Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proanthocyanidin composition of Bordeaux CS and M grapes (vintages 2006 to 2009)
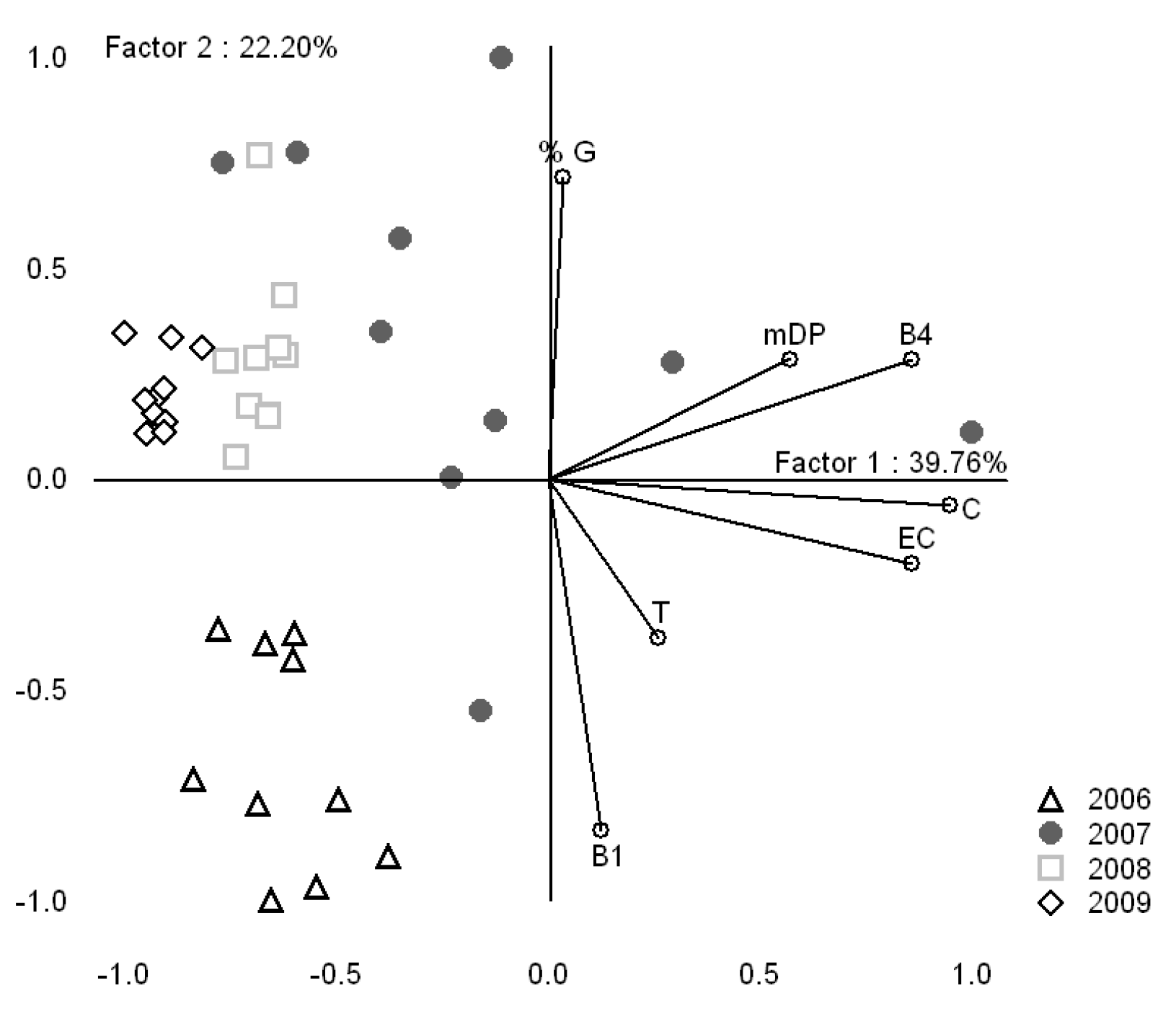
2.1.1. Grape variety effect on proanthocyanin composition of Bordeaux grapes
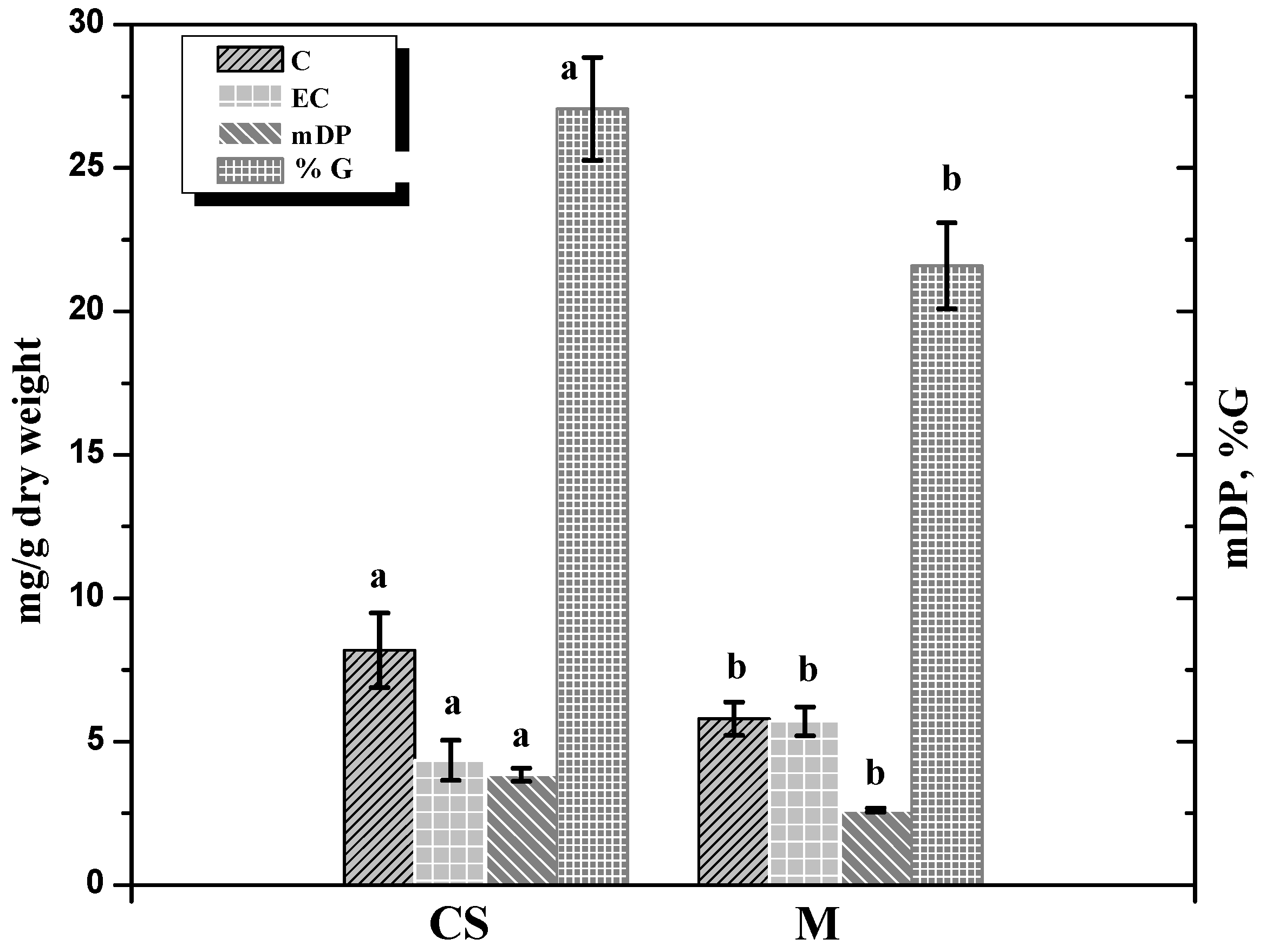
| Merlot-Seeds | Cabernet-Sauvignon-Seeds | |||||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2006 | 2007 | 2008 | 2009 | |
| C* | 5.107b** | 11.726c | 3.618ab | 1.806a | 8.468a | 17.415b | 3.968a | 2.048a |
| StdErr | 0.842 | 0.707 | 0.254 | 0.206 | 0.984 | 3.612 | 0.208 | 0.346 |
| EC | 6.067b | 10.652c | 3.062a | 2.354a | 5.166ab | 8.537b | 2.065a | 1.615a |
| StdErr | 0.924 | 0.171 | 0.219 | 0.207 | 0.864 | 2.036 | 0.163 | 0.227 |
| ECG | 0.789a | 2.622b | 0.2689a | 0.271a | 0.470a | 2.28b | 0.205a | 0.090a |
| StdErr | 0.244 | 0.759 | 0.028 | 0.014 | 0.053 | 0.145 | 0.038 | 0.020 |
| B1 | 3.179b | 0.638a | 0.143a | 0.150a | 4.386c | 0.712b | 0.112a | 0.228ab |
| StdErr | 0.307 | 0.031 | 0.019 | 0.058 | 0.295 | 0.181 | 0.018 | 0.092 |
| B2 | 1.230a | 3.778b | 1.121a | 0.588a | 1.080a | 6.727b | 0.990a | 0.791a |
| StdErr | 0.143 | 0.391 | 0.127 | 0.039 | 0.149 | 1.740 | 0.118 | 0.264 |
| B3 | 0.672b | 1.283c | 0.066a | 0.323a | 1.499b | 1.675b | 0.087a | 0.275a |
| StdErr | 0.112 | 0.105 | 0.008 | 0.064 | 0.119 | 0.475 | 0.024 | 0.080 |
| B4 | 0.702a | 2.748b | 2.625b | 0.384a | 0.358a | 3.742c | 2.137b | 0.527ab |
| StdErr | 0.153 | 0.142 | 0.222 | 0.053 | 0.056 | 0.758 | 0.165 | 0.095 |
| T | 0.806a | 3.477b | 0.080a | 0.120a | 2.298b | 0.091a | 0.103a | 0.044a |
| StdErr | 0.116 | 1.114 | 0.038 | 0.035 | 0.488 | 0.031 | 0.020 | 0.009 |
| mDP | 2.407a | 2.806b | 2.962b | 2.091a | 3.620a | 5.564b | 3.377a | 2.310a |
| StdErr | 0.051 | 0.155 | 0.100 | 0.048 | 0.295 | 0.543 | 0.195 | 0.078 |
| %G | 9.279a | 34.468c | 19.633b | 22.518b | 18.700a | 35.153b | 25.174ab | 27.160ab |
| StdErr | 1.541 | 2.345 | 1.552 | 0.869 | 1.701 | 4.683 | 2.599 | 1.000 |
2.1.2. Vintage effect on proanthocyanidin composition of Bordeaux grapes
| Merlot-Skins | Cabernet-Sauvignon-Skins | |||||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2006 | 2007 | 2008 | 2009 | |
| C* | 0.312b** | 0.243b | 0.025a | 0.032a | 0.127a | 0.762b | 0.045a | 0.018a |
| StdErr | 0.049 | 0.063 | 0.002 | 0.005 | 0.017 | 0.108 | 0.011 | 0.003 |
| EC | 0.031a | 0.481b | 0.014a | 0.029a | 0.098a | 0.625b | 0.036a | 0.006a |
| StdErr | 0.006 | 0.079 | 0.002 | 0.008 | 0.011 | 0.196 | 0.009 | 0.002 |
| B1 | 0.015a | 0.082a | 0.074a | 0.027a | 0.034a | 0.036a | 0.070a | 0.007a |
| StdErr | 0.002 | 0.038 | 0.005 | 0.003 | 0.010 | 0.023 | 0.015 | 0.002 |
| B3 | 0.014a | 0.484b | 0.070a | 0.009a | 0.085a | 1.502a | 0.158a | 0.004a |
| StdErr | 0.004 | 0.166 | 0.008 | 0.001 | 0.017 | 0.708 | 0.022 | 0.001 |
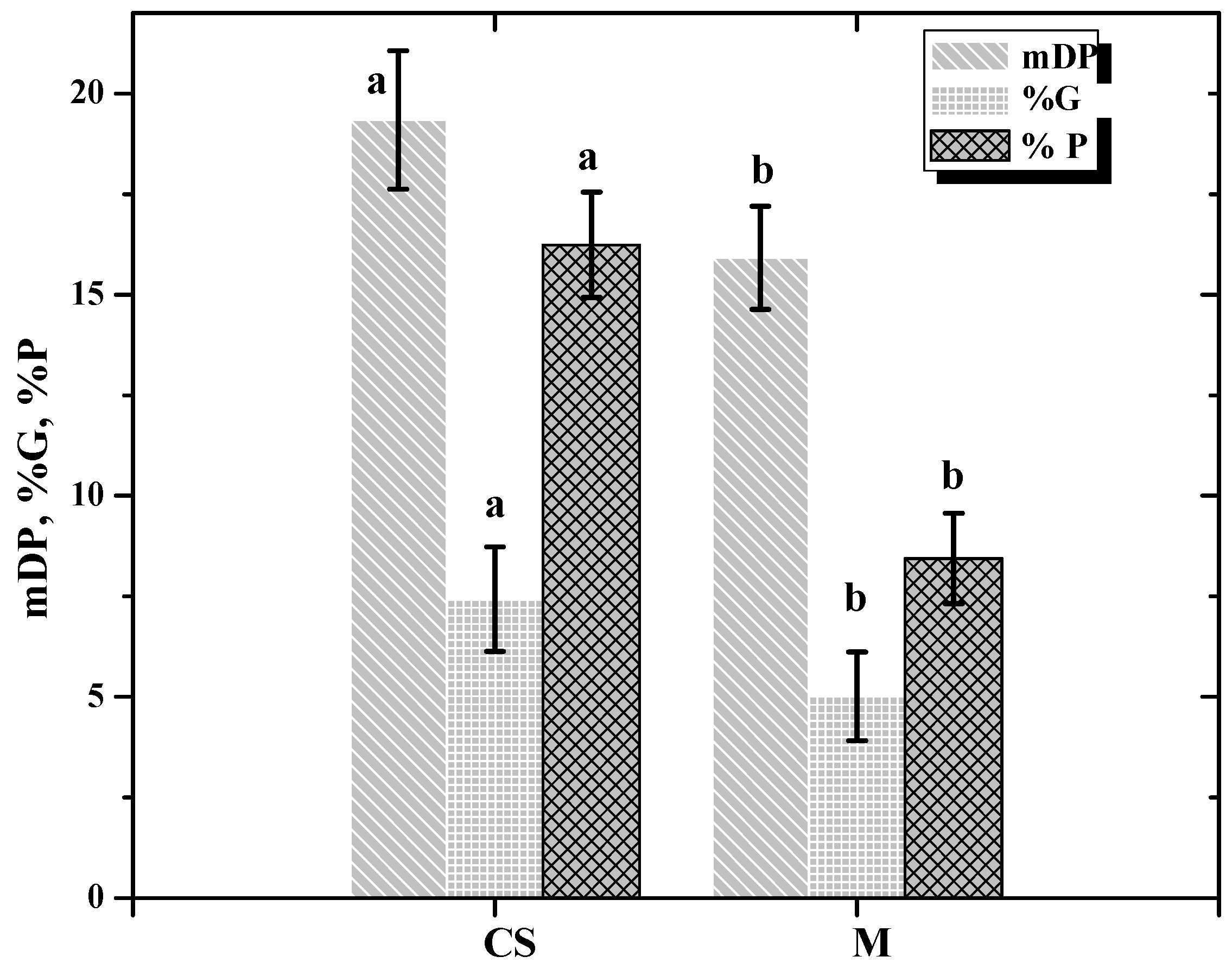
| mDP | 24.117c | 15.113b | 16.566b | 3.910a | 21.955b | 26.623b | 19.882b | 3.97a |
| StdErr | 1.997 | 1.970 | 1.823 | 0.331 | 1.597 | 3.762 | 2.299 | 0.316 |
| %G | 1.405a | 1.398a | 1.086a | 21.360b | 2.721a | 4.388a | 2.656a | 23.800b |
| StdErr | 0.225 | 0.183 | 0.118 | 1.427 | 0.287 | 0.698 | 0.330 | 2.660 |
| %P | 2.419a | 3.528a | 9.950b | 22.730c | 10.191a | 11.012a | 23.508b | 25.880b |
| StdErr | 0.266 | 0.643 | 0.716 | 2.284 | 1.217 | 1.813 | 1.628 | 2.400 |
2.2. Proanthocyanidin composition of Mediterranean grapes (2009 Vintage)
2.2.1. Grape variety effect on the proanthocyanidin composition of Mediterranean grapes
| CS* | M | Gre1 | Gre2 | Syrah | Carignan | Mour | Counoise | |
|---|---|---|---|---|---|---|---|---|
| C** | 2.048a*** | 1.806a | 2.330a | 1.141a | 1.807a | 0.700a | 0.433a | 2.205a |
| StdErr | 0.346 | 0.206 | 0.001 | 0.127 | 0.000 | 0.009 | 0.163 | 0.036 |
| EC | 1.615abc | 2.354c | 1.008abc | 0.912abc | 2.313abc | 0.615ab | 0.377a | 2.516bc |
| StdErr | 0.227 | 0.207 | 0.000 | 0.105 | 0.000 | 0.002 | 0.139 | 0.055 |
| B1 | 0.228a | 0.150a | 0.145a | 0.131a | 0.129a | 0.102a | 0.081a | 0.139a |
| StdErr | 0.092 | 0.058 | 0.003 | 0.015 | 0.000 | 0.007 | 0.029 | 0.004 |
| B2 | 0.791a | 0.588a | 0.432a | 0.428a | 0.338a | 0.373a | 0.183a | 0.567a |
| StdErr | 0.264 | 0.039 | 0.000 | 0.053 | 0.000 | 0.006 | 0.066 | 0.008 |
| B3 | 0.275a | 0.323a | 0.259a | 0.175a | 0.136a | 0.128a | 0.112a | 0.144a |
| StdErr | 0.080 | 0.064 | 0.001 | 0.017 | 0.000 | 0.010 | 0.041 | 0.000 |
| B4 | 0.527a | 0.384a | 0.189a | 0.174a | 0.153a | 0.153a | 0.110a | 0.190a |
| StdErr | 0.095 | 0.053 | 0.001 | 0.016 | 0.000 | 0.002 | 0.042 | 0.004 |
| mDP | 2.310ab | 2.091a | 2.732bc | 2.600bc | 2.242ab | 3.106c | 4.558d | 2.112ab |
| StdErr | 0.078 | 0.048 | 0.017 | 0.096 | 0.032 | 0.052 | 0.206 | 0.000 |
| %G | 27.160b | 22.518a | 37.485c | 34.898c | 35.269c | 36.051c | 51.884d | 28.697abc |
| StdErr | 1.000 | 0.869 | 0.856 | 1.448 | 1.499 | 0.085 | 0.708 | 0.361 |
2.2.2. Comparison of proanthocyanidin composition between Bordeaux and Mediterranean grapes
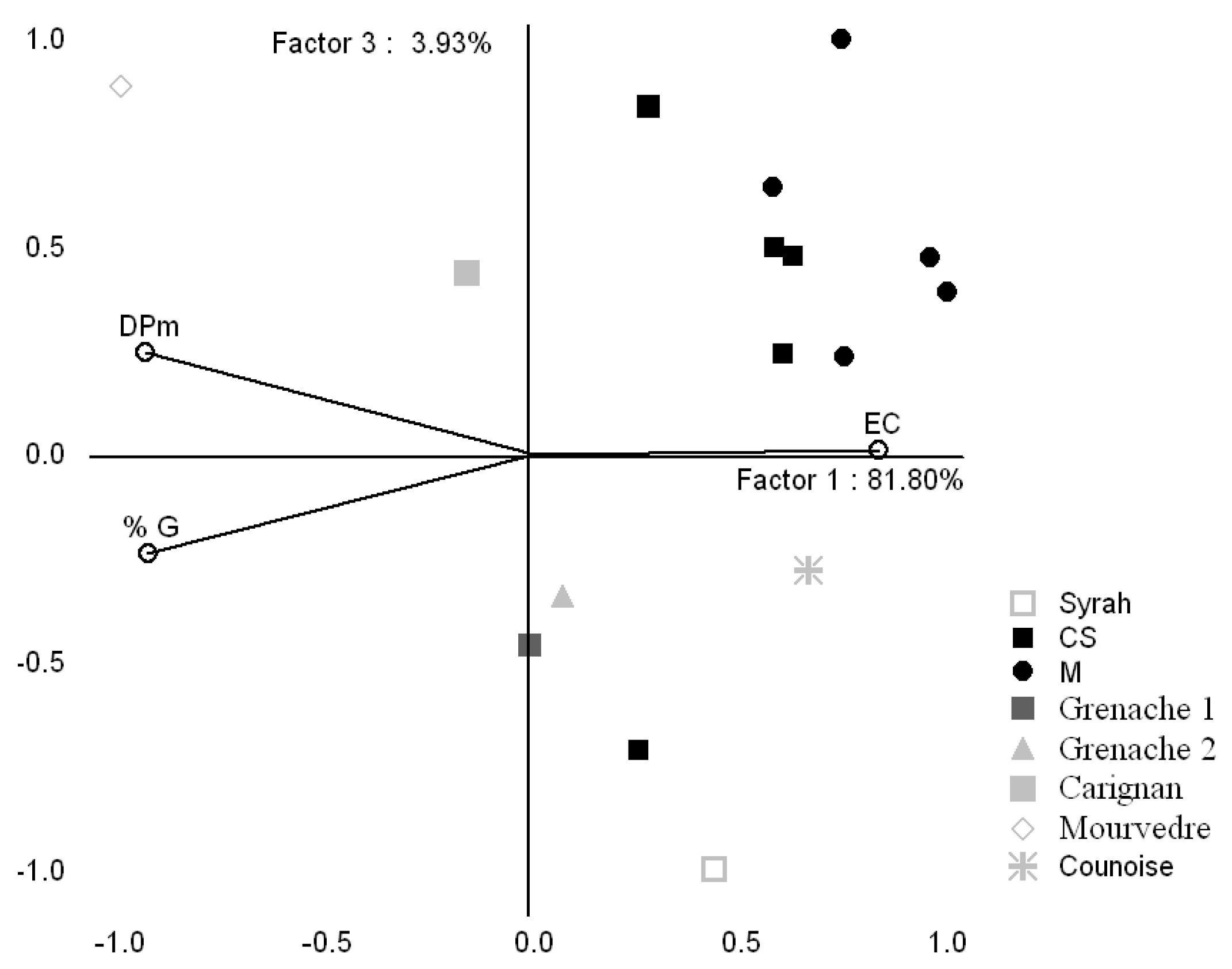
| CS* | M | Gre1 | Gre2 | Syrah | Carignan | Mour | Counoise | |
|---|---|---|---|---|---|---|---|---|
| C** | 0.018a | 0.032a | 0.144c | 0.014a | 0.276d | 0.076b | 0.035ab | 0.030a |
| StdErr | 0.003 | 0.005 | 0.007 | 0.001 | 0.016 | 0.014 | 0.008 | 0.002 |
| EC | 0.006a | 0.029ab | 0.056ab | 0.001ab | 0.079b | 0.021ab | 0.013ab | 0.016ab |
| StdErr | 0.002 | 0.008 | 0.004 | 0.000 | 0.059 | 0.005 | 0.002 | 0.001 |
| B1 | 0.007a | 0.027a | 0.050a | 0.017a | 0.919b | 0.037a | 0.029a | 0.021a |
| StdErr | 0.002 | 0.003 | 0.008 | 0.001 | 0.062 | 0.007 | 0.010 | 0.001 |
| B3 | 0.004a | 0.009bc | 0.021d | 0.003a | 0.027d | 0.012c | 0.005ab | 0.003a |
| StdErr | 0.001 | 0.001 | 0.003 | 0.000 | 0.002 | 0.003 | 0.001 | 0.000 |
| mDP | 3.970ab | 3.910ab | 5.682b | 4.052ab | 1.674a | 1.366a | 5.343b | 2.816ab |
| StdErr | 0.316 | 0.331 | 0.278 | 1.909 | 0.023 | 0.042 | 0.537 | 0.019 |
| %G | 23.800a | 21.360a | 29.863ab | 28.299a | 29.917ab | 18.851a | 18.317a | 50.666b |
| StdErr | 2.660 | 1.427 | 8.463 | 1.025 | 0.206 | 2.735 | 1.131 | 0.733 |
| %P | 25.880c | 22.730bc | 47.411d | 16.15abc | 11.269abc | 8.550ab | 52.120d | 5.754a |
| StdErr | 2.400 | 2.284 | 5.077 | 1.056 | 1.098 | 0.383 | 0.825 | 0.237 |

3. Experimental
3.1. Reagents
3.2. Selection of experimental area and samples
3.3. Samples collection and preparation
3.4. Proanthocyanidins analysis
3.4.1. Fractionation of seeds and skinsproanthocyanidins
3.4.2. HPLC Analysis of monomeric and oligomeric flavan-3-ols
3.4.3.HPLC-MSanalysis of mean degree of polymerization (mDP), %P, %G
3.5. Data analysis
4. Conclusions
Acknowledgements
References
- CIVB, Volet Viticole: Charte Terroir; CIVB: Bordeaux, France, 2006.
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar]
- Souquet, J.M.; Cheynier, V.; Brossaud, F.; Moutounet, M. Polymeric proanthocyanidins from grape skins. Phytochemistry 1996, 43, 509–512. [Google Scholar]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agr. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 73–95. [Google Scholar]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta 2002, 458, 147–155. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agr. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Viticult. 2006, 57, 298–305. [Google Scholar]
- Obreque-Slier, E.; Pena-Neira, A.; Lopes-Solis, R.; Zamora-Marin, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative Study of the Phenolic Composition of Seeds and Skins from Carménère and Cabernet Sauvignon Grape Varieties (Vitis vinifera L.) during Ripening. J. Agr. Food Chem. 2010, 58, 3591–3599. [Google Scholar]
- Hernández-Jiménez, A.; Gómez-Plaza, E.; Martínez-Cutillas, A.; Kennedy, J.A. Grape Skin and Seed Proanthocyanidins from Monastrell x Syrah Grapes. J. Agr. Food Chem. 2009, 57, 10798–10803. [Google Scholar]
- Monagas, M.; Gomez-Cordoves, C.; Bartolome, B.; Laureano, O.; Ricardo Da Silva, J.M. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agr. Food Chem. 2003, 51, 6475–6481. [Google Scholar]
- Bozan, B.; Tosun, G.; Özcan, D. Study of polyphenol content in the seeds of red grape (Vitis vinifera L.) varieties cultivated in Turkey and their antiradical activity. Food Chem. 2008, 109, 426–430. [Google Scholar] [CrossRef]
- Sun, B.S.; Pinto, T.; Leandro, M.C.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. Am. J. Enol. Viticult. 1999, 50, 179–184. [Google Scholar]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Lorrain, B.; Chira, K.; Teissedre, P.-L. Phenolic composition of Merlot and Cabernet-Sauvignon grapes from Bordeaux vineyard for the 2009-vintage: Comparison to 2006, 2007 and 2008 vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef]
- Tounsi, M.S.; Ouerghemmi, I.; Wannes, W.A.; Ksouri, R.; Zemni, H.; Marzouk, B.; Kchouk, M.E. Valorization of three varieties of grape. Ind. Crops Prod. 2009, 30, 292–296. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-Da-Silva, J.M.; Laureano, O. Tannin profiles of Vitis vinifera L. cv. red grapesa growing in Lisbon and from their monovarietal wines. Food Chem. 2009, 112, 197–204. [Google Scholar] [CrossRef]
- Guendez, R.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Tarascou, I.; Barathieu, K.; Andre, Y.; Pianet, I.; Dufourc, E.J.; Fouquet, E. An improved synthesis of procyanidin dimers: Regio- and stereocontrol of the interflavan bond. Eur. J. Org. Chem. 2006, 5367–5377. [Google Scholar]
- Drinkine, J.; Lopes, P.; Kennedy, J.A.; Teissedre, P.L.; Saucier, C. Ethylidene-bridged flavan-3-ols in red wine and correlation with wine age. J. Agr. Food Chem. 2007, 55, 6292–6299. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Hayasaka, Y.; Vidal, S.; Waters, E.J.; Jones, G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agr. Food Chem. 2001, 49, 5348–5355. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chira, K.; Lorrain, B.; Ky, I.; Teissedre, P.-L. Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties. Molecules 2011, 16, 1519-1532. https://doi.org/10.3390/molecules16021519
Chira K, Lorrain B, Ky I, Teissedre P-L. Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties. Molecules. 2011; 16(2):1519-1532. https://doi.org/10.3390/molecules16021519
Chicago/Turabian StyleChira, Kleopatra, Bénédicte Lorrain, Isabelle Ky, and Pierre-Louis Teissedre. 2011. "Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties" Molecules 16, no. 2: 1519-1532. https://doi.org/10.3390/molecules16021519
APA StyleChira, K., Lorrain, B., Ky, I., & Teissedre, P.-L. (2011). Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties. Molecules, 16(2), 1519-1532. https://doi.org/10.3390/molecules16021519




