Assessment of the In Vivo Genotoxicity of New Lead Compounds to Treat Sickle Cell Disease
Abstract
:1. Introduction
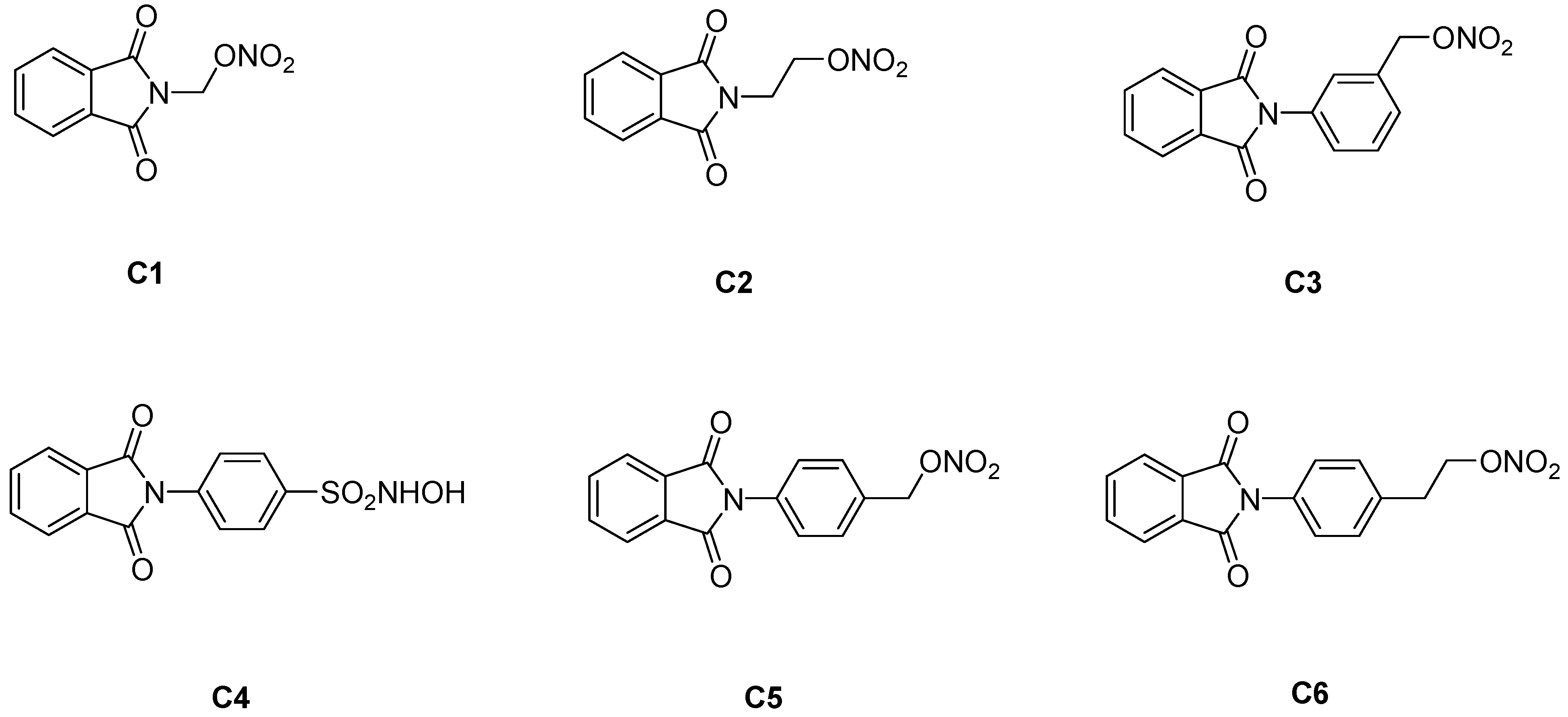
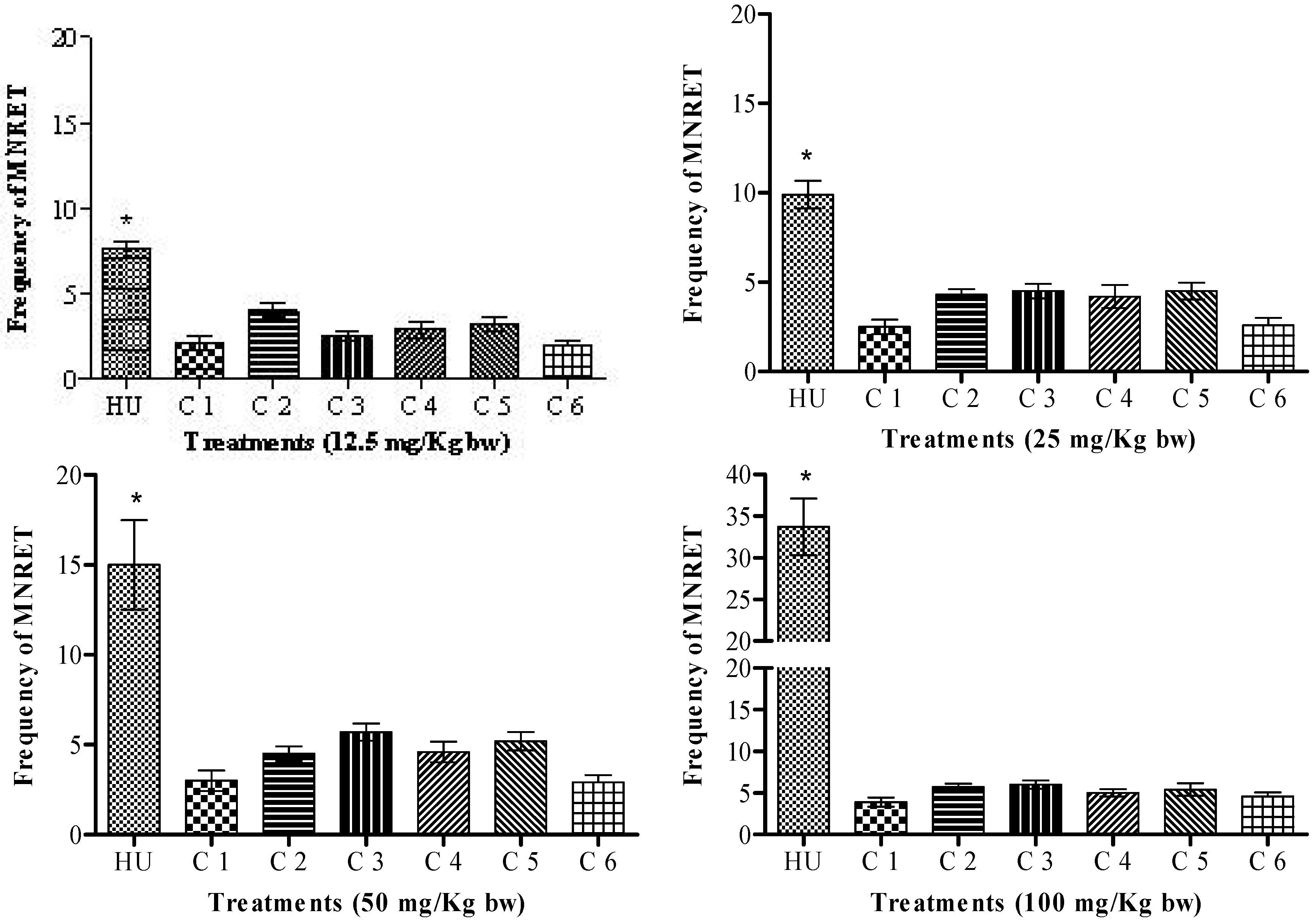
2. Results and Discussion
3. Experimental
3.1. Chemicals
3.2. Preparation of compounds C1–C6
3.3. Animals
3.4. Evaluation of the mutagenicity of HU and the synthesized compounds with a micronucleus test in peripheral blood cells of mice
4. Conclusions
Acknowledgements
References and Notes
- Steinberg, M.H. Pathophysiologically based drug treatment of sickle cell disease. Trends Pharmacol. Sci. 2006, 27, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hanft, V.N.; Fruchtman, S.R.; Pickens, C.V.; Rosse, W.F.; Howard, T.A.; Ware, R.E. Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood 2000, 95, 3589–3593. [Google Scholar] [PubMed]
- Lou, T.F.; Singh, M.; Mackie, A.; Li, W.; Pace, B.S. Hydroxyurea generates nitric oxide in human erythroid cells: Mechanisms for gamma-globin gene activation. Exp. Biol. Med. 2009, 234, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Conran, N.; Oresco-santos, C.; Acosta, H.C.; Fattori, A.; Saad, S.T.; Costa, F.F. Increased soluble guanylate cyclase activity in the red blood cells of sickle cell patients. Br. J. Haematol. 2004, 124, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Haywood, C., Jr.; Beach, M.C.; Bediako, S.; Carroll, C.P.; Lattimer, L.; Jarrett, D.; Lanzkron, S. Examining the characteristics and beliefs of hydroxyurea users and nonusers among adults with sickle cell disease. Am. J. Hematol. 2011, 86, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, Z.Y.; Tumblin, A.R.; Kato, G.J. Current therapy of sickle cell disease. Haematologica 2005, 90, 7–12. [Google Scholar]
- Santos, J.L.; Chung, M.C.; Lima, L.M.; Lanaro, C.; Costa, F.F. Use of phthalimide and/or sulphonamide derivatives in the treatment of diseases which require reducing the TNF-alpha levels and exogenous source of nitric oxide, phthalimide derivatives, sulphonamide derivatives, and a method for obtaining a sulphonamide derivative. PCT Int Appl. BR2008/000386, 12 December 2008. [Google Scholar]
- Santos, J.L.; Chung, M.C. Design, synthesis and pharmacological evaluation of hybrid compounds that are potentially active in the treatment of sickle cell disease. Rev. Bras. Hematol. Hemoter. 2010, 32, 341–342. [Google Scholar] [CrossRef]
- Santos, J.L.; Varanda, E.A.; Lima, L.M.; Chung, M.C. Mutagenicity of new lead compounds to treat sickle cell disease symptoms in Salmonella/microsome assay. Int. J. Mol. Sci. 2010, 11, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kwon, J.; Chung, M.K. Enhanced prediction of potential rodent carcinogenicity by utilizing comet assay and apoptotic assay in combination. Mutat. Res. 2003, 541, 9–19. [Google Scholar] [CrossRef]
- Juul, T.; Malolepszy, A.; Dybkaer, K.; Kidmose, R.; Rasmussen, J.T.; Andersen, G.R.; Jonhsen, H.E.; Jorgensen, J.E.; Andresen, S.U. The in vivo toxicicty of hydroxyurea depends on its direct target catalase. J. Biol. Chem. 2010, 285, 21411–21415. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.M.; Howard, T.A.; Mortier, N.; Avlasevich, S.L.; Smeltzer, M.P.; Wu, S.; Dertinger, S.D.; Ware, R.E. Assessment of genotoxicity associated with hydroxyurea therapy in children with sickle cell anemia. Mutat. Res. 2010, 698, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Burney, S.; Caulfield, J.; Niles, J.; Wishnok, J.; Tannenbaum, S. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 1999, 424, 37–49. [Google Scholar] [CrossRef]
- Wink, D.A.; Vodovotz, Y.; Laval, J.; Laval, F.; Dewhirst, W.; Mitchell, J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis 1998, 19, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Felley-Bosco, E. Role of nitric oxide in genotoxicity: Implication for carcinogenesis. Cancer Metastasis Rev. 1998, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xue, H.; Liu, S.; He, Y.; Fu, J.; Zhou, Z. Genotoxicity of nitric oxide produced from sodium nitroprusside. Mutat. Res. 1998, 339, 73–89. [Google Scholar] [CrossRef]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate, M.J. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res. 1990, 245, 245–249. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dos Santos, J.L.; Longhin Bosquesi, P.; Varanda, E.A.; Moreira Lima, L.; Chung, M.C. Assessment of the In Vivo Genotoxicity of New Lead Compounds to Treat Sickle Cell Disease. Molecules 2011, 16, 2982-2989. https://doi.org/10.3390/molecules16042982
Dos Santos JL, Longhin Bosquesi P, Varanda EA, Moreira Lima L, Chung MC. Assessment of the In Vivo Genotoxicity of New Lead Compounds to Treat Sickle Cell Disease. Molecules. 2011; 16(4):2982-2989. https://doi.org/10.3390/molecules16042982
Chicago/Turabian StyleDos Santos, Jean Leandro, Priscila Longhin Bosquesi, Eliana Aparecida Varanda, Lídia Moreira Lima, and Man Chin Chung. 2011. "Assessment of the In Vivo Genotoxicity of New Lead Compounds to Treat Sickle Cell Disease" Molecules 16, no. 4: 2982-2989. https://doi.org/10.3390/molecules16042982
APA StyleDos Santos, J. L., Longhin Bosquesi, P., Varanda, E. A., Moreira Lima, L., & Chung, M. C. (2011). Assessment of the In Vivo Genotoxicity of New Lead Compounds to Treat Sickle Cell Disease. Molecules, 16(4), 2982-2989. https://doi.org/10.3390/molecules16042982



