6 Hz, 2H), 8.06 (d, J = 8.0 Hz, 1H), 7.85 (dd, J = 8.3 Hz, J = 7.0 Hz, 1H), 7.65–7.74 (m, 1H), 4.30 (sxt, J = 7.3 Hz, 1H), 1.85–2.01 (m, 2H), 1.48–1.74 (m, 6H); 13C-NMR (DMSO‑d6), δ: 163.59, 150.30, 145.97, 137.85, 130.46, 129.22, 128.77, 128.09, 128.00, 118.66, 50.72, 32.17, 23.60; HR-MS: for C15H17N2O [M+H]+ calculated 241.1335 m/z, found 241.1375 m/z.
N-Cyclohexylquinoline-2-carboxamide (
6) [
40]. Yield 40%; Mp. 98–99 °C; IR (Zn/Se ATR, cm
−1): 3,329
m, 3,313
m, 2,969
s, 2,933
m, 2,875
m, 1,648
s, 1,556
w, 1,522
s, 1,498
s, 1,470
m, 1,423
s, 1,382
w, 1,361
m, 1,341
w, 1,324
w, 1,201
w, 1,178
m, 1,130
m, 1,105
w, 903
w, 844
m, 776
w, 735
w, 674
w;
1H-NMR (DMSO-
d6), δ: 8.57 (d,
J = 8.5 Hz, 1H), 8.54 (d,
J = 8.5 Hz, 1H), 8.16 (d,
J = 8.3 Hz, 2H), 8.06 (d,
J = 8.0 Hz, 1H), 7.81–7.88 (m, 1H), 7.66–7.72 (m, 1H), 3.78–3.89 (m, 1H), 1.06–1.88 (m, 10H);
13C-NMR (DMSO-
d6), δ: 162.92, 150.27, 145.94, 137.84, 130.44, 129.20, 128.77, 128.06, 127.98, 118.64, 48.12, 32.26, 25.12, 24.83; HR-MS: for C
16H
19N
2O [M+H]
+ calculated 255.1492
m/z, found 255.1525
m/z.
N-Cycloheptylquinoline-2-carboxamide (7). Yield 39%; Mp. 72–73 °C; IR (Zn/Se ATR, cm−1): 3,056w, 2,958m, 2,864m, 1,615s, 1,552m, 1,469m, 1,436m, 1,417s, 1,373w, 1,332w, 1,205w, 1,181w, 1,156w, 842m, 764m, 731w, 657w; 1H-NMR (DMSO-d6), δ: 8.61 (d, J = 8.5 Hz, 1H), 8.54 (d, J = 8.3 Hz, 1H), 8.15 (d, J = 8.5 Hz, 2H), 8.06 (d, J = 7.5 Hz, 1H), 7.85 (ddd, J = 8.4 Hz, J = 6.9 Hz, J = 1.3 Hz, 1H), 7.69 (ddd, J = 8.0 Hz, J = 6.9 Hz, J = 1.1 Hz, 1H), 4.01 (qt, J = 9.0 Hz, J = 4.5 Hz, 1H), 1.81–1.93 (m, 2H), 1.37–1.72 (m, 10H); 13C-NMR (DMSO-d6), δ: 162.64, 150.30, 145.94, 137.85, 130.45, 129.21, 128.78, 128.06, 127.98, 118.63, 50.32, 34.30, 27.57, 23.88; HR-MS: for C17H21N2O [M+H]+ calculated 269.1648 m/z, found 269.1619 m/z.
N-Cyclooctylquinoline-2-carboxamide (
8) [
41]. Yield 44%; Mp. 72–73 °C; IR (Zn/Se ATR, cm
−1): 3,301
w, 2,954
m, 2,916
s, 2,848
s, 1,667
s, 1,649
s, 1,593
w, 1,532
s, 1,498
s, 1,462
m, 1,418
m, 1,373
w, 1,340
w, 1,315
w, 1,295
m, 1,209
w, 1,168
m, 1136
w, 1,111
w, 903
w, 850
s, 796
w, 772
s, 735
m, 715
w, 666
w;
1H-NMR (DMSO-
d6), δ: 8.59 (d,
J = 7.8 Hz, 1H), 8.55 (d,
J = 8.5 Hz, 1H), 8.15 (d,
J = 8.3 Hz, 2H), 8.06 (d,
J = 8.0 Hz, 1H), 7.81–7.88 (m, 1H), 7.66–7.73 (m, 1H), 4.00–4.12 (m, 1H), 1.45–1.81 (m, 14H);
13C-NMR (DMSO-
d6), δ: 162.60, 150.32, 145.94, 137.85, 130.45, 129.21, 128.78, 128.06, 127.98, 118.61, 49.11, 31.80, 26.71, 25.00, 23.50; HR-MS: for C
18H
23N
2O [M+H]
+ calculated 283.1805
m/z, found 283.1777
m/z.
N-Phenylquinoline-2-carboxamide (
9). Yield 59%; Mp. 139–140 °C (Mp. 139.5–140 °C [
42]); IR (Zn/Se ATR, cm
−1): 3,293
m, 2,927
s, 2,855
m, 1,643
s, 1,562
m, 1,530
s, 1,500
s, 1,426
m, 1,315
w, 1,205
w, 1,185
w, 1,160
w, 1,070
w, 1,042
w, 910
m, 870
w, 841
m, 776
m, 731
w;
1H-NMR (DMSO-
d6), δ: 10.75 (bs, 1H), 8.62 (d,
J = 8.5 Hz, 1H), 8.22–8.30 (m, 2H), 8.11 (d,
J = 7.5 Hz, 1H), 7.94–7.99 (m, 2H), 7.91 (ddd,
J = 8.5 Hz,
J = 7.0 Hz,
J = 1.4 Hz, 1H), 7.75 (ddd,
J = 8.1 Hz,
J = 7.0 Hz,
J = 1.3 Hz, 1H), 7.38–7.45 (m, 2H), 7.12–7.19 (m, 1H);
13C-NMR (DMSO-
d6), δ: 162.71, 150.08, 145.88, 138.31, 138.22, 130.68, 129.35, 128.94, 128.78, 128.37, 128.15, 124.05, 120.29, 118.77; HR-MS: for C
16H
13N
2O [M+H]
+ calculated 249.1022
m/z, found 249.1015
m/z.
N-Benzylquinoline-2-carboxamide (
10). Yield 60%; Mp. 123–124 °C (Mp. 124–125 °C [
43]); IR (Zn/Se ATR, cm
−1): 3,273
m, 2,952
m, 2,856
m, 1,657
s, 1,643
s, 1,589
w, 1,562
m, 1,527
s, 1,498
s, 1,446
w, 1,424
m, 1,315
w, 1,299
w, 1,205
m, 1,181
w, 1,152
w, 1,143
w, 956
w, 899
m, 846
s, 792
m, 770
m, 776
m, 739
m, 686
m;
1H-NMR (DMSO-
d6), δ: 9.50 (t,
J = 6.4 Hz, 1H), 8.56 (d,
J = 8.3 Hz, 1H), 8.19 (d,
J = 8.5 Hz, 1H), 8.14 (d,
J = 8.5 Hz, 1H), 8.07 (dd,
J = 8.3 Hz,
J = 0.8 Hz, 1H), 7.86 (ddd,
J = 8.4 Hz,
J = 6.9 Hz,
J = 1.5 Hz, 1H), 7.70 (ddd,
J = 8.2 Hz,
J = 6.9 Hz,
J = 1.0 Hz, 1H), 7.36–7.41 (m, 2 H), 7.29–7.35 (m, 2H), 7.20–7.26 (m, 1H), 4.59 (d,
J = 6.5 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 164.13, 150.15, 146.04, 139.52, 137.88, 130.52, 129.19, 128.84, 128.30, 128.11, 128.07, 127.46, 126.82, 118.74, 42.56; HR-MS: for C
17H
15N
2O [M+H]
+ calculated 263.1179
m/z, found 263.1202
m/z.
N-(2-Phenylethyl)quinoline-2-carboxamide (
11) [
44]. Yield 38%; Mp. 80–81 °C; IR (Zn/Se ATR, cm
−1): 3,236
w, 2,935
s, 2,852
m, 1,633
s, 1,552
w, 1,467
m, 1,450
m, 1,422
m, 1,344
w, 1,279
m, 1,103
w, 1,025
w, 1,005
w, 952
w, 890
w, 841
m, 760
m, 731
w, 682
w;
1H-NMR (DMSO-
d6), δ: 9.00 (t,
J = 5.90 Hz, 1H), 8.54 (d,
J = 8.5 Hz, 1H), 8.17 (d,
J = 8.5 Hz, 1H), 8.11 (d,
J = 8.5 Hz, 1H), 8.06 (d,
J = 8.0 Hz, 1H), 7.85 (td,
J = 7.7 Hz,
J = 1.3 Hz, 1H), 7.66–7.73 (m, 1H), 7.25–7.33 (m, 4H), 7.16–7.22 (m, 1H), 3.58–3.65 (m, 2H), 2.92 (t,
J = 7.5 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 163.88, 150.14, 145.96, 139.36, 137.83, 130.48, 129.13, 128.78, 128.64, 128.38, 128.08, 128.00, 126.14, 118.59, 40.58, 35.23; HR-MS: for C
18H
17N
2O [M+H]
+ calculated 277.1335
m/z, found 277.1384
m/z.
N-(2-Hydroxyphenyl)quinoline-2-carboxamide (
12a) [
45]. Yield 27%; Mp. 219–220 °C;
1H-NMR (DMSO-
d6), δ: 10.67 (bs, 1H), 10.44 (bs, 1H), 8.63 (d,
J = 8.5 Hz, 1H), 8.44 (d,
J = 7.8 Hz, 1H), 8.29 (d,
J = 8.3 Hz, 1H), 8.15 (d,
J = 8.3 Hz, 1H), 8.10 (d,
J = 8.0 Hz, 1H), 7.88 (t,
J = 7.3 Hz, 1H), 7.73 (t,
J = 7.2 Hz, 1H), 7.00 (m, 2H), 6.89 (m, 1H);
13C-NMR (DMSO-
d6), δ: 161.26, 149.65, 146.77, 145.74, 138.59, 130.91, 129.29, 129.08, 128.46, 128.22, 126.20, 124.29, 119.37, 119.26, 118.42, 114.84; HR-MS: for C
16H
13N
2O
2 [M+H]
+ calculated 265.0977
m/z, found 265.0983
m/z.
N-(3-Hydroxyphenyl)quinoline-2-carboxamide (12b). Yield 52%; Mp. 154–155 °C; IR (Zn/Se ATR, cm−1): 3,338w, 2,973w, 1,760m, 1,680m, 1,605w, 1,530s, 1,504s, 1,462w, 1,425m, 1,263m, 1,235m, 1,216m, 1,171w, 1,145s, 1,096m, 1,054w, 1,002w, 964w, 908m, 879w, 841m, 773s, 754s, 685m, 665m; 1H-NMR (DMSO-d6), δ: 10.98 (bs, 1H), 8.62 (d, J = 8.7 Hz, 1H), 8.20–8.36 (m, 3H), 8.06–8.18 (m, 1H), 7.87–7.97 (m, 2H), 7.71–7.83 (m, 2H), 7.54 (t, J = 8.2 Hz, 1H), 7.18 (dd, J = 8.0 Hz, J = 1.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.05, 150.91, 149.86, 147.01, 145.90, 139.63, 138.29, 130.94, 129.99, 129.19, 128.49, 128.16, 121.35, 118.83, 117.99, 113.65; HR-MS: for C16H13N2O2 [M+H]+ calculated 265.0977 m/z, found 265.0985 m/z.
N-(4-Hydroxyphenyl)quinoline-2-carboxamide (
12c) [
46]. Yield 48%; Mp. 230–231 °C;
1H-NMR (DMSO-
d6), δ: 10.34 (bs, 1H), 8.28–8.48 (m, 3H), 8.21 (d,
J = 8.5 Hz, 1H), 7.88–8.04 (m, 3H), 7.77–7.87 (m, 1H), 7.69 (m, 1H), 7.39 (d,
J = 9.0 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 162.11, 149.40, 147.23, 146.23, 137.94, 135.82, 130.84, 129.62, 129.45, 128.95, 127.60, 122.38, 120.66, 118.73; HR-MS: for C
16H
13N
2O
2 [M+H]
+ calculated 265.0977
m/z, found 265.0971
m/z.
N-(2-Methoxyphenyl)quinoline-2-carboxamide (
13a) [
47]. Yield 37%; Mp. 111–112 °C; IR (Zn/Se ATR, cm
−1): 3,382
w, 1,676
s, 1,596
m, 1,532
s, 1,485
w, 1,454
m, 1,426
m, 1,334
w, 1,288
w, 1,253
m, 1,138
m, 1,129
m, 1,093
w, 1,020
s, 951
w, 908
m, 873
w, 840
m, 820
w, 770
s, 732
s;
1H-NMR (DMSO-
d6), δ: 10.68 (bs, 1H), 8.59 (d,
J = 8.5 Hz, 1H), 8.49 (d,
J = 7.8 Hz, 1H), 8.25 (d,
J = 8.5 Hz, 1H), 8.15 (d,
J = 8.5 Hz, 1H), 8.07 (d,
J = 8.3 Hz, 1H), 7.87 (t,
J = 7.3 Hz, 1H), 7.67–7.75 (m, 1H), 7.11 (d,
J = 4.0 Hz, 2H), 7.01 (dt,
J = 8.2 Hz,
J = 4.2 Hz, 1H), 3.98 (s, 3H);
13C-NMR (DMSO-
d6), δ: 161.25, 149.34, 148.51, 145.62, 138.55, 130.82, 129.30, 129.06, 128.44, 128.14, 126.87, 124.25, 120.68, 118.84, 118.27, 110.91, 56.05; HR-MS: for C
17H
15N
2O
2 [M+H]
+ calculated 279.1134
m/z, found 279.1148
m/z.
N-(3-Methoxyphenyl)quinoline-2-carboxamide (13b). Yield 47%; Mp. 117–118 °C; IR (Zn/Se ATR, cm−1): 3,352w, 1,687m, 1,589m, 1,524m, 1,503m, 1,456m, 1,425 m, 1,334w, 1,284m, 1,203 m, 1,157m, 1,128m, 1,049s, 906w, 876m, 854 m, 823 w, 798w, 762s, 740s, 685m; 1H-NMR (DMSO-d6), δ: 10.73 (bs, 1H), 8.58 (d, J = 8.5 Hz, 1H), 8.19–8.32 (m, 2H), 8.07 (d, J = 8.0 Hz, 1H), 7.82–7.96 (m, 1H), 7.65–7.79 (m, 2H), 7.59 (dd, J = 8.0 Hz, J = 1.0 Hz, 1H), 7.29 (t, J = 8.2 Hz, 1H), 6.72 (dd, J = 8.3 Hz, J = 2.01 Hz, 1H), 3.78 (s, 3H); 13C-NMR (DMSO-d6), δ: 162.70, 159.61, 149.99, 145.88, 139.53, 138.23, 130.67, 129.61, 129.37, 128.97, 128.37, 128.16, 118.75, 112.47, 109.68, 105.91, 55.09; HR-MS: for C17H15N2O2 [M+H]+ calculated 279.1134 m/z, found 279.1129 m/z.
N-(4-Methoxyphenyl)quinoline-2-carboxamide (
13c) [
48]. Yield 53%; Mp. 130–131 °C;
1H-NMR (DMSO-
d6), δ: 10.65 (bs, 1H), 8.57 (d,
J = 8.5 Hz, 1H), 8.24 (d,
J = 8.5 Hz, 2H), 8.07 (d,
J = 7.8 Hz, 1H), 7.82–7.95 (m, 3H), 7.63–7.78 (m, 1H), 6.97 (d,
J = 9.0 Hz, 2H), 3.75 (s, 3H);
13C-NMR (DMSO-
d6), δ: 162.28, 155.80, 150.25, 145.90, 138.09, 131.47, 130.59, 129.32, 128.87, 128.22, 128.11, 121.85, 118.73, 113.87, 55.17; HR-MS: for C
17H
15N
2O
2 [M+H]
+ calculated 279.1134
m/z, found 279.1145
m/z.
N-(2-Methylphenyl)quinoline-2-carboxamide (14a). Yield 40%; Mp. 100–101 °C; IR (Zn/Se ATR, cm−1): 3,334w, 1,686s, 1,587s, 1,528s, 1,498m, 1,454s, 1,427s, 1,422 m, 1,373w, 1,305m, 1,249w, 1,201w, 1,132m, 1,091w, 1,040w, 1,013w, 981w, 954m, 932w, 907m, 872m, 842s, 793w, 765s, 750s, 731s, 681s; 1H-NMR (DMSO-d6), δ: 10.45 (bs, 1H), 8.60 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 8.5 Hz, 1H), 8.17 (d, J = 8.3 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.83–7.91 (m, 1H), 7.69–7.77 (m, 1H), 7.22–7.31 (m, 2H), 7.08–7.16 (m, 1H), 2.37 (s, 3H); 13C-NMR (DMSO-d6), δ: 161.92, 149.67, 145.74, 138.35, 135.97, 130.72, 130.42, 130.01, 129.38, 129.01, 128.38, 128.11, 126.40, 124.96, 122.65, 118.49, 17.49; HR-MS: for C17H15N2O [M+H]+ calculated 263.1184 m/z, found 263.1182 m/z.
N-(3-Methylphenyl)quinoline-2-carboxamide (14b). Yield 43%; Mp. 82–83 °C; IR (Zn/Se ATR, cm−1): 3,355w, 1,685m, 1,592 m, 1,527s, 1,503s 1,457w, 1,424 m, 1,300w, 1,171w, 1,125m, 908w, 852m, 773s, 740w, 690s; 1H-NMR (DMSO-d6), δ: 10.66 (bs, 1H), 8.61 (d, J = 8.5 Hz, 1H), 8.25 (dd, J = 7.9 Hz, J = 5.40 Hz, 2H), 8.10 (d, J = 8.0 Hz, 1H), 7.90 (t, J = 7.5 Hz, 1H), 7.67–7.84 (m, 3H), 7.27 (t, J = 7.7 Hz, 1H), 6.96 (d, J = 7.3 Hz, 1H), 2.32 (s, 3H); 13C-NMR (DMSO-d6), δ: 162.54, 150.03, 145.88, 138.22, 138.20, 138.03, 130.68, 129.35, 128.94, 128.65, 128.35, 128.15, 124.75, 120.72, 118.70, 117.35, 21.23; HR-MS: for C17H15N2O [M+H]+ calculated 263.1184 m/z, found 263.1191 m/z.
N-(4-Methylphenyl)quinoline-2-carboxamide (
14c). Yield 44%; Mp. 107–108 °C (Mp. 109.5–110 °C [
39]);
1H-NMR (DMSO-
d6), δ: 10.67 (bs, 1H), 8.59 (d,
J = 8.5 Hz, 1H), 8.18–8.30 (m, 2H), 8.08 (d,
J = 8.0 Hz, 1H), 7.79–7.94 (m, 3H), 7.72 (t,
J = 7.4 Hz, 1H), 7.18 (d,
J = 8.3 Hz, 2H), 2.27 (s, 3H);
13C-NMR (DMSO-
d6), δ: 162.51, 150.17, 145.93, 138.21, 135.86, 133.09, 130.69, 129.39, 129.24, 128.94, 128.35, 128.18, 120.27, 118.77, 20.59; HR‑MS: for C
17H
15N
2O [M+H]
+ calculated 263.1184
m/z, found 263.1193
m/z.
N-(2-Fluorophenyl)quinoline-2-carboxamide (15a). Yield 30%; Mp. 116–117 °C; IR (Zn/Se ATR, cm−1): 3,328w, 1,691m, 1,615m, 1,591w, 1,530s, 1,504m, 1,477w, 1,454m, 1,428m, 1,317w, 1,247w, 1,185w, 1,126m, 1,088w, 910w, 837m, 772s, 746s, 683m; 1H-NMR (DMSO-d6), δ: 10.48 (bs, 1H), 8.57 (d, J = 8.5 Hz, 1H), 8.17–8.25 (m, 2H), 8.13 (d, J = 8.5 Hz, 1H), 8.05 (d, J = 8.0 Hz, 1H), 7.85 (t, J = 7.3 Hz, 1H), 7.65–7.76 (m, 1H), 7.28–7.40 (m, 1H), 7.13–7.27 (m, 2H); 13C-NMR (DMSO-d6), δ: 162.00, 153.58 (d, 1JFC = 244 Hz), 148.95, 145.67, 138.39, 130.76, 129.24, 129.15 (d, 2JFC = 19.1 Hz), 128.45, 128.08, 125.70 (d, 3JFC = 11.0 Hz), 125.53 (d, 3JFC = 7.3 Hz), 124.63 (d, 4JFC = 3.7 Hz), 122.91, 118.37, 115.43 (d, 2JFC = 19.1 Hz); HR-MS: for C16H12FN2O [M+H]+ calculated 267.0934 m/z, found 267.0950 m/z.
N-(3-Fluorophenyl)quinoline-2-carboxamide (15b). Yield 38%; Mp. 126–127 °C; IR (Zn/Se ATR, cm−1): 3,343w, 1,690s, 1,588m, 1,531s, 1,504m, 1,481s, 1,409s, 1,170m, 1,137m, 899m, 841s, 791m, 768s, 738m, 682s; 1H-NMR (DMSO-d6), δ: 10.91 (bs, 1H), 8.58 (d, J = 8.5 Hz, 1H), 8.16–8.31 (m, 2H), 8.08 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 11.8 Hz, 1H), 7.86–7.92 (m, 1H), 7.79 (d, J = 8.3 Hz, 1H), 7.68–7.75 (m, 1H), 7.35–7.49 (m, 1H), 6.96 (td, J = 8.4 Hz, J = 2.0 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.01, 162.15 (d, 1JFC = 241 Hz), 149.73, 145.86, 140.11 (d, 3JFC = 11.0 Hz), 138.18, 130.65, 130.31 (d, 3JFC = 9.5 Hz), 129.33, 128.97, 128.39, 128.12, 118.77, 116.15 (d, 4JFC = 2.9 Hz), 110.47 (d, 2JFC = 21.3 Hz), 107.09 (d, 2JFC = 26.4 Hz); HR-MS: for C16H12FN2O [M+H]+ calculated 267.0934 m/z, found 267.0953 m/z.
N-(4-Fluorophenyl)quinoline-2-carboxamide (
15c) [
46,
48]. Yield 33%; Mp. 115–116 °C;
1H-NMR (DMSO-
d6), δ: 10.83 (bs, 1H), 8.57 (d,
J = 8.3 Hz, 1H), 8.17–8.29 (m, 2H), 8.06 (d,
J = 8.0 Hz, 1H), 7.94–8.02 (m, 2H), 7.87 (td,
J = 7.7 Hz,
J = 1.3 Hz, 1H), 7.66–7.76 (m, 1H), 7.17–7.28 (m, 2H);
13C-NMR (DMSO-
d6), δ: 162.76, 158.58 (d,
1JFC = 237 Hz), 150.03, 145.95, 138.18, 134.81 (d,
4JFC = 2.2 Hz), 130.67, 129.39, 128.98, 128.37, 128.17, 122.31 (d,
3JFC = 7.3 Hz), 118.83, 115.26 (d,
2JFC = 22.7 Hz); HR-MS: for C
16H
12FN
2O [M+H]
+ calculated 267.0934
m/z, found 267.0954
m/z.
N-(2-Chlorophenyl)quinoline-2-carboxamide (
16a) [
48]. Yield 39%; Mp. 130–131 °C;
1H-NMR (DMSO-
d6), δ: 10.77 (bs, 1H), 8.58 (d,
J = 8.5 Hz, 1H), 8.43 (d,
J = 8.0 Hz, 1H), 8.21 (d,
J = 8.5 Hz, 1H), 8.10 (d,
J = 8.5 Hz, 1H), 8.05 (d,
J = 8.3 Hz, 1H), 7.85 (t,
J = 7.5 Hz, 1H), 7.64–7.75 (m, 1H), 7.54 (d,
J = 7.8 Hz, 1H), 7.39 (t,
J = 7.7 Hz, 1H), 7.10–7.24 (m, 1H);
13C-NMR (DMSO-
d6), δ: 161.54, 148.70, 145.47, 138.50, 134.21, 130.75, 129.29, 129.20, 129.07, 128.46, 128.00, 127.88, 125.23, 123.38, 121.27, 118.15; HR-MS: for C
16H
12ClN
2O [M+H]
+ calculated 283.0638
m/z, found 283.0652
m/z.
N-(3-Chlorophenyl)quinoline-2-carboxamide (
16b) [
48]. Yield 46%; Mp. 127–128 °C;
1H-NMR (DMSO-
d6), δ: 10.90 (bs, 1H), 8.58 (d,
J = 8.5 Hz, 1H), 8.18–8.31 (m, 2H), 8.15 (s, 1H), 8.07 (d,
J = 8.0 Hz, 1H), 7.82–7.97 (m, 2H), 7.66–7.78 (m, 1H), 7.40 (t,
J = 8.0 Hz, 1H), 7.11–7.23 (m, 1H);
13C-NMR (DMSO-
d6), δ: 163.02, 149.67, 145.87, 139.85, 138.20, 133.12, 130.68, 130.36, 129.34, 128.98, 128.43, 128.14, 123.70, 119.82, 118.77; HR-MS: for C
16H
12ClN
2O [M+H]
+ calculated 283.0638
m/z, found 283.0648
m/z.
N-(4-Chlorophenyl)quinoline-2-carboxamide (
16c). Yield 34%; Mp. 134–135 °C (Mp. 135–135.5 °C [
39]);
1H-NMR (DMSO-
d6), δ: 10.88 (bs, 1H), 8.58 (d,
J = 8.5 Hz, 1H), 8.17–8.30 (m, 2H), 8.08 (d,
J = 8.0 Hz, 1H), 8.01 (d,
J = 8.8 Hz, 2H), 7.84–7.93 (m, 1H), 7.68–7.77 (m, 1H), 7.43 (d,
J = 8.8 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 162.87, 149.85, 145.88, 138.16, 137.34, 130.65, 129.34, 128.95, 128.62, 128.37, 128.14, 127.69, 121.92, 118.78; HR-MS: for C
16H
12ClN
2O [M+H]
+ calculated 283.0638
m/z, found 283.0631
m/z.
N-(2-Bromophenyl)quinoline-2-carboxamide (17a). Yield 26%; Mp. 134–135 °C; IR (Zn/Se ATR, cm−1): 3,277w, 1,689s, 1,588m, 1,579m, 1,543m, 1,530s, 1,496m, 1,440m, 1,427m, 1,302w, 1,132w, 1,204m, 908w, 842m, 768s, 736m, 698m; 1H-NMR (DMSO-d6), δ: 10.82 (bs, 1H), 8.60 (d, J = 8.5 Hz, 1H), 8.44 (d, J = 8.3 Hz, 1H), 8.23 (d, J = 8.5 Hz, 1H), 8.13 (d, J = 8.5 Hz, 1H), 8.07 (d, J = 8.3 Hz, 1H), 7.87 (t, J = 7.5 Hz, 1H), 7.64–7.77 (m, 2H), 7.44 (t, J = 7.8 Hz, 1H), 7.10 (t, J = 7.7 Hz, 1H); 13C-NMR (DMSO-d6), δ: 161.61, 148.71, 145.50, 138.60, 135.46, 132.58, 130.83, 129.26, 129.13, 128.55, 128.53, 128.08, 125.74, 121.39, 118.19, 114.08; HR-MS: for C16H12BrN2O [M+H]+ calculated 327.0133 m/z, found 327.0138 m/z.
N-(3-Bromophenyl)quinoline-2-carboxamide (17b). Yield 35%; Mp. 139–140 °C; IR (Zn/Se ATR, cm−1): 3,318w, 1,687m, 1,581m, 1,519m, 1,478w, 1,408m, 1,296w, 1,124m, 1,067w, 912w, 847m, 764s, 685m; 1H-NMR (DMSO-d6), δ: 10.89 (bs, 1H), 8.60 (d, J = 8.3 Hz, 1H), 8.19-8.32 (m, 3H), 8.09 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 7.5 Hz, 1H), 7.87–7.93 (m, 1H), 7.68–7.78 (m, 1H), 7.27–7.40 (m, 2H); 13C-NMR (DMSO-d6), δ: 163.02, 149.67, 145.86, 139.98, 138.21, 130.69, 130.67, 129.32, 128.97, 128.44, 128.14, 126.59, 122.66, 121.55, 119.14, 118.77; HR-MS: for C16H12BrN2O [M+H]+ calculated 327.0133 m/z, found 327.0143 m/z.
N-(4-Bromophenyl)quinoline-2-carboxamide (17c). Yield 57%; Mp. 157–158 °C; IR (Zn/Se ATR, cm−1): 3,355w, 1,693s, 1,581m, 1,522s, 1,496s, 1,423w, 1,389m, 1,305w, 1,120m, 1,095w, 1,068m, 998w, 907w, 839s, 807s, 769s, 693w; 1H-NMR (DMSO-d6), δ: 10.84 (bs, 1H), 8.58 (d, J = 8.5 Hz, 1H), 8.18–8.30 (m, 2H), 8.07 (d, J = 8.3 Hz, 1H), 7.95 (d, J = 8.8 Hz, 2H), 7.86–7.92 (m, 1H), 7.67–7.78 (m, 1H), 7.56 (d, J = 8.8 Hz, 2H); 13C-NMR (DMSO-d6), δ: 162.86, 149.82, 145.87, 138.16, 137.74, 131.53, 130.64, 129.33, 128.94, 128.37, 128.12, 122.28, 118.77, 115.80; HR-MS: for C16H12BrN2O [M+H]+ calculated 327.0133 m/z, found 327.0129 m/z.
N-(2-Trifluoromethylphenyl)quinoline-2-carboxamide (18a). Yield 32%; Mp. 120–121 °C; IR (Zn/Se ATR, cm−1): 3,316w, 1,698s, 1,590s, 1,537s, 1,498w, 1,452m, 1,423m, 1,320m, 1,288m, 1,244w, 1,202w, 1,165m, 1,124m, 1,094m, 1,054m, 1,026m, 953w, 906w, 871w, 836m, 792w, 763s, 676m; 1H-NMR (DMSO-d6), δ: 10.78 (bs, 1H), 8.61 (d, J = 8.3 Hz, 1H), 8.36 (d, J = 8.3 Hz, 1H), 8.23 (d, J = 8.3 Hz, 1H), 8.07 (t, J = 8.3 Hz, 2H), 7.87 (t, J = 7.5 Hz, 1H), 7.64–7.81 (m, 3H), 7.38 (t, J = 7.7 Hz, 1H); 13C-NMR (DMSO-d6), δ: 162.05, 148.48, 145.53, 138.74, 135.14, 133.57, 131.01, 129.48 (q, 2JFC = 37 Hz), 129.21, 129.17, 128.68, 128.16, 126.41 (q, 3JFC = 5.1 Hz), 125.05, 124.10 (q, 1JFC = 274 Hz), 123.89 (q, 3JFC = 5.9 Hz), 118.31; HR-MS: for C17H12F3N2O [M+H]+ calculated 317.0896 m/z, found 317.0891 m/z.
N-(3-Trifluoromethylphenyl)quinoline-2-carboxamide (18b). Yield 31%; Mp. 121–122 °C; IR (Zn/Se ATR, cm−1): 3,339w, 1,692s, 1,614w, 1,536m, 1,490m, 1,424w, 1,330s, 1,223w, 1,166m, 1,109s, 1,091s, 1,065m, 952w, 933w, 874s, 844m, 808s, 771s, 744w, 698s; 1H-NMR (DMSO-d6), δ: 11.08 (bs, 1H), 8.59 (d, J = 8.5 Hz, 1H), 8.46 (s, 1H), 8.17–8.31 (m, 3H), 8.08 (d, J = 8.0 Hz, 1H), 7.89 (t, J = 7.4 Hz, 1H), 7.68–7.78 (m, 1H), 7.61 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 7.5 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.26, 149.64, 145.90, 139.23, 138.21, 130.69, 129.86, 129.35 (q, 2JFC = 32 Hz), 129.34, 129.03, 128.45, 128.16, 124.20 (q, 1JFC = 273 Hz), 123.91, 120.24 (q, 3JFC = 3.7 Hz), 118.78, 116.61 (q, 3JFC = 3.7 Hz); HR-MS: for C17H12F3N2O [M+H]+ calculated 317.0896 m/z, found 317.0892 m/z.
N-(4-Trifluoromethylphenyl)quinoline-2-carboxamide (
18c) [
41,
44]. Yield 43%; Mp. 147–148 °C;
1H-NMR (DMSO-
d6), δ: 11.02 (bs, 1H), 8.59 (d,
J = 8.3 Hz, 1H), 8.26 (d,
J = 8.5 Hz, 1H), 8.23 (d,
J = 8.3 Hz, 1H), 8.19 (d,
J = 8.5 Hz, 2H), 8.08 (d,
J = 8.0 Hz, 1H), 7.86–7.93 (m, 1H), 7.69–7.77 (m, 3H);
13C-NMR (DMSO-
d6), δ: 163.27, 149.63, 145.88, 141.95, 138.23, 130.69, 129.38, 129.03, 128.48, 128.14, 125.96 (q,
3JFC = 3.7 Hz), 124.39 (q,
1JFC = 271 Hz), 124.00 (q,
2JFC = 32 Hz), 120.29, 118.81; HR-MS: for C
17H
12F
3N
2O [M+H]
+ calculated 317.0896
m/z, found 317.0890
m/z.
N-(2-Nitrophenyl)quinoline-2-carboxamide (
19a). Yield 29%; Mp. 181–182 °C (Mp. 179.5–180 °C [
39]);
1H-NMR (DMSO-
d6), δ: 12.55 (bs, 1H), 8.75 (d,
J = 8.5 Hz, 1H), 8.60 (d,
J = 8.3 Hz, 1H), 8.17–8.28 (m, 2H), 8.09 (dd,
J = 13.9, 8.4 Hz, 2H), 7.86–7.95 (m, 1H), 7.78–7.85 (m, 1H), 7.70–7.77 (m, 1H), 7.34 (t,
J = 7.8 Hz, 1H);
13C-NMR (DMSO-
d6), δ: 162.61, 148.50, 145.57, 138.55, 137.44, 135.73, 133.47, 130.94, 129.20, 129.16, 128.70, 128.09, 125.80, 123.97, 121.77, 118.38; HR-MS: for C
16H
12N
3O
3 [M+H]
+ calculated 294.0879
m/z, found 294.0888
m/z.
N-(3-Nitrophenyl)quinoline-2-carboxamide (19b). Yield 30%; Mp. 189–190 °C; IR (Zn/Se ATR, cm−1): 3,305w, 1,687m, 1,620w, 1,591w, 1,529m, 1,496w, 1,427m, 1,344m, 1,202w, 1,128m, 1,068w, 939w, 291m, 837m, 798m, 773s, 733s, 675s; 1H-NMR (DMSO-d6), δ: 11.14 (bs, 1H), 8.95 (t, J = 1.9 Hz, 1H), 8.57 (d, J = 8.5 Hz, 1H), 8.34 (dd, J = 8.0 Hz, J = 1.0 Hz, 1H), 8.16–8.28 (m, 2H), 8.06 (d, J = 8.0 Hz, 1H), 7.83–7.99 (m, 2H), 7.69–7.77 (m, 1H), 7.64 (t, J = 8.2 Hz, 1H); 13C-NMR (DMSO-d6), δ: 163.35, 149.40, 147.88, 145.85, 139.56, 138.16, 130.64, 129.94, 129.30, 128.99, 128.44, 128.10, 126.34, 118.74, 118.34, 114.54; HR-MS: for C16H12N3O3 [M+H]+ calculated 294.0879 m/z, found 294.0871 m/z.
N-(4-Nitrophenyl)quinoline-2-carboxamide (
19c) [
48,
49]. Yield 29%; Mp. 227–228 °C;
1H-NMR (DMSO-
d6), δ: 11.29 (bs, 1H), 8.64 (d,
J = 8.5 Hz, 1H), 8.18–8.35 (m, 6H), 8.12 (d,
J = 8.0 Hz, 1H), 7.88–7.99 (m, 1H), 7.78 (d,
J = 7.8 Hz, 1H);
13C-NMR (DMSO-
d6), δ: 163.61, 149.43, 145.88, 144.58, 142.77, 138.38, 130.83, 129.40, 129.11, 128.68, 128.21, 124.81, 120.17, 118.92; HR-MS: for C
16H
12N
3O
3 [M+H]
+ calculated 294.0879
m/z, found 294.0870
m/z.
3.2.2. General Procedure for Synthesis of Carboxamide Derivatives 20–38c
2-Naphthoic acid (1 g, 5.8 mmol) was suspended in dry toluene (40 mL) and SOCl
2 (0.6 mL, 8 mmol, 1.4 eq.) was added dropwise. The mixture was refluxed for 2 h and then evaporated to dryness. The crystalline residue was washed with dry toluene and used directly in the next step. Yield: 99%; Mp. 46–47 °C (Mp. 43 °C [
50]). Into the solution of 2-naphtoyl chloride (1.1 g, 5.8 mmol) in dry CH
2Cl
2 (30 mL), triethylamine (1.2 mL, 0.88 g, 8.66 mmol, 1.5 eq.) and corresponding substituted amine (5.8 mmol) were added dropwise. The mixture was stirred at room temperature for 20 h after which the solvent was removed under reduced pressure. The solid residue was washed with 10% HCl and the crude product was recrystallized from EtOH, cyclohexan, CHCl
3 or acetone. The studied compounds
20–
38c are presented in
Table 2.
N-Isopropyl-2-naphthamide (
20). Yield 18%; Mp. 174–176 °C (Mp. 170 °C [
51]); IR (Zn/Se ATR, cm
−1): 3,246
m, 3,054
w, 2,969
w, 2,935
w, 1,632
m, 1,618s, 1,543
s 1,499
w, 1,352
w, 1,327
w, 1,294
m, 1,201
w, 1,168
m, 1,136
w, 1,106
w, 953
w, 911
m, 835
m, 781
m 766
w, 736
m, 690
w;
1H-NMR (DMSO-
d6), δ: 8.37–8.51 (m, 2H), 7.88–8.09 (m, 4H), 7.47–7.67 (m, 2H), 4.18 (dsxt,
J = 13.8 Hz,
J = 6.7 Hz, 1H), 1.21 (d,
J = 6.5 Hz, 6H);
13C-NMR (DMSO-
d6), δ: 165.42, 134.05, 132.23, 132.16, 128.79, 127.72, 127.60, 127.44, 127.33, 126.66, 124.38, 41.14, 22.41; HR-MS: for C
14H
16NO [M+H]
+ calculated 214.1226
m/z, found 214.1187
m/z.
N-Dodecyl-2-naphthamide (21). Yield 15%; Mp. 102–103 °C; IR (Zn/Se ATR, cm−1): 3,259m, 2,915s, 2,848s, 1,643m, 1,619s, 1,552s, 1,501w, 1,467w, 1,432w, 1,317m, 1,204w, 1,146w, 954w, 912w, 871w, 839w, 779w, 734w; 1H-NMR (DMSO-d6), δ: 8.61 (t, J = 5.8 Hz, 1H), 8.37–8.52 (m, 1H), 7.88–8.10 (m, 4H), 7.46–7.69 (m, 2H), 3.26–3.34 (m, 2H), 1.55 (quin, J = 7.2 Hz, 2H), 1.13–1.37 (m, 18H), 0.78-0.87 (m, 3H); 13C-NMR (DMSO-d6), δ: 166.06, 134.06, 132.17, 132.08, 128.78, 127.76, 127.59, 127.43, 127.28, 126.63, 124.20, 39.34, 31.32, 29.17, 29.16, 29.09, 29.06, 29.05, 28.84, 28.75, 26.55, 22.11, 13.95; HR-MS: for C23H34NO [M+H]+ calculated 340.2635 m/z, found 340.2655 m/z.
1-(2-Naphthoyl)pyrrolidine (
22). Yield 12%; Mp. 76–77 °C (Mp. 75.5–76.5 °C [
52]); IR (Zn/Se ATR, cm
−1): 3,050
m, 2,963
s, 2,863
s, 1,629
m, 1,608
s, 1,469
m, 1,434
w, 1,417
m, 1,353
w, 1,337
w;
1H-NMR (DMSO-
d6), δ: 8.10 (s, 1H), 7.97–8.03 (m, 1H), 7.91–7.97 (m, 2H), 7.51–7.64 (m, 3H), 3.51 (t,
J = 6.9 Hz, 2H), 3.37–3.45 (m, 2H), 1.82–1.92 (m, 2H), 1.69-1.82 (m, 2H);
13C-NMR (DMSO-
d6), δ: 168.18, 134.50, 133.23, 132.15, 128.47, 127.77, 127.60, 127.10, 126.62, 126.59, 124.61, 48.97, 46.01, 25.99, 23.97; HR-MS: for C
15H
16NO [M+H]
+ calculated 226.1226
m/z, found 226.1200
m/z.
1-(2-Naphthoyl)piperidine (
23) [
53]. Yield 40%; Mp. 97–98 °C; IR (Zn/Se ATR, cm
−1): 3,050
w, 2,933
m, 2,852
m, 1,633
w, 1,612
s, 1,476
m, 1,432
m, 1,347
w, 1,278
m, 1,269
m, 1,229
w, 1,199
w, 1,126
w, 1,100
w, 1,007
w, 954
w, 906
w, 833
w, 807
w, 758
m;
1H-NMR (DMSO-
d6), δ: 7.86–8.08 (m, 4H), 7.39–7.67 (m, 4H), 3.09–3.85 (m, 4H), 1.26–1.77 (m, 6H);
13C-NMR (DMSO-
d6), δ: 168.84, 133.91, 132.96, 132.31, 128.27, 127.98, 127.65, 126.93, 126.66, 125.96, 124.29, 48.95, 43.22, 26.51, 26.01, 24.05; HR-MS: for C
16H
18NO [M+H]
+ calculated 240.1383
m/z, found 240.1349
m/z.
N-Cyclopentyl-2-naphthamide (24). Yield 21%; Mp. 190–191 °C; IR (Zn/Se ATR, cm−1): 3,245s, 2,954m, 2,867w, 1,618m, 1,545m; 1H-NMR (DMSO-d6), δ: 8.37–8.56 (m, 2H), 7.89–8.11 (m, 4H), 7.48–7.67 (m, 2H), 4.31 (sxt, J = 7.0 Hz, 1H), 1.42–2.05 (m, 8H); 13C-NMR (DMSO-d6), δ: 166.00, 134.04, 132.20, 132.15, 128.78, 127.68, 127.60, 127.41, 127.38, 126.62, 124.45, 51.08, 32.20, 23.70; HR-MS: for C16H18NO [M+H]+ calculated 240.1383 m/z, found 240.1372 m/z.
N-Cyclohexyl-2-naphthamide (
25). Yield 45%; Mp. 184–185 °C (Mp. 183–184 °C [
52]); IR (Zn/Se ATR, cm
−1): 3,316
m, 3,051
w, 2,933
s, 2,917
m, 2,850
m, 1,635
m, 1,622
s, 1,601
m, 1,531
s, 1,446
w, 1,318
m, 1,288
m, 1,231
m, 1,202
w, 1,151
w, 1,081
m, 890
m, 859
w, 819
m, 779
m, 758
m, 728
w, 667
w;
1H-NMR (DMSO-
d6), δ: 8.30–8.54 (m, 2H), 7.85–8.12 (m, 4H), 7.46–7.71 (m, 2H), 3.70–3.97 (m, 1H), 1.87 (d,
J = 9.8 Hz, 2H), 1.74 (dd,
J = 9.8 Hz,
J = 2.5 Hz, 2H), 1.61 (d,
J = 12.6 Hz, 1H), 1.23–1.43 (m, 4H), 1.04–1.21 (m, 1H);
13C-NMR (DMSO-
d6), δ: 165.42, 134.04, 132.26, 132.15, 128.78, 127.68, 127.59, 127.41, 127.34, 126.63, 124.42, 48.49, 32.50, 25.31, 25.00; HR-MS: for C
17H
20NO [M+H]
+ calculated 254.1539
m/z, found 254.1493
m/z.
N-Cycloheptyl-2-naphthamide (26). Yield 18%; Mp. 157–158 °C; IR (Zn/Se ATR, cm−1): 3,318m, 2,918m, 2,655m, 1,635m, 1,623s, 1,600w, 1,529s, 1,504m, 1,446w, 1,314m, 1,234w, 1,201w, 1,186w, 1,053w, 907w, 819w, 777w, 758w, 729w, 679w; 1H-NMR (DMSO-d6), δ: 8.34–8.56 (m, 2H), 7.87–8.12 (m, 4H), 7.45–7.71 (m, 2H), 4.04 (qt, J = 8.8 Hz, J = 4.6 Hz, 1H), 1.81–2.03 (m, 2H), 1.29–1.77 (m, 10H); 13C-NMR (DMSO-d6), δ: 165.20, 134.05, 132.35, 132.17, 128.78, 127.69, 127.61, 127.41, 127.35, 126.63, 124.47, 50.59, 34.42, 27.89, 24.00; HR-MS: for C18H22NO [M+H]+ calculated 268.1696 m/z, found 268.1621 m/z.
N-Cyclooctyl-2-naphthamide (27). Yield 17%; Mp. 145–146 °C; IR (Zn/Se ATR, cm−1): 3,252s, 2,921s, 2,849m, 1,634s, 1,623s, 1,548s, 1,446w, 1,348w, 1,326m, 1,279w, 1,243w, 1,199w, 1,066m, 897w, 864w, 825w, 774m, 762w, 737m, 712m; 1H-NMR (DMSO-d6), δ: 8.30–8.55 (m, 2H), 7.87–8.10 (m, 4H), 7.46–7.69 (m, 2H), 4.09 (qt, J = 8.3 Hz, J = 4.2 Hz, 1H), 1.37–1.92 (m, 14H); 13C-NMR (DMSO-d6), δ: 165.18, 134.03, 132.37, 132.15, 128.76, 127.66, 127.59, 127.38, 127.32, 126.60, 124.47, 49.39, 31.72, 26.86, 25.14, 23.60; HR-MS: for C19H24NO [M+H]+ calculated 282.1852 m/z, found 282.1873 m/z.
N-Phenyl-2-naphthamide (
28). Yield 96%; Mp. 175–176 °C (170 °C [
50], Mp. 160 °C [
54]); IR (Zn/Se ATR, cm
−1): 3,360
m, 3,256
w, 3,053
w, 2,989
w, 1,640
s, 1,595
s, 1,519
s, 1,489
s, 1,431
s, 1,313
m, 1,245
w, 1,131
w, 1,076
w, 956
w, 912
m, 870
w, 824
w, 777
w, 749
s, 731
m, 689
m;
1H-NMR (DMSO-
d6) [
55,
56], δ: 10.48 (bs, 1H), 8.61 (s, 1H), 7.96–8.17 (m, 4H), 7.88 (d,
J = 8.0 Hz, 2H), 7.56–7.71 (m, 2H), 7.39 (t,
J = 7.5 Hz, 2H), 7.05–7.19 (m, 1H);
13C-NMR (DMSO-
d6), δ: 165.66, 139.31, 134.30, 132.35, 132.12, 128.98, 128.69, 128.04, 128.03, 127.83, 127.71, 126.87, 124.53, 123.72, 120.42; HR-MS: for C
17H
14NO [M+H]
+ calculated 248.1070
m/z, found 248.1095
m/z.
N-Benzyl-2-naphthamide (
29). Yield 42%; Mp. 138–139 °C (Mp. 140–143 °C [
57]); IR (Zn/Se ATR, cm
−1): 3,287
s, 3,054
w, 3,027
w, 2,920
w, 1,635
s, 1,622
s, 1,600
w, 1,543
s, 1,504
m, 1,495
m, 1,449
w, 1,414
m, 1,359
w, 1,316
m, 1,300
m, 1,263
m, 1,206
w, 1,146
w, 1,119
w, 1,047
w, 996
w, 910
m, 869
m, 835
m, 777
m, 737
w, 719
m, 693
m;
1H-NMR (DMSO-
d6), δ: 9.25 (t,
J = 6.02 Hz, 1H), 8.53 (s, 1H), 7.91–8.11 (m, 4H), 7.53–7.70 (m, 2H), 7.29–7.46 (m, 4H), 7.21–7.28 (m, 1H), 4.56 (d,
J = 6.02 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 166.29, 139.70, 134.17, 132.18, 131.72, 128.87, 128.32, 127.92, 127.63, 127.61, 127.55, 127.30, 126.77, 126.74, 124.22, 42.77; HR-MS: for C
18H
16NO [M+H]
+ calculated 262.1226
m/z, found 262.1257
m/z.
N-(2-Phenylethyl)-2-naphthamide (
30). Yield 26%; Mp. 133–134 °C (Mp. 133–134 °C [
52]); IR (Zn/Se ATR, cm
−1): 3,325
s, 3,054
w, 3,027
w, 2,933
w, 2,900
w, 1,639
s, 1,626
m, 1,600
m, 1,541
s, 1,452
m, 1,302
w, 1,211
w, 1,074
w, 829
w, 779
w, 756
w, 692
w;
1H-NMR (DMSO-
d6), δ: 8.78 (t,
J = 5.5 Hz, 1H), 8.45 (s, 1H), 7.86–8.11 (m, 4H), 7.48–7.71 (m, 2H), 7.12–7.41 (m, 5H), 3.49–3.66 (m, 2H), 2.91 (t,
J = 7.5 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 166.23, 139.58, 134.11, 132.17, 131.99, 128.84, 128.71, 128.38, 127.87, 127.63, 127.54, 127.37, 126.73, 126.13, 124.17, 41.06, 35.19; HR-MS: for C
19H
18NO [M+H]
+ calculated 276.1383
m/z, found 276.1353
m/z.
N-(3-Hydroxyphenyl)-2-naphthamide (31b). Yield 69%; Mp. 194 °C; IR (Zn/Se ATR, cm−1): 3,349s, 1,712s, 1,663s, 1,603s, 1,530s, 1,432s, 1,277s, 1,220s, 1,192s, 1,144s, 1,081s, 970s, 913s, 861s, 820s, 772s, 760s, 679s; 1H-NMR (DMSO-d6), δ: 10.32 (s, 1H), 8.57 (s, 1H), 8.11–7.99 (m, 5H), 7.69–7.60 (m, 2H), 7.43 (t, J = 1.8 Hz, 1H), 7.25–7.10 (m, 2H), 6.54 (dt, J = 7.7 Hz, J = 2.2 Hz, 1H); 13C-NMR (DMSO-d6), δ: 165.36, 157.44, 140.13, 134.10, 132.34, 131.97, 129.04, 128.76, 127.75, 127.73, 127.58, 127.50, 126.62, 124.35, 111.09, 110.74, 107.51; HR-MS: for C17H14NO2 [M+H]+ calculated 264.1019 m/z, found 264.1012 m/z.
N-(4-Hydroxyphenyl)-2-naphthamide (31c). Yield 66%; Mp. 250–255 °C; IR (Zn/Se ATR, cm−1): 3,376s, 3,055w, 1,731s, 1,660s, 1,520s, 1,311s, 1,267s, 1,221s, 1,202s, 1,081s, 953s, 802s, 775s, 760s, 754s; 1H-NMR (DMSO-d6), δ: 10.58 (s, 1H), 8.61 (s, 1H), 8.14–7.99 (m, 5H), 7.70–7.61 (m, 2H), 7.40–7.34 (m, 2H), 6.79–6.74 (m, 2H); 13C-NMR (DMSO-d6), δ: 165.53, 153.65, 146.38, 134.21, 132.04, 131.36, 128.84, 127.93, 127.90, 127.66, 127.57, 124.32, 121.91, 121.31, 114.91; HR-MS: for C17H14NO2 [M+H]+ calculated 264.1019 m/z, found 264.1010 m/z.
N-(2-Methoxyphenyl)-2-naphthamide (32a). Yield 46%; Mp. 82 °C; IR (Zn/Se ATR, cm−1): 3,274s, 3,060w, 2,997w, 2,973w, 1,647s, 1,598m, 1,535s, 1,493s, 1,460s, 1,433s, 1,336s, 1,259s, 1,026m, 749s; 1H-NMR (DMSO-d6), δ: 9.62 (s, 1H), 8.62 (s, 1H), 8.10–8.08 (m, 1H), 8.04 (d, J = 1.0 Hz, 2H), 8.01 (dd, J = 8.2 Hz, J = 1.1 Hz, 1H), 7.85 (dd, J = 7.9 Hz, J = 1.3 Hz, 1H), 7.66–7.60 (m, 2H), 7.22–7.18 (m, 1H), 7.12–7.10 (m, 1H), 7.00 (td, J = 7.7 Hz, J = 1.2 Hz, 1H), 3.86 (s, 3H); 13C-NMR (DMSO-d6), δ: 165.08, 151.52, 134.30, 132.16, 131.84, 129.02, 128.12, 127.92, 127.84, 127.66, 126.89, 126.83, 125.76, 124.36, 124.24, 120.24, 111.41, 55.73; HR-MS: for C18H16NO2 [M+H]+ calculated 278.1176 m/z, found 278.1179 m/z.
N-(3-Methoxyphenyl)-2-naphthamide (32b). Yield 47%; Mp. 142 °C; IR (Zn/Se ATR, cm−1): 3,243s, 3,005w, 1,640s, 1,591s, 1,530s, 1,451s, 1,429s, 1,301s, 1,282s, 1,158s, 1,049s, 771s; 1H-NMR (DMSO-d6), δ: 10.43 (s, 1H), 8.59 (s, 1H), 8.11–8.07 (m, 1H), 8.05 (s, 1H), 8.04 (d, J = 1.5 Hz, 1H), 8.02–8.00 (m, 1H), 7.66–7.60 (m, 2H), 7.55 (t, J = 2.3 Hz, 1H), 7.45 (dd, J = 8.0 Hz, J = 1.0 Hz, 1H), 7.28 (t, J = 8.2 Hz, 1H), 6.71 (dd, J = 8.2 Hz, J = 1.8 Hz, 1H); 3.78 (s, 3H); 13C-NMR (DMSO-d6), δ: 165.44, 159.38, 140.30, 134.13, 132.16, 131.95, 129.20, 128.75, 127.81, 127.75, 127.61, 127.49, 126.64, 124.26, 112.51, 109.10, 106.06, 54.90; HR-MS: for C18H16NO2 [M+H]+ calculated 278.1176 m/z, found 278.1175 m/z.
N-(4-Methoxyphenyl)-2-naphthamide (32c). Yield 65%; Mp. 182 °C; IR (Zn/Se ATR, cm−1): 3,371s, 3,049w, 2,949w, 2,835w, 1,656s, 1,598s, 1,527s, 1,511s, 1,463s, 1,414s, 1,314s, 1,303s, 1,220s, 1,182s, 1,033s, 820s, 761s; 1H-NMR (DMSO-d6), δ: 10.34 (s, 1H), 8.58 (s, 1H), 8.08 (d, J = 8.8 Hz, 1H), 8.04 (s, 2H), 8.00 (d, J = 8.3 Hz, 1H), 7.75 (d, J = 8.3 Hz, 2H), 7.66–7.59 (m, 2H), 6.69 (d, J = 8.3 Hz, 2H), 3.76 (s, 3H); 13C-NMR (DMSO-d6), δ: 165.15, 155.57, 134.21, 132.39, 132.33, 132.12, 128.92, 127.97, 127.81, 127.72, 127.67, 126.81, 124.46, 121.99, 113.78, 55.18; HR-MS: for C18H16NO2 [M+H]+ calculated 278.1176 m/z, found 278.1173 m/z.
N-(2-Methylphenyl)-2-naphthamide (33a). Yield 33%; Mp. 146 °C; IR (Zn/Se ATR, cm−1): 3,239s, 3,057w, 1,639s, 1,584s, 1,531s, 1,454s, 1,315s, 1,134s, 916s, 777s, 749s, 731s, 692m; 1H-NMR (DMSO-d6), δ: 10.09 (s, 1H), 8.63 (s, 1H), 8.10–8.08 (m, 1H), 8.08–8.06 (m, 2H), 8.04–8.01 (m, 1H), 7.67–7.60 (m, 2H), 7.41 (d, J = 6.7 Hz, 1H), 7.30 (d, J = 7.3 Hz, 1H), 7.28 (td, J = 7.5 Hz, J = 1.5 Hz, 1H), 7.19 (td, J = 7.3 Hz, J = 1.3 Hz, 1H); 2.29 (s, 3H); 13C-NMR (DMSO-d6), δ: 166.05, 137.16, 134.95, 134.41, 132.82, 132.55, 131.02, 129.62, 128.69, 128.68, 128.42, 128.33, 127.49, 127.27, 126.70, 126.67, 125.12, 18.66; HR-MS: for C18H16NO [M+H]+ calculated 262.1226 m/z, found 262.1225 m/z.
N-(3-Methylphenyl)-2-naphthamide (33b). Yield 59%; Mp. 160 °C; IR (Zn/Se ATR, cm−1): 3,246s, 3,055w, 1,641s, 1,590s, 1,550s, 1,432s, 1,309s, 1,130s, 777s, 749s, 731s, 690s; 1H-NMR (DMSO-d6), δ: 10.37 (s, 1H), 8.59 (s, 1H), 8.10–8.07 (m, 1H), 8.04–8.02 (m, 2H), 8.02–8.00 (m, 1H), 7.69 (s, 1H), 7.66–7.60 (m, 3H), 7.26 (t, J = 7.8 Hz, 1H), 6.94 (d, J = 7.3 Hz, 1H), 2.33 (s, 3H); 13C-NMR (DMSO-d6), δ: 165.35, 139.02, 137.61, 134.12, 132.22, 131.97, 128.75, 128.28, 127.81, 127.72, 127.60, 127.49, 126.64, 124.29, 124.23, 120.84, 117.47, 21.03; HR-MS: for C18H16NO [M+H]+ calculated 262.1226 m/z, found 262.1224 m/z.
N-(4-Methylphenyl)-2-naphthamide (
33c). Yield 67%; Mp. 195 °C (Mp. 191 °C [
50]; IR (Zn/Se ATR, cm
−1): 3,254
s, 3,056
w, 2,917
w, 1,640
s, 1,602
s, 1,538
s, 1,513
s, 1,404
s, 1,329
s, 1,132
s, 914
s, 811
s, 754
s, 733
s, 707
m;
1H-NMR (DMSO-
d6) [
56], δ: 10.38 (s, 1H), 8.59 (s, 1H), 8.10–8.06 (m, 1H), 8.04 (s, 2H), 8.02–8.00 (m, 1H), 7.73 (d,
J = 8.5 Hz, 2H), 7.66–7.60 (m, 2H), 7.18 (d,
J = 8.3 Hz, 2H), 2.29 (s, 3H);
13C-NMR (DMSO-
d6), δ: 165.22, 136.59, 134.10, 132.51, 132.27, 132.00, 128.85, 128.75, 127.79, 127.69, 127.57, 127.50, 126.64, 124.30, 120.34, 20.34; HR-MS: for C
18H
16NO [M+H]
+ calculated 262.1226
m/z, found 262.1225
m/z.
N-(2-Fluorophenyl)-2-naphthamide (34a). Yield 59%; Mp. 122 °C; IR (Zn/Se ATR, cm−1): 3,316s, 3,054w, 3,023w, 1,648s, 1,541s, 1,489s, 1,456s, 1,321s, 1,257s, 1,192m, 829m, 750s; 1H-NMR (DMSO-d6), δ: 10.33 (s, 1H), 8.64 (s, 1H), 8.11–8.08 (m, 1H), 8.06 (d, J = 1.0 Hz, 2H), 8.03–8.01 (m, 1H), 7.70–7.60 (m, 3H), 7.36–7.23 (m, 3H); 13C-NMR (DMSO-d6), δ: 165.38, 155.64 (d, J = 245.8 Hz), 134.27, 131.98, 131.21, 128.82, 128.13, 127.88, 127.73, 127.50, 126.72 (d, J = 8.8 Hz), 126.68, 126.68 (d, J = 7.6 Hz), 125.75 (d, J = 11.4 Hz), 124.24, 124.13 (d, J = 3.8 Hz), 115.65 (d, J = 20.5 Hz); HR-MS: for C17H13NOF [M+H]+ calculated 266.0976 m/z, found 266.0974 m/z.
N-(3-Fluorophenyl)-2-naphthamide (34b). Yield 21%; Mp. 167 °C; IR (Zn/Se ATR, cm−1): 3,267s, 3,140w, 3,058w, 1,642s, 1,604s, 1,541s, 1,444s, 1,322s, 1,301s, 1,142m, 775m, 765m, 682m; 1H-NMR (DMSO-d6), δ: 10.64 (s, 1H), 8.59 (s, 1H), 8.11-8.09 (m, 1H), 8.08–8.01 (m, 3H), 7.83 (dt, J = 11.8 Hz, J = 2.5 Hz, 1H), 7.68–7.61 (m, 3H), 7.42 (dt, J = 8.2 Hz, J = 7.0 Hz, 1H), 6.95 (tdd, J = 8.5 Hz, J = 2.5 Hz, J = 0.75 Hz, 1H); 13C-NMR (DMSO-d6), δ: 165.71, 161.98 (d, J = 239.7 Hz), 140.89 (d, J = 11.4 Hz), 134.23, 131.93, 131.83, 130.06 (d, J = 9.9 Hz), 128.79, 127.93, 127.90, 127.75, 127.53, 126.73, 124.21, 115.90 (d, J = 3.1 Hz), 109.94 (d, J = 20.5 Hz), 106.90 (d, J = 25.8 Hz); HR-MS: for C17H13NOF [M+H]+ calculated 266.0976 m/z, found 266.0972 m/z.
N-(4-Fluorophenyl)-2-naphthamide (
34c). Yield 70%; Mp. 191 °C; IR (Zn/Se ATR, cm
−1): 3,380
s, 3,063
w, 1,654
s, 1,527
s, 1,506
s, 1,405
s, 1,313
m, 1,212
s, 1,197
s, 826
s, 814
s, 764
s;
1H-NMR (DMSO-
d6) [
56], δ: 10.51 (s, 1H), 8.58 (s, 1H), 8.10–8.08 (m, 1H), 8.07–8.00 (m, 3H), 7.88–7.84 (m, 2H), 7.66–7.60 (m, 2H), 7.25–7.20 (m, 2H);
13C-NMR (DMSO-
d6), δ: 165.36, 158.22 (d,
J = 238.9 Hz), 135.46 (d,
J = 2.3 Hz), 134.17, 132.03, 131.98, 128.75, 127.84, 127.78, 127.64, 127.50, 126.67, 124.24, 122.13 (d,
J = 8.0 Hz), 115.01 (d,
J = 22.0 Hz); HR-MS: for C
17H
13NOF [M+H]
+ calculated 266.0976
m/z, found 266.0975
m/z.
N-(2-Chlorophenyl)-2-naphthamide (
35a) [
58]. Yield 45%; Mp. 116 °C; IR (Zn/Se ATR, cm
−1): 3,281
s, 3,061
w, 1,653
s, 1,583
s, 1,523
s, 1,439
s, 1,305
s, 1,053
m, 777
s, 759
s, 746
s;
1H-NMR (DMSO-
d6), δ: 10.26 (s, 1H), 8.65 (s, 1H), 8.11–8.09 (m, 1H), 8.09–8.05 (m, 2H), 8.04–8.00 (m, 1H), 7.68–7.61 (m, 3H), 7.59 (dd,
J = 7.9 Hz,
J = 1.3 Hz, 1H), 7.42 (td,
J = 7.7 Hz,
J = 1.5 Hz, 1H), 7.32 (td,
J = 7.8 Hz,
J = 1.5 Hz, 1H);
13C-NMR (DMSO-
d6), δ: 165.48, 135.16, 134.42, 132.11, 131.30, 129.61, 129.55, 129.02, 128.50, 128.25, 128.15, 127.96, 127.70, 127.52, 127.51, 126.92, 124.34; HR-MS: for C
17H
13NOCl [M+H]
+ calculated 282.0680
m/z, found 282.0679
m/z.
N-(3-Chlorophenyl)-2-naphthamide (35b). Yield 67%; Mp. 180 °C; IR (Zn/Se ATR, cm−1): 3,262s, 3,236s, 3,110w, 3,055w, 1,644s, 1,591s, 1,531s, 1,420s, 1,316s, 1,301s, 1,133m, 778s, 765s, 695m; 1H-NMR (DMSO-d6), δ: 10.61 (s, 1H), 8.59 (s, 1H), 8.11–8.09 (m, 1H), 8.08–8.01 (m, 4H), 7.78 (ddd, J = 8.3 Hz, J = 1.8 Hz, J = 0.8 Hz, 1H), 7.68–7.61 (m, 2H), 7.41 (t, J = 8.2 Hz, 1H), 7.18 (ddd, J = 8.0 Hz, J = 1.9 Hz, J = 0.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 165.69, 140.61, 134.24, 132.86, 131.93, 131.77, 130.14, 128.81, 127.95, 127.92, 127.78, 127.53, 126.75, 124.21, 123.21, 119.68, 118.55; HR-MS: for C17H13NOCl [M+H]+ calculated 282.0680 m/z, found 282.0681 m/z.
N-(4-Chlorophenyl)-2-naphthamide (
35c). Yield 81%; Mp. 218 °C; IR (Zn/Se ATR, cm
−1): 3,377
s, 3,058
w, 1,657
s, 1,628
s, 1,593
s, 1,514
s, 1,492
s, 1,398
s, 1,310
s, 1,097
m, 823
s, 760
s;
1H-NMR (DMSO-
d6) [
56], δ: 10.58 (s, 1H), 8.59 (s, 1H), 8.10–8.08 (m, 1H), 8.08–8.01 (m, 3H), 7.91–7.87 (m, 2H), 7.67–7.60 (m, 2H), 7.46–7.42 (m, 2H);
13C-NMR (DMSO-
d6), δ: 165.54, 138.10, 134.21, 131.95, 131.92, 128.78, 128.38, 127.89, 127.88, 127.72, 127.52, 127.20, 126.72, 124.24, 121.78; HR-MS: for C
17H
13NOCl [M+H]
+ calculated 282.0680
m/z, found 282.0679
m/z.
N-(2-Bromophenyl)-2-naphthamide (
36a) [
58]. Yield 70%; Mp. 123 °C; IR (Zn/Se ATR, cm
−1): 3,277
s, 3,060
w, 1,652
s, 1,629
m, 1,578
m, 1,523
s, 1,433
s, 1,304
s, 1,028
m, 777
m, 759
m, 745
s;
1H-NMR (DMSO-
d6), δ: 10.25 (s, 1H), 8.65 (s, 1H), 8.10-8.08 (m, 1H), 8.07 (s, 2H), 8.03-8.01 (m, 1H), 7.75 (dd,
J = 7.8 Hz,
J = 0.8 Hz, 1H), 7.68–7.61 (m, 3H), 7.46 (td,
J = 7.5 Hz,
J = 1.0 Hz, 1H), 7.25 (td,
J = 7.7 Hz,
J = 1.4 Hz, 1H);
13C-NMR (DMSO-
d6), δ: 165.43, 136.70, 134.41, 132.73, 132.13, 131.41, 129.01, 128.93, 128.22, 128.15, 128.15, 127.95, 127.72, 126.94, 124.37, 124.34, 120.68; HR-MS: for C
17H
13NOBr [M+H]
+ calculated 326.0175
m/z, found 326.0175
m/z.
N-(3-Bromophenyl)-2-naphthamide (
36b). Yield 69%; Mp. 193 °C; IR (Zn/Se ATR, cm
−1): 3,261
s, 3,233
s, 3,106
w, 3,056
w, 1,644
s, 1,590
s, 1,526
s, 1,415
s, 1,313
s, 1,300
s, 1,133
m, 778
s, 765
s, 686
m;
1H-NMR (DMSO-
d6) [
56], δ: 10.59 (s, 1H), 8.59 (s, 1H), 8.18 (t,
J = 1.9 Hz, 1H), 8.11–8.08 (m, 1H), 8.08–8.00 (m, 3H), 7.83 (dt,
J = 7.7 Hz,
J = 1.6 Hz, 1H), 7.68–7.61 (m, 2H), 7.37–7.30 (m, 2H);
13C-NMR (DMSO‑
d6), δ: 165.63, 140.74, 134.23, 131.92, 131.72, 130.43, 128.78, 127.93, 127.90, 127.75, 127.52, 126.73, 126.08, 124.20, 122.51, 121.25, 118.92; HR-MS: for C
17H
13NOBr [M+H]
+ calculated 326.0175
m/z, found 326.0174
m/z.
N-(4-Bromophenyl)-2-naphthamide (
36c). Yield 55%; Mp. 231 °C; IR (Zn/Se ATR, cm
−1): 3,375
s, 3,283
w, 3,056
w, 1,658
s, 1,591
s, 1,516
s, 1,489
s, 1,395
s, 1,310
s, 1,073
m, 1,009
m, 820
s, 760
s;
1H-NMR (DMSO-
d6) [
56], δ: 10.57 (s, 1H), 8.58 (s, 1H), 8.10–8.08 (m, 1H), 8.08–7.99 (m, 3H), 7.83 (d,
J = 8.5 Hz, 2H), 7.67–7.61 (m, 2H) 7.57 (d,
J = 8.5 Hz, 2H);
13C-NMR (DMSO-
d6), δ: 165.54, 138.52, 134.21, 131.93, 131.90, 131.30, 128.79, 127.90, 127.72, 127.52, 126.72, 124.24, 122.16, 122.15, 115.21; HR-MS: for C
17H
13NOBr [M+H]
+ calculated 326.0175
m/z, found 326.0173
m/z.
N-(3-Trifluoromethylphenyl)-2-naphthamide (37b). Yield 82%; Mp. 150 °C; IR (Zn/Se ATR, cm−1): 3,261s, 3,100w, 3,062w, 1,648s, 1,602s, 1,552s, 1,445s, 1,434s, 1,324s, 1,309s, 1,165s, 1,107s, 1,069s, 778m, 697m; 1H-NMR (DMSO-d6), δ: 10.75 (s, 1H), 8.63 (s, 1H), 8.32 (s, 1H), 8.15–8.01 (m, 5H), 7.69–7.60 (m, 3H), 7.47 (d, J = 7.8 Hz, 1H); 13C-NMR (DMSO-d6), δ: 165.85, 139.95, 134.29, 131.93, 131.66, 129.72, 129.31 (q, J = 32.1 Hz), 128.83, 128.02, 127.97, 127.82, 127.55, 126.78, 124.21, 124.06 (q, J = 273.1 Hz), 123.68, 119.79 (q, J = 3.8 Hz), 116.31 (q, J = 3.8 Hz); HR-MS: for C18H13NOF3 [M+H]+ calculated 316.0944 m/z, found 316.0942 m/z.
N-(4-Trifluoromethylphenyl)-2-naphthamide (37c). Yield 80%; Mp. 224 °C; IR (Zn/Se ATR, cm−1): 3,372s, 3,073w, 1,662s, 1,600s, 1,556s, 1,514s, 1,408s, 1,316s, 1,159s, 1,112s, 1,067s, 1,018m, 836s, 826s, 764m; 1H-NMR (DMSO-d6), δ: 10.78 (s, 1H), 8.62 (s, 1H), 8.12–8.01 (m, 6H), 7.76 (d, J = 8.8 Hz, 2H), 7.69–7.61 (m, 2H); 13C-NMR (DMSO-d6), δ: 165.97, 142.80, 134.32, 131.93, 131.71, 128.87, 128.13, 127.97, 127.87, 127.58, 126.81, 125.80 (q, J = 3.8 Hz), 124.29, 124.29 (q, J = 270.9 Hz), 123.57 (q, J = 32.1 Hz), 120.07; HR-MS: for C18H13NOF3 [M+H]+ calculated 316.0944 m/z, found 316.0942 m/z.
N-(2-Nitrophenyl)-2-naphthamide (
38a). Yield 66%; Mp. 136 °C (Mp. 138–139 °C [
59]); IR (Zn/Se ATR, cm
−1): 3,383
s, 3,124
w, 3,054
w, 1,678
s, 1,582
s, 1,493
s, 1,448
s, 1,427
s, 1,335
s, 1,284
s, 1,273
s, 1,195
s, 759
s, 738
s;
1H-NMR (DMSO-
d6), δ: 10.97 (s, 1H), 8.62 (s, 1H), 8.15–8.00 (m, 5H), 7.86–7.82 (m, 1H), 7.81–7.76 (m, 1H), 7.70–7.62 (m, 2H), 7.44 (t,
J = 7.7 Hz, 1H);
13C-NMR (DMSO-
d6), δ: 165.32, 142.68, 134.45, 133.94, 131.98, 131.63, 130.83, 128.92, 128.28, 128.20, 128.04, 127.60, 126.91, 125.80, 125.43, 124.88, 123.92; HR-MS: for C
17H
13N
2O
3 [M+H]
+ calculated 293.0921
m/z, found 293.0919
m/z.
N-(3-Nitrophenyl)-2-naphthamide (
38b). Yield 68%; Mp. 177 °C; IR (Zn/Se ATR, cm
−1): 3,269
s, 3,093
w, 1,648
s, 1,626
s, 1,522
s, 1,429
s, 1,341
s, 1,321
s, 1,288
s, 1,272
s, 761
s, 736
s, 669
s;
1H-NMR (DMSO-
d6) [
56], δ: 10.88 (s, 1H), 8.85 (t,
J = 2.1 Hz, 1H), 8.63 (s, 1H), 8.26 (ddd,
J = 8.2 Hz,
J = 2.0 Hz,
J = 0.9 Hz, 1H), 8.11–7.99 (m, 4H), 7.97 (ddd,
J = 8.2 Hz,
J = 2.0 Hz,
J = 0.9 Hz, 1H), 7.69–7.61 (m, 3H);
13C-NMR (DMSO-
d6), δ: 165.94, 147.88, 140.34, 134.35, 131.92, 131.42, 129.92, 128.85, 128.14, 128.02, 127.91, 127.58, 126.82, 126.06, 124.20, 117.96, 114.30; HR-MS: for C
17H
13N
2O
3 [M+H]
+ calculated 293.0921
m/z, found 293.0917
m/z.
N-(4-Nitrophenyl)-2-naphthamide (
38c). Yield 46%; Mp. 200 °C; IR (Zn/Se ATR, cm
−1): 3,410
s, 3,054
w, 1,679
s, 1,611
s, 1,595
s, 1,538
s, 1,499
s, 1,481
s, 1,324
s, 1,302
s, 1,284
s, 1,243
s, 1,109
s, 849
s, 771
s, 750
s;
1H-NMR (DMSO-
d6) [
56], δ: 10.99 (s, 1H), 8.62 (s, 1H), 8.29 (dd,
J = 9.3 Hz,
J = 2.3 Hz, 2H), 8.13–8.01 (m, 6H), 7.69–7.61 (m, 2H);
13C-NMR (DMSO-
d6), δ: 166.20, 145.45, 142.43, 134.41, 131.90, 131.43, 128.92, 128.37, 128.07, 128.02, 127.61, 126.88, 124.67, 124.27, 119.80; HR-MS: for C
17H
13N
2O
3 [M+H]
+ calculated 293.0921
m/z, found 293.0918
m/z.