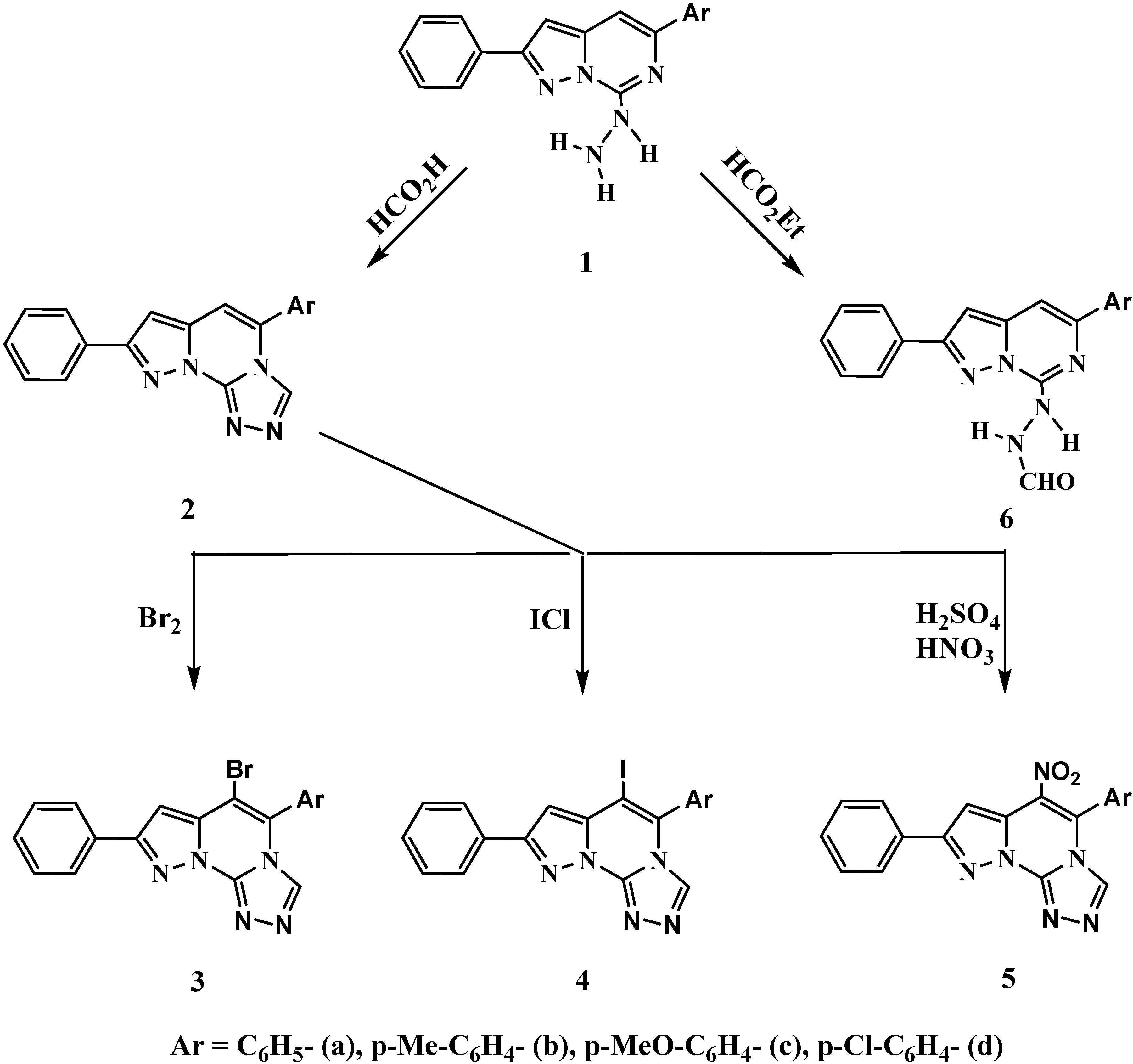
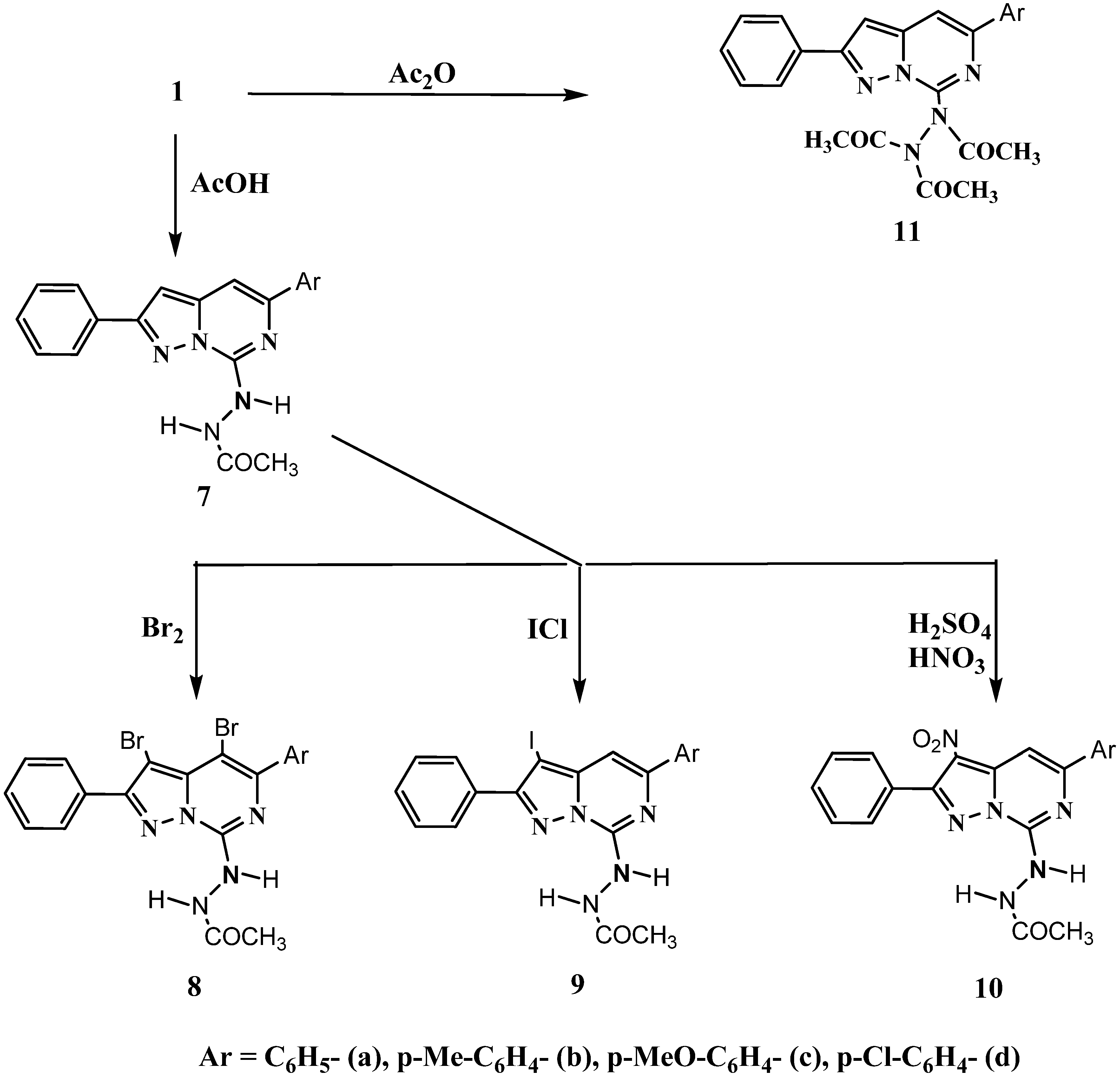
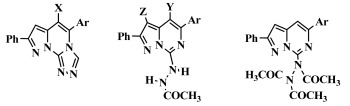
3.2. Synthesis of Compounds
3.2.1. 5-Aryl-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines 2a-d
A mixture of 5-aryl-7-hydrazino-2-phenylpyrazolo[1,5-c]pyrimidines (1a-d, 1 mmol) and formic acid (10 mL, 99%) was heated under reflux for 10 h. The mixture was evaporated under reduced pressure and the obtained residue was triturated with water, filtered, washed with EtOH and crystallized from EtOH to give the 5-aryl-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines 2a-d as colorless needles.
5,8-Diphenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (2a). Yield 81%, 0.25 g, mp 245–246 °C; IR (υmax, cm−1): 1649 (pyrazole ring C=N), 1580 (triazole ring C=N), and 1476 (C=C); 1H-NMR (CDCl3, δH, ppm): 6.91 (s, 1H, pyrazole-H), 7.43 (s, 1H, pyrimidine-H), 7.45–7.64 (m, 8H, aromatic- H), 8.05 (d, 2H, aromatic-H) and 8.53 (s, 1H, triazole-H); MS, m/z (%) = 312 (M.++1, 100,), 285 (M.+- CN, 4), 283 (M.+-N2, 33), 257 (M.+-CN3, 3), 255 (M.+-CH2N3, 16) and 227 (M.+-CH2N5, 8); Anal. Calc. for C19H13N5 (311.34): C, 73.30; H, 4.21; N, 22.49%, found: C, 73.27; H, 4.22; N, 22.53%.
8-Phenyl-5-p-tolylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (2b). Yield 76%, 0.25 g, mp 247–248 °C; IR (υmax, cm−1): 1643 (pyrazole ring C=N), 1577 (triazole ring C=N), and 1454 (C=C); 1H NMR (DMSO-d6, δH, ppm): 2.39 (s, 3H, CH3), 7.03 (d, 2H, aromatic-H), 7.26 (s, 1H, pyrazole-H),7.39–7.51 (m, 5H, aromatic-H), 7.44 (s, 1H, pyrimidine-H), 7.65 (d, 2H, aromatic-H) and 8.96 (s, 1H, triazole-H); MS, m/z (%) = 327 (M.++2, 17), 325 (M.+, 100), 297 (M.+-N2, 24), 282 (M.+-CH3N2, 8),255 (M.+-C2H4N3, 7) and 227 (M.+-C2H4N5, 10); Anal. Calc. for C20H15N5 (325.37): C, 73.83; H, 4.65;N, 21.52%, found: C, 73.87; H, 4.62; N, 21.55%.
5-(p-Methoxyphenyl)-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (2c). Yield 74%, 0.25 g, mp 237–238 °C; IR (υmax, cm−1): 1643 (pyrazole ring C=N), 1587 (triazole ring C=N), and 1454 (C=C); 1H NMR (CDCl3, δH, ppm): 3.92 (s, 3H, CH3), 6.93 (s, 1H, pyrazole-H), 7.10 (d, 2H, aromatic- H), 7.40–7.48 (m, 3H, aromatic-H), 7.44 (s, 1H, pyrimidine-H), 7.57 (d, 2H, aromatic-H), 8.05 (d, 2H, aromatic-H) and 8.55 (s, 1H, triazole-H); MS, m/z (%) = 343 (M.++2, 15), 341 (M.+, 100), 326 (M.+- CH3, 3), 313(M.+-N2, 10), 299 (M.+-CH2N2, 8), 285 (M.+-C2H4N2, 2) and 270 (M.+-C2H3N2O, 11); Anal. Calc. for C20H15N5O (341.37): C, 70.37; H, 4.43; N, 20.52%, found: C, 70.40; H, 4.40; N, 20.55%.
5-(p-Chlorophenyl)-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (2d). Yield 71%, 0.25 g, mp 299–300 °C; IR (υmax, cm−1): 1643 (pyrazole ring C=N), 1583 (triazole ring C=N), and 1467 (C=C); 1H NMR (DMSO-d6, δH, ppm): 7.33 (s, 1H, pyrazole-H), 7.43 (s, 1H, pyrimidine-H), 7.44–7.52 (m, 3H, aromatic-H), 7.66 (d, 2H, aromatic-H), 7.79 (d, 2H, aromatic-H), 8.04 (d, 2H, aromatic-H) and 9.00 (s, 1H, triazole-H); MS, m/z (%) = 347 (M.++1, 52), 345 (M.+-1, 100), 319 (M.+-HCN, 8), 317 (M.+-HN2, 17), 289 (M.+-C2H5N2, 6), 282 (M.+-HClN2, 13), 255 (M.+-C2H4ClN2, 16) and 227 (M.+- C3H6ClN3, 8); Anal. Calc. for C19H12ClN5 (345.79): C, 66.00; H, 3.50; N, 20.25%, found: C, 59.98; H, 3.50; N, 20.22%.
3.2.2. 5-Aryl-6-bromo-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines 3a-d
A solution of bromine (0.06 mL, 1.2 mmol) in acetic acid (10 mL) was gradually added to a suspension of 5-aryl-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines 2a-d (1 mmol) in acetic acid (10 mL) with stirring for 3 h at room temperature. The precipitated 5-aryl-6-bromo-8- phenylpyrazolo[1,5-c]-1,2,4-triazolo-[4,3-a]pyrimidines 3a-d were filtered, washed with water, dried and crystallized from EtOH as colorless needles.
6-Bromo-5,8-diphenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (3a). Yield 75%, 0.30 g, mp 235–236 °C; IR (υmax, cm−1): 1640 (pyrazole ring C=N), 1580 (triazole ring C=N), and 1455 (C=C); 1H NMR (CDCl3, δH, ppm): 6.92 (s, 1H, pyrazole-H), 7.43–7.69 (m, 8H, aromatic-H), 8.14 (d, 2H, aromatic-H) and 8.58 (s, 1H, triazole-H); MS, m/z (%) = 390 (M.+, 100), 362 (M.+-N2, 13), 310 (M.+- Br, 2), 282 (M.+-BrN2, 13), 255 (M.+-CHBrN3, 12) and 227 (M.+-CHBrN5, 6); Anal. Calc. for C19H12BrN5 (390.24): C, 58.48; H, 3.10; N, 17.95%, found: C, 58.52; H, 3.08; N, 17.90%.
6-Bromo-8-phenyl-5-p-tolylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (3b). Yield 75%, 0.30 g, mp 215–216 °C; IR (υmax, cm−1): 1636 (pyrazole ring C=N), 1578 (triazole ring C=N), and 1421 (C=C); 1H NMR (CDCl3, δH, ppm): 2.49 (s, 3H, CH3), 6.90 (s, 1H, pyrazole-H), 7.41–7.57 (m, 7H, aromatic-H), 8.14 (d, 2H, aromatic-H) and 8.60 (s, 1H, triazole-H); MS, m/z (%) = 407 (M.++3, 8), 405 (M.++1, 100), 377 (M.+-HCN, 8), 324 (M.+-Br, 2), 281 (M.+-CH3BrN2, 6) and 254 (M.+-C2H4BrN3, 5); Anal. Calc. for C20H14BrN5 (404.26): C, 59.42; H, 3.49; N, 17.32%, found: C, 59.39; H, 3.45; N, 17.27%.
6-Bromo-5-(p-methoxyphenyl)-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (3c). Yield 71%, 0.30 g, mp 213–214 °C; IR (υmax, cm−1): 1632 (pyrazole ring C=N), 1587 (triazole ring C=N), and 1427 (C=C); 1H NMR (CDCl3, δH, ppm): 3.90 (s, 3H, OCH3), 6.81 (s, 1H, pyrazole-H),7.09 (d, 2H, aromatic-H), 7.44–7.56 (m, 3H, aromatic-H),7.59 (d, 2H, aromatic-H), 8.10 (d, 2H, aromatic-H) and 8.57 (s, 1H, triazole-H); MS, m/z (%) = 422 (M.++2, 100), 420 (M.+, 80), 405 (M.+-CH3, 3), 391 (M.+- HN2, 8), 378 (M.+-CH2N2, 5), 350 (M.+- C2H4N3, 6), 340 (M.+-Br, 3), 313 (M.+-CHBrN, 4), 281 (M.+- CH3BrN2O, 5) and 269 (M.+-C2H3BrN2O, 13); Anal. Calc. for C20H14BrN5O (420.26): C, 57.16; H, 3.36; N, 16.66%, found: C, 57.20; H, 3.38; N, 16.70%.
6-Bromo-5-(p-chlorophenyl)-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (3d). Yield 71%, 0.30 g, mp 238–239 °C; IR (υmax, cm−1): 1637 (pyrazole ring C=N), 1580 (triazole ring C=N), and 1414 (C=C); 1H NMR (CDCl3, δH, ppm): 6.92 (s, 1H, pyrazole-H), 7.47–7.66 (m, 7H, aromatic-H), 8.14 (d, 2H, aromatic-H) and 8.55 (s, 1H, triazole-H); MS, m/z (%) = 429 (M.++4, 3), 428 (M.++3, 38), 426 (M.++1, 100), 424 (M.+-1, 76), 398 (M.+-HCN, 11), 317 (M.+-BrN2, 8), 289 (M.+-CH2BrN3, 8), 281 (M.+- HBrClN2, 15), 253 (M.+-C2H5BrClN2, 10) and 226 (M.+-C3H6BrClN3, 7); Anal. Calc. for C19H11BrClN5 (424.68): C, 53.74; H, 2.61; N, 16.49%; found: C, 53.72; H, 2.57; N, 16.50%.
3.2.3. 5-Aryl-6-iodo-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines 4a-d
A solution of iodine monochloride (0.2 g, 1.2 mmol) in acetic acid (10 mL) was gradually added to a suspension of 5- aryl-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[3,4-a]pyrimidines 2a-d (1mmol) in acetic acid (10 mL) with stirring for 3 h at room temperature. The reaction mixture was then poured onto crushed ice and the precipitated 5-aryl-6-iodo-8-phenylpyrazolo[1,5,c]-1,2,4-triazolo[3,4- a]pyrimidines 4a-d were filtered, washed with water, dried and crystallized from EtOH as colorless needles.
6-Iodo-5,8-diphenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (4a). Yield 91%, 0.40 g, mp 281–282 °C; IR (υmax, cm-1): 1640 (pyrazole ring C=N), 1575 (triazole ring C=N), and 1405 (C=C); 1H NMR (CDCl3, δH, ppm): 6.94 (s, 1H, pyrazole-H), 7.50-7.69 (m, 8H, aromatic-H), 8.10 (d, 2H, aromatic-H) and 8.61 (s, 1H, triazole-H); MS, m/z (%) = 437 (M.+, 100), 409 (M.+-N2, 5), 310 (M.+-I,7), 282 (M.+-IN2, 10), 242 (M.+-C2H2IN3, 4) and 227 (M.+-C3H5IN3, 8); Anal. Calc. for C19H12IN5 (437.24): C, 52.19; H, 2.77; N, 16.02%, found: C, 52.17; H, 2.80; N, 16.00%.
6-Iodo-8-phenyl-5-p-tolylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (4b). Yield 89%, 0.40 g, mp 245–246 °C; IR (υmax, cm−1): 1633 (pyrazole ring C=N), 1576 (triazole ring C=N), and 1416 (C=C); 1H NMR (CDCl3, δH, ppm): 2.49 (s, 3H, CH3), 6.90 (s, 1H, pyrazole-H), 7.41-7.69 (m, 7H, aromatic-H), 8.08 (d, 2H, aromatic-H) and 8.60 (s, 1H, triazole-H); MS, m/z (%) = 453 (M.++2, 9), 452 (M.++1, 100), 423 (M.+-N2, 5), 325 (M.++1-I, 12), 296 (M.+-IN2, 9) and 269 (M.+-CHIN3, 10); Anal. Calc. for C20H14IN5 (451.26): C, 53.23; H, 3.13; N, 15.52%, found: C, 53.27; H, 3.15; N, 15.50%.
6-Iodo-5-(p-methoxyphenyl)-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (4c). Yield 85%, 0.40 g, mp 225–226 °C; IR (υmax, cm−1): 1623 (pyrazole ring C=N), 1572 (triazole ring C=N), and 1458 (C=C); 1H NMR (CDCl3, δH, ppm): 3.93 (s, 3H, OCH3), 6.86 (s, 1H, pyrazole-H), 7.12 (d, 2H, aromatic-H), 7.48–7.51 (m, 3H, aromatic-H), 7.61 (d, 2H, aromatic-H), 8.10 (d, 2H, aromatic-H) and 8.61 (s, 1H, triazole-H); MS, m/z (%) = 470 (M.++3, 3), 468 (M.++1, 100),439 (M.+-N2, 6), 341 (M.++1-I, 12), 313 (M.+-CHIN, 4), 285 (M.+-CHIN3, 7), 269 (M.+-C2H3IN2O, 12) and 255 (M.+- C2H3IN3O, 4); Anal. Calc. for C20H14IN5O (467.26): C, 51.41; H, 3.02; N, 14.99%, found: C, 51.44; H, 3.00; N, 15.02%.
5-(p-Chlorophenyl)-6-iodo-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (4d). Yield 85%, 0.40 g, mp 270–271 °C; IR (υmax, cm−1): 1632 (pyrazole ring C=N), 1576 (triazole ring C=N), and 1410 (C=C); 1H NMR (DMSO-d6, δH, ppm): 7.02 (s, 1H, pyrazole-H), 7.49–7.54 (m, 3H, aromatic-H), 7.65 (d, 2H, aromatic-H), 7.80 (d, 2H, aromatic-H), 7.92 (d, 2H, aromatic-H) and 9.04 (s, 1H, triazole-H); MS, m/z (%) = 475 (M.++3, 3), 472 (M.+, 100), 443 (M.+-HN2, 3), 345 (M.+-I, 7), 309 (M.+-ClI, 2), 289 (M.+-CH2IN3, 5), 282 (M.+-CHClIN, 8) and 241 (M.+-C2H2ClIN3, 9); Anal. Calc. for C19H11ClIN5 (471.68): C, 48.38; H, 2.35; N, 14.85%, found: C, 48.40; H, 2.40; N, 14.90%.
3.2.4. 5-Aryl-6-nitro-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidines 5a-d
A mixture of nitric acid (d 1.41, 1 mL) and sulfuric acid (d 1.84, 1 mL) in glacial acetic acid (10 mL) was gradually added to a suspension of 5-aryl-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3- a]pyrimidines 2a-d (1 mmol) in glacial acetic acid (10 mL) with stirring for 3 h at room temperature. The reaction mixture was then poured onto cold water with stirring and the yellow precipitated solids were filtered, washed with cold water, dried and crystallized from EtOH to give the title compounds 5a-d as yellow needles.
6-Nitro-5,8-diphenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (5a). Yield 83%, 0.30 g, mp 241–242 °C; IR (υmax, cm−1): 1649 (pyrazole ring C=N), 1579 (triazole ring C=N), and 1462 (C=C); 1H NMR (CDCl3, δH, ppm): 6.93 (s, 1H, pyrazole-H), 7.40–7.44 (m, 6H, aromatic-H), 7.62 (d, 2H, aromatic-H), 8.03 (d, 2H, aromatic-H) and 8.52 (s, 1H, triazole-H); MS, m/z (%) = 356 (M.+, 7), 326 (M.+-H2N2, 4), 311 (M.++1-NO2, 100), 283 (M.+-CHN2O2, 27), 271 (M.+ +1-CN3O2, 2) and 255 (M.+-CHN4O2, 13); Anal. Calc. for C19H12N6O2 (356.34): C, 64.04; H, 3.39; N, 23.58%, found: C, 64.00; H, 3.40; N, 23.60%.
6-Nitro-8-phenyl-5-p-tolylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (5b). Yield 81%, 0.30 g, mp 243–244 °C; IR (υmax, cm−1): 1643 (pyrazole ring C=N), 1578 (triazole ring C=N), and 1415 (C=C); 1H NMR (CDCl3, δH, ppm): 2.47 (s, 3H, CH3), 6.90 (s, 1H, pyrazole-H), 7.37–7.45 (m, 5H, aromatic-H), 7.51 (d, 2H, aromatic-H), 8.03 (d, 2H, aromatic-H) and 8.54 (s, 1H, triazole-H); MS, m/z (%) = 370 (M.+, 4), 340 (M.+-H2N2, 2), 325 (M.++1-NO2, 100), 309 (M.+-CH3NO2, 1), 297 (M.+-CHN2O2, 22) and 269 (M.+-C2H3N3O2, 9); Anal. Calc. for C20H14N6O2 (370.36): C, 64.86; H, 3.81; N, 22.69%, found: C, 64.90; H, 3.80; N, 22.72%.
5-(p-Methoxyphenyl)-6-nitro-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (5c), Yield 77%, 0.30 g, mp 250–251 °C; IR (υmax, cm−1): 1637 (pyrazole ring C=N), 1585 (triazole ring C=N), and 1420 (C=C); 1H NMR (CDCl3, δH, ppm): 3.89 (s, 3H, OCH3), 6.97 (s, 1H, pyrazole-H), 7.45–7.47 (m, 3H, aromatic-H), 7.62 (d, 2H, aromatic-H), 7.79 (d, 2H, aromatic-H), 8.02 (d, 2H, aromatic-H) and 8.75 (s, 1H, triazole-H); MS, m/z (%) = 388 (M.++2, 15), 387 (M.++1, 100), 356 (M.+-CH2O, 7), 339 (M.+-HNO2, 7), 312 (M.+-CH2N2O2, 4), 310 (M.+-CH4N2O2, 11), 284 (M.+-C3H6N2O2, 5) and 251 (M.+-C7H7N2O, 16); Anal. Calc. for C20H14N6O3 (386.36): C, 62.17; H, 3.65; N, 21.75%, found: C, 62.20; H, 3.61; N, 21.73%.
5-(p-Chlorophenyl)-6-nitro-8-phenylpyrazolo[1,5-c]-1,2,4-triazolo[4,3-a]pyrimidine (5d). Yield 77%, 0.30 g, mp 306–307 °C; IR (υmax, cm−1): 1641 (pyrazole ring C=N), 1583 (triazole ring C=N), and 1416 (C=C); 1H NMR (DMSO-d6, δH, ppm): 7.33 (s, 1H, pyrazole-H), 7.43 (t, 1H, aromatic-H), 7.50 (t, 2H, aromatic-H), 7.66 (d, 2H, aromatic-H), 7.79 (d, 2H, aromatic-H), 8.03 (d, 2H, aromatic-H) and 8.99 (s, 1H, triazole-H); MS, m/z (%) = 391 (M.+, 3), 349 (M.+-CH2N2, 3), 347 (M.+-N2O, 46), 345 (M.+-NO2, 100), 317 (M.+-N3O2, 17), 289 (M.+-C2H4N3O2, 7), 282 (M.+-C6H5O2, 15), 254 (M.+-C7H4ClN, 22) and 227 (M.+-C7H3ClN3, 12); Anal. Calc. for C19H11ClN6O2 (390.78): C, 58.40; H, 2.84; N, 21.51%, found: C, 58.44; H, 2.80; N, 21.53%.
3.2.5. 5-Aryl-7-formylhydrazino-2-phenylpyrazolo[1,5-c]pyrimidines 6a-d
A suspension of 5-aryl-7-hydrazino-2-phenylpyrazolo[1,5-c]pyrimidines (1a-d, 1mmol) and ethyl formate (5 mL) was heated under reflux for 3 h. The product which separated upon cooling was filtered, washed with EtOH and crystallized from EtOH to give the title compounds 6a-d as colorless needles.
7-Formylhydrazino-2,5-Diphenylpyrazolo[1,5-c]pyrimidine (6a). Yield 76%, 0.25 g, mp 177–178 °C; IR (υmax, cm−1): 3368 (NH), 1700 (C=O), 1624 (pyrazole ring C=N), 1568 (pyrimidine ring C=N), and 1455 (C=C); 1H NMR (CDCl3, δH, ppm): 4.80 (s, 2H, exchangeable 2NH), 6.72 (s, 1H, pyrazole-H), 7.29 (s, 1H, pyrimidine-H), 7.30-7.49 (m, 8H, aromatic-H), 7.98 (d, 2H, aromatic-H) and 8.08 (s, 1H, CHO); MS, m/z (%) = 330 (M.++1, 2), 302 (M.++1-CO, 100), 286 (M.+-CHNO, 23), 272 (M.+-CHN2O, 81), 257 (M.+-C2H4N2O, 2), 244 (M.+-C2H3N3O, 17) and 228 (M.+-C2H5N4O, 6); Anal. Calc. for C19H15N5O (329.36): C, 69.29; H, 4.59; N, 21.26%, found: C, 69.30; H, 4.62; N, 21.30%.
7-Formylhydrazino-2-phenyl-5-p-tolylpyrazolo[1,5-c]pyrimidine (6b). Yield 71%, 0.25 g, mp 132–133 °C; IR (υmax, cm−1): 3315 (NH), 1701 (C=O), 1610 (pyrazole ring C=N), 1572 (pyrimidine ring C=N), and 1447 (C=C); 1H NMR (CDCl3, δH, ppm): 2.42 (s, 3H, CH3), 4.77 (s, 2H, exchangeable 2NH), 6.70 (s, 1H, pyrazole-H), 7.28 (s, 1H, pyrimidine-H), 7.29-7.49 (m, 7H, aromatic-H), 7.96 (d, 2H, aromatic- H) and 7.98 (s, 1H, CHO); MS, m/z (%) = 344 (M.++1, 2), 316 (M.++1-CO, 100), 300 (M.+-CHNO, 33), 286 (M.+-CHN2O, 85), 270 (M.+-C2H5N2O, 4), 259 (M.+-C2H2N3O, 7) and 227 (M.+-C8H6N, 10); Anal. Calc. for C20H17N5O (343.38): C, 69.96; H, 4.99; N, 20.40%, found: C, 70.02; H, 5.02; N, 20.44%
7-Formylhydrazino-5-(p-methoxyphenyl)-2-phenylpyrazolo[1,5-c]pyrimidine (6c). Yield 69%, 0.25 g, mp 157–158 °C; IR (υmax, cm−1): 3265(NH), 1708 (C=O), 1667 (pyrazole ring C=N), 1585 (pyrimidine ring C=N), and 1448 (C=C); 1H NMR (CDCl3, δH, ppm): 3.87 (s, 3H, OCH3), 4.73 (s, 2H, exchangeable 2NH), 6.68 (s, 1H, pyrazole-H), 7.19 (s, 1H, pyrimidine-H), 7.20–7.46 (m, 3H, aromatic- H), 7.95–7.97 (m, 4H, aromatic-H), 7.98 (d, 2H, aromatic-H) and 8.00 (s, 1H, CHO); MS, m/z (%) = 361 (M.++2, 5), 359 (M.+, 30), 332 (M.++1-CO, 100), 316 (M.+-CHNO, 23), 302 (M.+-CHN2O, 78), 287 (M.+-C2H4N2O, 12) and 275 (8, M.+-C2H2N3O); Anal. Calc. for C20H17N5O2 (359.38): C, 66.84; H, 4.77; N, 19.49%, found: C, 66.80; H, 4.82; N, 19.50%.
5-(p-Chlorophenyl)-7-formylhydrazino-2-phenylpyrazolo[1,5-c]pyrimidine (6d). Yield 69%, 0.25 g, mp 175–176 °C; IR (υmax, cm−1): 3315 (NH), 1700 (C=O), 1615 (pyrazole ring C=N), 1575 (pyrimidine ring C=N), and 1450 (C=C); 1H NMR (CDCl3, δH, ppm): 4.76 (s, 2H, exchangeable 2NH), 6.72 (s, 1H, pyrazole-H), 7.28 (s, 1H, pyrimidine-H), 7.41–7.46 (m, 5H, aromatic-H), 7.95–7.99 (m, 4H, aromatic- H) and 8.00 (s, 1H, CHO); MS, m/z (%) = 361 (M.+-3, 2,), 335 (M.++1-CO, 19), 320 (M.+-CHNO, 100), 305 (M.+-CH2N2O, 47), 294 (M.+-C2HN2O, 10), 269 (M.+-C6H6O, 5) and 242 (M.+-C7H7NO, 5); Anal. Calc. for C19H14ClN5O (363.80): C, 62.73; H, 3.88; N, 19.25%, found: C, 62.70; H, 3.90; N, 19.30%.
3.2.6. 7-Acetylhydrazino-5-aryl-2-phenylpyrazolo[1,5-c]pyrimidines 7a-d
5-Aryl-7-hydrazino-2-phenylpyrazolo[1,5-c]pyrimidines 1a-d (1mmol) and glacial acetic acid (10 mL) was heated at reflux for 3 h. The reaction mixture was poured onto crushed ice and the product which separated was filtered, washed with water, dried and crystallized from EtOH to give the title compounds 7a-d as colorless needles.
7-Acetylhydrazino-2,5-diphenylpyrazolo[1,5-c]pyrimidine (7a). Yield 88%, 0.30 g, mp 217–218 °C; IR (υmax, cm−1): 3150 (NH), 1661 (C=O), 1628 (pyrazole ring C=N), 1568 (pyrimidine ring C=N), and 1459 (C=C); 1H NMR (CDCl3, δH, ppm): 2.21(s, 3H, COCH3), 6.67 (s, 1H, pyrazole-H), 7.21 (s, 1H, pyrimidine-H), 7.30–7.51 (m, 5H, aromatic-H), 7.84–7.96 (m, 5H, aromatic-H), 8.01 (s, 1H, exchangeable NH) and 8.48 (s, 1H, exchangeable NHCO); MS, m/z (%) = 343 (M.+, 100), 328 (M.+-CH3, 1), 301 (M.+-COCH2, 59), 286 (M.+-NCOCH3, 18) and 243 (M.+-C3H6N3O, 11); Anal. Calc. for C20H17N5O (343.38): C, 69.96; H, 4.99; N, 20.40%, found: C, 70.00; H, 5.02; N, 20.43%.
7-Acetylhydrazino-2-phenyl-5-p-tolylpyrazolo[1,5-c]pyrimidine (7b). Yield 83%, 0.30 g, mp 224–225 °C; IR (υmax, cm−1): 3252 (NH), 1659 (C=O), 1618 (pyrazole ring C=N), 1556 (pyrimidine ring C=N), and 1445 (C=C); 1H NMR (CDCl3, δH, ppm): 2.23(s, 3H, CH3), 2.36 (s, 3H, COCH3), 6.70 (s, 1H, pyrazole-H), 7.19 (d, 2H, aromatic-H), 7.26 (s, 1H, pyrimidine-H), 7.36–7.48 (m, 3H, aromatic-H), 7.79 (d, 2H, aromatic-H), 7.97 (d, 2H, aromatic-H), 8.14 (s, 1H, exchangeable NH) and 8.31 (s, 1H, exchangeable NHCO); MS, m/z (%) = 358 (M.++1, 100), 315 (M.+-COCH2, 63), 300 (M.+-NCOCH3, 19), 288 (M.+-C3H3NO, 6) and 259 (M.+-C3H4N3O, 5); Anal. Calc. for C21H19N5O (357.41): C, 70.57; H, 5.36; N, 19.59%, found: C, 70.60; H, 5.32; N, 19.55%.
7-Acetylhydrazino-5-p-methoxyphenyl-2-phenylpyrazolo[1,5-c]pyrimidine (7c). Yield 81%, 0.30 g, mp 193–194 °C; IR (υmax, cm−1): 3283 (NH), 1664 (C=O), 1618 (pyrazole ring C=N), 1564 (pyrimidine ring C=N), and 1448 (C=C); 1H NMR (CDCl3, δH, ppm): 2.24 (s, 3H, COCH3), 3.78 (s, 3H, OCH3), 6.63 (s, 1H, pyrazole-H), 6.88 (d, 2H, aromatic-H), 7.07 (s, 1H, pyrimidine-H), 7.36–7.50 (m, 3H, aromatic-H), 7.76 (d, 2H, aromatic-H), 7.91 (d, 2H, aromatic-H), 8.13 (s, 1H, exchangeable NH) and 8.91 (s, 1H, exchangeable NHCO); MS, m/z (%) = 374 (M.++1, 100), 331 (M.+-COCH2, 38), 316 (M.+-NCOCH3, 16), 302 (M.+-NNCOCH3, 56), 287 (M.+-C3H6N2O, 8) and 259 (M.+- C4H8N3O, 19); Anal. Calc. for C21H19N5O2 (373.41): C, 67.55; H, 5.13; N, 18.76%, found: C, 67.58; H, 5.16; N, 18.80%.
7-Acetylhydrazino-5-p-Chlorophenyl-2-phenylpyrazolo[1,5-c]pyrimidine (7d). Yield 79%, 0.30 g, mp 246–247 °C; IR (υmax, cm−1): 3306 (NH), 1664 (C=O), 1613 (pyrazole ring C=N), 1572 (pyrimidine ring C=N), and 1447 (C=C); 1H NMR (DMSO-d6, δH, ppm): 2.04 (s, 3H, COCH3), 7.08 (s, 1H, pyrazole-H), 7.67 (s, 1H, pyrimidine-H), 7.41–7.52 (m, 5H, aromatic-H), 8.09 (d, 2H, aromatic-H), 9.81 (s, 1H, exchangeable NH) and 10.14 (s, 1H, exchangeable NHCO); MS, m/z (%) = 382 (M.++4, 1), 381 (M.++3, 4), 379 (M.++1, 44), 378 (M.+, 100), 336 (M.+-COCH2, 65), 320 (M.+-NHCOCH3, 16), 306 (M.+-NNHCOCH3, 77) and 270 (M.+-C2H5ClN2O, 10); Anal. Calc. for C20H16ClN5O (377.83): C, 63.58; H, 4.27; N, 18.54%, found: C, 63.61; H, 4.26; N, 18.60%.
3.2.7. 7-Acetylhydrazino-5-aryl-3,4-dibromo-2--phenylpyrazolo[1,5-c]pyrimidines 8a-d
A solution of bromine (0.12 mL, 2.4 mmol) in acetic acid (10 mL) was gradually added to a suspension of 7-acetylhydrazino-5-aryl-2-phenylpyrazolo[1,5-c]pyrimidines 7a-d (1 mmol) in acetic acid (10 mL) with stirring for 3 h at room temperature. The precipitated 7-acetylhydrazino-5-aryl-3,4- dibromo-2-phenylpyrazolo[1,5-c]pyrimidines 8a-d were filtered, washed with water, dried and crystallized from EtOH as colorless needles.
7-Acetylhydrazino-3,4-dibromo-2,5-diphenylpyrazolo[1,5-c]pyrimidine (8a). Yield 80%, 0.40 g, mp 229–230 °C; IR (υmax, cm−1): 3451 (NH), 1657 (C=O), 1644 (pyrazole ring C=N), 1560 (pyrimidine ring C=N), and 1439 (C=C); 1H NMR (CDCl3, δH, ppm): 2.09 (s, 3H, COCH3), 7.44–7.53 (m, 6H, aromatic-H), 7.63 (d, 2H, aromatic-H), 7.94 (d, 2H, aromatic-H) and 8.01 (s, 2H, exchangeable NH and exchangeable NHCO); MS, m/z (%) = 505(M.++4, 4), 503 (M.++2, 38), 501 (M.+, 100), 459 (M.+- COCH2, 80), 444 (M.+-NCOCH3, 14), 429 (M.+-NNHCOCH3, 40), 423 (M.++2-Br, 21), 421 (M.+-Br, 19) and 341 (M.+-Br2, 2); Anal. Calc. for C20H15Br2N5O (501.17): C, 47.93; H, 3.02; N, 13.97%, found: C, 47.95; H, 3.06; N, 14.00%.
7-Acetylhydrazino-3,4-dibromo-2-phenyl-5-p-tolylpyrazolo[1,5-c]pyrimidine (8b). Yield 77%, 0.40 g, mp 212–213 °C; IR (υmax, cm−1): 3280 (NH), 1664 (C=O), 1618 (pyrazole ring C=N), 1562 (pyrimidine ring C=N), and 1439 (C=C); 1H NMR (CDCl3, δH, ppm): 2.10 (s, 3H, CH3), 2.40 (s, 3H, COCH3), 7.46–7.55 (m, 5H, aromatic-H), 7.86 (d, 2H, aromatic-H), 7.93 (d, 2H, aromatic-H), 8.04 (s, 1H, exchangeable NH) and 8.06 (s, 1H, exchangeable NHCO); MS, m/z (%) = 519 (M.++4, 1), 517 (M.++2, 15), 515 (M.+, 33), 473 (M.+-COCH2, 18), 458 (M.+-NCOCH3, 2), 444 (M.+-NNCOCH3, 7), 442 (M.+-NHNHCOCH3, 5), 437 (M.++2-Br, 100), 435 (M.+-Br, 84) and 355 (M.+-Br2, 1); Anal. Calc. for C21H17Br2N5O (515.20): C, 48.96; H, 3.33; N, 13.59%, found: C, 49.00; H, 3.36; N, 13.60%.
7-Acetylhydrazino-3,4-dibromo-5-(p-methoxyphenyl)-2-phenylpyrazolo[1,5-c]-pyrimidine (8c). Yield 75%, 0.40 g, mp 182–183 °C; IR (υmax, cm−1): 3269 (NH), 1668 (C=O), 1612 (pyrazole ring C=N), 1535 (pyrimidine ring C=N), and 1443 (C=C); 1H NMR (CDCl3, δH, ppm): 2.47 (s, 3H, COCH3), 3.79 (s, 3H, OCH3), 7.48–7.62 (m, 3H, aromatic-H), 7.91 (d, 2H, aromatic-H), 8.04 (d, 2H, aromatic-H), 8.08 (d, 2H, aromatic-H), 10.05 (s, 1H, exchangeable NH) and 10.10 (s, 1H, exchangeable NHCO); MS, m/z (%) = 535(M.++4, 1), 533 (M.++2, 6), 531 (M.+, 10), 489 (M.+-COCH2, 6), 475 (M.+-NCOCH2, 13), 459 (M.+-NNHCOCH3, 23), 453 (M.++2-Br, 73), 451 (M.+-Br, 61) and 370 (M.++1-Br2, 1); Anal. Calc. for C21H17Br2N5O2 (531.20): C, 47.48; H, 3.23; N, 13.18%, found: C, 47.50; H, 3.26; N, 13.20%.
7-Acetylhydrazino-5-(p-chlorophenyl)-3,4-dibromo-2-phenylpyrazolo[1,5-c]-pyrimidine (8d). Yield 74%, 0.40 g, mp 226–227 °C; IR (υmax, cm−1): 3267 (NH), 1664 (C=O), 1620 (pyrazole ring C=N), 1570 (pyrimidine ring C=N), and 1437 (C=C); 1H NMR (DMSO-d6, δH, ppm): 2.47 (s, 3H, COCH3), 7.49–7.57 (m, 5H, aromatic-H), 8.04 (d, 2H, aromatic-H), 8.15 (d, 2H, aromatic-H), 10.06 (s, 1H, exchangeable NH) and 10.17 (s, 1H, exchangeable NHCO); MS, m/z (%) = 538 (M.++2, 2), 535 (M.+-1, 4), 493 (M.+- COCH3, 4), 463 (M.+-NHNHCOCH3, 3), 458 (M.++2-Br, 20), 456 (M.+-Br, 100) and 376 (M.+-Br2, 1); Anal. Calc. for C20H14Br2ClN5O (535.62): C, 44.85; H, 2.63; N, 13.08%, found: C, 44.81; H, 2.60; N, 13.11%.
3.2.8. 7-Acetylhydrazino-5-aryl-3-iodo-2--phenylpyrazolo[1,5-c]pyrimidines 9a-d
A solution of iodine monochloride (0.2 g, 1.2 mmol) in acetic acid (10 mL) was gradually added to a suspension of 7-acetylhydrazino-5-aryl-2-phenylpyrazolo[1,5-c]pyrimidines 7a-d (1 mmol) in acetic acid (10 mL) with stirring for 3 h at room temperature. The reaction mixture was then poured onto crushed ice and the precipitated 7-acetylhydrazino-5-aryl-3-iodo-2-phenylpyrazolo[1,5-c]pyrimidines 9a-d were filtered, washed with water, dried and crystallized from EtOH as colorless needles.
7-Acetylhydrazino-3-iodo-2,5-diphenylpyrazolo[1,5-c]pyrimidine (9a). Yield 85%, 0.40 g, mp 229–230 °C; IR (υmax, cm−1): 3433 (NH), 1680 (C=O), 1618 (pyrazole ring C=N), 1549 (pyrimidine ring C=N), and 1438 (C=C); 1H NMR (DMSO-d6, δH, ppm): 2.05 (s, 3H, COCH3), 7.29–7.80 (m, 10H, aromatic-H), 7.48 (s, 1H, pyrimidine-H), and 8.06 (s, 2H, exchangeable NHCO and exchangeable NH); MS, m/z (%) = 469 (M.+, 2), 451 (M.+- H2O, 4), 437 (M.+- CH4O, 2), 343 (M.++1-I, 5), 300 (M.+- I-CH2CO, 32) and 385 (M.+ - CH3INO, 100); Anal. Calc. for C20H16IN5O (469.28): C, 51.19; H, 3.44; N, 14.92%, found: C, 51.20; H, 3.40; N, 14.88%.
7-Acetylhydrazino-3-iodo-2-phenyl-5-p-tolylpyrazolo[1,5-c]pyrimidine (9b). Yield 83%, 0.40 g, mp 204–205 °C; IR (υmax, cm−1): 3273 (NH), 1661 (C=O), 1610 (pyrazole ring C=N), 1564 (pyrimidine ring C=N), and 1433 (C=C); 1H NMR (CDCl3, δH, ppm): 2.26 (s, 3H, CH3), 2.42 (s, 3H, COCH3), 7.18 (s, 1H, pyrimidine-H), 7.28–7.30 (m, 3H, aromatic-H), 7.49–7.52 (m, 4H, aromatic-H), 7.84 (d, 2H, aromatic-H), 7.97 (s, 1H, exchangeable NH), and 7.99 (s, 1H, exchangeable NHCO); MS, m/z (%) = 484 (M.++1, 83), 441 (M.+-COCH2, 34), 426 (M.+-NCOCH3, 12), 412 (M.+-NNCOCH3, 12), 410 (M.+ -NHNHCOCH3, 1) and 357 (M.++1- I, 16); Anal. Calc. for C21H18IN5O (483.30): C, 52.19; H, 3.75; N, 14.49%, found: C, 52.50; H, 3.79; N, 14.52%.
7-Acetylhydrazino-3-iodo-5-(p-methoxyphenyl)-2-phenylpyrazolo[1,5-c]-pyrimidine (9c). Yield 80%, 0.40 g, mp 124–125 °C; IR (υmax, cm−1): 3290 (NH), 1711 (C=O), 1610 (pyrazole ring C=N), 1560 (pyrimidine ring C=N), and 1448 (C=C); 1H NMR (CDCl3, δH, ppm): 2.16 (s, 3H, COCH3), 3.87 (s, 3H, OCH3), 6.99–7.49 (m, 7H, aromatic-H), 7.69 (s, 1H, pyrimidine-H), 8.01 (d, 2H, aromatic-H) and 9.28 (s, 2H, exchangeable NH and exchangeable NHCO); MS, m/z (%) = 484 (M.+-CH3, 1), 343 (M.+- NCOCH2, 3), 373 (M.++1-I, 6) and 372 (M.+- I, 1); Anal. Calc. for C21H18IN5O2 (499.30): C, 50.52; H, 3.63; N, 14.03%. Found: C, 50.55; H, 3.59; N, 14.06%.
7-Acetylhydrazino-5-(p-chlorophenyl)-3-iodo-2-phenylpyrazolo[1,5-c]pyrimidine (9d). Yield 80%, 0.40 g, mp 207–208 °C; IR (υmax, cm−1): 3273 (NH), 1662 (C=O), 1616 (pyrazole ring C=N), 1566 (pyrimidine ring C=N), and 1439 (C=C); 1H NMR (DMSO-d6, δH, ppm): 2.01 (s, 3H, COCH3), 7.14 (d, 2H, aromatic-H), 7.40 (s, 1H, pyrimidine-H), 7.49–7.54 (m, 3H, aromatic-H), 7.68 (d, 1H, aromatic-H), 7.80 (d, 1H, aromatic-H), 7.98 (d, 2H, aromatic-H),. 10.00 (s, 1H, exchangeable NH), and 10.16 (s, 1H, exchangeable NHCO); MS, m/z (%) = 506 (M.++2, 45), 504 (M.+, 100), 463 (M.++2-COCH3, 22), 461 (M.+-COCH3, 57), 446 (M.+-NHCOCH3, 15), 432 (M.+ -NNHCOCH3, 17), 379 (M.++2-I, 10) and 377 (M.+-I, 24); Anal. Calc. for C20H15ClIN5O (503.72): C, 47.69; H, 3.00; N, 13.90%, found: C, 47.72; H, 2.99; N, 13.92%.
3.2.9. 7-Acetylhydrazino-5-aryl-3-nitro-2-phenylpyrazolo[1,5-c]pyrimidines 10a-d
A mixture of nitric acid (d 1.41, 1 mL) and sulfuric acid (d 1.84, 1 mL) in glacial acetic acid (10 mL) was gradually added to a suspension of 7-acetylhydrazino-5-aryl-2-phenylpyrazolo[1,5- c]pyrimidines 7a-d (1 mmol) in glacial acetic acid (10 mL) with stirring for 3 h at room temperature. The reaction mixture was then poured onto cold water with stirring and the yellow precipitated solid were filtered, washed with cold water, dried and crystallized from EtOH to give the title compounds 10a-d as yellow needles.
7-Acetylhydrazino-3-nitro-2,5-diphenylpyrazolo[1,5-c]pyrimidine (10a). Yield 77%, 0.30 g, mp 191–192 °C; IR (υmax, cm−1): 3436 (NH), 1743 (C=O), 1633 (pyrazole ring C=N), 1551 (pyrimidine ring C=N), and 1462 (C=C); 1H NMR (CDCl3, δH, ppm): 1.90 (s, 3H, COCH3), 7.37 (s, 1H, pyrimidine-H), 7.44–7.68 (m, 6H, aromatic-H), 7.97 (d, 2H, aromatic-H), 8.03 (d, 2H, aromatic-H), 8.46 (s, 1H, exchangeable NH), and 9.26 (s, 1H, exchangeable NHCO); MS, m/z (%) = 389 (M.++1, 4), 360 (M.+- CO, 7), 346 (M.+-COCH2, 2), 332 (M.+-NCOCH2, 100), 316 (M.+-NNHCOCH3, 4), 302 (M.+-C3H6N2O, 17), 286 (M.+-C2H2N2O3, 10) and 258 (M.+-C3H4N3O3, 25); Anal. Calc. for C20H16N6O3 (388.38): C, 61.85; H, 4.15; N, 21.64%, found: C, 61.89; H, 4.12; N, 21.60%.
7-Acetylhydrazino-3-nitro-2-phenyl-5-p-tolylpyrazolo[1,5-c]pyrimidine (10b). Yield 75%, 0.30 g, mp 194–195 °C; IR (υmax, cm−1): 3371 (NH), 1745 (C=O), 1601 (pyrazole ring C=N), 1533 (pyrimidine ring C=N), and 1443 (C=C); 1H NMR (CDCl3, δH, ppm): 1.96 (s, 3H, CH3), 2.44 (s, 3H, COCH3), 7.30–7.71 (m, 10H, aromatic-H and pyrimidine-H) and 8.44 (s, 2H, exchangeable NH and exchangeable NHCO); MS, m/z (%) = 403 (M.++1, 2), 402 (M.+, 3), 401 (M.+-1, 16), 374 (M.+-CO, 8) and 346 (M.+-C2H2NO, 6); Anal. Calc. for C21H18N6O3 (402.41): C, 62.68; H, 4.51; N, 20.88%, found: C, 62.71; H, 4.50; N, 20.91%.
7-Acetylhydrazino-5-(p-methoxyphenyl)-3-nitro-2-phenylpyrazolo[1,5-c]-pyrimidine (10c). Yield 71%, 0.30 g, mp 132–133 °C; IR (υmax, cm−1): 3464 (NH), 1747 (C=O), 1696 (pyrazole ring C=N), 1531 (pyrimidine ring C=N), and 1454 (C=C); 1H NMR (CDCl3, δH, ppm): 1.81 (s, 3H, COCH3), 3.89 (s, 3H OCH3), 7.00–7.71 (m, 10H, aromatic-H and pyrimidine-H) and 8.43 (s, 2H, exchangeable NH and exchangeable NHCO); MS, m/z (%) = 420 (M.++2, 1), 358 (M.+-C2H6NO, 1), 334 (M.+ -C3H4N2O, 1) and 300 (M.+-C2H4N3O3, 3); Anal. Calc. for C21H18N6O4 (418.41): C, 60.28; H, 4.34; N, 20.09%, found: C, 60.31; H, 4.32; N, 20.13%.
7-Acetylhydrazino-5-(p-chlorophenyl)-3-nitro-2-phenylpyrazolo[1,5-c]-pyrimidine (10d). Yield 71%, 0.30 g, mp 174–175 °C; IR (υmax, cm−1): 3371 (NH), 1749 (C=O), 1597 (pyrazole ring C=N), 1533 (pyrimidine ring C=N), and 1443 (C=C); 1H NMR (CDCl3, δH, ppm): 2.13 (s, 3H, COCH3), 7.34–7.73 (m, 10H, aromatic-H and pyrimidine-H) and 8.45 (s, 2H, exchangeable NH and exchangeable NHCO); MS, m/z (%) = 423 (M.+, 1), 381 (M.+-COCH2, 1), 366 (M.+- NCOCH3, 1), 324 (M.+-C3H5N3O, 1) and 300 (M.+-C6H5NO2, 2); Anal. Calc. for C20H15ClN6O3 (422.82): C, 56.81; H, 3.58; N, 19.88%, found: C, 56.77; H, 3.61; N, 19.85%.
3.2.10. 7-Triacetylhydrazino-5-aryl-2-phenylpyrazolo[1,5-c]pyrimidines 11a-d
A suspension of 5-aryl-7-hydrazino-2-phenylpyrazolo[1,5-c]pyrimidines 1a-d (1 mmol) in acetic anhydride (5 mL) was heated under reflux for 1 h and the mixture was cooled and poured onto crushed ice. The product that separated out was filtered off, washed with water and then dried. It was crystallized from EtOH to give the title compounds 11a-d as colorlees needles.
7-Triacetylhydrazino-2,5-diphenylpyrazolo[1,5-c]pyrimidine (11a). Yield 83%, 0.35 g, mp 179–180 °C; IR (υmax, cm−1): 1736 (C=O), 1623 (pyrazole ring C=N), 1542 (pyrimidine ring C=N) and 1458 (C=C); 1H NMR (DMSO-d6, δH, ppm): 2.45 (s,3H, COCH3), 2.48 (s, 3H, COCH3), 2.50 (s, 3H, COCH3), 7.28 (s, 1H, pyrazole-H), 7.44 (t, 2H, aromatic-H), 7.50 (t, 4H, aromatic-H), 7.99 (d, 2H, aromatic-H) , 8.05 (d, 2H, aromatic-H) and 8.24 (s, 1H, pyrimidine-H); MS, m/z (%) = 428 (M.++1, 15), 385 (M.+-CH2CO, 15), 343 (M.+-2 CH2CO, 100), 301 (M.+-3 CH2CO, 65), 286 (M.+- C6H6NO2, 24), 272 (M.+-C6H6N2O2, 88) and 270 (M.+-C6H8N2O2, 26); Anal. Calc. for C24H21N5O3 (427.46): C, 67.44; H, 4.95; N, 16.38%, found: C, 67.40; H, 5.00; N, 16.40%.
7-Triacetylhydrazino-2-phenyl-5-p-tolylpyrazolo[1,5-c]pyrimidine (11b). Yield 80%, 0.35 g, mp 194–195 °C; IR (υmax, cm−1): 1726 (C=O), 1622 (pyrazole ring C=N), 1520 (pyrimidine ring C=N) and 1450 (C=C); 1H NMR (CDCl3, δH, ppm): 2.17(s, 3H, CH3), 2.42 (s,3H, COCH3), 2.54 (s, 3H, COCH3), 2.56 (s, 3H, COCH3),6.90 (s, 1H, pyrazole-H), 7.28 (d, 2H, aromatic-H), 7.42–7.50 (m, 3H, aromatic-H), 7.72 (s, 1H, pyrimidine-H), 7.87 (d, 2H, aromatic-H) and 7.95 (d, 2H, aromatic-H); MS, m/z (%) = 443 (M.++2, 2), 441 (M.+, 10), 399 (M.+-COCH2, 11), 357 (M.+-2COCH2, 100), 315 (M.+-3COCH2, 44) and 300 (M.+-C6H7NO3, 21); Anal. Calc. for C25H23N5O3 (441.48): C, 68.01; H, 5.25; N, 15.86%, found: C, 68.12; H, 5.22; N, 15.81%.
7-Triacetylhydrazino-5-p-methoxyphenyl-2-phenylpyrazolo[1,5-c]pyrimidine (11c). Yield 76%, 0.35 g, mp 132–133 °C; IR (υmax, cm−1): 1720 (C=O), 1614 (pyrazole ring C=N), 1510 (pyrimidine ring C=N), and 1448 (C=C); 1H NMR (CDCl3, δH, ppm): 2.53(s, 9H, 3COCH3), 3.86 (s, 3H, OCH3), 6.87 (s, 1H, pyrazole-H), 6.98 (d, 2H, aromatic-H), 7.41–7.49 (m, 3H, aromatic-H), 7.65 (s, 1H, pyrimidine- H) and 7.91–7.95 (m, 4H, aromatic-H); MS, m/z (%) = 459 (M.++2, 2), 457 (M.+, 17), 415 (M.+- COCH2, 19), 373 (M.+-2COCH2, 100), 331 (M.+-3COCH2, 39), 316 (M.+-C6H7NO3, 17) and 302 (M.+-C7H9NO3, 41); Anal. Calc. for C25H23N5O4 (457.48): C, 65.63; H, 5.07; N, 15.31%, found: C, 65.51; H, 5.05; N, 15.28%.
7-Triacetylhydrazino-5-p-chlorophenyl-2-phenylpyrazolo[1,5-c]pyrimidine (11d). Yield 76%, 0.35 g, mp 174–175 °C; IR (υmax, cm−1): 1722 (C=O), 1616 (pyrazole ring C=N), 1533 (pyrimidine ring C=N), and 1443 (C=C); 1H NMR (CDCl3, δH, ppm): 2.52(s, 3H, COCH3), 2.56 (s, 6H, 2COCH3), 6.91 (s, 1H, pyrazole-H), 7.41–7.49 (m, 5H, aromatic-H), 7.70 (s, 1H, pyrimidine-H), 7.88 (d, 2H, aromatic-H) and 7.93 (d, 2H, aromatic-H); MS, m/z (%) = 464 (M.++2, 1), 462 (M.+, 5), 420 (M.+-COCH2, 8), 378 (M.+-2COCH2, 58), 336 (M.+-3COCH2, 30), 320 (M.+-C6H8NO3, 13) and 306 (M.+-C6H8N2O3, 24); Anal. Calc. for C24H20ClN5O3 (461.90): C, 62.41; H, 4.36; N, 15.16%, found: C, 62.40; H, 4.32; N, 15.20%.