The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest
Abstract
:1. Introduction
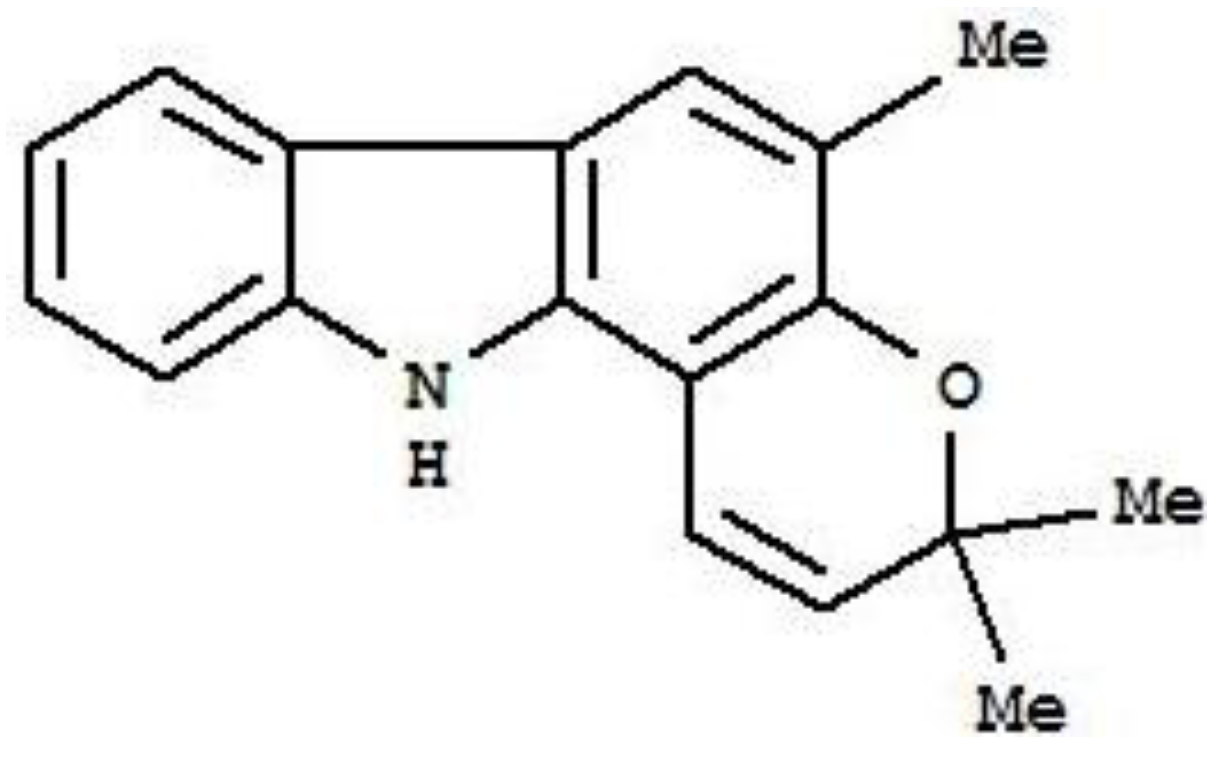
2. Results
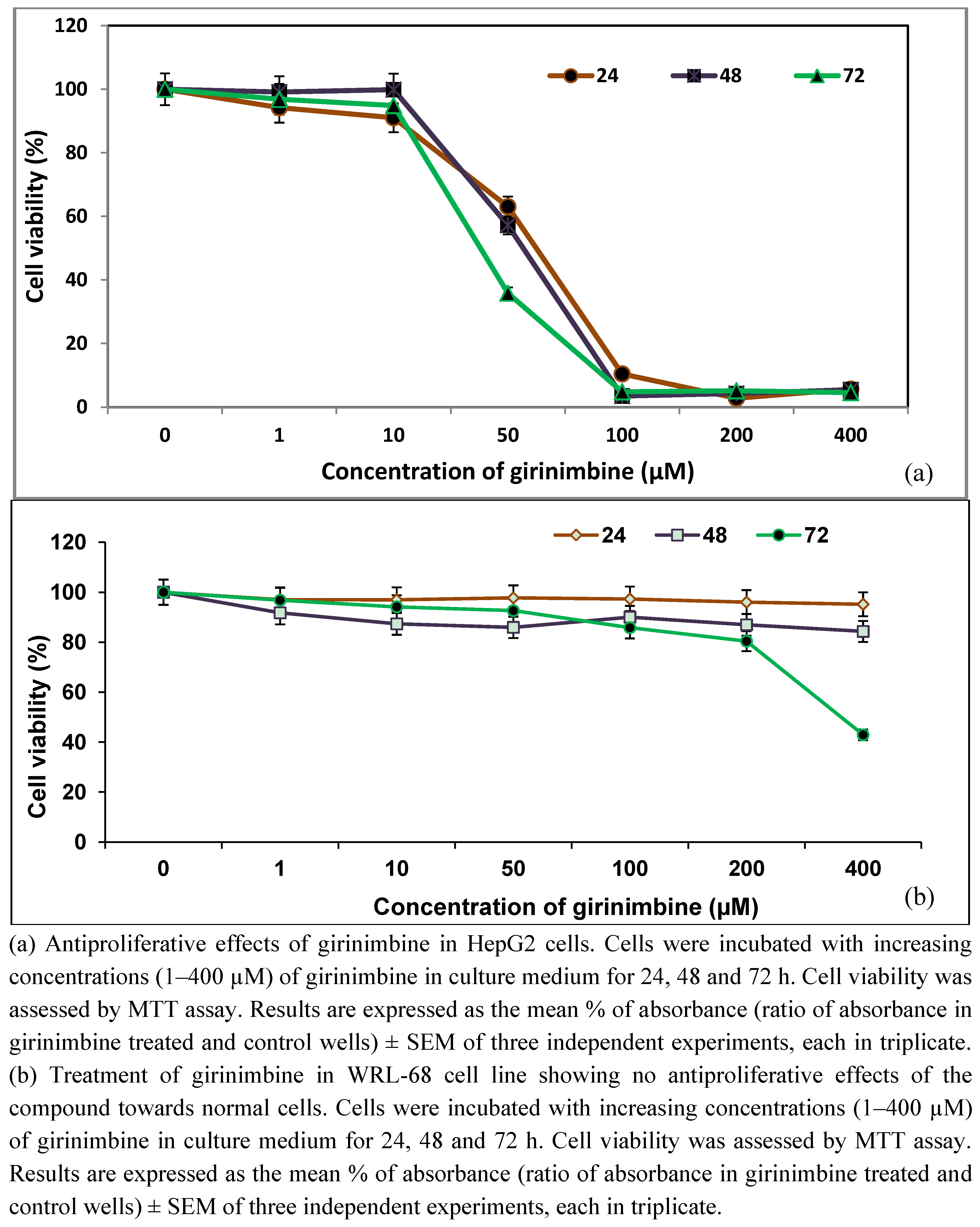
2.1. Girinimbine Inhibits the Proliferation of HepG2 Cells
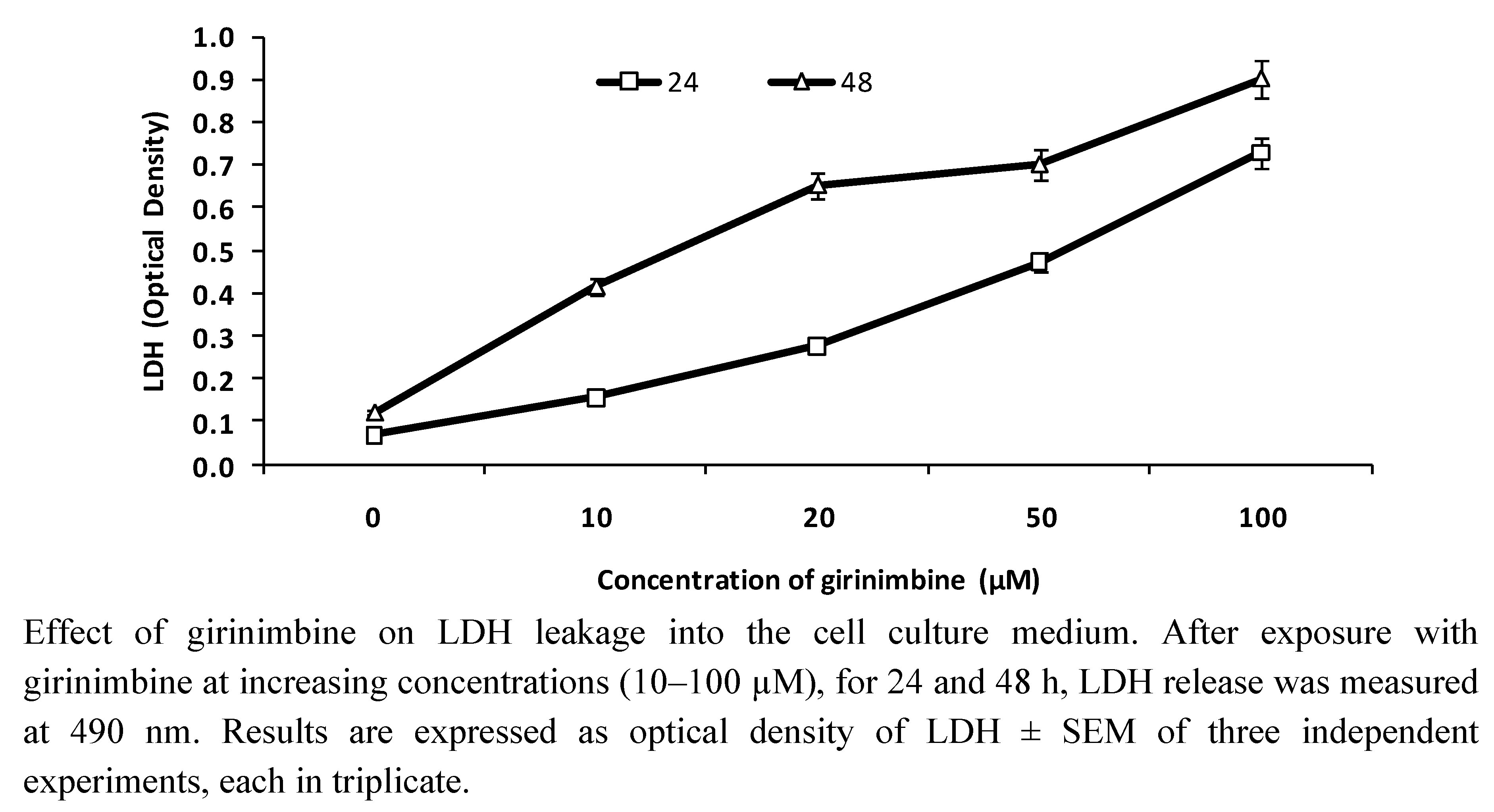
2.2. Girinimbine Increased LDH Release from HepG2 Cells
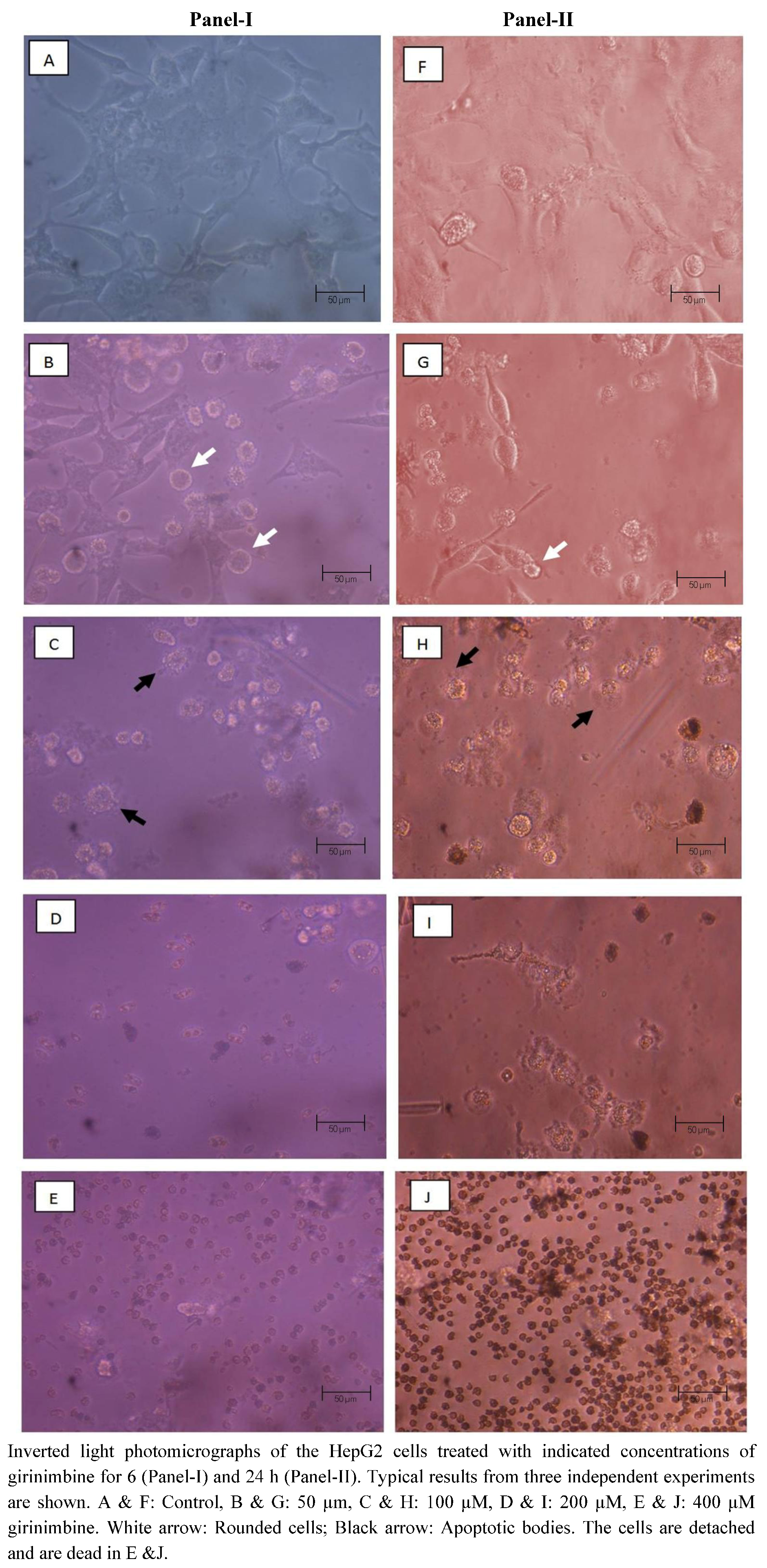
2.3. Girinimbine Induces Morphological Changes in HepG2 Cells Prior to Cell Death
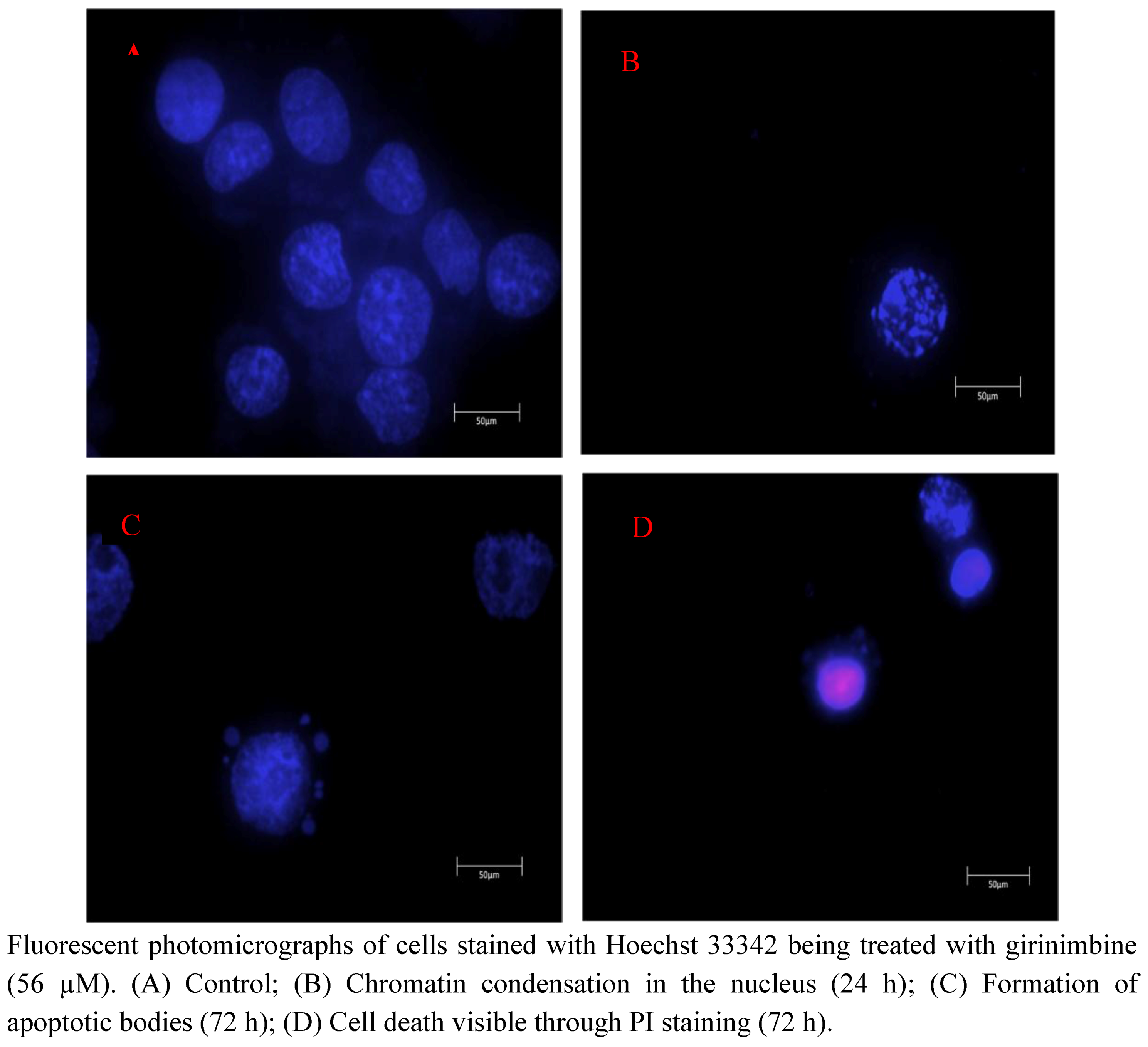
2.4. Girinimbine Showed Biochemical Features of Apoptosis
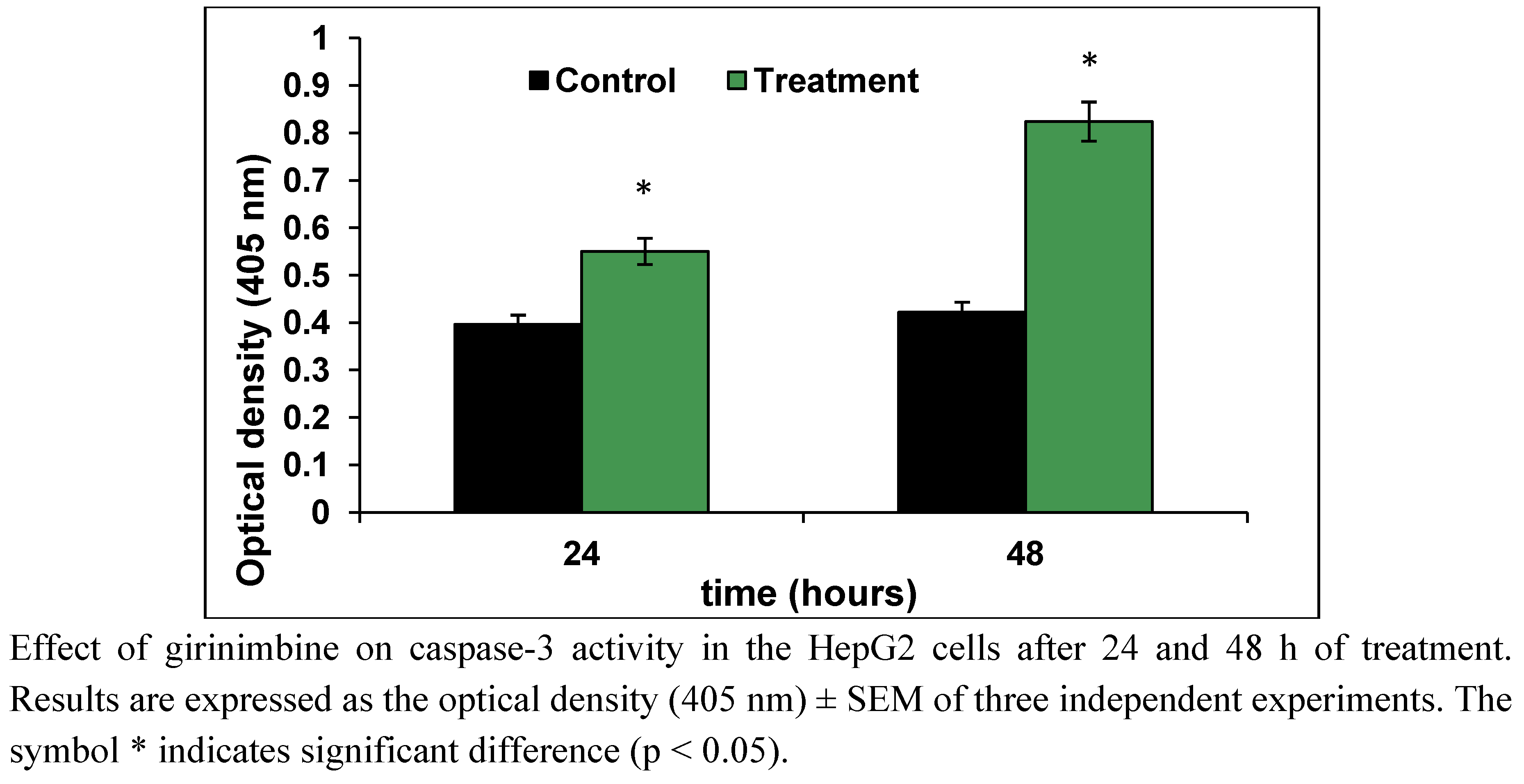
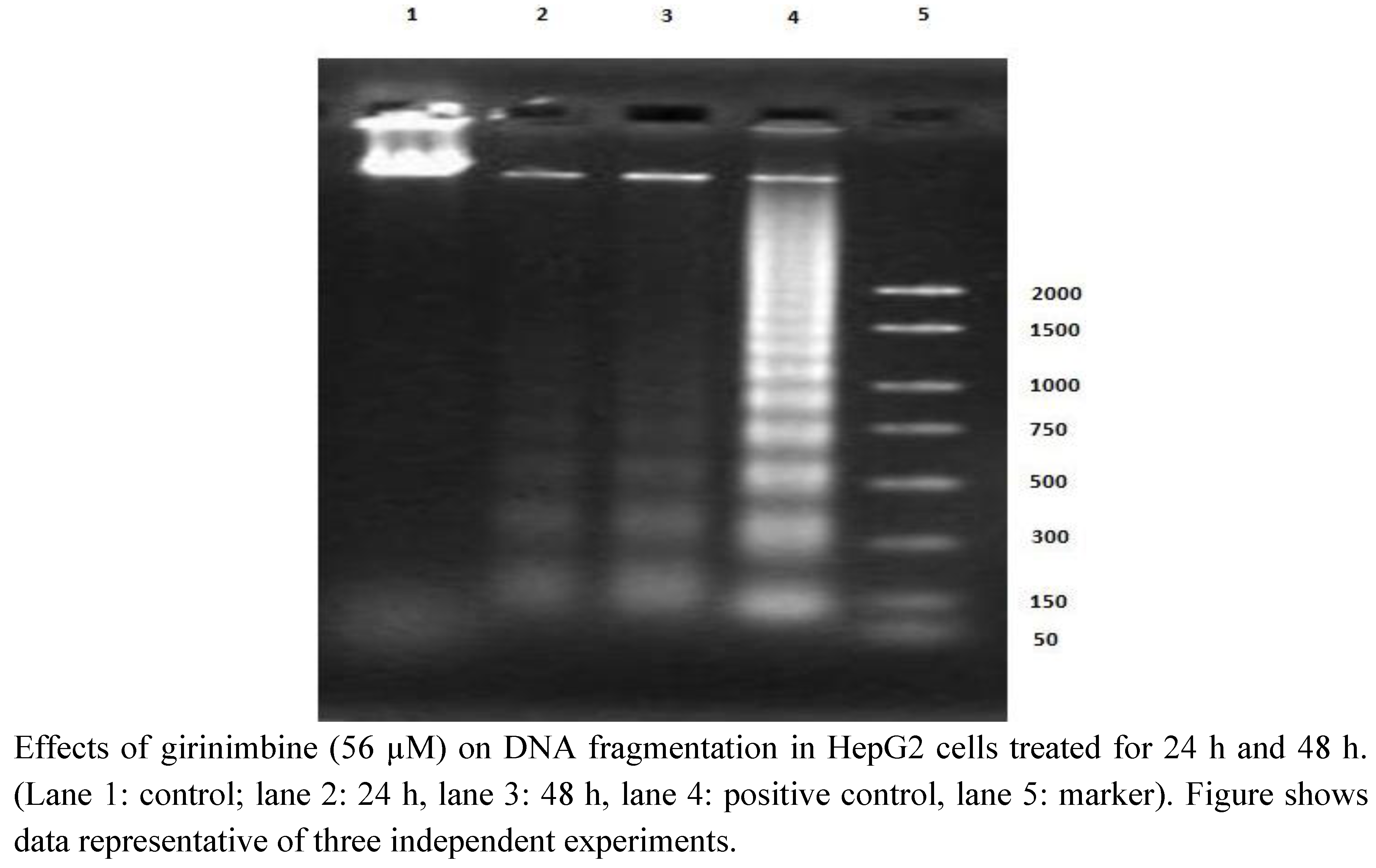
2.5. Girinimbine Induces G0/G1-Phase Arrest in HepG2 Cells
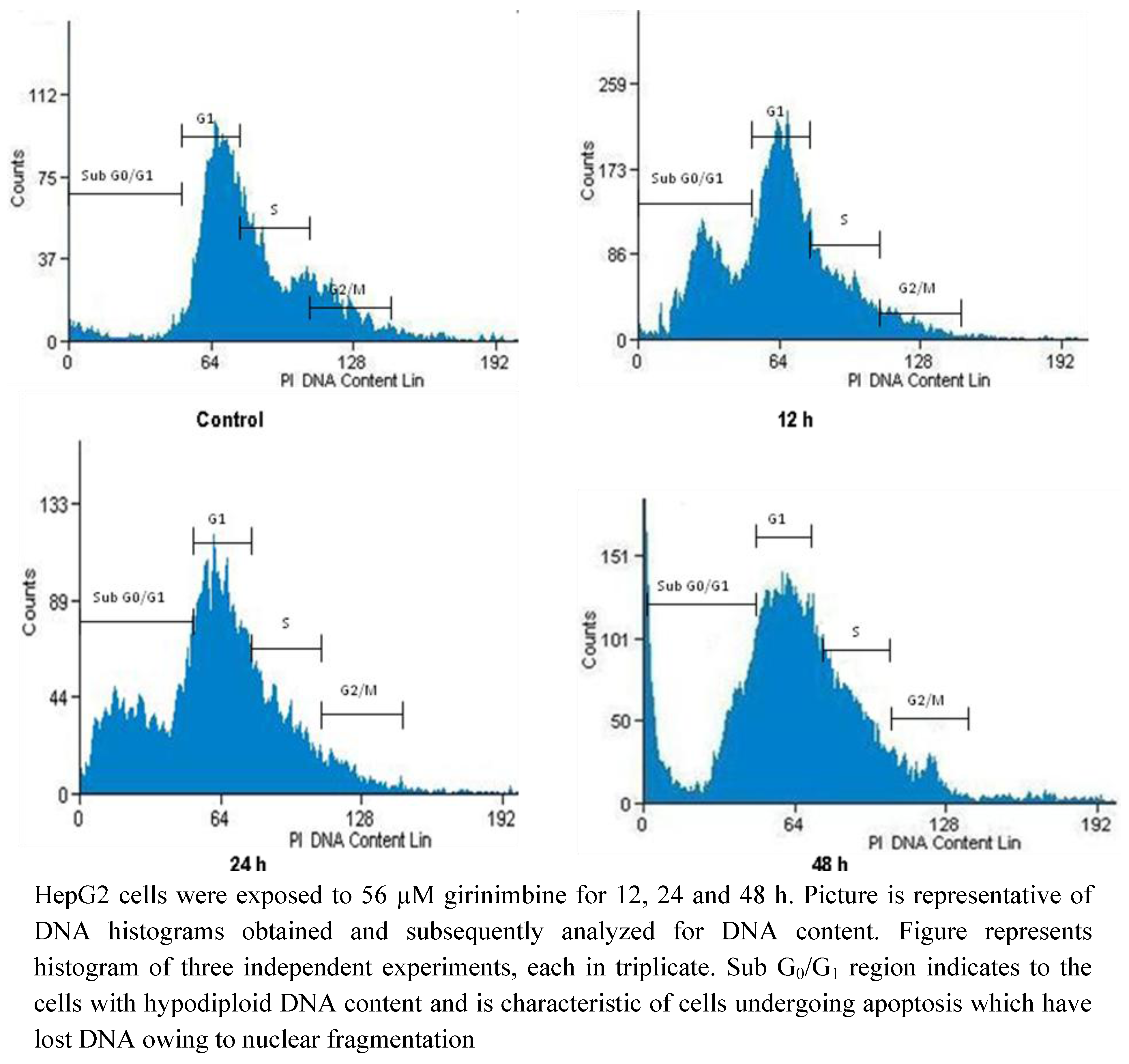
| Sub G0/G1 | G0/G1 | S | G2/M | |
|---|---|---|---|---|
| Control | 3.81 ± 0.41 | 67.86 ± 7.8 | 8.93 ± 0.1 | 19.4 ± 1.2 |
| 12 h | 6.56 ± 0.53 * | 69.63 ± 6.44 * | 6.77 ± 0.21 * | 17.04 ± 1.5 * |
| 24 h | 7.62 ± 0.19 * | 72.09 ± 8 .1 * | 5.21 ± 0.43 * | 15.08 ± 2.1 * |
| 48 h | 10.34 ± 0.84 * | 75.32 ± 7.9 * | 5.74 ± 0.4 * | 8.6 ± 0.9 * |
3. Discussion
4. Experimental
4.1. Drugs and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. LDH Release Assay
4.5. Evaluation of Morphology by Light Microscopy
4.6. Chromatin Condensation Assay
4.7. Colourimetric Assay of Caspase-3
4.8. DNA Laddering
4.9. Flow Cytometric Analysis of DNA Cell Cycle
5. Conclusions
Acknowledgments
Conflict of Interest
- Sample Availability: Samples of the compound girinimbine are available from the authors.
References
- Barshack, I.; Meiri, E.; Rosenwald, S.; Lebanony, D.; Bronfeld, M.; Aviel-Ronen, S.; Rosenblatt, K.; Polak-Charcon, S.; Leizerman, I.; Ezagouri, M. Differential diagnosis of hepatocellular carcinoma from metastatic tumors in the liver using microRNA expression. Int. J. Biochem. Cell Biol. 2010, 42, 1355–1362. [Google Scholar] [CrossRef]
- Capocaccia, R.; Sant, M.; Berrino, F.; Simonetti, A.; Santi, V.; Trevisani, F. Hepatocellular carcinoma: Trends of incidence and survival in Europe and the United States at the end of the 20th century. Am. J. Gastroenterol. 2007, 102, 1661–1670. [Google Scholar] [CrossRef]
- Yuen, M.F.; Hou, J.L.; Chutaputti, A. Hepatocellular carcinoma in the Asia pacific region. J. Gastroenterol. Hepatol. 2009, 24, 346–353. [Google Scholar] [CrossRef]
- Ferenci, P.; Fried, M.; Labrecque, D.; Bruix, J.; Sherman, M.; Omata, M.; Heathcote, J.; Piratsivuth, T.; Kew, M.; Otegbayo, J.A. Hepatocellular Carcinoma (HCC): A Global Perspective. J. Clin. Gastroenterol. 2010, 44, 239–245. [Google Scholar] [CrossRef]
- Guglielmi, A.; Ruzzenente, A.; Valdegamberi, A.; Pachera, S.; D’Onofrio, M.C.T.; Martone, E.; Nicoli, P.; Iacono, C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J. Gastrointest. Surg. 2008, 12, 192–198. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.F.; Li, Y.; Chan, T.H.M.; Zheng, B.J.; Huang, J.; Guan, X.Y. Clinical significance of CHD1L in hepatocellular carcinoma and therapeutic potentials of virus-mediated CHD1L depletion. Gut 2011, 60, 534–543. [Google Scholar] [CrossRef]
- Knolker, H.J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428. [Google Scholar] [CrossRef]
- Itoigawa, M.; Kashiwada, Y.; Ito, C.; Furukawa, H.; Tachibana, Y.; Bastow, K.F.; Lee, K.H. Carbazole alkaloid murrayaquinone A and related synthetic carbazolequinones as cytotoxic agents. J. Nat. Prod. 2000, 63, 893–897. [Google Scholar] [CrossRef]
- Asche, C.; Demeunynck, M. Antitumor carbazoles. Anticancer Agents Med. Chem. 2007, 7, 247–267. [Google Scholar] [CrossRef]
- Knolker, H. Occurrence, biological activity, and convergent organometallic synthesis of carbazole alkaloids. Top. Curr. Chem. 2005, 24, 115–148. [Google Scholar]
- Cui, C.B.; Yan, S.Y.; Cai, B.; Yao, X.S. Carbazole alkaloids as new cell cycle inhibitor and apoptosis inducers from Clausena dunniana Levl. J. Asian Nat. Prod. Res. 2002, 4, 233–241. [Google Scholar] [CrossRef]
- Joshi, B.S.; Kamat, V.N.; Gawad, D.H.; Govindachari, T.R. Structure and synthesis of heptaphylline. Phytochemistry 1972, 11, 2065–2071. [Google Scholar] [CrossRef]
- Joseph, S.; Peter, K.V. Curry leaf (Murraya koenigii), perennial, nutritious, leafy vegetable. Econ. Bot. 1985, 39, 68–73. [Google Scholar] [CrossRef]
- Adebajo, A.C.; Ayoola, O.F.; Iwalewa, E.O.; Akindahunsi, A.A.; Omisore, N.O.A.; Adewunmi, C.O.; Adenowo, T.K. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 2006, 13, 246–254. [Google Scholar] [CrossRef]
- Ko, F.N.; Lee, Y.S.; Wu, T.S.; Teng, C.M. Inhibition of cyclooxygenase activity and increase in platelet cyclic AMP by girinimbine, isolated from Murraya euchrestifolia. Biochem. Pharmacol. 1994, 48, 353–360. [Google Scholar] [CrossRef]
- Thevissen, K.; Marchand, A.; Chaltin, P.; Meert, E.M.K.; Cammue, B. Antifungal carbazoles. Curr. Med. Chem. 2009, 16, 2205–2211. [Google Scholar] [CrossRef]
- Wang, S.; Cai, B.; Cui, C.; Yan, S.; Wu, C. Study on induction of apoptosis by girinimbine in HCT-15 cell in vitro. Chin. J. Pharm. Anal. 2008, 28, 176–181. [Google Scholar]
- Wang, S.; Cai, B.; Cui, C. Induction of apoptosis by girinimbine in K562 cell. Chin. Tradit. Herb. Drugs 2007, 38, 1677–1680. [Google Scholar]
- Abubakar, N.H.; Sukari, M.A.; Rahmani, M.; Sharif, M.A.; Khalid, K.; Yusof, U.K. Chemical Constituents from Stem Barks and Roots of Murraya koenigii (Rutaceae). MJAS 2007, 11, 173–176. [Google Scholar]
- Rowinsky, E.K.; Donehower, R.C. Paclitaxel (taxol). N. Eng. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef]
- Rosenkranz, V.; Wink, M. Alkaloids induce programmed cell death in bloodstream forms of Trypanosomes (Trypanosoma b. Brucei). Molecules 2008, 13, 2462–2473. [Google Scholar] [CrossRef]
- Khan, B.A.; Abraham, A.; Lelamma, S. Murraya koenigii and Brassica juncea-Alterations on lipid profile in 1-2 dimethyl hydrazine induced colon carcinogenesis. Invest. New Drugs 1996, 14, 365–369. [Google Scholar]
- Haslizawati, A.B.N. Chemical constituents, bioactivity and HPLC profiling of microwave-assisted normal extraction of Murraya koenigii. Masters thesis, Universiti Putra Malaysia, Selangor, Malaysia, 30 April 2010. [Google Scholar]
- Roy, M.K.; Thalang, V.N.; Trakoontivakorn, G.; Nakahara, K. Mahanine, a carbazole alkaloid from Micromelum minutum, inhibits cell growth and induces apoptosis in U937 cells through a mitochondrial dependent pathway. Br. J. Pharmacol. 2005, 145, 145–155. [Google Scholar] [CrossRef]
- Kok, Y.Y. Cytotoxicity of mahanimbine, murryafoline A and S-benzyldithiocarbazate on human leukemic cell line. Masters thesis, Universiti Putra Malaysia, Selangor, Malaysia, 23 November 2010. [Google Scholar]
- Jiang, H.; Zhang, L.; Kuo, J.; Kuo, K.; Gautam, S.C.; Groc, L.; Rodriguez, A.I.; Koubi, D.; Hunter, T.J.; Corcoran, G.B. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol. Cancer Ther. 2005, 4, 554–561. [Google Scholar] [CrossRef]
- Liu, J.; Shen, H.M.; Ong, C.N. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Lett. 2000, 153, 85–93. [Google Scholar] [CrossRef]
- Zain, W.N. Antiproliferative properties of Clausine-B against cancer cell lines. Malays. J. Med. Sci. 2009, 16, 31–36. [Google Scholar]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Chang, C.H.; Lin, C.C. The mechanism of ellipticine-induced apoptosis and cell cycle arrest in human breast MCF-7 cancer cells. Cancer Lett. 2005, 223, 293–301. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Kuo, Y.C.; Chang, C.H.; Lin, C.C. The anti-proliferative inhibition of ellipticine in human breast MDA-MB-231 cancer cells is through cell cycle arrest and apoptosis induction. Anti-cancer Drug. 2005, 16, 789–795. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar]
- Collins, J.A.; Schandl, C.A.; Young, K.K.; Vesely, J.; Willingham, M.C. Major DNA Fragmentation Is a Late Event in Apoptosis. J. Histochem. Cytochem. 1997, 45, 923–934. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Nakao, K.; Murata, T.; Tsuboi, M.; Kaneda, N.; Furukawa, H. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine 2006, 13, 359–365. [Google Scholar] [CrossRef]
- Elankumaran, S.; Rockemann, D.; Samal, S.K. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 2006, 80, 7522–7534. [Google Scholar] [CrossRef]
- Liger, F.; Popowycz, F.; Besson, T.; Picot, L.; Galmarini, C.M.; Joseph, B. Synthesis and antiproliferative activity of clausine E, mukonine, and koenoline bioisosteres. Bioorg. Med. Chem. 2007, 15, 5615–5619. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Syam, S.; Abdul, A.B.; Sukari, M.A.; Mohan, S.; Abdelwahab, S.I.; Wah, T.S. The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest. Molecules 2011, 16, 7155-7170. https://doi.org/10.3390/molecules16087155
Syam S, Abdul AB, Sukari MA, Mohan S, Abdelwahab SI, Wah TS. The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest. Molecules. 2011; 16(8):7155-7170. https://doi.org/10.3390/molecules16087155
Chicago/Turabian StyleSyam, Suvitha, Ahmad Bustamam Abdul, Mohd. Aspollah Sukari, Syam Mohan, Siddig Ibrahim Abdelwahab, and Tang Sook Wah. 2011. "The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest" Molecules 16, no. 8: 7155-7170. https://doi.org/10.3390/molecules16087155
APA StyleSyam, S., Abdul, A. B., Sukari, M. A., Mohan, S., Abdelwahab, S. I., & Wah, T. S. (2011). The Growth Suppressing Effects of Girinimbine on Hepg2 Involve Induction of Apoptosis and Cell Cycle Arrest. Molecules, 16(8), 7155-7170. https://doi.org/10.3390/molecules16087155






