Microwave Assisted Organic Synthesis (MAOS) of Small Molecules as Potential HIV-1 Integrase Inhibitors
Abstract
:1. Introduction

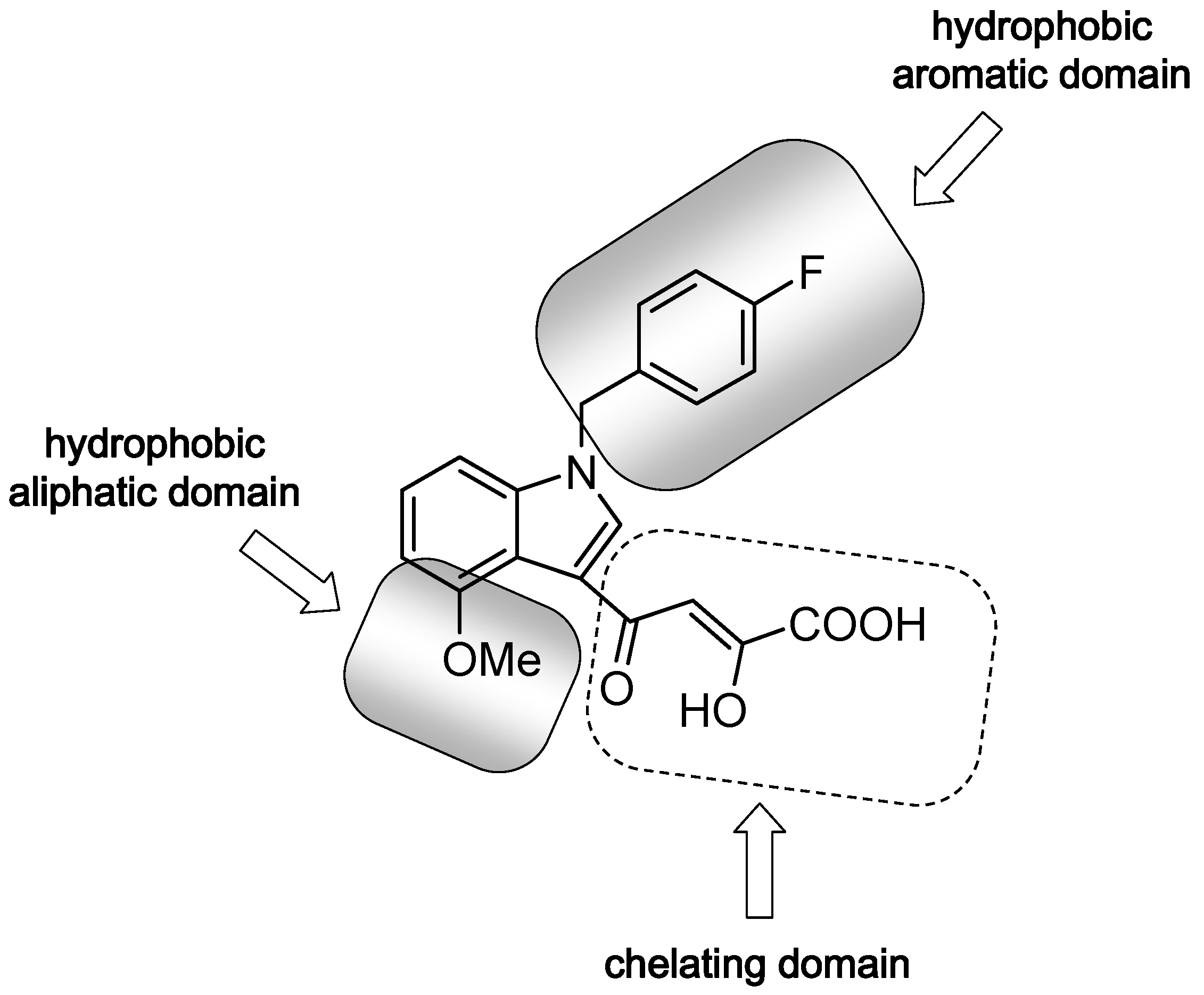
2. Results and Discussion
| Compound | IN enzymatic activity (IC50-µM) | |
|---|---|---|
| a Overall | b ST | |
| 7a | 1.74 ± 1.32 | 1.07 ± 0.76 |
| 7b | 0.42 ± 0.29 | 1.36 ± 0.64 |
| 7c | 5.76 ± 1.82 | 8.84 ± 5.60 |
| 7d | 2.61 ± 1.17 | 6.79 ± 0.64 |
| 7e | 51.76 ± 33.81 | 55.85 ± 43.83 |
| 7f | 3.83 ± 2.21 | 9.47 ± 1.06 |
| 7g | 7.34 ± 1.32 | 23.54 ± 17.78 |
| 7h | 7.69 ± 5.59 | 5.49 ± 2.67 |
| 7i | 2.17 ± 1.65 | 5.24 ± 1.34 |
| CHI-1043 | 0.08 ± 0.003 | 0.14 ± 0.11 |
3. Experimental
3.1. Chemistry
3.1.1. General
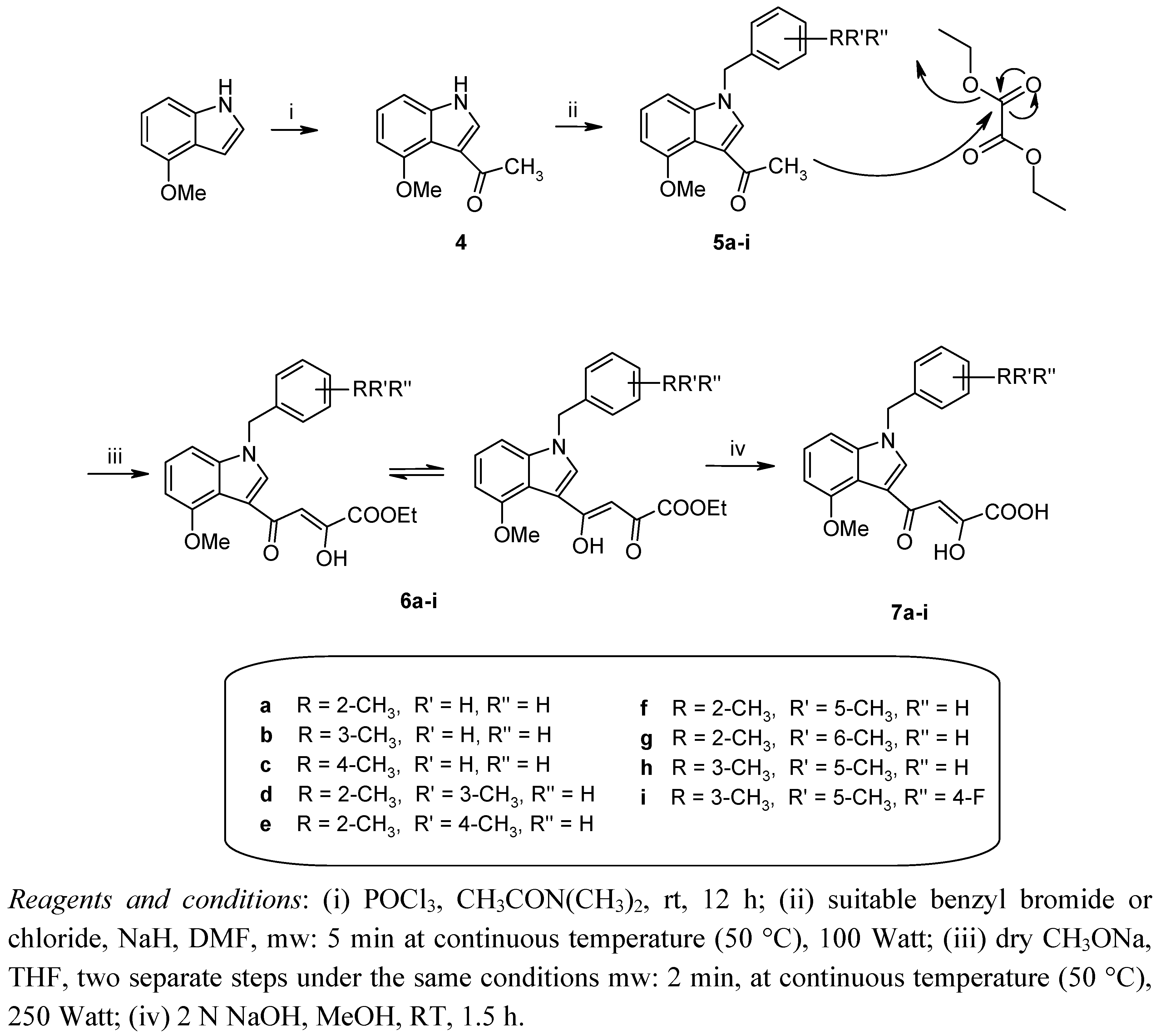
3.1.2. General procedure for the synthesis of 3-acetyl-4-methoxy-1-benzyl-1H-indoles 5a–i
3.1.3. General procedure for the synthesis of 4-[1-benzyl-4-methoxy-1H-indol-3-yl]-2-hydroxy-4-oxobut-2-enoates 6a–i
3.1.4. General procedure for the synthesis of 4-[1-benzyl-4-methoxy-1H-indol-3-yl]-2-hydroxy-4-oxobut-2-enoic acids 7a–i
3.2. Biological Assays
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Mehellou, Y.; De Clercq, E. Twenty-six years of anti-hiv drug discovery: Where do we stand and where do we go? J. Med. Chem. 2010, 53, 521–538. [Google Scholar] [CrossRef]
- Katlama, C.; Murphy, R. Emerging role of integrase inhibitors in the management of treatment-experienced patients with HIV infection. Ther. Clin. Risk Manag. 2009, 5, 331–340. [Google Scholar] [CrossRef]
- Sechi, M.; Rizzi, G.; Bacchi, A.; Carcelli, M.; Rogolino, D.; Pala, N.; Sanchez, T.W.; Taheri, L.; Dayam, R.; Neamati, N. Design and synthesis of novel dihydroquinoline-3-carboxylic acids as HIV-1 integrase inhibitors. Bioorg. Med. Chem. 2009, 17, 2925–2935. [Google Scholar]
- Esposito, D.; Craigie, R. HIV integrase structure and function. Adv. Virus Res. 1999, 52, 319–333. [Google Scholar] [CrossRef]
- Tang, J.; Maddali, K.; Metifiot, M.; Sham, Y.Y.; Vince, R.; Pommier, Y.; Wang, Z. 3-hydroxypyrimidine-2,4-diones as an inhibitor scaffold of HIV integrase. J. Med. Chem. 2011, 54, 2282–2292. [Google Scholar] [CrossRef]
- Lewinski, M.K.; Bushman, F.D. Retroviral DNA integration—Mechanism and consequences. Adv. Genet. 2005, 55, 147–181. [Google Scholar] [CrossRef]
- d'Angelo, J.; Mouscadet, J.F.; Desmaële, D.; Zouhiri, F.; Leh, H. HIV-1 integrase: The next target for aids therapy? Pathol. Biol. (Paris) 2001, 49, 237–246. [Google Scholar] [CrossRef]
- Yang, W.; Steitz, T.A. Recombining the structures of HIV integrase, ruvc and rnase h. Structure 1995, 3, 131–134. [Google Scholar] [CrossRef]
- Delelis, O.; Carayon, K.; Saib, A.; Deprez, E.; Mouscadet, J.F. Integrase and integration: Biochemical activities of HIV-1 integrase. Retrovirology 2008, 5, 114. [Google Scholar] [CrossRef]
- Mouscadet, J.F.; Desmaele, D. Chemistry and structure-activity relationship of the styrylquinoline-type HIV integrase inhibitors. Molecules 2010, 15, 3048–3078. [Google Scholar] [CrossRef]
- Chirch, L.M.; Morrison, S.; Steigbigel, R.T. Treatment of HIV infection with raltegravir. Expert Opin. Pharmacother. 2009, 10, 1203–1211. [Google Scholar] [CrossRef]
- Vandeckerckhove, L. Gsk-1349572, a novel integrase inhibitor for the treatment of HIV infection. Curr. Opin. Investig. Drugs 2010, 11, 203–212. [Google Scholar]
- Pommier, Y.; Johnson, A.A.; Marchand, C. Integrase inhibitors to treat HIV/Aids. Nat. Rev. Drug Discov. 2005, 4, 236–248. [Google Scholar] [CrossRef]
- Sechi, M.; Azzena, U.; Delussu, M.P.; Dallocchio, R.; Dessi, A.; Cosseddu, A.; Pala, N.; Neamati, N. Design and synthesis of bis-amide and hydrazide-containing derivatives of malonic acid as potential HIV-1 integrase inhibitors. Molecules 2008, 13, 2442–2461. [Google Scholar] [CrossRef]
- Barreca, M.L.; Ferro, S.; Rao, A.; De Luca, L.; Zappalà, M.; Monforte, A.M.; Debyser, Z.; Witvrouw, M.; Chimirri, A. Pharmacophore-based design of HIV-1 integrase strand-transfer inhibitors. J. Med. Chem. 2005, 48, 7084–7088. [Google Scholar] [CrossRef]
- Ferro, S.; Barreca, M.L.; De Luca, L.; Rao, A.; Monforte, A.M.; Debyser, Z.; Witvrouw, M.; Chimirri, A. New 4-[(1-benzyl-1h-indol-3-yl)carbonyl]-3-hydroxyfuran-2(5h)-ones, beta-diketo acid analogs as HIV-1 integrase inhibitors. Arch. Pharm. (Weinheim) 2007, 340, 292–298. [Google Scholar] [CrossRef]
- Ferro, S.; De Luca, L.; Barreca, M.L.; Iraci, N.; De Grazia, S.; Christ, F.; Witvrouw, M.; Debyser, Z.; Chimirri, A. Docking studies on a new human immunodeficiency virus integrase-Mg-DNA complex: Phenyl ring exploration and synthesis of 1H-benzylindole derivatives through fluorine substitutions. J. Med. Chem. 2009, 52, 569–573. [Google Scholar] [CrossRef]
- Ferro, S.; De Luca, L.; Barreca, M.L.; De Grazia, S.; Christ, F.; Debyser, Z.; Chimirri, A. New chloro, fluorobenzylindole derivatives as integrase strand-transfer inhibitors (INSTIs) and their mode of action. Bioorg. Med. Chem. 2010, 18, 5510–5518. [Google Scholar] [CrossRef]
- De Luca, L.; De Grazia, S.; Ferro, S.; Gitto, R.; Christ, F.; Debyser, Z.; Chimirri, A. HIV-1 integrase strand-transfer inhibitors: Design, synthesis and molecular modeling investigation. Eur. J. Med. Chem. 2011, 46, 756–764. [Google Scholar] [CrossRef]
- De Luca, L.; Barreca, M.L.; Ferro, S.; Iraci, N.; Michiels, M.; Christ, F.; Debyser, Z.; Witvrouw, M.; Chimirri, A. A refined pharmacophore model for HIV-1 integrase inhibitors: Optimization of potency in the 1H-benzylindole series. Bioorg. Med. Chem. Lett. 2008, 18, 2891–2895. [Google Scholar]
- Hombrouck, A.; Van Remoortel, B.; Michiels, M.; Noppe, W.; Christ, F.; Eneroth, A.; Sahlberg, B.L.; Benkestock, K.; Vrang, L.; Johansson, N.G.; et al. Preclinical evaluation of 1H-benzylindole derivatives as novel human immunodeficiency virus integrase strand transfer inhibitors. Antimicrob. Agents Chemother. 2008, 52, 2861–2869. [Google Scholar]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef]
- Hwang, Y.; Rhodes, D.; Bushman, F. Rapid microtiter assays for poxvirus topoisomerase, mammalian type ib topoisomerase and HIV-1 integrase: Application to inhibitor isolation. Nucleic Acids Res. 2000, 28, 4884–4892. [Google Scholar] [CrossRef]
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; De Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 1988, 20, 309–321. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7a–i are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferro, S.; Grazia, S.D.; Luca, L.D.; Gitto, R.; Faliti, C.E.; Debyzer, Z.; Chimirri, A. Microwave Assisted Organic Synthesis (MAOS) of Small Molecules as Potential HIV-1 Integrase Inhibitors. Molecules 2011, 16, 6858-6870. https://doi.org/10.3390/molecules16086858
Ferro S, Grazia SD, Luca LD, Gitto R, Faliti CE, Debyzer Z, Chimirri A. Microwave Assisted Organic Synthesis (MAOS) of Small Molecules as Potential HIV-1 Integrase Inhibitors. Molecules. 2011; 16(8):6858-6870. https://doi.org/10.3390/molecules16086858
Chicago/Turabian StyleFerro, Stefania, Sara De Grazia, Laura De Luca, Rosaria Gitto, Caterina Elisa Faliti, Zeger Debyzer, and Alba Chimirri. 2011. "Microwave Assisted Organic Synthesis (MAOS) of Small Molecules as Potential HIV-1 Integrase Inhibitors" Molecules 16, no. 8: 6858-6870. https://doi.org/10.3390/molecules16086858
APA StyleFerro, S., Grazia, S. D., Luca, L. D., Gitto, R., Faliti, C. E., Debyzer, Z., & Chimirri, A. (2011). Microwave Assisted Organic Synthesis (MAOS) of Small Molecules as Potential HIV-1 Integrase Inhibitors. Molecules, 16(8), 6858-6870. https://doi.org/10.3390/molecules16086858




