Abstract
In this work, an efficient microwave-assisted methodology for the esterification of unprotected α-amino acids is described. Ionic esterified amino acids were synthesized in satisfactory yields in a facile one-pot solventless protocol from unprotected amino acids and alcohols under acid catalysis (MsOH or p-TsOH) to afford the pure products after a simple work-up procedure. This procedure can also be extended to the preparation of long and short chain alkyl and benzyl esters.
1. Introduction
Microwave technology has become a powerful tool in organic synthesis which is able to grant access to a wide range of organic compounds in a very simple, swift and efficient way The use of microwave dielectric heating have been shown to dramatically reduce processing time and often leading to high purities and better yields to products as compared to conventional methods [1,2,3,4].
Parallel synthesis of combinatorial libraries can be described as synthetic sequences using an ordered array of spatially separated reaction vessels under the same reaction conditions which generally yield a more or less extensive library of compounds [5]. Parallel synthesis has been reported under both conventional heating conditions and, more recently, under microwave irradiation for combinatorial chemistry [6]. In this regard, microwaves can allow a quick and simple optimization of reaction conditions including time, suppression of byproducts, improved yields which can significantly simplify the synthesis and/or development of novel organic compounds in an efficient fashion.
The synthesis of carboxylic esters is one of the most fundamental protocols for producing natural and synthetically useful chemicals in peptide chemistry [7]. Particularly, long alkyloyl amino acids are important compounds due to their nutritional [8] and surfactant properties [9]. Some of them also show antisickling activity [10], protective properties against microorganism growth, as well as in the preservation of perishable food products [11].
Synthetic routes to prepare such compounds are rather limited to date, in spite of the wide applications of amino acid esters. These methods often rely on the use of the alcohol components in the liquid phase (as both solvent and reagent) so they are therefore not readily applicable to long chain solid alcohols [12]. Furthermore, most reported protocols to date lack green credentials and either require the presence of strong acids such as HCl or H2SO4 [13,14], hazardous reagents including p-toluenesulfonyl chloride [15], diazomethane [16] or orthoesters [17]. In some other cases, coupling agents (e.g., carbodiimide) [18] need to be employed or poorly atom efficient protocols with several reaction steps (protection, esterification and deprotection) as well as the use of expensive N-protected amino acids starting materials [19,20,21]. Many of such protocols also have a limited scope [22]. Comparatively, few heterogeneous catalysts including ionic liquids have been described as efficient systems to prepare short alkyl amino acid esters via esterification of amino acids [23,24] but these approaches have a limited applicability (e.g., nitrogen atmosphere) and/or suffer from many of the aforementioned drawbacks.
In this work, we report a one-pot, solventless and highly versatile microwave-assisted methodology for the esterification and simultaneous salt formation of unprotected α-amino acids using long chain alcohols and simple organic acid catalysts [methanesulfonic acid (MsOH) and p-toluenesulfonic acid (p-TsOH)] to obtain ionic amino acids ester with organic anions. The proposed approach could be efficiently extended to the synthesis of long and short chain alkyl and aryl esters. To the best of our knowledge, this is the first report of a general procedure for the esterification of unprotected amino acids under microwave irradiation, despite other investigations related to the esterification of amino acids under microwave irradiation [19,20,21,22].
2. Results and Discussion
2.1. Microwave-Assisted Parallel Synthesis of Ionic-Esterified Amino Acids
The esterification reaction of carboxylic acids under microwave irradiation has been widely study [25,26,27], however the esterification of amino acids is comparatively more difficult to that of ordinary carboxylic acids as a consequence of their zwitterionic structure.
After several experiments to establish the optimum conditions (data not shown), a versatile procedure was obtained for the parallel synthesis of ionic-esterified α-amino acids from unprotected amino acids, alcohols and acid catalysts (MsOH or p-TsOH) under microwave irradiation. Reactions were carried out employing glycine (entries 1–8), D-alanine (entries 9–14) and DL-tryptophan (entries 15–21, Table 1).

Table 1.
Parallel synthesis of ionic-esterified amino acids under microwave irradiation. 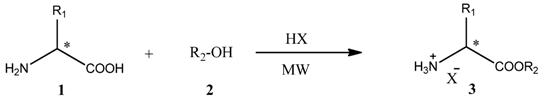

| Entry | R1 | R2 | X− | Product | Yield (%) a |
|---|---|---|---|---|---|
| 1 | H | i-C3H7 | MsO | 3a | 75 |
| 2 | H | C4H9 | MsO | 3b | 78 |
| 3 | H | C8H17 | p-TsO | 3c | 63 |
| 4 | H | C10H21 | MsO | 3d | 78 |
| 5 | H | C12H25 | p-TsO | 3e | 62 |
| 6 | H | C14H29 | MsO | 3f | 79 |
| 7 | H | C16H33 | MsO | 3g | 78 |
| 8 | H | C18H37 | MsO | 3h | 84 |
| 9 | Me | Bz | MsO | 3i | 76 |
| 10 | Me | C2H5 | p-TsO | 3j | 73 |
| 11 | Me | C8H17 | p-TsO | 3k | 21 |
| 12 | Me | C10H21 | MsO | 3l | 74 |
| 13 | Me | C12H25 | p-TsO | 3m | 24 |
| 14 | Me | C14H29 | MsO | 3n | 76 |
| 15 | 3-methylene-1H-indole (3MIn) | C8H17 | MsO | 3o | 77 |
| 16 | 3MIn | C10H21 | MsO | 3p | 74 |
| 17 | 3MIn | C12H25 | MsO | 3q | 75 |
| 18 | 3MIn | C14H29 | MsO | 3r | 75 |
| 19 | 3MIn | C16H33 | MsO | 3s | 78 |
| 20 | 3MIn | C18H37 | MsO | 3t | 74 |
| 21 | 3MIn | C18H37 | p-TsO | 3u | 62 |
a Isolated yields.
Table 1 proves ionic-esterified amino acids (ionic liquids analogues) could be obtained in one-step by simultaneous esterification of the carboxylic group and protonation of the amine group (with subsequent formation of the organic salt end). The ionic product is more stable than the neutral and consequently inter- and intramolecular reactions could be avoided in our proposed approach. Due to their ionic character, amino acids interact very efficiently with microwave irradiation providing a fast and homogeneous increase in temperature.
Simultaneous cooling with compressed air (5 bar) was applied in order to prevent the strong exotherm in the synthesis, avoiding overheating and abrupt temperature overshoot (presented when reaction was carried out without air cooling) and allows a higher level of irradiation power at the established temperature [28,29].
In these reactions the temperature profiles were obtained simultaneously by both external IR and external FO sensor [30]. Due to the strong microwave absortivity of our ionic products and the delay experience in monitoring temperature on the outer surface of a heavy-walled vessel and especially in our experiments with simultaneous cooling, the magnetron output power was controlled by the most precise internal FO sensor (IR as slave). In all experiments significant differences between FO and IR measurement (~40–50 °C) were observed, as can be seen as example in FO/IR temperature (T) and power (P) profiles in the synthesis of the compound 3b (Figure 1).
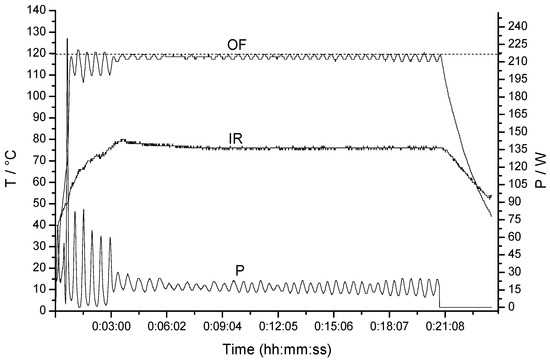
Figure 1.
FO/IR temperature (T) and power (P) profiles for the reaction to obtain compound 3b.
Particularly related to this work, our efforts have been especially focused in the synthesis of ionic amphiphilic amino acids with long alkyl chains (C8–C18, Table 1). However, it is worth pointing out that the proposed versatile approach can also be extended to the preparation of short chain (entries 1, 2 and 10, Table 1) and benzyl (entry 9, Table 1) esterified ionic amino acids.
Good yields to products could be obtained for all investigated amino acids and alcohols, regardless of their structure and composition, except for products 3k and 3m where ionic product from acid-base reaction without esterification, was curiously the main product. Products were obtained in acceptable purities (>95% as seen by NMR) after a simple workup comprising of crystallization via EtOH addition and subsequent washing with ethyl ether.
MsOH and p-TsOH were found to be suitable catalysts for the reactions under the investigated reaction conditions but yields were higher for the case of MsOH as compared to those obtained with p-TsOH. An additional advantage of these reactions is that a high alcohol excess was not required to obtained high yields to products as compared to most literature reported protocols [19,20,21,23]. The data collected shows that no significant racemization (ratio ~ D:L, 5:1) take place during the synthesis for products obtained from D-alanine.
2.2. Amphiphilic Properties of Ionic Amino Acids with Long Alkyl Chains
The critical miscellar concetration (CMC) is an important characteristic of a surfactant. Besides the essential contribution of the hydrophobic interactions, the micellization of ionic surfactants in aqueous solutions is influenced considerably by the electrostatic interactions between the ionized head-groups and their interactions with the surrounding counterions and water molecules.
The surfactant CMC determination is indeed done in an aqueous system where the surfactant forms micelles with polar heads oriented toward the aqueous medium. The amphiphilic properties of water soluble long alkyl chain (C8–C14) synthesized compounds were determined for measuring the CMCs for compounds 3c–3f obtained from glycine. CMCs were determined by two methods, using the classical method by interfacial tensiometry and by Steady-state fluorescence measurements [31]. The results are showed in Table 2.

Table 2.
CMCs values of 3c–3f determined by interfacial tensiometry (IT) and by Steady-state fluorescence (F) measurements.
| Compound | CMC (ppm) by IT | CMC (ppm) by F |
|---|---|---|
| 3c | 151.2 | 151.4 |
| 3d | 75.7 | 60.0 |
| 3e | 9.8 | 12.1 |
| 3f | 2.9 | 4.0 |
As can be observed in Table 2, good agreement in the values was obtained by both methods. The carbon chain length of the hydrophobe was found to be a determining factor in the value of CMC, for the prepared series of amino acid esters surfactants, we found that was a marked decline in CMC values with increase the number of carbon atoms in chain length. CMC for C8 is higher than C10, whereas C10 is higher than C12. The CMC values decreases as hydrophobic group size increases. Similar behavior is typically for other surfactants series, such as alkylpolyglucosydes [32,33] and N-acetylated cationic surfactants [34]. These results point out that these compounds could be employed as emulsifiers for oilfield applications.
3. Experimental
3.1. General
All Aldrich reagents were used without previous purification. p-TsOH was dehydrated by heating (100 °C) y vacuum during 4 h. Melting points were measured in a Fisher Scientific apparatus with a 300 °C thermometer. 1H-NMR (300 MHz) and 13C-NMR (75.4 MHz) spectra were obtained with a Varian-Gemini-300 equipment using TMS as internal standard and the solvent specified in each case at room temperature, as shown below. IR spectra were recorded on a Nicolet spectrometer Nexus 470 FT-IR (KBr powder, diffuse reflectance mode). Microwave reaction were performed using a commercially available mono-mode microwave, Monowave 300 manufactured by Anton Paar [35], employing a 10 mL Pyrex vial in a closed vessel mode. Reactions were carried out with simultaneous cooling with compressed air (5 bar) and stirring at a fixed rate of 800 rpm. The reaction temperatures were monitored by both, an external infrared sensor (IR) and by an internal fiber-optic (FO) temperature probe (ruby thermometer) protected by a borosilicate immersion well inserted directly in the reaction mixtures. The magnetron output power was controlled by the FO probe (IR as slave) and heating rates was controlled by selecting the “as-fast-as-possible” mode. Pressure sensing is achieved by a hydraulic sensor integrated in the swiveling cover of the instrument. Critical Micellar Concentrations (CMC) were determined by tensiometry and by fluorometry methods. Different concentrations of each sample were prepared and the surface tension at 25 °C was measured using a platinum ring on a tensiometer DuNoüy. Several concentrations were prepared by diluting the stock solution with distilled water (interfacial tension = 72 dyne/cm) to the appropriate concentration to be used in the determination of the critical micelle concentration (CMC). The surfactants CMC determinations by fluorometric spectroscopy were performed on a RF-5301PC Shimadzu spectrofluorometer equipped with a 150 W Xe lamp and a cell temperature controller using pyrene as a fluorophore probe. The emission spectra of pyrene and assays with surfactants were recorded between 350 and 600 nm using a λexc of 334 nm at 25 °C. The calculations for CMC were determined from the emission spectra of each surfactant as described before [31].
3.2. General Procedure for Microwave-Assisted Synthesis of Ionic-Esterified Amino Acids Salts
In a reaction vessel provided with reflux condenser, alcohols (5 mmol) were heated for 30 s at 70 °C with magnetic stirring under microwave irradiation at 70 W, to better homogenize compounds at the bottom of the microwave tube in the case of solid alcohols. The corresponding amino acids (2.5 mmol) and the catalyst (MsOH or p-TsOH, 6 mmol) were then added and the mixture was irradiated for 20 min under simultaneous cooling with air (20 psi). Upon reaction completion, the final obtained product was diluted with hot ethanol (10 mL) and ether (20 mL). The mixture was allowed to stand overnight at 5 °C. A mixture of the corresponding amino acid (2.5 mmol), alcohol (5.0 mmol) and the catalyst (MsOH or p-TsOH, 6 mmol) were placed in a 10 mL Pyrex reaction vessel equipped with a magnetical stir bar and fitted with an immersion well for the ruby thermometer. The reaction mixture was first irradiated 30 s at 70 °C and then for 20 min under simultaneous cooling with compressed air (5 bar). Upon reaction completion, the final obtained product was diluted with hot ethanol (10 mL) and ether (20 mL). The mixture was allowed to stand 1 h at 5 °C. The precipitate product was vacuum-filtered, washed with ethyl ether and dried under vacuum. Products were characterized by IR, 1H and 13C-NMR.
2-Oxo-2-(propan-2-yloxy)ethanaminium methanesulfonate (3a). White solid; Mp. 99–104 °C; 1H-NMR (δ, DMSO-d6): 8.36 (ws), 7.46 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.21 (dd, J = 6.8, 2.1 Hz, 1H), 7.05 (td, J = 6.8, 2.1 Hz, 1H), 6.97 (td, J = 6.8, 1.0 Hz, 1H), 4.17 (s, 1H), 4.01 (m, 2H), 3.27 (m, 2H), 2.46 (s, 3H), 1.45 (qi, J = 6.7 Hz, 2H), 1.20 (m, 10H), 0.85 (t, J = 7.1 Hz, 3H); 13C-NMR: 167.5, 135.4, 134.4 124.9, 122.9, 119.2, 116.7, 115.9, 109.7, 104.4, 77.4, 76.9, 76.5, 63.8, 50.9, 29.42, 26.8, 26.7 25.9, 24.4, 23.3, 20.3, 12.0; IR, RD-KBr (cm−1): 3315, 3055, 2983, 2935, 1740, 1597, 1516, 1493, 1439, 1464, 1383, 1356, 1338, 1290, 1205, 1176, 1119, 1072, 1045, 957, 899, 779, 742, 557.
2-(Butyloxy)-2-oxoethanaminium methanesulfonate (3b). White solid; Mp. 117–119 °C; 1H-NMR (δ, DMSO-d6): 8.36 (ws), 7.46 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.21 (dd, J = 6.8, 2.1 Hz, 1H), 7.05 (td, J = 6.8, 2.1 Hz, 1H), 6.97 (td, J = 6.8, 1.0 Hz, 1H), 4.17 (s, 1H), 4.01 (m, 2H), 3.27 (m, 2H), 2.46 (s, 3H), 1.45 (qi, J = 6.7 Hz, 2H), 1.20 (m, 10H), 0.85 (t, J = 7.1 Hz, 3H); 13C-NMR: 167.5, 1354.5, 134.4 124.9, 122.9, 119.2, 116.7, 115.9, 109.7, 104.4, 77.4, 76.9, 76.5, 63.8, 50.9, 29.42, 26.8, 26.7 25.9, 24.4, 23.3, 20.3, 12.0; IR, RD-KBr (cm−1): 3421, 3024, 2966, 2881, 1747, 1610, 1506, 1435, 1319, 1207, 1169, 1045, 906, 864, 781, 557.
2-(Octyloxy)-2-oxoethanaminium p-toluenesulfonate (3c). White solid; Mp. 200–201 °C; 1H-NMR (δ, CD3OD): 8.24 (ws), 7.73 (d, J = 7.7 Hz, 2H), 7.22 (d, J = 7.9 Hz, 2H), 3.79 (s, 2H), 3.56 (t, J = 7.0 Hz, 2H), 2.37 (s, 3H), 1.54 (m, 2H), 1.29 (m, 10H), 0.89 (t, J = 7.0 Hz, 3H); 13C-NMR: 167.7, 140.8, 140.0, 128.2, 125.0, 77.4, 76.9, 76.5, 39.2, 20.2; IR, RD-KBr (cm−1): 3460, 3061, 2954, 2848, 1755, 1595, 1503, 1443, 1242, 1161, 1038, 1012, 928, 860.
2-(Decyloxy)-2-oxoethanaminium methanesulfonate (3d). White solid; Mp. 67–68 °C; 1H-NMR (δ, CD3OD): 8.34 (ws), 4.24 (t, J = 6.7 Hz, 2H), 3.83 (s, 2H), 2.72 (s, 3H), 1.69 (qi, J = 6.7 Hz, 2H), 1.29 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H); 13C-NMR: 168.3, 67.2, 40.9, 39.4, 32.8, 30.5, 30.4, 30.2, 30.1, 26.7, 23.6, 14.5; IR, RD-KBr (cm−1): 3454, 3016, 2956, 2924, 1749, 1593, 1500, 1468, 1421, 1379, 1207, 1192, 1051, 904, 785.
2-(Dodecyloxy)-2-oxoethanaminium p-toluenesulfonate (3e). White solid; Mp. 195–196 °C; 1H-NMR (δ, CD3OD): 8.27 (ws), 7.73 (d, J = 7.7 Hz, 2H), 7.22 (d, J = 7.2 Hz, 2H), 4.19 (t, J = 6.8 Hz, 2H), 3.78 (s, 2H), 2.37 (s, 3H), 1.66 (qi, J = 6.7 Hz, 2H), 1.28 (m, 18H), 0.89 (t, J = 6.9 Hz, 3H); 13C-NMR: 167.6, 140.9, 139.8, 128.0, 124.9, 77.4, 76.9, 76.6, 39.0, 28.8, 28.7, 28.5, 19.9; IR, RD-KBr (cm−1): 3467, 3223, 3059, 2954, 2920, 1751, 1595, 1506, 1443, 1242, 1161, 1124, 1038, 1012, 926, 860.
2-(Tetradecyloxy)-2-oxoethanaminium methanesulfonate (3f). White solid; Mp. 78–79 °C. 1H-NMR (δ, CD3OD): 8.32 (ws), 4.23 (t, J = 6.7 Hz, 2H), 3.83 (s, 2H), 2.71 (s, 3H), 1.69 (qi, J = 6.7 Hz, 2H), 1.28 (m, 22H), 0.89 (t, J = 7.0 Hz, 3H); 13C-NMR: 168.5, 67.3, 40.9, 40.7, 39.46, 32.9, 30.7, 30.68, 30.63, 30.5, 30.4, 30.3, 29.49; IR, RD-KBr (cm−1): 3466, 3008, 2960, 2918, 2850, 1745, 1541, 1466, 1433, 1387, 1240, 1192, 1109, 1059, 891, 783, 723.
2-(Hexadecyloxy)-2-oxoethanaminium methanesulfonate (3g). White solid; Mp. 85–86 °C; 1H-NMR (δ, CD3OD): 8.32 (ws), 4.24 (t, J = 6.7 Hz, 2H), 3.81 (s, 2H), 2.74 (s, 3H), 1.69 (qi, J = 7.0 Hz, 2H), 1.28 (m, 26H), 0.89 (t, J = 7.0 Hz, 3H); 13C-NMR: 166.4, 77.4, 76.6, 65.5, 39.0, 38.9, 37.7, 31.6, 31.0, 28.8, 28.7, 28.6, 28.6, 28.5, 28.3, 27.5, 24.92, 24.8, 21.7, 12.7; IR, RD-KBr (cm−1): 3390, 3024, 2924, 2914, 2850, 1747, 1514, 1473, 1439, 1387, 1242, 1174, 1051, 918, 864, 715.
2-(Octadecyloxy)-2-oxoethanaminium methanesulfonate (3h). White solid; Mp. 72 °C (dec.); 1H-NMR (δ, CD3OD): 8.36 (ws), 4.24 (t, J = 6.7 Hz, 2H), 3.81 (s, 1H), 3.75 (s, 1H), 2.74 (s, 3H), 1.70 (qi, J = 7.0 Hz, 2H), 1.28 (m, 30H), 0.89 (t, J = 6.9 Hz, 3H); 13C-NMR: 166.8, 77.4, 76.9, 76.5, 65.4, 61.1, 38.9, 38.8, 37.6, 31.6, 31.0, 28.8, 28.7, 28.6, 28.5, 28.4, 28.3, 27.5, 24.9, 24.8, 21.7, 12.68; IR, RD-KBr (cm−1): 3388, 3088, 2918, 2850, 1747, 1514, 1473, 1383, 1240, 1176, 1051, 912, 781, 715.
(S)-1-(Benzyloxy)-1-oxopropan-2-aminium methanesulfonate (3i). Brown oil; 1H-NMR (δ, CD3OD): 7.38 (s, 5H), 5.26 (s, 2H), 4.08 (q, J = 7.3 Hz, 1H), 2.74 (s, 3H), 1.58 (d, J = 7.3 Hz, 3H); 13C-NMR: δ 169.2, 134.2, 128.3, 128.2, 127.9, 77.4, 76.9, 76.5, 67.6, 38.3, 15.2, 15.1; IR, RD (cm−1): 3431, 3032, 2954, 2843, 22748, 1747, 1525, 1498, 1456, 1329, 1213, 1151, 1113, 1038, 958, 910, 777.
(S)-1-(Ethyloxy)-1-oxopropan-2-aminium p-toluenesulfonate (3j). White solid; Mp. 218–220 °C; 1H-NMR (δ, CD3OD): 8.33 (ws), 7.78 (d, J = 8.2 Hz, 2H), 7.29 (d, J = 8.2 Hz, 2H), 4.25 (q, J = 7.0 Hz, 2H), 3.61 (q, J = 7.1 Hz, 1H), 2.36 (s, 3H), 1.74 (d, J = 7.2 Hz, 3H), 1.18 (t, J = 7.0 Hz, 3H); 13C-NMR: 170.3, 140.9, 139.8, 128.0, 124.9, 77.4, 76.9, 76.5, 76.5, 19.8, 14.6; IR, RD-KBr (cm−1): 3469, 3066, 2951, 2851, 1749, 1518, 1460, 1203, 1126, 1113, 1043, 1014, 814, 688, 567.
(S)-1-(Octyloxy)-1-oxopropan-2-aminium p-toluenesulfonate (3k). White solid; Mp. 203–205 °C; 1H-NMR (δ, CD3OD): 8.32 (ws), 7.73 (d, J = 8.2 Hz, 2H), 7.22 (d, J = 8.1 Hz, 2H), 3.95 (qi, J = 7.3 Hz, 1H), 3.33 (m, 2H), 2.37(s, 3H), 1.54 (d, J = 7.3 Hz, 3H), 1.46 (m, 2H), 1.31 (m, 10H), 0.89 (t, J = 6.7 Hz, 3H); 13C-NMR: 170.4, 141.0, 139.8, 130.2, 128.0, 124.9, 77.4, 76.9, 76.6, 48.1, 47.8, 47.5, 47.2, 46.9, 19.9, 19.96, 14.6; IR, RD-KBr (cm−1): 3477, 3066, 2900, 2825, 1749, 1601, 1520, 1462, 1244, 1203, 1163, 1126, 1043, 1012, 860, 814, 688, 656.
(S)-1-(Decyloxy)-1-oxopropan-2-aminium methanesulfonate (3l). White solid; Mp. 94–95 °C; 1H-NMR (δ, CD3OD): 8.43 (ws), 4.24 (qd, J = 4.5, 2.2 Hz, 2H), 4.05 (q, J = 7.2 Hz, 1H), 2.74 (s, 3H), 1.69 (qi, J = 7.1 Hz, 2H), 1.57 (d, J = 7.3 Hz, 3H), 1.29 (m, 14H), 0.89 (t, J = 6.9 Hz, 3H); 13C-NMR: 169.1, 77.4, 76.9, 76.5, 65.7, 37.8, 31.0, 28.7, 28.6, 28.5, 28.4, 27.6, 24.9, 21.8, 14.7, 14.6, 12.8; IR, RD-KBr (cm−1): 3491, 3059, 2958, 2918, 2852, 1751, 1523, 1470, 1336, 1244, 1205, 1157, 1119, 1043, 1005, 775, 750, 555.
(S)-1-(Dodecyloxy)-1-oxopropan-2-aminium p-toluenesulfonate (3m). White solid; Mp. 224–225 °C. 1H-NMR (δ, CD3OD): 8.31 (ws), 7.73 (d, J = 8.2 Hz, 2H) 7.22 (d, J = 8.0 Hz, 2H), 3.95 (qi, J = 7.3 Hz, 1H), 3.33 (m, 2H), 2.37 (s, 3H), 1.54 (d, J = 7.3 Hz, 3H), 1.46 (m, 2H), 1.31 (m, 18H), 0.89 (t, J = 6.7 Hz, 3H); 13C-NMR: 170.4, 140.9, 139.8, 128.1, 124.9, 77.4, 76.9, 76.5, 20.1, 20.0, 14.7; IR, RD-KBr (cm−1): 3471, 3068, 2949, 2906, 2829, 1749, 1520, 1205, 1126, 1113, 1043, 1014, 816, 688, 567.
(S)-1-(Tetradecyloxy)-1-oxopropan-2-aminium methanesulfonate (3n). White solid; Mp. 86–87 °C; 1H-NMR (δ, CD3OD): 4.24 (qd, J = 4.0, 2.2 Hz, 2H), 4.04 (q, J = 7.3 Hz, 1H), 2.75 (s, 3H), 1.70 (qi, J = 7.0 Hz, 2H), 1.57 (d, J = 7.3 Hz, 3H), 1.27 (m, 22H), 0.89 (t, J = 7.0 Hz, 3H); 13C-NMR: 169.2, 77.4, 76.9, 76.6, 65.9, 38.0, 31.8, 31.2, 28.9, 28.95, 28.9, 28.8, 28.8, 28.6, 28.5, 27.7, 25.0, 21.9, 14.9, 14.8, 13.0; IR, RD-KBr (cm−1): 3491, 3145, 2958, 2918, 2850, 1751, 1531, 1470, 1207, 1157, 1120, 1043, 775, 721, 555.
3-(1H-Indol-3-yl)-1-(octyloxy)-1-oxopropan-2-aminium methanesulfonate (3o). White solid; Mp. 119–121 °C; 1H-NMR (δ, DMSO-d6): 10.99 (s, NH), 8.36 (ws, 3H), 7.46 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.21 (dd, J = 6.8 Hz, J = 2.1 Hz, 1H), 7.05 (td, J = 6.8, 2.1 Hz, 1H), 6.97 (td, J = 6.8, 1.0 Hz, 1H), 4.17 (s, 1H), 4.01 (m, 2H), 3.27 (m,2H), 2.46 (s, 3H), 1.45 (qi, J = 6.7 Hz, 2H), 1.20 (m, 10H), 0.85 (t, J = 7.1 Hz, 3H); 13C-NMR: 167.5, 135.4, 134.4 124.9, 122.9, 119.2, 116.7, 115.9, 109.7, 104.4, 77.4, 76.9, 76.5, 63.8, 50.9, 29.42, 26.8, 26.7 25.9, 24.4, 23.3, 20.3, 12.0; IR, RD-KBr (cm−1): 3331, 3062, 2953, 2870, 2854, 1998, 1747, 1522, 1458, 1437, 1362, 1288, 1209, 1039, 947, 781, 737, 557.
3-(1H-Indol-3-yl)-1-(decyloxy)-1-oxopropan-2-aminium methanesulfonate (3p). White solid; Mp. 124–125 °C; 1H-NMR (δ, DMSO-d6): 11.04 (ws), 8.40 (ws), 7.49 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.23 (dd, J = 6.5,2.4 Hz, 1H), 7.09 (td, J = 7.0, 1.0 Hz, 1H), 7.00 (td, J = 7.0, 1.0 Hz, 1H), 4.21 (s, 1H), 4.03 (m, J = 4.7 Hz, 2H), 3.30 (dd, J = 6.4, 4.0 Hz, 2H), 2.47 (s, 3H), 1.46 (qi, J = 6.8 Hz, 2H), 1.24 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H); 13C-NMR: 172.2, 139.1, 129.6, 127.6, 123.9, 121.3, 120.6, 114.4, 109.0, 82.1, 81.7, 81.3, 68.4, 55.6, 34.1, 31.9, 31.8, 31.6, 31.5, 30.6, 29.1, 27.9, 24.9, 16.8; IR, RD-KBr (cm−1): 3331, 3068, 2953, 2920, 2848, 2000, 1749, 1524, 1458, 1437, 1362, 1290, 1211, 1173, 1120, 1078, 1038, 947, 837, 779, 746, 557.
3-(1H-Indol-3-yl)-1-(dodecyloxy)-1-oxopropan-2-aminium methanesulfonate (3q). White solid; Mp. 122–123 °C; 1H-NMR (δ, DMSO-d6): 11.02 (ws), 8.38 (ws), 7.48 (d, J = 7.8 Hz, 1H), 7.38 (d, J = 8.0 Hz, 1H), 7.23 (dd, J = 6.6, 2.3 Hz, 1H), 7.08 (td, J = 6.9, 1.0 Hz, 1H), 7.00 (td, J = 7.0, 1.0 Hz, 1H), 4.21 (s, 1H), 4.04 (m, J = 4.1 Hz, 2H), 3.30 (dd, J = 5.8, 1.8 Hz, 2H), 2.47 (s, 3H), 1.48 (qi, J = 6.8 Hz, 2H), 1.25 (m, 18H), 0.87 (t, J = 7.0 Hz, 3H); 13C-NMR: 167.5, 134.4, 124.9, 122.9, 119.2, 116.7, 115.9, 109.7, 104.3, 77.4, 76.9, 76.5, 63.8, 50.9, 29.5, 27.3, 27.2, 27.2, 27.1, 26.9, 26.9, 25.9, 24.4, 23.3, 20.3, 12.1; IR, RD-KBr (cm−1): 3331 (νNH), 3068, 3028, 2953, 2920, 2848 (νCH), 2000, 1749 (νC=O), 1595, 1524, 1458, 1437, 1362, 1290, 1213 (νSO3), 1173, 1120, 1078, 1038, 947, 837, 779, 746, 557.
3-(1H-Indol-3-yl)-1-(tetradecyloxy)-1-oxopropan-2-aminium methanesulfonate (3r). White solid; Mp. 118–119 °C; 1H-NMR (δ, DMSO-d6): 11.05 (ws), 8.38 (ws), 7.48 (d, J = 7.7 Hz, 1H), 7.38 (d, J = 8.0 Hz, 1H), 7.23 (dd, J = 5.9, 2.3 Hz, 1H), 7.09 (td, J = 7.0, 1.0 Hz, 1H), 6.99 (td, J = 7.0, 2.1 Hz, 1H), 4.22 (s, 1H), 4.02 (m, J = 4.4 Hz, 2H), 3.30 (d, J = 5.5 Hz, 2H), 2.45 (s, 3H), 1.45(q, J = 6.4 Hz, 2H), 1.24 (m, 18H), 0.86 (t, J = 6.4 Hz, 3H); 13C-NMR: 167.5, 134.3, 124.9, 122.9, 119.2, 116.6, 115.9, 109.6, 104.3, 77.4, 76.9, 76.5, 63.7, 50.8, 29.4, 27.2, 27.1, 27.1, 27.0, 26.7, 26.6, 25.8, 24.3, 23.2, 20.2, 12.0; IR, RD-KBr (cm−1): 3331, 3070, 3028, 2953, 2920, 2848, 1998, 1751, 1593, 1524, 1458, 1437, 1362, 1290, 1213, 1173, 1122, 1078, 1038, 947, 837, 779, 744, 555.
3-(1H-Indol-3-yl)-1-(hexadecyloxy)-1-oxopropan-2-aminium methanesulfonate (3s). White solid; Mp. 120–121 °C; 1H-NMR (δ, DMSO-d6): 11.05(ws), 8.38 (ws), 7.48 (d, J = 7.8 Hz, 1H), 7.38 (d, J = 8.0 Hz, 1H), 7.23 (dd, J = 5.7, 2.3 Hz, 1H), 7.08 (td, J = 7.0, 1.0 Hz, 1H), 7.00 (td, J = 7.0, 0.9 Hz, 1H), 4.22 (s, 1H), 4.02 (m, J = 6.5 Hz, 2H), 3.30 (dd, J = 3.3 Hz, J = 5.8 Hz, 2H), 2.44 (s, 3H), 1.46 (qi, J = 6.5 Hz, 2H), 1.24 (m, 26H), 0.86 (t, J = 6.5 Hz, 3H); 13C-NMR: 167.5, 134.3, 124.9, 122.9, 119.2, 116.6, 115.9, 109.6, 104.3, 77.4, 76.9, 76.5, 63.7, 50.8, 38.4, 38.1, 37.8, 37.6, 37.5, 37.3, 36.9, 29.4, 28.7, 27.2, 27.1, 27.0, 26.9, 26.8, 25.9, 24.4, 23.2, 20.2, 12.0; IR, RD-KBr (cm−1): 3331, 3068, 3028, 2953, 2920, 2848, 1751, 1595, 1524, 1464, 1437, 1362, 1290, 1213, 1173, 1120, 1038, 949, 837, 779, 744, 555.
3-(1H-Indol-3-yl)-1-(octadecyloxy)-1-oxopropan-2-aminium methanesulfonate (3t). White solid; Mp. 114–116 °C (dec); 1H-NMR (δ, DMSO-d6): 10.99 (ws), 8.36 (ws), 7.46 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.21 (dd, J = 6.8 Hz, J = 2.1 Hz, 1H), 7.05 (td, J = 6.8, 2.1 Hz, 1H), 6.97 (td, J = 6.8, 1.0 Hz, 1H), 4.17 (s, 1H), 4.01 (m, 2H), 3.27 (m, 2H), 2.46 (s, 3H), 1.45 (qi, J = 6.7 Hz, 2H), 1.20 (m, 10H), 0.85 (t, J = 7.1 Hz, 3H); 13C-NMR: 167.5, 1354.5, 134.4 124.9, 122.9, 119.2, 116.7, 115.9, 109.7, 104.4, 77.4, 76.9, 76.5, 63.8, 50.9, 29.42, 26.8, 26.7 25.9, 24.4, 23.3, 20.3, 12.0; IR, RD-KBr (cm−1): 3331, 3068, 2920, 2848, 1751, 1597, 1524, 1464, 1377, 1290, 1213, 1173, 1120, 1038, 947, 837, 779, 744, 555.
3-(1H-Indol-3-yl)-1-(octadecyloxy)-1-oxopropan-2-aminium p-toluenesulfonate (3u). White solid; 92 °C (dec.); 1H-NMR (δ, DMSO-d6): 10.91 (ws), 8.36 (ws), 7.64 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 7.8 Hz, 1H), 7.38 (d, J = 7.9 Hz, 1H), 7.23 (dd, J = 9.2, 2.3 Hz, 1H), 7.13 (d, J = 7.9 Hz, 2H), 7.08 (t, J = 7.2 Hz, 1H), 7.02(t, J = 7.2 Hz, 1H), 4.15 (3, 1H), 4.07 (m, 2H), 3.32 (d, J = 6.2 Hz, 2H), 2.33 (s, 3H), 1.53 (qi, J = 6.4 Hz, 2H), 1.25 (m, 30H), 0.87 (t, J = 6.4 Hz, 3H); 13C-NMR: 167.7, 142.7, 136.8, 134.8, 126.6, 125.2, 124.0, 123.3, 119.7, 119.6, 117.1, 116.1, 110.0, 109.9, 104.5, 77.4, 76.9, 76.5, 64.3, 51.3, 51.2, 29.9, 27.7, 27.6, 27.5, 27.4, 27.3, 26.4, 23.8, 20.7, 19.4, 12.4; IR, RD-KBr (cm−1): 3431, 3269, 3240 (νNH), 2951, 2916, 2848, 1741, 1578, 1504, 1471, 1437, 1346, 1290, 1165, 1126, 1036, 1012, 814, 731, 575, 561.
4. Conclusions
The simultaneous esterification and acid-base reaction of unprotected α-amino acids to obtain ionic amino acid esters was demonstrated to be efficiently promoted in a one-pot, solventless approach using a wide range of alcohols and amino acids using MsOH or p-TsOH as acid catalysts under microwave irradiation. The proposed versatile protocol may be in principle easily extended to a range of other compounds, paving the way to the facile preparation of related relevant organic compounds of industrial important as surfactants and emulsifiers. Further investigations of these reactions are currently being ongoing in our laboratories.
Acknowledgments
RC-C and LEM thank to CONACyT and IMP for the awarded postdoctoral fellowship, respectively. We acknowledge Irina Likhanova for the use of the Monowave microwave reactor from her laboratory. We appreciate the financial support of project D.01225 (IMP).
- Sample Availability: Samples of the compounds 3a–3u are available from the authors.
References
- Loupy, A. Microwaves in Organic Synthesis, 2nd ed; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Larhed, M.; Olofsson, K. Microwave Methods in Organic Synthesis; Springer: Berlin, Germany, 2006. [Google Scholar]
- Martínez-Palou, R. Química en Microondas; CEM Publishing: Mattews, NC, USA, 2006; (E-book in spanish). [Google Scholar]
- Leadbeater, N.E. Microwave Heating as a Tool for Sustainable Chemistry; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Nüechter, M.; Ondruschka, B. Tools for microwave-assisted parallel syntheses and combinatorial chemistry. Mol. Diver. 2003, 7, 253–264. [Google Scholar] [CrossRef]
- Martínez-Palou, R. Advances in microwave-assisted combinatorial chemistry without polymer-supported reagents. Mol. Diver. 2006, 10, 435–462. [Google Scholar] [CrossRef]
- Kiso, H.; Yajima, H. Peptides. Synthesis, Structures and Applications; Guue, B., Ed.; Academic Press: New York, NY, USA, 1995; Volume 40. [Google Scholar]
- Paquet, A. Preparation of some long-chain N-acyl derivatives of essential amino-acids for nutritional studies. Can. J. Biochem. 1980, 58, 573–576. [Google Scholar] [CrossRef]
- Kawase, T.; Nishioka, Y.; Oida, T. A novel synthesis of N-alkoxycarbonyl amino acids and surfactant. Properties of their sodium salts. J. Oleo Sci. 2010, 59, 191–201. [Google Scholar] [CrossRef]
- Gorecki, M.; Acquaye, C.T.A.; Wilchek, M.; Votano, J.R.; Rich, A. Antisickling activity of amino acid benzyl esters. Proc. Natl. Acad. Sci. USA 1980, 77, 181–185. [Google Scholar]
- Beltran, J.B.U.; Bonaventura, J.S. Use of cationic preservative in food products. U.S. Patent 7407679 B2, 2008. [Google Scholar]
- Brook, M.; Chan, T.H. A simple procedure for the esterification of carboxylic-acids. Synthesis-Stuttgart 1983, 201-203. [Google Scholar]
- Yang, Q.; Wang, X.-J.; Li, Z.Y.; Sun, L.; You, Q.D. Microwave-assisted esterification of diverse carboxylic acids and chiral amino acids. Synth. Commun. 2008, 38, 4107–4115. [Google Scholar] [CrossRef]
- Penney, C.L.; Shah, P.; Landi, S. A simple method for the synthesis of long-chain alkyl esters of amino-acids. J. Org. Chem. 1985, 50, 1457–1459. [Google Scholar] [CrossRef]
- Arai, I.; Muramatsu, I. A simple and convenient method for esterification of tryptophan and other amino-acids. J. Org. Chem. 1983, 48, 121–123. [Google Scholar] [CrossRef]
- Leyendecker, F.; Jesser, F.; Laucher, D. Ligand effects in enantioface differentiating 1,4 addition to 1,3-diphenyl-2-propen-1-one. Tetrahedron Lett. 1983, 24, 3513–3516. [Google Scholar] [CrossRef]
- Gibson, S.; Romero, D.; Jacobs, H.K.; Gopalan, A.S. Concurrent esterification and N-acetylation of amino acids with orthoesters: A useful reaction with interesting mechanistic implications. Tetrahedron Lett. 2010, 51, 6737–6740. [Google Scholar]
- Hassner, A.; Alexanian, V. Synthetic methods. 12. Direct room-temperature esterification of carboxylic-acids. Tetrahedron Lett. 1978, 46, 4475–4478. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Z.; Cowins, J.V.; Olubajo, O. Microwave-assisted esterification of N-acetyl-L-phenylalanine using modified Mukaiyama's reagents: A new approach involving ionic liquids. Int. J. Mol. Sci. 2008, 9, 33–44. [Google Scholar] [CrossRef]
- Sureshbabu, V.V.; Kantharaju; Krishna, G.C. Microwave irradiation accelerated rapid, efficient and high yield esterification of Boc-amino acid to Merrifield resin mediated by KF. Int. J. Chem. B 2007, 46, 1466–1469. [Google Scholar]
- Zhang, S.; Arvidsson, P.I. Facile synthesis of N-protected amino acids assisted by microwave irradiation. Int. J. Pept. Res. Ther. 2008, 14, 219–222. [Google Scholar] [CrossRef]
- Vasanthakumar, G.R.; Patil, B.S.; Babu, V.V.S. Microwave-assisted facile synthesis of amino acid benzyl ester p-toluenesulfonate and hydrochloride salts. Lett. Pept. Sci. 2002, 9, 207–209. [Google Scholar]
- Sathe, M.; Kaushik, M.P. An efficient method for the esterification of amino acids using silica chloride. Catal. Commun. 2006, 7, 644–646. [Google Scholar]
- Biondini, D.; Brinchi, L.; Germani, R.; Goracci, L.; Savelli, G. Esterification of unprotected alpha-amino acids in ionic liquids as the reaction media. Lett. Org. Chem. 2010, 7, 39–44. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.; Kim, G.-J.; Seol, S.K.; Jung, S. Accelerated esterification of free fatty acid using pulsed microwaves. Biores. Technol. 2011, 102, 7229–7231. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.; Kim, G.J.; Seol, S.K.; Ha, Y.C.; Vijayan, M.; Jung, S.; Kim, B.H.; Lee, G.D.; Park, S.S. Microwave-accelerated energy-efficient esterification of free fatty acid with a heterogeneous catalyst. Biores. Technol. 2011, 102, 3639–3641. [Google Scholar]
- Melo, C.A.R.; Alburquerque, C.E.R.; Carneiro, J.S.A.; Dariva, C.; Fortuny, M.; Santos, A.F.; Egues, S.M.S.; Ramos, A.L.D. Solid-acid-catalyzed esterification of oleic acid assisted by microwave heating. Ing. Eng. Chem. Res. 2010, 49, 12135–12139. [Google Scholar]
- Herrero, M.A.; Krensner, J.M.; Kappe, C.O. Nonthermal microwave efffects revisited: On the importance of internal temperature monitoring and agitation microwave chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef]
- Hosseini, M.; Stiasni, N.; Barbieri, V.; Kappe, C.O. Microwave-assisted asymmetric organocatalysis. A probe for nonthermal microwave effects and the concept of simultaneous cooling. J. Org. Chem. 2007, 72, 1417–1424. [Google Scholar]
- Obermayer, D.; Kappe, C.O. On the importance of simultaneous infrared/fiber-optic temperature monitoring in the microwave-assisted synthesis of ionic liquids. Org. Biomol. Chem. 2010, 8, 114–121. [Google Scholar] [CrossRef]
- Murillo-Hernández, J.A.; García-Cruz, I.; López-Ramirez, S.; Durán-Valencia, C.; Domínguez, J.M.; Aburto, J. Aggregation behavior of heavy crude oil-ionic liquids solutions by fluorescence spectroscopy. Energy Fuels 2009, 23, 4584–4592. [Google Scholar]
- Ware, A.M.; Waghmare, J.T.; Momin, S.A. Alkylpolyglycoside: Carbohydrate based surfactant. J. Dispersion Sci. Technol. 2007, 28, 437–444. [Google Scholar]
- El-Sukkary, M.M.A.; Syed, N.A.; Aiad, I.; El-Azab, W.I.M. Synthesis and characterization of some alkyl polyglycosides surfactants. J. Surfactants Detergents. 2008, 11, 129–137. [Google Scholar] [CrossRef]
- Murguía, M.C.; Machuca, L.M.; Lurá, M.C.; Cabrera, M.I.; Grau, R.J. Synthesis and properties of novel antifungal gemini compounds derived from N-acetyl diethanolamines. J. Surfactants Detergents 2008, 11, 223–230. [Google Scholar] [CrossRef]
- Synthesis to the Power of 3, Anton-Paar website. Available online: http://www.anton-paar.com/monowave300/ (accessed date: 11.oct. 2011).
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).