Daphnoretin Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma (HOS) Cells
Abstract
:1. Introduction
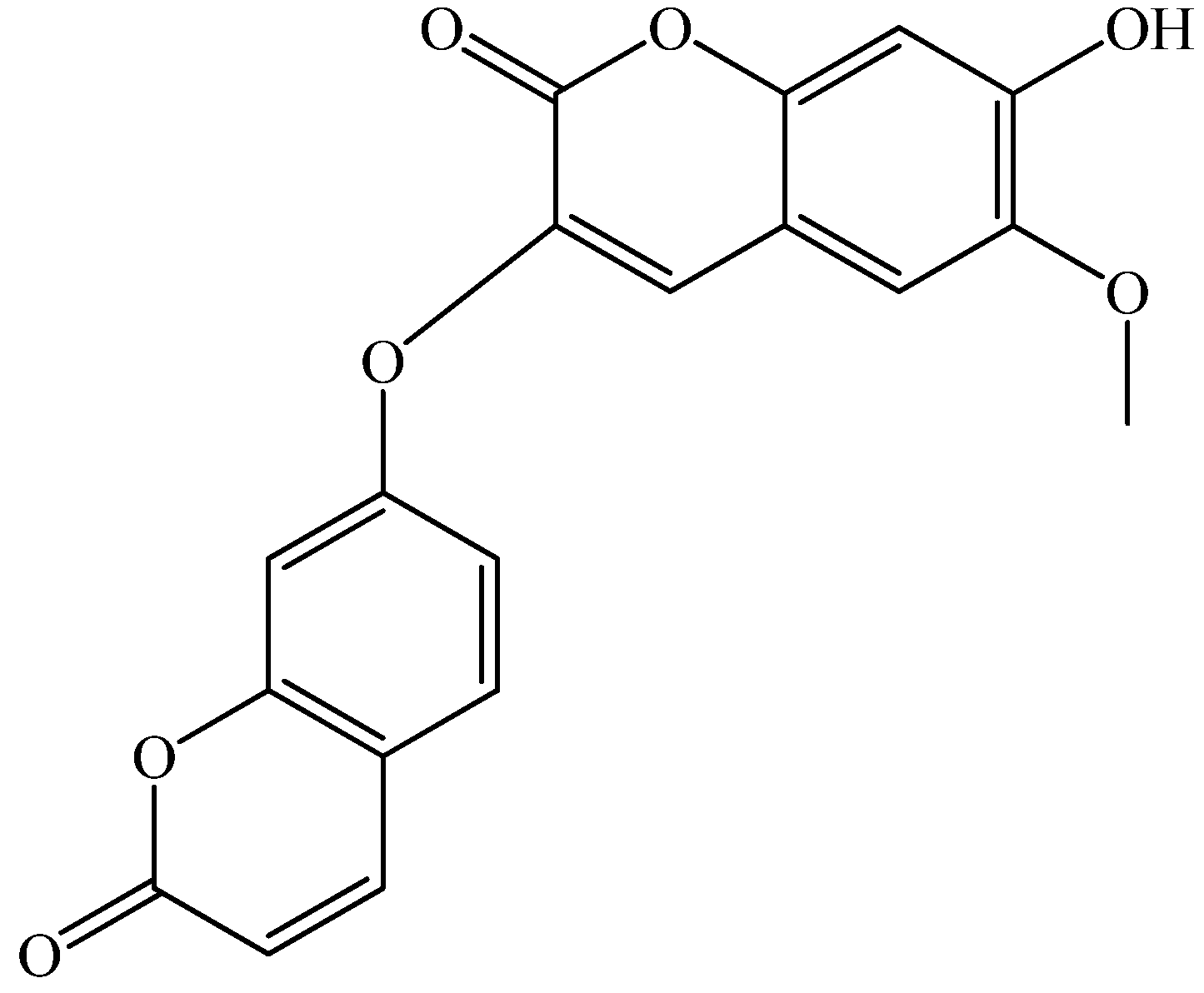
2. Results and Discussion
2.1. Cytotoxicity Assays
| Cell lines | IC50 (µM) | |
|---|---|---|
| Daphnoretin | Taxol | |
| HOS | 3.89 * | 3.01 |
| U2-OS | 8.72 * | 5.35 |
| MG-63 | 5.11 * | 3.74 |
| Normal human osteoblast cells | 8.57 * | 3.20 |
2.2. Induction of Apoptosis by Daphnoretin
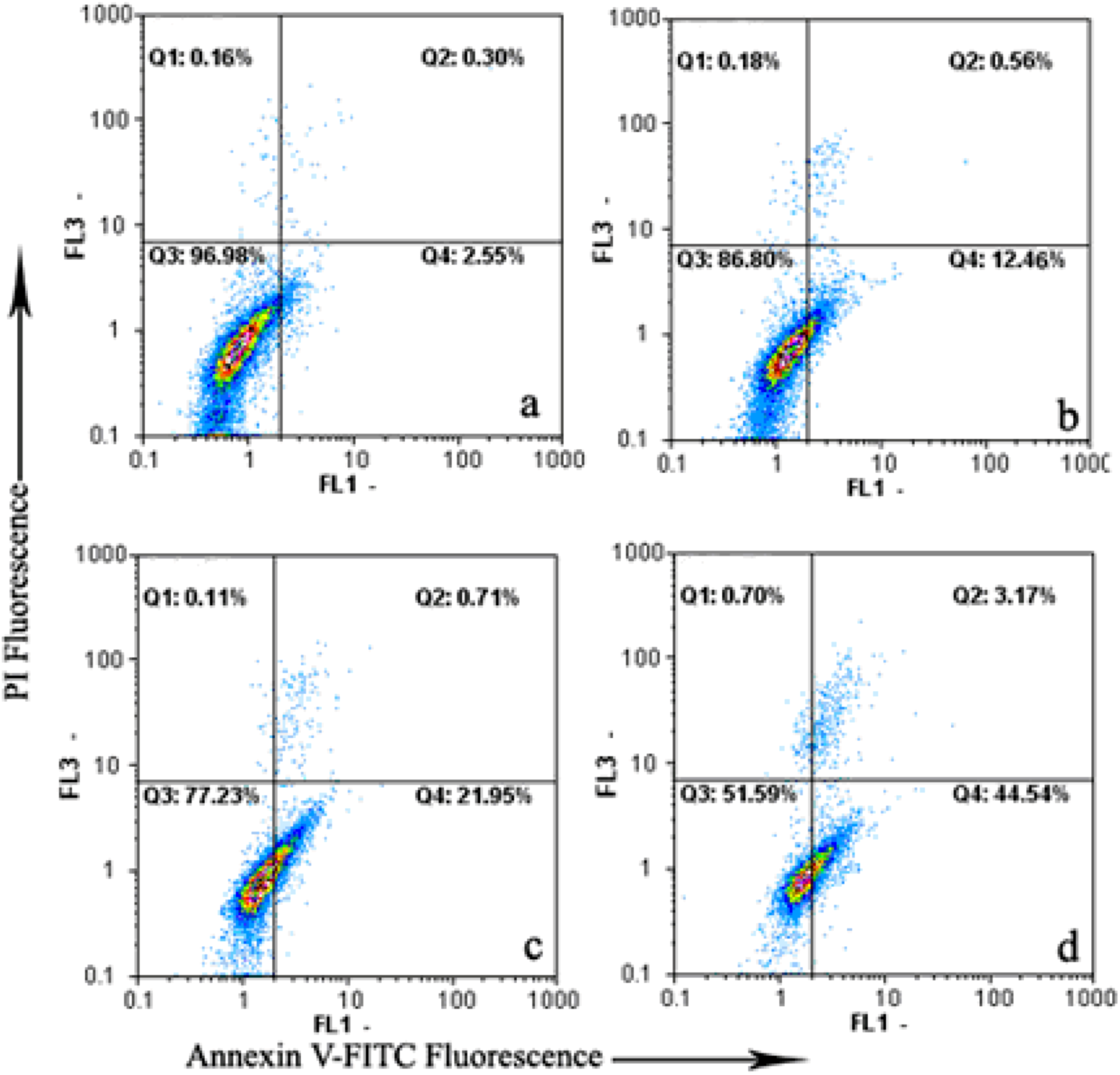
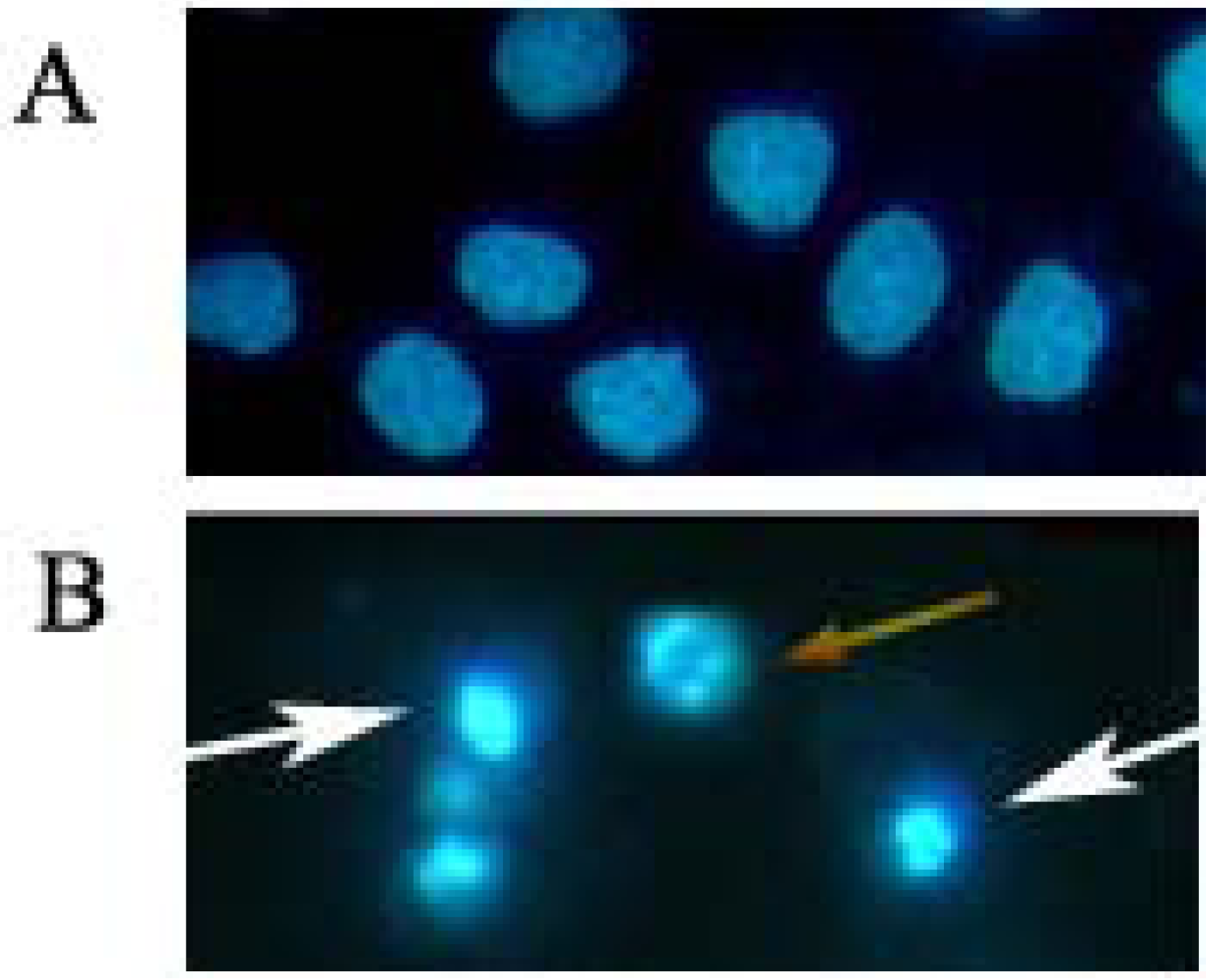
2.3. Disruption of Δψm and Cytochrome c Release During Daphnoretin Induced Apoptosis
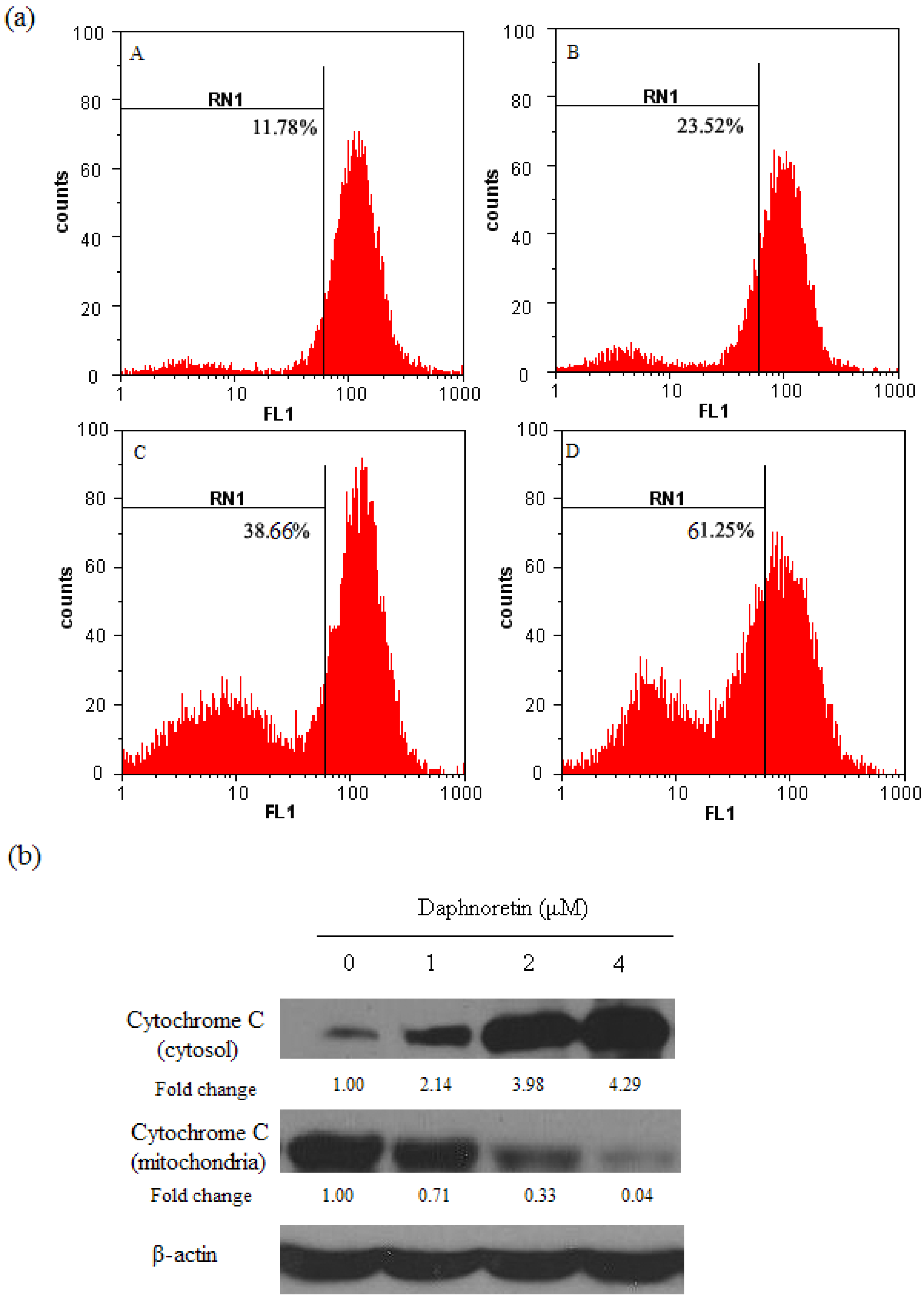
2.4. Flow-Cytometric Analysis
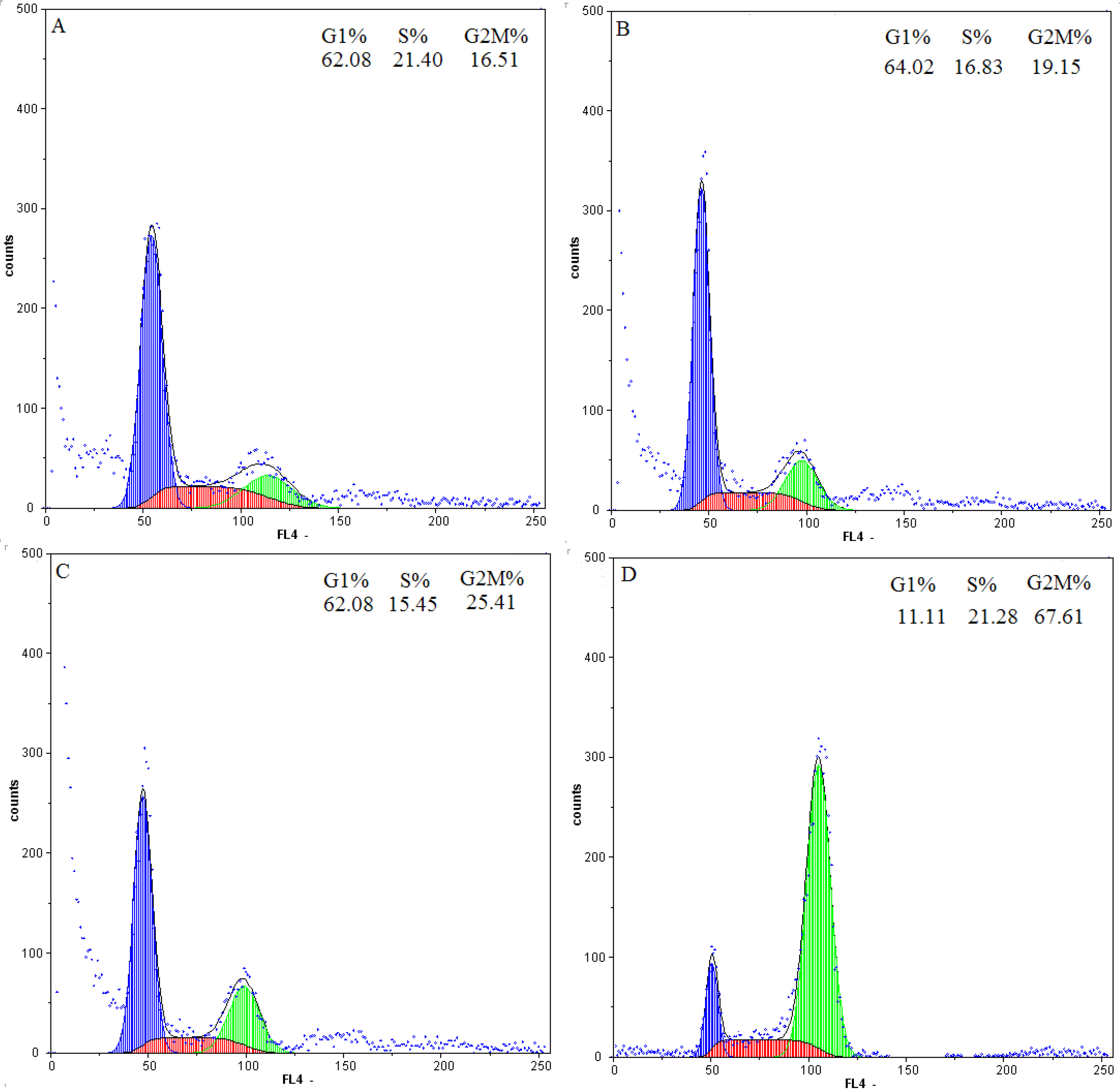
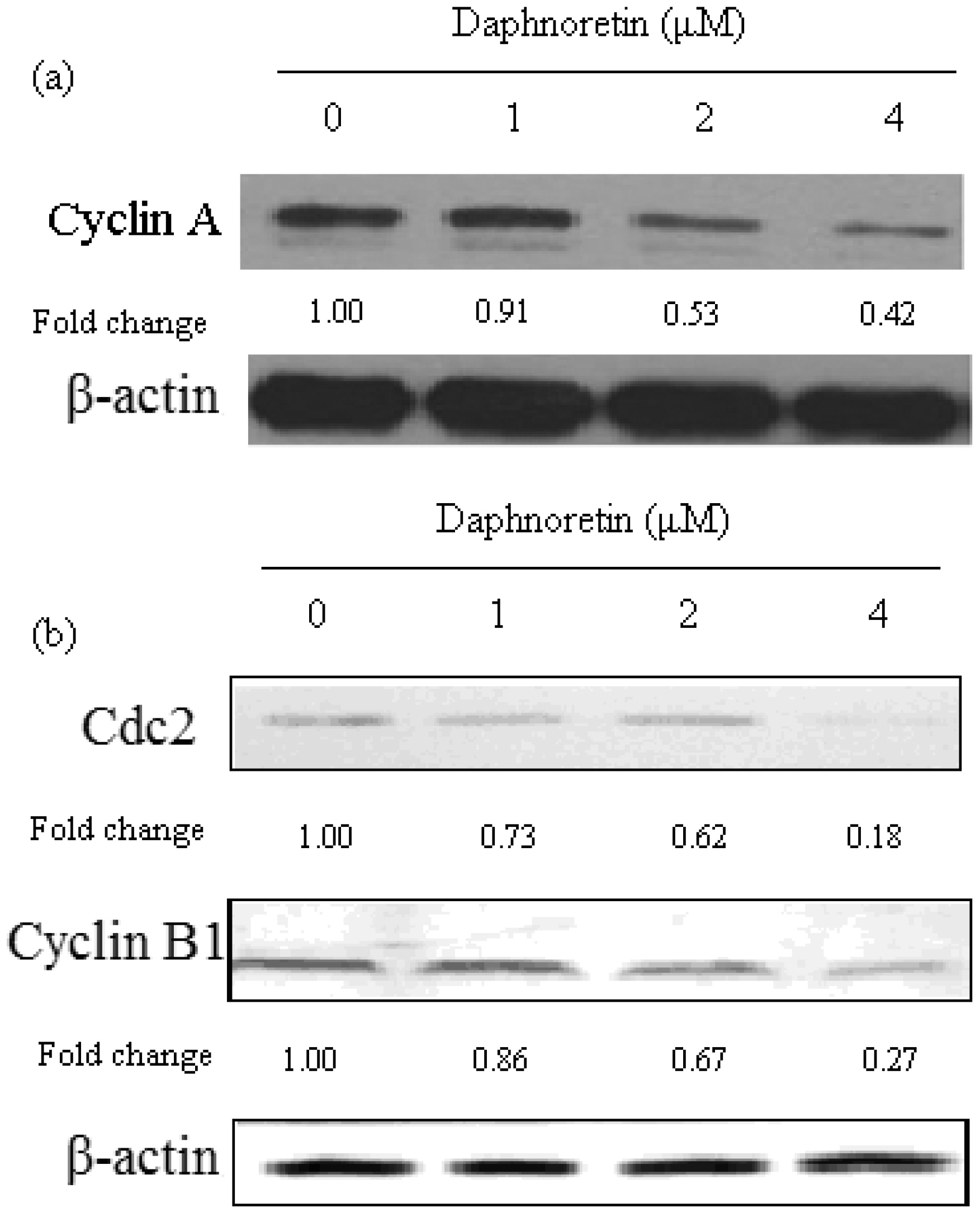
2.5. Effect of Daphnoretin on Cell Cycle-Related Proteins
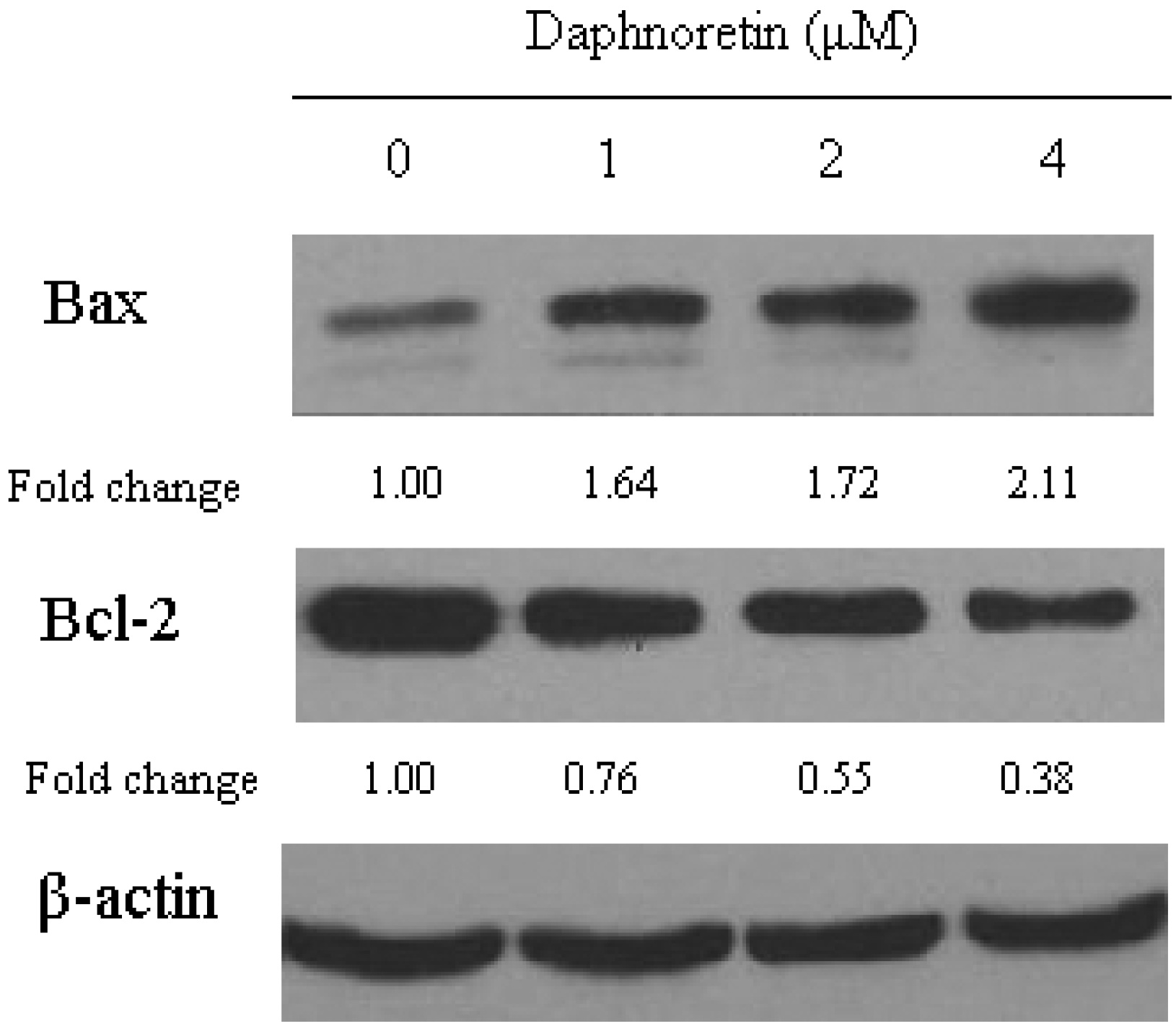
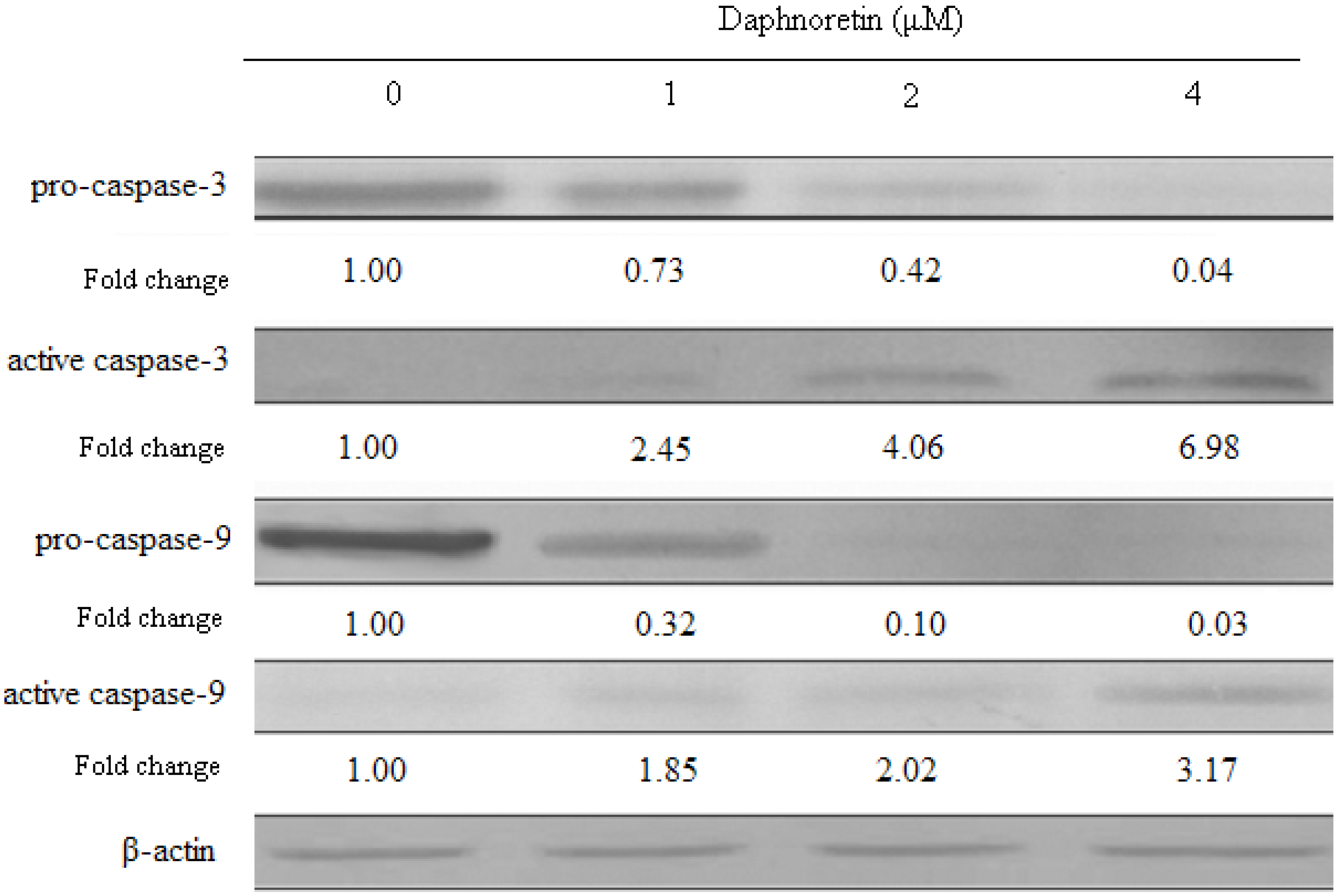
2.6. Effect of Daphnoretin on Apoptosis-Related Proteins
3. Experimental
3.1. Materials
3.2. Growth of Cells
3.3. Cytotoxicity Assays
3.4. Flow Cytometric Analysis of Apoptosis
3.5. Morphological Observation of Nuclear Change
3.6. Cell Cycle Analysis
3.7. Changes of Mitochondrial Membrane Potential (Δψm)
3.8. Western Blot Assay
3.9. Statistical Analysis
4. Conclusions
- Sample Availability: Not available
References and Notes
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Unni, K.K.; Dahlin, D.C. Dahlin's Bone Tumors: General Aspects and Data on 11,087 Cases, 5th ed; Lippincott-Raven: Philadelphia, PA, USA, 1996. [Google Scholar]
- Ferrari, S.; Briccoli, A.; Mercuri, M.; Bertoni, F.; Picci, P. Postrelapse survival in osteosarcoma of the extremities: Prognostic factors for long-term survival. J. Clin. Oncol. 2003, 21, 710–715. [Google Scholar] [CrossRef]
- Ferrari, S.; Smeland, S.; Mercuri, M.; Bertoni, F.; Longhi, A. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: A joint study by the Italian and Scandinavian Sarcoma Groups. J. Clin. Oncol. 2005, 23, 8845–8852. [Google Scholar] [CrossRef]
- Jaffe, N.; Gorlick, R. High-dose methotrexate in osteosarcoma: Let the questions surcease time for final acceptance. J. Clin. Oncol. 2008, 26, 4365–4366. [Google Scholar] [CrossRef]
- Anderson, P. Chemotherapy for osteosarcoma with high-dose methotrexate is effective and outpatient therapy is now possible. Nat. Clin. Pract. Oncol. 2007, 4, 624–625. [Google Scholar]
- Tang, Q.L.; Liang, Y.; Xie, X.B.; Yin, J.Q.; Zou, C.Y.; Zhao, Z.Q.; Shen, J.N.; Wang, J. Enrichment of osteosarcoma stem cells by chemotherapy. Chin. J. Cancer 2011, 30, 426–432. [Google Scholar] [CrossRef]
- Vinatier, D.; Dufour, P.; Subtil, D. Apoptosis: A programmed cell death involved in ovarian and uterine physiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 67, 85–102. [Google Scholar] [CrossRef]
- Das, G.C.; Holiday, D.; Gallardo, R.; Haas, C. Taxol-induced cell cycle arrest and apoptosis: Dose-response relationship in lung cancer cells of different wild-type p53 status and under isogenic condition. Cancer Lett. 2001, 165, 147–153. [Google Scholar] [CrossRef]
- Debatin, K.M. Activation of apoptosis pathways by anticancer treatment. Toxicol. Lett. 2000, 113, 41–48. [Google Scholar] [CrossRef]
- da Silva, C.P.; de Oliveira, C.R.; de Lima, M.C.P. Apoptosis as a mechanism of cell death induced by different chemotherapeutic drugs in human leukemic T-lymphocytes. Biochem. Pharmacol. 1996, 51, 1331–1340. [Google Scholar] [CrossRef]
- Pulido, M.D.; Parrish, A.R. Metal-induced apoptosis: Mechanisms. Mutat. Res. 2003, 533, 227–241. [Google Scholar] [CrossRef]
- Sarraf, C.E.; Bowen, I.D. Proportions of mitotic and apoptotic cells in a range of untreated experimental tumours. Cell Tissue Kinet. 1988, 21, 45–49. [Google Scholar]
- Carson, D.A.; Ribeiro, J.M. Apoptosis and disease. Lancet 1993, 341, 1251–1254. [Google Scholar] [CrossRef]
- Cordon-Cardo, C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am. J. Pathol. 1995, 147, 545–560. [Google Scholar]
- Park, H.R.; Park, Y.K. Expression of p53 protein, PCNA, and Ki-67 in osteosarcomas of bone. J. Korean Med. Sci. 1995, 10, 360–367. [Google Scholar]
- Hall, M.; Peters, G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv. Cancer Res. 1996, 68, 67–108. [Google Scholar] [CrossRef]
- Junior, A.T.; de Abreu Alves, F.; Pinto, C.A.; Carvalho, A.L.; Kowalski, L.P.; Lopes, M.A. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. 2003, 39, 521–530. [Google Scholar] [CrossRef]
- Kamel, O.W.; LeBrun, D.P.; Davis, R.E.; Berry, G.J.; Warnke, R.A. Growth fraction estimation of malignant lymphomas in formalinfixed paraffin-embedded tissue using anti-PCNA/Cyclin 19A2. Correlation with Ki-67 labeling. Am. J. Pathol. 1991, 138, 1471–1477. [Google Scholar]
- Rosa, J.C.; Mendes, R.; Filipe, M.I.; Morris, R.W. Measurement of cell proliferation in gastric carcinoma: Comparative analysis of Ki-67 and proliferative cell nuclear antigen (PCNA). Histochem. J. 1992, 24, 93–101. [Google Scholar] [CrossRef]
- Rieger, E.; Hofmann-Wellenhof, R.; Soyer, H.P.; Kofler, R.; Cerroni, L.; Smolle, J. Comparison of proliferative activity as assessed by proliferating cell nuclear antigen (PCNA) and Ki-67 monoclonal antibodies in melanocytic skin lesions. A quantitative immunohistochemical study. J. Cutan. Pathol. 1993, 20, 229–236. [Google Scholar] [CrossRef]
- Tuccari, G.; Rizzo, A.; Muscara, M.; Giuffre, G.; Barresi, G. PCNA/cyclin expression in breast carcinomas: Its relationships with Ki-67, ER, PgR immunostainings and clinico-pathologic aspects. Pathologica 1993, 85, 47–55. [Google Scholar]
- Kushner, J.; Bradley, G.; Young, B.; Jordan, R.C. Aberrant expression of cyclin A and cyclin B1 proteins in oral carcinoma. J. Oral Pathol. Med. 1999, 28, 77–81. [Google Scholar]
- Sherr, C.J. G1 phase progression: Cycling on cue. Cell 1994, 79, 551–555. [Google Scholar] [CrossRef]
- Jiang, S.G.; Zu, Y.G.; Fu, Y.J.; Zhang, Y.; Efferth, T. Activation of the mitochondria drivenpathway of apoptosis in human PC-3 prostate cancer cells by a novel hydrophilic paclitaxelderivative, 7-xylosyl-10-deacetylpaclitaxel. Int. J. Oncol. 2008, 33, 103–111. [Google Scholar]
- Holloway, S.L.; Glotzer, M.; King, R.W.; Murray, A.W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 1993, 73, 1393–1402. [Google Scholar] [CrossRef]
- Shi, Z.C. The Poisonous Plants in Chinese Pasture; China Agricultural Press: Beijing, China, 1997. [Google Scholar]
- Zhao, S.H.; Wang, S.Q. Progress of investigations and application of insecticide plants. Guangdong Agric. Sci. 1997, 26–28. [Google Scholar]
- Feng, W.; Tetsuro, I.; Mitsuzi, Y. The antitumor activities of gnidimacrin isolated from Stellera chamaejasme L. Chin. J. Cancer Res. 1995, 17, 24–26. [Google Scholar]
- Narantuya, S.; Batsurén, D.; Rashkes, Y.V.; Mil'grom, E.G. Chemical study of plants of the Mongolian flora coumarins ofStellera chamaejasme: The structure of chamaejasmoside—A new bicoumarin glycoside. Chem. Nat. Compd. 1994, 30, 197–199. [Google Scholar] [CrossRef]
- Jin, C.; Michetich, R.G.; Daneshtalab, M. Flavonoids from Stellera chamaejasme. Phytochemistry 1999, 50, 505–508. [Google Scholar]
- Yu, M.H.; Hou, A.J. Anti-HBV constituents from Euphorbia fischeriana. Zhongguo Zhong Yao Za Zhi 2010, 35, 3002–3006. [Google Scholar]
- Chen, H.C.; Chou, C.K.; Kuo, Y.H.; Yeh, S.F. Identification of a protein kinase C (PKC) activator, daphnoretin, that suppresses hepatitis B virus gene expression in human hepatoma cells. Biochem. Pharmacol. 1996, 52, 1025–1032. [Google Scholar]
- Jiang, Z.H.; Tanaka, T.; Sakamoto, T.; Kouno, I.; Duan, J.A.; Zhou, R.H. Biflavanones, diterpenes, and coumarins from the roots of Stellera chamaejasme L. Chem. Pharm. Bull. (Tokyo) 2002, 50, 137–139. [Google Scholar] [CrossRef]
- Fujimori, J.; Matsuo, T.; Shimose, S.; Kubo, T.; Ishikawa, M.; Yasunaga, Y.; Ochi, M. Antitumor effects of telomerase inhibitor TMPyP4 in osteosarcoma cell lines. J. Orthop. Res. 2011, 29, 1707–1711. [Google Scholar] [CrossRef]
- Kostova, I.; Momekov, G. New zirconium (IV) complexes of coumarins with cytotoxic activity. Eur. J. Med. Chem. 2006, 41, 717. [Google Scholar] [CrossRef]
- Kostova, I. Anti Cancer Agents. Curr. Med. Chem. 2005, 5, 29. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.Z.; Zhao, Y.L.; Fan, D.W.; Ding, L.; Zhang, S.D. Cytotoxic Activity of Some Novel Dicoumarin Derivatives in vitro. Chem. Res. Chin. Univ. 2009, 25, 644–647. [Google Scholar]
- Kaufmann, S.H.; Hengartner, M.O. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef]
- Jeong, H.W.; Han, D.C.; Son, K.H.; Han, M.Y.; Lim, J.S.; Ha, J.H.; Lee, C.W.; Kim, H.M.; Kim, H.C.; Kwon, B.M. Antitumor effect of the cinnamaldehyde derivative CB403 through the arrest of cell cycle progression in the G2/M phase. Biochem. Pharmacol. 2003, 65, 1343–1350. [Google Scholar]
- Buolamwini, J.K. Cell cycle molecular targets in novel anticancer drug discovery. Curr. Pharm. Des. 2000, 6, 379–392. [Google Scholar] [CrossRef]
- McDonald, E.R.; EI-Deiry, W.S. Cell cycle control as a basis for cancer drug development. Int. J. Oncol. 2000, 16, 871–886. [Google Scholar]
- Morgan, D.O. Principles of CDK regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef]
- Pines, J. Cyclins and cyclin-dependent kinases: A biochemical view. Biochem. J. 1995, 308, 697–711. [Google Scholar]
- Nurse, P. Universal control mechanism regulating onset of Mphase. Nature 1990, 344, 503–508. [Google Scholar] [CrossRef]
- Piao, W.; Yoo, J.; Lee, D.K.; Hwang, H.J.; Kim, J.H. Induction of G2/M phase arrest and apoptosis by a new synthetic anticancer agent, DW2282, in promyelocytic leukemia 6 (HL-60) cell. Biochem. Pharmacol. 2001, 62, 1439–1447. [Google Scholar]
- Petros, A.M.; Olejniczak, E.T.; Fesik, S.W. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 2004, 1644, 83–94. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yang, X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821. [Google Scholar] [CrossRef]
- Stennicke, H.R.; Salvesen, G.S. Properties of the caspases. Biochim. Biophys. Acta 1998, 1387, 17. [Google Scholar] [CrossRef]
- Cain, K.; Brown, D.G.; Langlais, C.; Cohen, G.M. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 1999, 274, 22686–22692. [Google Scholar] [CrossRef]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptor. Cell 1998, 94, 481. [Google Scholar] [CrossRef]
- Soeda, J.; Miyagawa, S.; Sano, K.; Masumoto, J.; Taniguchi, S.; Kawasaki, S. Cytochrome c release into cytosol with subsequent caspase activation during warm ischemia in rat liver. Am. J. Physiol. Gastrointest Liver Physiol. 2001, 281, G1115–G1123. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gu, S.; He, J. Daphnoretin Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma (HOS) Cells. Molecules 2012, 17, 598-612. https://doi.org/10.3390/molecules17010598
Gu S, He J. Daphnoretin Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma (HOS) Cells. Molecules. 2012; 17(1):598-612. https://doi.org/10.3390/molecules17010598
Chicago/Turabian StyleGu, Shoubin, and Jinhai He. 2012. "Daphnoretin Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma (HOS) Cells" Molecules 17, no. 1: 598-612. https://doi.org/10.3390/molecules17010598
APA StyleGu, S., & He, J. (2012). Daphnoretin Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma (HOS) Cells. Molecules, 17(1), 598-612. https://doi.org/10.3390/molecules17010598




