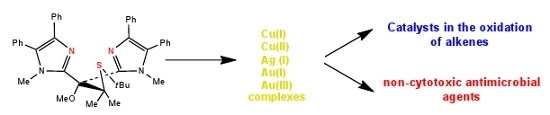
Group 11 Metal Compounds with Tripodal Bis(imidazole) Thioether Ligands. Applications as Catalysts in the Oxidation of Alkenes and as Antimicrobial Agents
Abstract
:1. Introduction
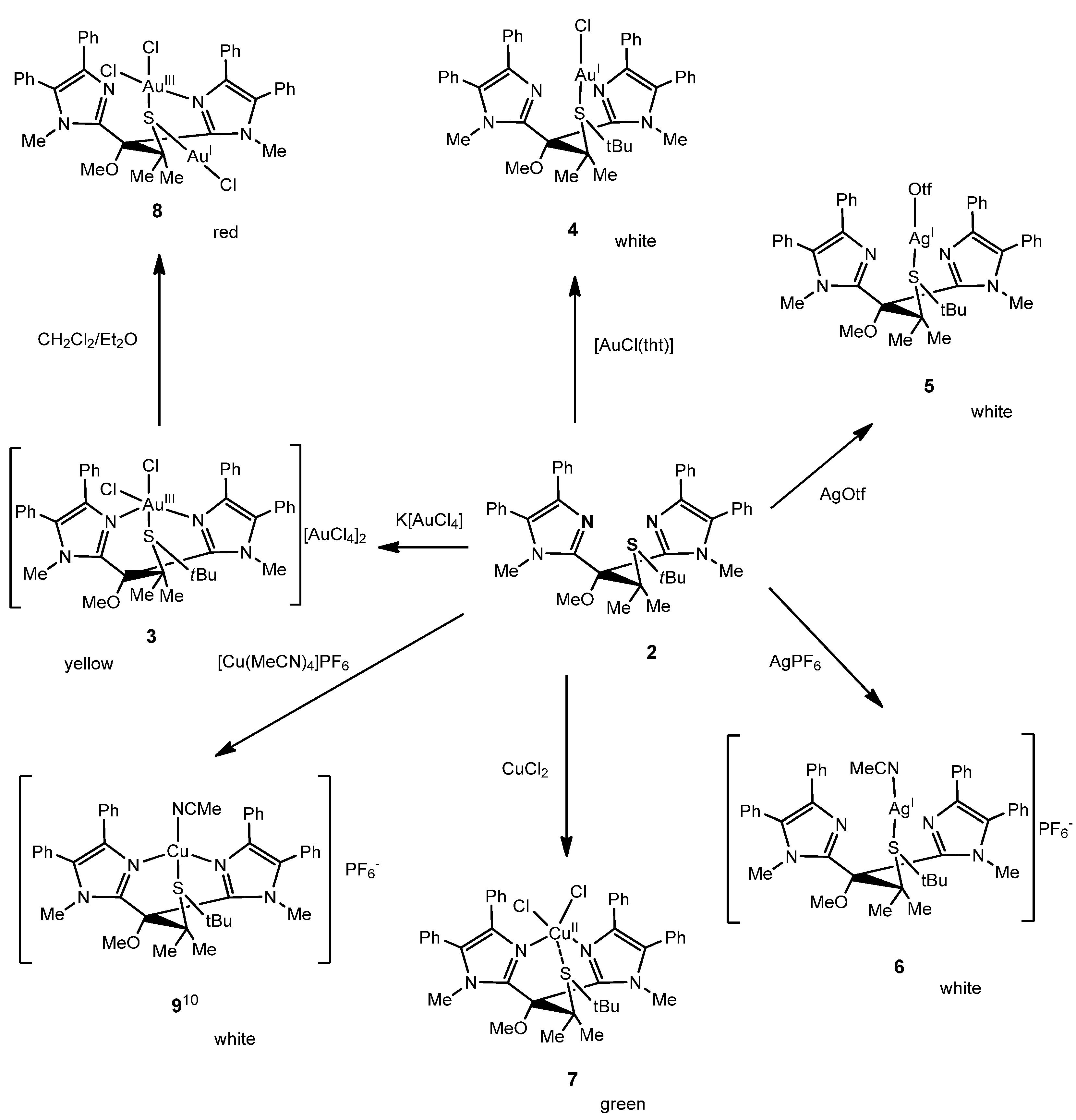
2. Results and Discussion
2.1. Chemistry
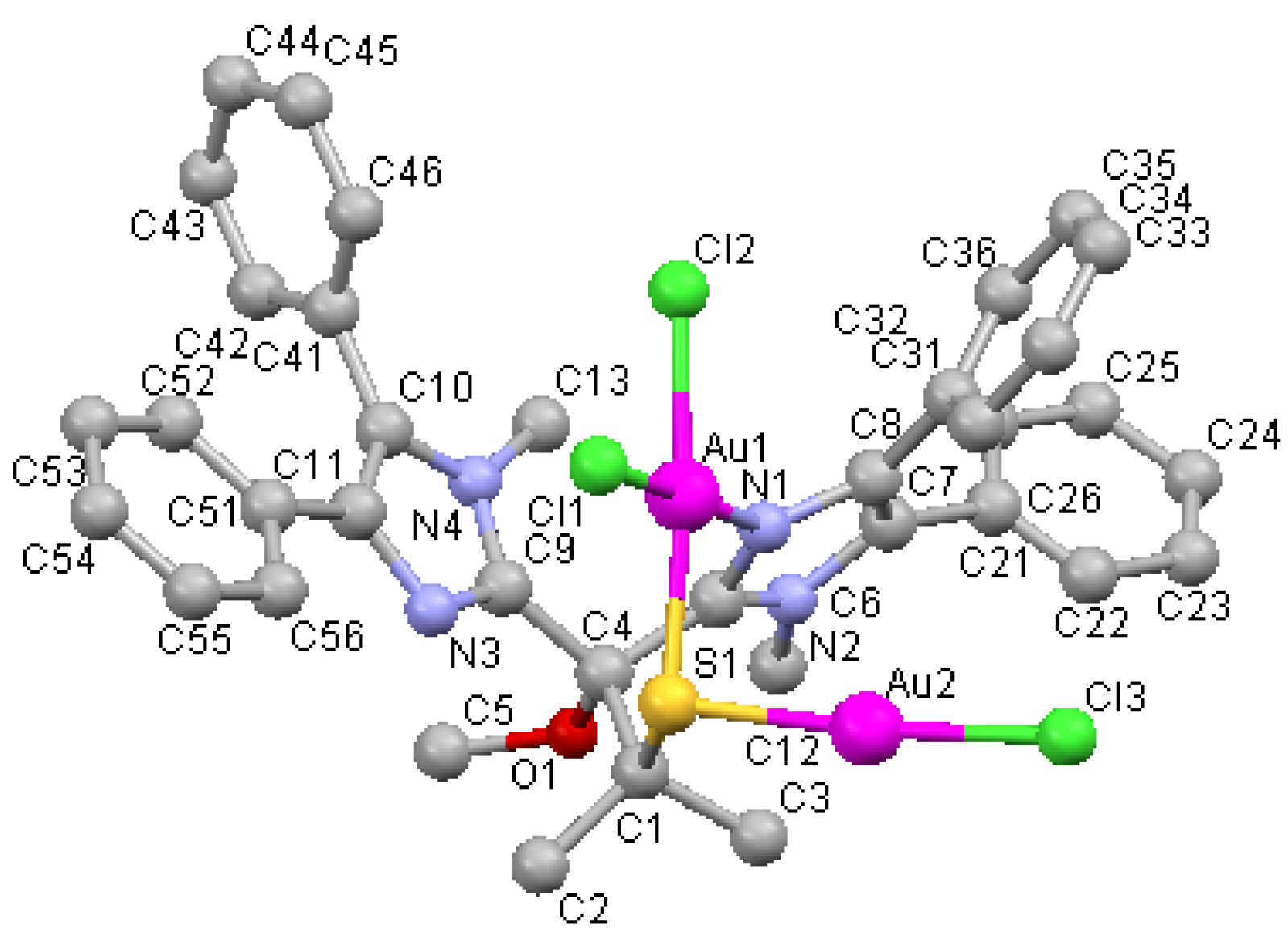
| Au1 N1 2.038(5) Å | N1 Au1 Cl1 177.4(2)° |
| Au1 Cl1 2.3066(18) Å | Cl2 Au1 S1 174.35(8)° |
| Au1 Cl2 2.3119(19) Å | N1 Au1 Cl2 91.01(17)° |
| Au1 S1 2.327(2) Å | Cl1 Au1 Cl2 90.66(7)° |
| Au2 S1 2.248(2) Å | N1 Au1 S1 89.42(17) |
| Au2 Cl3 2.258(2) Å | Cl1 Au1 S1 89.11(7)° |
| Au1 Au2 3.383 Å | S1 Au2 Cl3 174.81(8)° |
| Au2 S1 Au1 95.37(6)° |
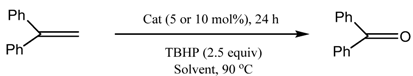
2.2. Catalysis
| Run | Catalyst | Solvent | Temp. (°C) | Cat. mol % | Conversion % a |
|---|---|---|---|---|---|
| 1 | 3 | Toluene / Acetonitrile | 90 | 5 | 26 |
| 2 | 3 | Toluene / Acetonitrile | 90 | 10 | 31 |
| 3 | 4 | Toluene | RT | 5 | 0 |
| 4 | 4 | Toluene | RT | 10 | 0 |
| 5 | 4 | Toluene | 90 | 5 | 43 |
| 6 | 4 | Toluene | 90 | 10 | 60 |
| 7 | 5 | Toluene / Acetonitrile | 90 | 5 | 42 |
| 8 | 5 | Toluene / Acetonitrile | 90 | 10 | 48 |
| 9 | 6 | Toluene / Acetonitrile | 90 | 5 | 42 |
| 10 | 6 | Toluene / Acetonitrile | 90 | 10 | 80 |
| 11 | 7 | Toluene / Acetone | 90 | 5 | 0 |
| 12 | 7 | Toluene / Acetone | 90 | 10 | 7 |
| 13 | 9 | Toluene | 90 | 10 | 10 |
2.3. Biological Activity
2.3.1. Antimicrobial Activity
| Compound | Yeast | Gram-negative | Gram-positive | ||
|---|---|---|---|---|---|
| S. Cerevisiae (X2180-1A) | E. Coli | S. Tiphymurium | B. Cereus | S. Aureus | |
| 2 b | --- | --- | --- | --- | --- |
| 3 (AuIII) c | 100 | 100 | 100 | 10 | 10 |
| 4 (AuI) c | 100 | 0.1 | 100 | --- | 100 |
| 5 (AgI) c | 100 | 10 | 10 | 10 | 10 |
| 7 (CuII) c | --- | --- | --- | 10 | --- |
| 9 (CuI) c | --- | --- | --- | --- | --- |
2.3.2. Cytotoxicity
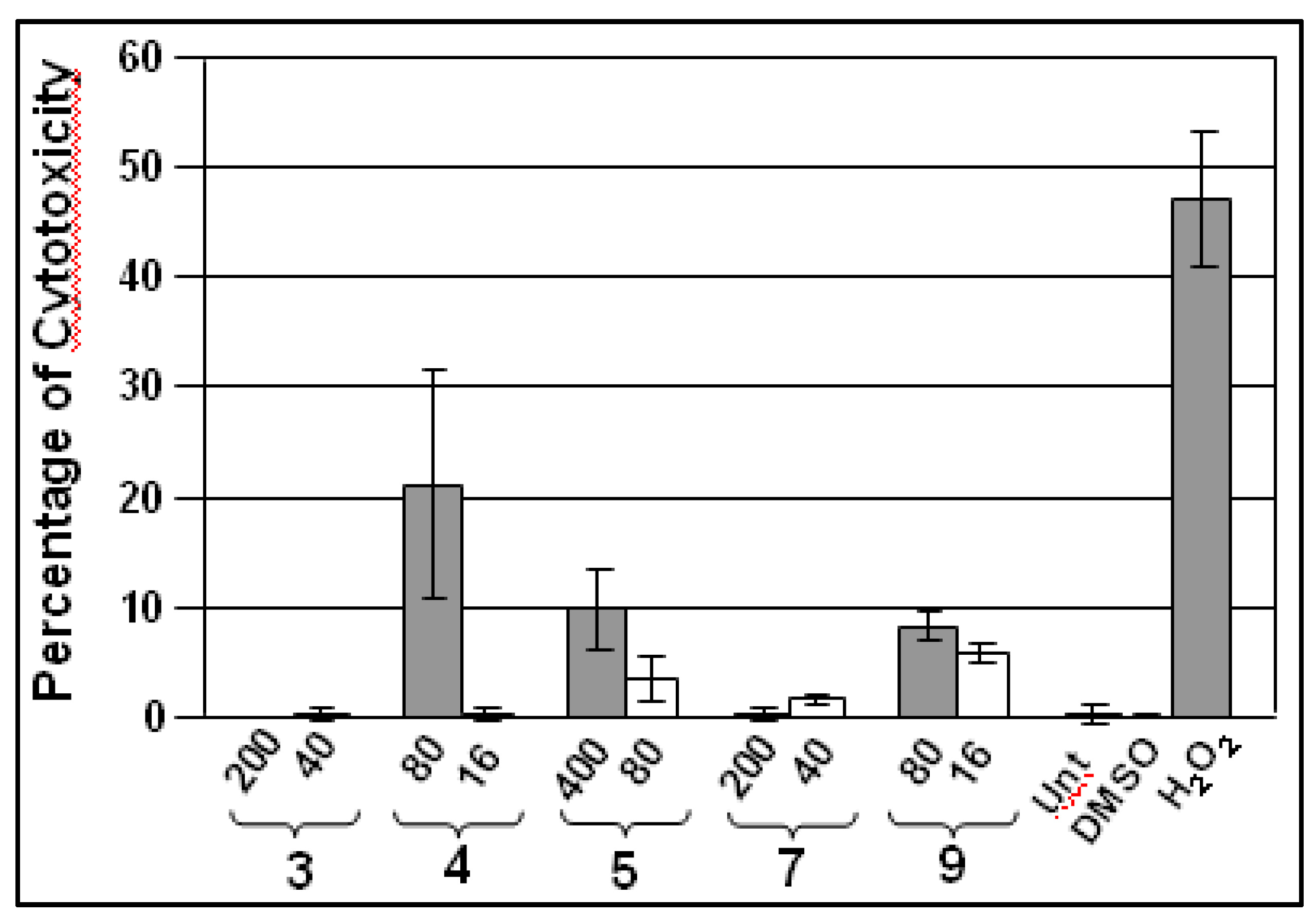
| IC50(μM) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell line | Cisplatin | 2 b | 3 | 4 | 5 | 6 b | 7c | 9 c |
| HeLa-GPF | 14.9 | ---- | 155 | 155 | 73.6 | --- | --- | --- |
3. Experimental
3.1. General
3.2. Catalytic Experiments
3.3. Single-Crystal X-ray Diffraction Studies
3.4. EPR studies
3.5. Antimicrobial Assays
3.6. Cytotoxicity Assays
3.7. Cytotoxicity against normal primary culture cells
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Chomitz, W.A.; Arnold, J. Use of tetradentate monoanionic ligands for stabilizing reactive metal complexes. Chem. Eur. J. 2009, 15, 2020–2030. [Google Scholar] [CrossRef]
- Latest review: Liang, L.C. Metal complexes of chelating diarylamido phosphine ligands. Coord. Chem. Rev. 2006, 250, 1152–1177. [Google Scholar]
- For example: Lewis, F.A.; Tolman, W.B. Reactivity of dioxygen-copper systems. Chem. Rev. 2004, 104, 1047–1076. [Google Scholar]
- Bai, S.-Q.; Koh, L.L.; Hor, T.S.A. Structures of copper complexes of the hybrid [SNS] ligand of bis(2-(benzylthio)ethyl)amine and facile catalytic formation of 1-benzyl-4-phenyl-1H-1,2,3-triazole through click reaction. Inorg. Chem. 48, 1207–1213, and references therein.
- Zablotskaya, A.; Segal, I.; Lukevics, E.; Belyakov, S.; Spies, H. Tetrahydroquinoline and tetrahydroisoquinoline mixed ligand rhenium complexes with the SNS/S donor atom set. Appl. Organometal. Chem. 2007, 21, 288–293. [Google Scholar]
- Zhang, J.-A.; Pan, M.; Zhang, J.-Y.; Zhang, H.-K.; Fan, Z.-J.; Kang, B.-S.; Su, C.-Y. Syntheses, structures and bioactivities of silver(I) complexes with a tridentate heterocyclic N- and S-ligand. Polyhedron 2009, 28, 145–149, and references therein. [Google Scholar]
- Aragoni, M.C.; Arca, M.; Bencini, A.; Biagini, S.; Blake, A.J.; Caltagirone, C.; Demartin, F.; De Filippo, G.; Devillanova. F.A.; Garau, A.; et al. Interaction of mixed-donor macrocycles containing the 1,10-phenanthroline subunit with selected transition and post-transition metal ions: metal ion recognition in competitive liquid-liquid solvent extraction of CuII, ZnII, PbII, CdII, AgI, and HgII. Inorg. Chem. 2008, 47, 8391–8404, and references therein. [Google Scholar]
- For example: Lee, Y.; Lee, D.-H.; Narducci-Sarjeant, A.A.; Zakharov, L.N.; Rheingold, A.L.; Karlin, K.D. Thioether sulfur oxygenation from O2 or H2O2 reactivity of copper complexes with tridentate N2S thioether ligands. Inorg. Chem. 2006, 45, 10098–10107. [Google Scholar] [CrossRef]
- Zhou, L.; Powell, D.; Nicholas, K.M. Tripodal bis(imidazole) thioether copper(I) complexes: mimics of the Cu(B) site of hydroxylase enzymes. Inorg. Chem. 2006, 45, 3840–3842. [Google Scholar] [CrossRef]
- Zhou, L.; Powell, D.; Nicholas, K.M. Tripodal bis(imidazole) thioether copper(I) complexes: Mimics of the CuM site of copper hydroxylase enzymes. Inorg. Chem. 2007, 46, 7789–7799. [Google Scholar] [CrossRef]
- Zhou, L.; Nicholas, K.M. Imidazole substituent effects on oxidative reactivity of tripodal(imid)2(thioether)CuI complexes. Inorg. Chem. 2008, 47, 4356–4367. [Google Scholar] [CrossRef]
- Laguna, M.; Villacampa, M.D.; Contel, M.; Garrido, J. Hexanuclear mercury-silver complexes. Novel coordination for bridging mesityl and triflate Groups. Inorg. Chem. 1998, 37, 133–135, and references therein. [Google Scholar] [CrossRef]
- Contel, M.; Villuendas, P.R.; Fernández-Gallardo, J.; Alonso, P.J.; Vincent, J.-M.; Fish, R.H. Fluorocarbon soluble copper(II) carboxylate complexes with nonfluoroponytailed nitrogen ligands as precatalysts for the oxidation of alkenols and alcohols under fluorous biphasic or thermomorphic modes: structural and mechanistic aspects. Inorg. Chem. 2005, 44, 9771–9778, and references therein. [Google Scholar]
- Dell’Amico, D.B.; Claderazzo, F.; Pasqualetti, N.; Hubener, R.; Maichele-Mossmer, C.; Strahle, J. Reactions of carbonylchlorogold(I) with thiolates and thiols. Crystal and molecular structures of [NaL(H2O)][(AuCl)3SBut], [NaL][AuCl2], [NaL]4[AuCl2]2[Au4Cl6S2] and [NaL]2[Au4Cl6S2] (L = 1,4,7,10,13-pentaoxacyclopentadecane). J. Chem. Soc., Dalton Trans. 1995, 3917–3924. [Google Scholar]
- Wang, S.; Fackler, J.P., Jr. Gold thiolate complexes with short intermolecular gold-gold distances from reactions of organic disulfides with gold(I) complexes. Syntheses and crystal structures of [AuI2(PPh3)2(μ-SCH2Ph)](NO3) and [AuIII2Cl4(μ-SPh)2]. Inorg. Chem. 1990, 29, 4404–4407. [Google Scholar] [CrossRef]
- Bardaji, M.; Calhorda, M.J.; Costa, P.J.; Jones, P.G.; Laguna, A.; Pérez, M.R.; Villacampa, M.D. Synthesis, structural characterization and theoretical studies of gold(I) and gold(I)-gold(III) thiolate complexes: quenching of gold(I) thiolate luminescence. Inorg. Chem. 2006, 45, 1059–1068. [Google Scholar]
- Crespo, O.; Canales, F.; Gimeno, M.C.; Jones, P.G.; Laguna, A. Synthesis of [Au2(SC6F5)2(m-dppf)] and [Au2(m -SC6F5)( m -dppf)] (dppf = 1,1’-bis(diphenylphosphino)ferrocene). Reactivity toward various metallic fragments. Organometallics 1999, 18, 3142–3148. [Google Scholar] [CrossRef]
- Calhorda, M.J.; Canales, F.; Gimeno, M.C.; Jones, P.G.; Jimenez, J.; Jones, P.G.; Laguna, A.; Verios, L.F. Gold(I)-Gold(III) interactions in polynuclear sulfur-centered complexes. Synthesis and structural characterization of [S(Au2dppf){Au(C6F5)3}] and [{S(Au2dppf)}2{Au(C6F5)2}]OTf (dppf = 1,1'-bis(diphenylphosphino)ferrocene). Organometallics 1997, 16, 3837–3844. [Google Scholar] [CrossRef]
- Canales, F.; Gimeno, M.C.; Laguna, A.; Jones, P.G. Synthesis and structural characterization of tetranuclear sulfur-centered complexes with mixed-valent gold atoms: [S(Au2dppf){Au(C6F5)3}2] (dppf = 1,1'-Bis(diphenylphosphino)ferrocene) and [S(AuPPh3)2{Au(C6F5)3}2]. Organometallics 1996, 15, 3412–3415. [Google Scholar] [CrossRef]
- Canales, S.; Crespo, O.; Gimeno, M.C.; Jones, P.G.; Laguna, A.; Mendizábal, F. Mixed Gold(I)-Gold(III) complexes with bridging selenido ligands. Theoretical studies of the Gold(I)-Gold(III) interactions. Organometallics 2001, 20, 4812–4818. [Google Scholar] [CrossRef]
- Von Petersen, A. Complexes of gold(I) with thioethers and chloride or phosphines as ligands. Archiv. Pharma. 1991, 324, 411. [Google Scholar] [CrossRef]
- Kostin, G.A.; Mashukov, V.I.; Torgov, V.G.; Kalchenko, V.I.; Drapaillo, A.B. Reduction kinetics of gold(III) complexes with calix[4]arenes modified by thioether groups on the upper rim. Z. Neorg. Khim. 2006, 51, 536–542. [Google Scholar]
- Zhong, C.J.; Porter, M.D. Evidence for carbon-sulfur bond cleavage in spontaneously adsorbed organosulfide-based monolayers at gold. J. Am. Chem. Soc. 1994, 116, 11616–11617, and references therein.. [Google Scholar] [CrossRef]
- Hughes, M.D.; Xu, Y.-J.; Jenkins, P.; McMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 2005, 437, 1132–1135. [Google Scholar]
- Guan, B.; Xing, D.; Cai, G.; Wan, X.; Yu, N.; Fang, Z.; Yang, L.; Shi, Z. Highly selective aerobic oxidation of alcohol catalyzed by a gold(I) complex with an anionic ligand. J. Am. Chem. Soc. 2005, 127, 18004–18005. [Google Scholar] [CrossRef]
- Xing, D.; Guan, B.; Cai, G.; Fang, Z.; Yang, L.; Shi, Z. Gold(I)-catalyzed oxidative cleavage of a C-C double bond in water. Org. Lett. 2006, 8, 693–696. [Google Scholar] [CrossRef]
- Das, A.; Chaudhury, R.; Liu, R.-S. Gold-catalyzed oxidative cleavage of aryl-substituted alkynyl ethers using molecular oxygen. Simultaneous degradation of C-H and single and triple carbon-carbon bonds under ambient conditions. Chem. Commun. 2009, 4046–4048. [Google Scholar]
- De Vos, D.E.; Sels, B.F. Gold Redox Catalysis for selective oxidation of methane to methanol. Angew. Chem. Int. Ed. 2004, 43, 2–4. [Google Scholar]
- Jones, C.J.; Taube, D.; Ziatdinov, V.R.; Periana, R.A.; Nielsen, R.J.; Oxgaard, J.; Goddard, W.A., III. Selective oxidation of methane to methanol catalyzed, with C-H activation, by homogeneous, cationic gold. Angew. Chem. Int. Ed. 2004, 43, 4626–4629. [Google Scholar] [CrossRef]
- Gasparrini, F.; Giovannoli, M.; Misiti, D.; Natile, G.; Palmieri, G. Gold(III) catalyzed oxidation of sulfides to sulfoxides by nitric acid under phase-transfer conditions: A new synthesis of sulfoxides. Tetrahedron 1983, 19, 3181–3184. [Google Scholar]
- Yuan, Y.; Bian, Y. Gold(III) catalyzed oxidation of sulfides to sulfoxides with hydrogen peroxide. Tetrahedron Lett. 2007, 48, 8518–8520. [Google Scholar] [CrossRef]
- Boring, E.; Geletii, Y.V.; Hill, C.L. A homogeneous catalyst for selective O2 oxidation at ambient temperature. Diversity-based discovery and mechanistic investigation of thioether oxidation by the Au(III)Cl2NO3(thioether)/O2 system. J. Am. Chem. Soc. 2001, 123, 1625–1635. [Google Scholar] [CrossRef]
- Boring, E.; Geletii, Y.V.; Hill, C.L. Catalytic aerobic oxidation of 2-chloroethyl ethylsulfide, a mustard stimulant, under ambient conditions. Effect of solvents, ligands, and transition metals on reactivity. J. Mol. Catal. A: Chem. 2001, 176, 49–63. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Shilov, A.E.; Süss-Fink, G. Alkane oxygenation catalysed by gold complexes. Tetrahedron Lett. 2001, 42, 7253–7256. [Google Scholar] [CrossRef]
- Li, X.-Q.; Li, C.; Song, F.-B.; Zhang, C.J. A first homogeneous gold(III)-catalysed epoxidation of aromatic alkenes. Chem. Res. 2007, 722–724. [Google Scholar]
- Aschwanden, L.; Mallat, T.; Grunwaldt, J.-D.; Krumeich, F.; Baiker, A. Gold-catalyzed aerobic oxidation of dibenzylamine: Homogeneous or heterogeneous catalysis? J. Mol. Catal A: Chem. 2009, 300, 111–115. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, L.; Wang, Y.; Zhang, L. Homogeneous gold-catalyzed oxidative carboheterofunctionalization of alkenes. J. Am. Chem. Soc. 2010, 132, 1474–1475, and references therein. [Google Scholar] [CrossRef]
- Liu, X.; Liu. D.; Yang, Y.; Yao, H. Complex catalyst for synthesis of carbonate ester by alcohol homogeneous phase oxidation of carbonyl. 2006; CN1792453(A). [Google Scholar]
- Sundermeyer, J.; Jost, C. Liquid phase oxidation of organic compounds using peroxides in the presence of homogeneous sol. gold or silver complex catalysts. 2001; DE10041510(A1). [Google Scholar]
- Elie, B.T.; Levine, C.; Ubarretxena-Belandia, I.; Varela-Ramirez, A.; Aguilera, R.J.; Ovalle, R.; Contel, M. Water-soluble (phosphane)gold(I) complexes - applications as recyclable catalysts in a three-component coupling reaction and as antimicrobial and anticancer agents. Eur. J. Inorg. Chem. 2009, 3421–3430, and references therein. [Google Scholar]
- Novelli, F.; Recine, M.; Sparatore, F.; Juliano, C. Gold(I) complexes as antimicrobial agents. Farmaco 1999, 232–236. [Google Scholar]
- Fillat, M.F.; Gimeno, M.C.; Laguna, A.; Latorre, E.; Ortego, L.; Villacampa, M.D. Synthesis, structure and bactericide activity of (aminophosphane)gold(I) thiolate complexes. Eur. J. Inorg. Chem. 2011, 1487–1495, and references therein. [Google Scholar]
- Sreekanth, A.; Fun, H.-K.; Kurup, M.R.P. Formation of first gold(III) complex of an N(4)-disubstituted thiosemicarbazone derived from 2-benzoylpyridine: structural and spectral studies. Inorg. Chem. Commun. 2004, 7, 1250, and references therein. [Google Scholar] [CrossRef]
- Ray, S.; Mohan, R.; Singh, J.K.; Samantaray, M.K.; Shaikh, M.M.; Panda, D.; Ghosh, P. Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexe. J. Am. Chem. Soc. 2007, 129, 15042–15043. [Google Scholar]
- Kasuga, N.C.; Sugie, A.; Nomiya, K. Syntheses, structures and antimicrobial activities of water-soluble silver(I)-oxygen bonding complexes with chiral and racemic camphanic acid (Hca) ligands. Dalton Trans. 2004, 3732–3740. [Google Scholar]
- Melaiye, A.; Simons, R.S.; Milsted, A.; Pingitore, F.; Wesdemiotis, C.; Tessier, C.A.; Youngs, W.J. Formation of water-soluble pincer silver(I)-carbene complexes: A novel antimicrobial agent. J. Med. Chem. 2004, 47, 973–977. [Google Scholar] [CrossRef]
- Melaiye, A.; Sun, Z.H.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D.H.; Tessier, C.A.; Youngs, W.J. Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005, 127, 2285–2291. [Google Scholar]
- Roland, S.; Jolivat, C.; Cresteil, T.; Eloy, L.; Bouhours, P.; Hequet, A.; Mansuy, V.; Vanucci, C.; Paris, J.-M. Investigation of a series of silver-N-Heterocyclic carbenes as antibacterial agents: Activity, synergistic effects, and cytotoxicity. Chem. Eur. J. 2011, 17, 1442–1446. [Google Scholar]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; et al. Synthesis from caffeine of a mixed N-heterocyclic carbene-silver acetate complex active against resistant respiratory pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar]
- Hindi, K.M.; Siciliano, T.J.; Durmus, S.; Panzner, M.J.; Medvetz, D.A.; Reddy, D.V.; Hogue, L.A.; Hovis, C.E.; Hilliard, J.K.; Mallet, R.J.; et al. Synthesis, stability, and antimicrobial studies of electronically tuned silver acetate N-heterocyclic carbenes. J. Med. Chem. 2008, 51, 1577–1583. [Google Scholar]
- Panzner, M.J.; Deeraksa, A.; Smith, A.; Wright, B.D.; Hindi, K.M.; Kascatan-Nebioglu, A.; Torres, A.G.; Judy, B.M.; Hovis, C.E.; Hilliard, J.K.; et al. Synthesis and in vitro efficacy studies of silver carbene complexes on biosafety level 3 bacteria. Eur. J. Inorg. Chem. 2009, 1739–1745. [Google Scholar]
- Zhang, J.-A.; Pan, M.; Zhang, J.-Y.; Zhagn, H.-K.; Fan, Z.-J.; Kang, B.-S. Syntheses, structures and bioactivities of silver(I) complexes with a tridentate heterocyclic N- and S-ligand. Polyhedron 2009, 28, 145–149. [Google Scholar]
- van Waasbergen, L.G.; Fajdetic, I.; Fianchini, M.; Dias, H.V.R. Antimicrobial properties of highly fluorinated tris(pyrazolyl)borates. J. Inorg. Biochem. 2007, 101, 1180–1183, and references therein. [Google Scholar] [CrossRef]
- Montoya, J.; Varela-Ramirez, A.; Estrada, A.; Martinez, L.E.; Garza, K.; Aguilera, R.J. A fluorescence-based rapid screening assay for cytotoxic compounds. Biochem. Biophys. Res. Commun. 2004, 325, 1517–1523. [Google Scholar] [CrossRef]
- Shaik, N.; Martinez, A.; Augustin, I.; Giovinazzo, H.; Varela, A.; Aguilera, R.; Sanaú, M.; Contel, M. Synthesis of apoptosis-inducing iminophosphorane organogold(III) complexes and study of their interactions with biomolecular targets. Inorg. Chem. 2009, 48, 1577–1587. [Google Scholar] [CrossRef]
- Kanda, T.; Sullivan, K.F.; Wahl, G.M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998, 8, 377–385. [Google Scholar] [CrossRef]
- Askanas, V.; Engel, W.K. A new program for investigating adult human skeletal muscle grown aneurally in tissue culture. Neurology 1975, 25, 58. [Google Scholar] [CrossRef]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell. Biochem. 2011. Submitted. [Google Scholar]
- Sample Availability: Samples of ligand 2 (250 mg) and silver derivatives 5 and 6 (20-30 mg) are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, F.; Anis, R.; Hwang, E.; Ovalle, R.; Varela-Ramírez, A.; Aguilera, R.J.; Contel, M. Group 11 Metal Compounds with Tripodal Bis(imidazole) Thioether Ligands. Applications as Catalysts in the Oxidation of Alkenes and as Antimicrobial Agents. Molecules 2011, 16, 6701-6720. https://doi.org/10.3390/molecules16086701
Liu F, Anis R, Hwang E, Ovalle R, Varela-Ramírez A, Aguilera RJ, Contel M. Group 11 Metal Compounds with Tripodal Bis(imidazole) Thioether Ligands. Applications as Catalysts in the Oxidation of Alkenes and as Antimicrobial Agents. Molecules. 2011; 16(8):6701-6720. https://doi.org/10.3390/molecules16086701
Chicago/Turabian StyleLiu, Fangwei, Reema Anis, Eunmi Hwang, Rafael Ovalle, Armando Varela-Ramírez, Renato J. Aguilera, and María Contel. 2011. "Group 11 Metal Compounds with Tripodal Bis(imidazole) Thioether Ligands. Applications as Catalysts in the Oxidation of Alkenes and as Antimicrobial Agents" Molecules 16, no. 8: 6701-6720. https://doi.org/10.3390/molecules16086701
APA StyleLiu, F., Anis, R., Hwang, E., Ovalle, R., Varela-Ramírez, A., Aguilera, R. J., & Contel, M. (2011). Group 11 Metal Compounds with Tripodal Bis(imidazole) Thioether Ligands. Applications as Catalysts in the Oxidation of Alkenes and as Antimicrobial Agents. Molecules, 16(8), 6701-6720. https://doi.org/10.3390/molecules16086701