Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes
Abstract
:1. Introduction

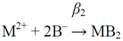
2. Methods
2.1. Calculation of topological indices

 (M),
(M),  (N), and
(N), and  (O).
(O).
 (M),
(M),  (N), and
(N), and  (O).
(O).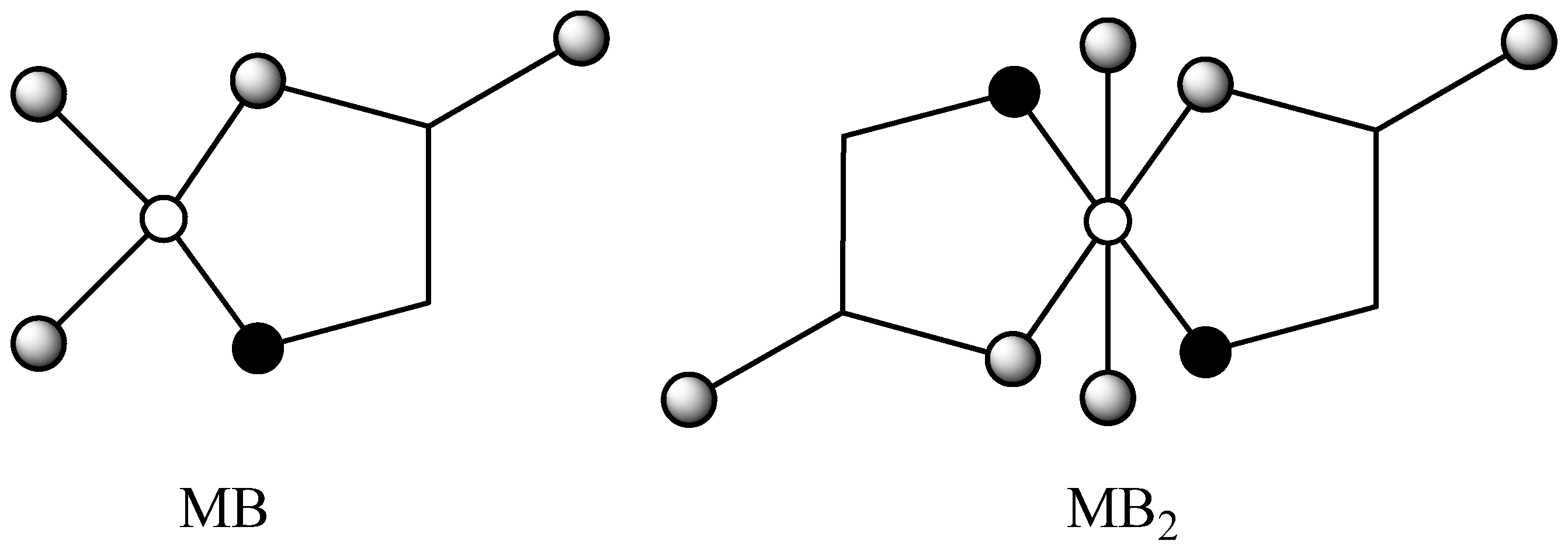
2.2. Regression calculations

2.3. Stability constants selection
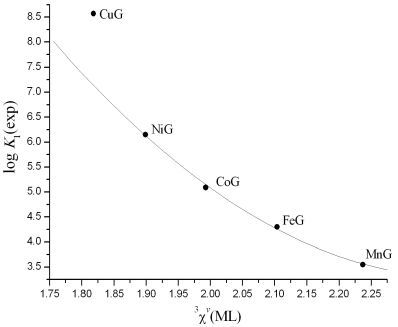
3. Results and Discussion
| Metal/Ligand | log K1 | log β2 | References |
|---|---|---|---|
| Cu/Glycine | 8.57 | 15.63 | [32,33,34,35] |
| Ni/Glycine | 6.15 | 11.15 | [32,33] |
| Co/Glycine | 5.09 | 9.10 | [32,33] |
| Fe/Glycine | 4.30 | 7.80 | [36] |
| Mn/Glycine | 3.55 | 6.63 | [32,33] |
| Cu/Alanine | 8.41 | 15.21 | [32,33,37,38] |
| Ni/Alanine | 5.96 | 10.66 | [33] |
| Co/Alanine | 4.83 | 8.55 | [32,33,39] |
| Fe/Alanine | 7.30 | [39] | |
| Mn/Alanine | 3.13 | 6.05 | [32,33] |
| Cu/Valine | 7.93 | 14.45 | [32] |
| Co/Valine | 4.57 | 8.24 | [32] |
| Fe/Valine | 6.80 | [39] | |
| Mn/Valine | 2.84 | 5.56 | [32] |
| Cu/Leucine | 7.89 | 14.34 | [32] |
| Ni/Leucine | 5.62 | 10.18 | [40] |
| Co/Leucine | 4.52 | 8.16 | [32,40] |
| Mn/Leucine | 2.78 | 5.45 | [32] |


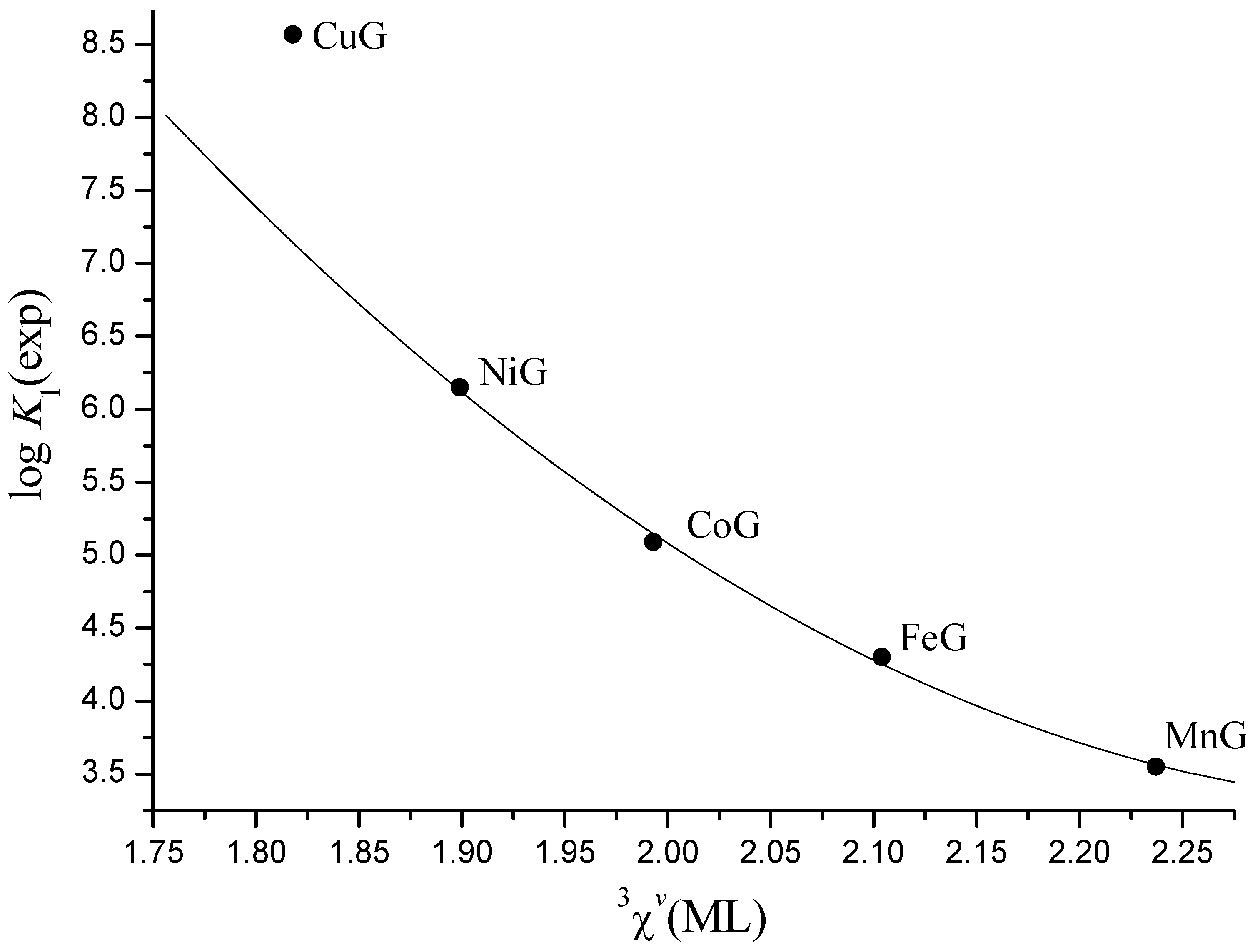
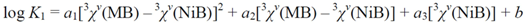
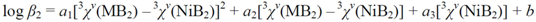
| Eq. | N | Dependentvariable | Regression coefficients | Intercept (S.E.) | r | S.E. | S.E.cv | ||
|---|---|---|---|---|---|---|---|---|---|
| a1(S.E.) | a2(S.E.) | a3(S.E.) | |||||||
| (6) | 12 | log K1 | 12.2(14) | −12.10(55) | −0.676(46) | 7.49(12) | 0.999 | 0.05 | 0.08 |
| (7) | 14 | log β 2 | 3.10(33) | −7.66(36) | −0.646(52) | 14.58(33) | 0.998 | 0.11 | 0.15 |
| Metal/Ligand | log K1 (cv) | log β2 (cv) | 3χv(MB) | 3χv(MB2) |
|---|---|---|---|---|
| Ni/Glycine | 6.21 | 11.11 | 1.90 | 5.37 |
| Co/Glycine | 5.18 | 9.25 | 1.99 | 5.65 |
| Fe/Glycine | 4.24 | 7.65 | 2.10 | 5.97 |
| Mn/Glycine | 3.51 | 6.58 | 2.24 | 6.36 |
| Ni/Alanine | 5.93 | 10.69 | 2.32 | 6.02 |
| Co/Alanine | 4.79 | 8.76 | 2.42 | 6.30 |
| Fe/Alanine | 7.13 | 2.55 | 6.64 | |
| Mn/Alanine | 3.09 | 6.10 | 2.70 | 7.05 |
| Ni/Valine | 2.75 | 6.89 | ||
| Co/Valine | 4.59 | 8.32 | 2.85 | 7.15 |
| Fe/Valine | 6.74 | 2.96 | 7.47 | |
| Mn/Valine | 2.91 | 5.64 | 3.10 | 7.85 |
| Ni/Leucine | 5.57 | 10.01 | 2.85 | 7.08 |
| Co/Leucine | 4.50 | 8.16 | 2.95 | 7.35 |
| Mn/Leucine | 2.81 | 5.48 | 3.20 | 8.06 |


4. Conclusions
Acknowledgment
References
- Irving, H.; Williams, R.J.P. Order of stability of metal complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar]
- Cannon, R.D. Stabilities of chromium(II) complexes. J. Inorg. Nucl. Chem. 1976, 38, 1222–1223. [Google Scholar] [CrossRef]
- Vinokurov, E.G.; Bondar, V.V. Prediction of stability constants for Cr(III) and Cr(II) complexes. Russ. J. Coord. Chem. 2003, 29, 66–72. [Google Scholar] [CrossRef]
- Miličević, A.; Raos, N. Prediction of stability constants. In Handbook of Inorganic Chemistry Research; Morrison D.A., Ed., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2010; pp. 267–294. [Google Scholar]
- Raos, N.; Miličević, A. Estimation of stability constants of coordination compounds using models based on topological indices. Arh. Hig. Rada Toksikol. 2009, 60, 123–128. [Google Scholar] [CrossRef]
- Miličević, A.; Raos, N. Estimation of stability constants of copper(II) and nickel(II) chelates with dipeptides by using topological indices. Polyhedron 2008, 27, 887–892. [Google Scholar] [CrossRef]
- Miličević, A.; Raos, N. Prediction of stability of copper(II) and nickel(II) complexes with fructose-amino acids from the molecular-graph models developed on amino-acid chelates. Croat. Chem. Acta 2007, 80, 557–563. [Google Scholar]
- Trinajstić, N. Chemical Graph Theory, 2nd ed; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Janežić, D.; Miličević, A.; Nikolić, S.; Trinajstić, N. Graph-Theoretical Matrices in Chemistry. In Mathematical Chemistry Monographs; Gutman, I., Ed.; University of Kragujevac and Faculty of Science: Kragujevac, Serbia, 2007; No. 3. [Google Scholar]
- Kier, L.B.; Hall, L.H. Molecular Connectivity and Drug Design; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Graovac, A.; Gutman, I.; Trinajstić, N. Topological Approach to the Chemistry of Conjugated Molecules. In Lecture Notes in Chemistry; Springer-Verlag: Berlin, Germany, 1977. [Google Scholar]
- Seybold, P.G.; May, M.; Bengal, U.A. Molecular structure-property relationships. J. Chem. Educ. 1987, 64, 575–581. [Google Scholar] [CrossRef]
- Miličević, A.; Nikolić, S.; Trinajstić, N. Toxicity of aliphatic ethers: A comparative study. Mol. Divers. 2006, 10, 95–99. [Google Scholar] [CrossRef]
- Grgas, B.; Nikolić, S.; Paulić, N.; Raos, N. Estimation of stability constants of copper(II) chelates with N-alkylated amino acids using topological indices. Croat. Chem. Acta 1999, 72, 885–895. [Google Scholar]
- Raos, N.; Branica, G.; Miličević, A. The use of graph-theoretical models to evaluate two electroanalytical methods for determination of stability constants. Croat. Chem. Acta 2008, 81, 511–517. [Google Scholar]
- Miličević, A.; Raos, N. Estimation of stability of coordination compounds by using topological indices. Polyhedron 2006, 25, 2800–2808. [Google Scholar] [CrossRef]
- Miličević, A.; Raos, N. Estimation of stability constants of mixed copper(II) chelates using valence connectivity index of the 3rd order derived from two molecular graph representations. Acta Chim. Slov. 2009, 56, 373–378. [Google Scholar]
- Miličević, A.; Raos, N. Estimation of stability constants of copper(II) chelates with triamines and their mixed complexes with amino acids by using topological indices and the overlapping spheres method. Polyhedron 2007, 26, 3350–3356. [Google Scholar] [CrossRef]
- Miličević, A.; Raos, N. Estimation of stability constants with connectivity index: Development of bivariate and multivariate linear models for copper(II) chelates with oligopeptides. Croat. Chem. Acta 2009, 82, 633–639. [Google Scholar]
- Miličević, A.; Raos, N. Estimation of stability constants of cadmium(II) bis-complexes with amino acids by model based on 3χv connectivity index. Acta Chim. Slov. 2010, 57, 866–871. [Google Scholar]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; Tanchuk, V.Y.; Prokopenko, V.V. Virtual computational chemistry laboratory: design and description. J. Comput-Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- VCCLAB, Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org (accessed on 1 September 2010).
- Todeschini, R.; Consonni, V. Handbook of Molecular Descriptors. In Methods and Principles in Medicinal Chemistry; Mannhold, R., Kubinyi, H., Timmerman, H., Eds.; Wiley-VCH: Weinheim, Germany, 2000; Volume 11. [Google Scholar]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics. In Methods and Principles in Medicinal Chemistry, 2nd; Mannhold, R., Kubinyi, H., Folkers, G., Eds.; Wiley-VCH: Weinheim, Germany, 2009; Volume 41. [Google Scholar]
- Available online: http://cactus.nci.nih.gov/services/translate/ (Accessed on 1 September 2010).
- Kier, L.B.; Hall, L.H. Molecular connectivity. J. Pharm. Sci. 1976, 65, 1806–1809. [Google Scholar] [CrossRef]
- Kier, L.B.; Hall, L.H. Molecular Connectivity in Structure-Activity Analysis; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Randić, M. On history of the Randić index and emerging hostility toward chemical graph theory. MATCH Commun. Math. Comput. Chem. 2008, 59, 5–124. [Google Scholar]
- Miličević, A.; Raos, N. Influence of chelate ring interactions on copper(II) chelate stability studied by connectivity index functions. J. Phys. Chem. A 2008, 112, 7745–7749. [Google Scholar] [CrossRef]
- Lučić, B.; Trinajstić, N. Multivariate regression outperforms several robust architectures of neural networks in QSAR modelling. J. Chem. Inf. Comput. Sci. 1999, 39, 121–132. [Google Scholar] [CrossRef]
- Maley, L.; Mellor, D. Metal derivatives of 8-hydroxyquinoline 5-sulphonic acid and series of monocarboxylic mono-α-amino acids including histidine. Australian J. Sci. Res. A 1949, 92, 579–594. [Google Scholar]
- Monk, C. Electolytes in solution of amino acids. Trans. Faraday Soc. 1951, 47, 285-291, 297-302. [Google Scholar] [CrossRef]
- Anderson, K.P.; Greenhalgh, W.O; Izatt, R.M. Formation constants and enthalpy and entropy values for the association of H+ and Cu2+ with glycinate and phenylalanate ions in aqueous solution at 10, 25, and 40°. Inorg. Chem. 1966, 5, 2106–2109. [Google Scholar] [CrossRef]
- Izatt, R.M.; Johnson, H.D.; Christensen, J.J. Log Ki, ΔH°i, and ΔS°i values for the interaction of glycinate ion with H+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+ at 10, 25, and 40°. J. Chem. Soc. Dalton Trans. 1972, 1152–1157. [Google Scholar]
- Albert, A. Quantitative studies of the avidity of naturally occurring substances for trace metals. Biochem. J. 1953, 54, 646–650. [Google Scholar]
- Neilands, J.B. Metal and hydrogen-ion binding properties of cycloserine. Arch. Biochem. Biophys. 1956, 62, 151–162. [Google Scholar] [CrossRef]
- Anderson, K.P.; Newel, D.A.; Izatt, R.M. Formation constant, enthalpy, and entropy values for the association of alanine with H+ and Cu2+ at 10, 25, and 40°. Inorg. Chem. 1966, 5, 62–65. [Google Scholar] [CrossRef]
- Albert, A. Quantitative studies on the avidity of naturally occurring substances for trace metals. J. Biochem. 1950, 47, 531–540. [Google Scholar]
- Datta, S.; Leberman, R.; Rabin, B. The chelation of metal ions by dipeptides and related substances. Trans. Faraday Soc. 1959, 55, 1982-1987, 2141-2151. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miličević, A.; Branica, G.; Raos, N. Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes. Molecules 2011, 16, 1103-1112. https://doi.org/10.3390/molecules16021103
Miličević A, Branica G, Raos N. Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes. Molecules. 2011; 16(2):1103-1112. https://doi.org/10.3390/molecules16021103
Chicago/Turabian StyleMiličević, Ante, Gina Branica, and Nenad Raos. 2011. "Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes" Molecules 16, no. 2: 1103-1112. https://doi.org/10.3390/molecules16021103
APA StyleMiličević, A., Branica, G., & Raos, N. (2011). Irving-Williams Order in the Framework of Connectivity Index 3χv Enables Simultaneous Prediction of Stability Constants of Bivalent Transition Metal Complexes. Molecules, 16(2), 1103-1112. https://doi.org/10.3390/molecules16021103