Direct One-Pot Synthesis of Primary 4-Amino-2,3-diaryl-quinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-azido-3-iodoquinolines with Arylboronic Acids
Abstract
:1. Introduction
2. Results and Discussion
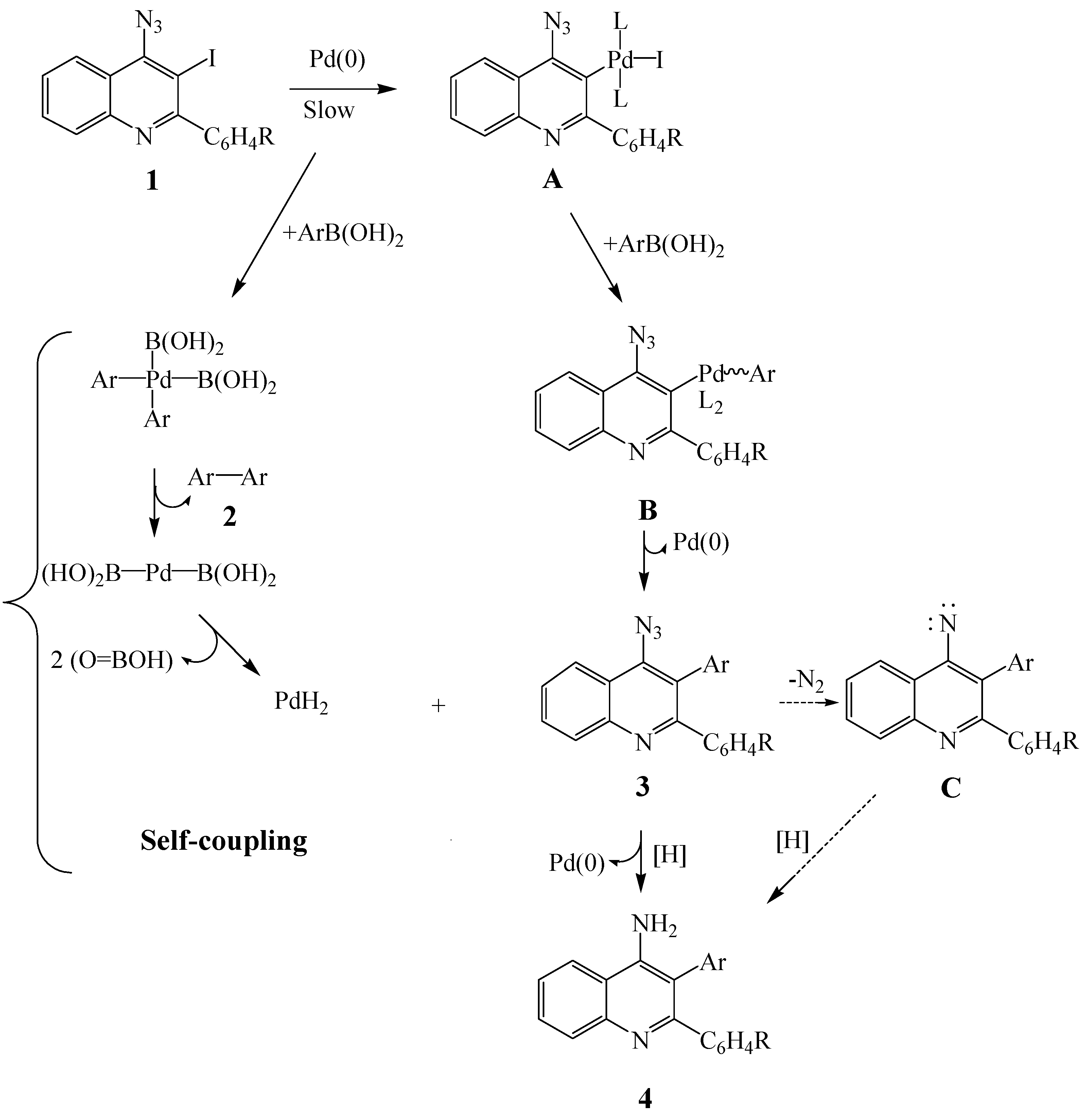
 SPd(0)(PPh3)2 + PPh3 (K2/[PPh3] << 1); S = solvent} to afford the low reactivity ligated 14-electron species (Pd(0)(PPh3)2) [24]. Conversely, the oxidative addition performed by the palladium(0) complex (Pd(0)(PPh3)2Cl−) generated by the reduction of dichlorobis(triphenylphosphine)palladium(II) (PdCl2(PPh3)2) is reported to be more than 30 times faster than that performed from Pd(0)(PPh3)4 [24]. Likewise, alkylphosphine ligands are known to coordinate with palladium and increase its electron density more than arylphosphines and, in turn, accelerate the oxidative addition and reductive elimination steps in the catalytic cycle [27,28]. Consequently, we subjected substrates 1a–d to 2 equiv. of phenylboronic acid in the presence of PdCl2(PPh3)2-tricyclohexylphosphine (PCy3) catalyst mixture and 2 M potassium carbonate in DMF under reflux (Scheme 1). The reaction in the presence of PdCl2(PPh3)2-PCy3 catalyst mixture was complete within 18 h. Analysis of the crude product mixtures by thin layer chromatography revealed in all cases three spots of different polarity and intensity with no traces of the spot corresponding to the starting material. The mixture was isolated by column chromatography on silica gel to afford the biphenyl 2a, 4-azido-2,3-diarylquinolines 3a–d (minor) and 4-amino-2,3-diarylquinolines 4a–d (major) in sequence. The reaction conditions were also extended to include 4-fluorophenylboronic and 4-methoxyphenylboronic acids as coupling partners. Although in all cases, traces of the 4-azido-2,3-diarylquinolines 3 (2nd spot) were detected by thin layer chromatography in the crude product mixture, careful column chromatographic separation on silica gel in most cases led to isolation of the self-coupled biaryl derivatives 2b,c (minor) and the 4-amino-2,3-diarylquinolines 4a–l as the major products.
SPd(0)(PPh3)2 + PPh3 (K2/[PPh3] << 1); S = solvent} to afford the low reactivity ligated 14-electron species (Pd(0)(PPh3)2) [24]. Conversely, the oxidative addition performed by the palladium(0) complex (Pd(0)(PPh3)2Cl−) generated by the reduction of dichlorobis(triphenylphosphine)palladium(II) (PdCl2(PPh3)2) is reported to be more than 30 times faster than that performed from Pd(0)(PPh3)4 [24]. Likewise, alkylphosphine ligands are known to coordinate with palladium and increase its electron density more than arylphosphines and, in turn, accelerate the oxidative addition and reductive elimination steps in the catalytic cycle [27,28]. Consequently, we subjected substrates 1a–d to 2 equiv. of phenylboronic acid in the presence of PdCl2(PPh3)2-tricyclohexylphosphine (PCy3) catalyst mixture and 2 M potassium carbonate in DMF under reflux (Scheme 1). The reaction in the presence of PdCl2(PPh3)2-PCy3 catalyst mixture was complete within 18 h. Analysis of the crude product mixtures by thin layer chromatography revealed in all cases three spots of different polarity and intensity with no traces of the spot corresponding to the starting material. The mixture was isolated by column chromatography on silica gel to afford the biphenyl 2a, 4-azido-2,3-diarylquinolines 3a–d (minor) and 4-amino-2,3-diarylquinolines 4a–d (major) in sequence. The reaction conditions were also extended to include 4-fluorophenylboronic and 4-methoxyphenylboronic acids as coupling partners. Although in all cases, traces of the 4-azido-2,3-diarylquinolines 3 (2nd spot) were detected by thin layer chromatography in the crude product mixture, careful column chromatographic separation on silica gel in most cases led to isolation of the self-coupled biaryl derivatives 2b,c (minor) and the 4-amino-2,3-diarylquinolines 4a–l as the major products.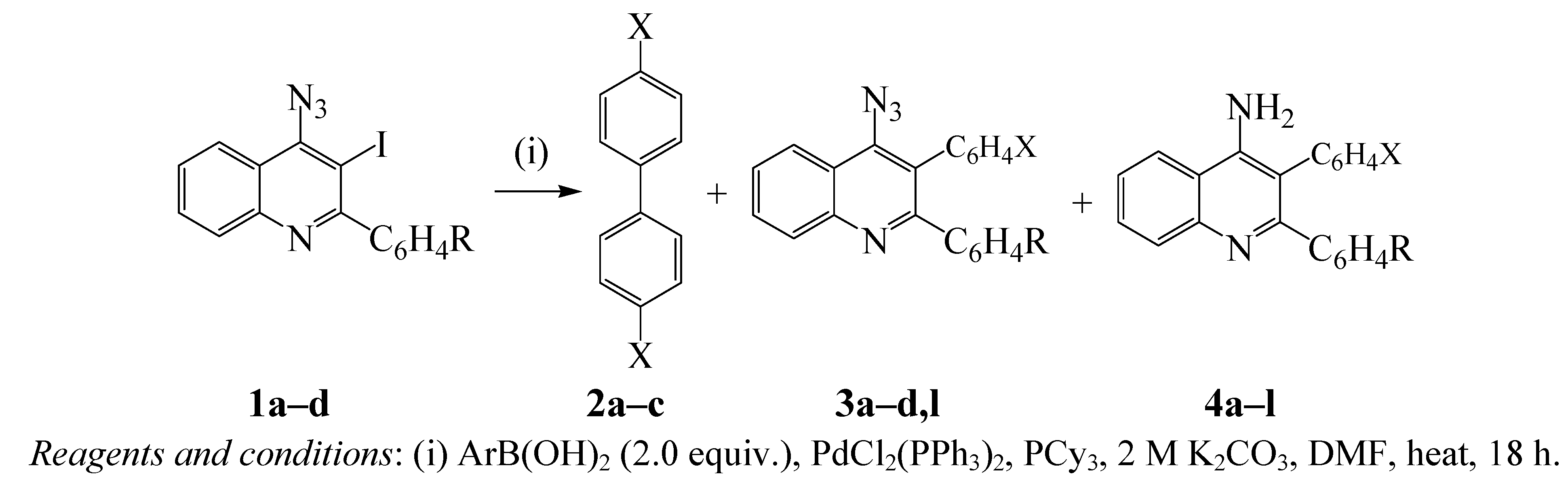
| 3/4 | 4-R | 4-X | % Yield 2 | % Yield 3 | % Yield 4 |
| a | 4-H | 4-H | 24 ( 2a) | 12 | 57 |
| b | 4-F | 4-H | 24 ( 2a) | 16 | 65 |
| c | 4-Cl | 4-H | 24 ( 2a) | 10 | 54 |
| d | 4-OMe | 4-H | 24 ( 2a) | 11 | 66 |
| e | 4-H | 4-F | 20 ( 2b) | - | 65 |
| f | 4-F | 4-F | 20 ( 2b) | - | 66 |
| g | 4-Cl | 4-F | 20 ( 2b) | - | 56 |
| h | 4-OMe | 4-F | 20 ( 2b) | - | 64 |
| i | 4-H | 4-MeO | 17 ( 2c) | - | 63 |
| j | 4-F | 4-MeO | 17 ( 2c) | 57 | |
| k | 4-Cl | 4-MeO | 17 ( 2c) | - | 60 |
| l | 4-OMe | 4-MeO | 17 ( 2c) | 9 | 68 |
3. Experimental
3.1. General
3.2. Typical Procedure for the PdCl2(PPh3)2-PCy3 Catalyzed Cross-Coupling Reactions of 1 with ArB(OH)2
3.2.1. Biphenyl (2a), 4-Azido-2,3-diphenylquinoline (3a) and 4-Amino-2,3-diphenylquinoline (4a)
3.2.2. Biphenyl (2a), 4-Azido-2-(4-fluorophenyl)-3-phenylquinoline (3b) and 4-Amino-2-(4-fluoro-phenyl)-3-phenylquinoline (4b)
3.2.3. Biphenyl (2a), 4-Azido-2-(4-chlorophenyl)-3-phenylquinoline (3c) and 4-Amino-2-(4-chloro-phenyl)-3-phenylquinoline (4c)
3.2.5. 4,4'-Difluoro-1,1'-biphenyl (2b) and 4-Amino-3-(4-fluorophenyl)-2-(phenyl)quinoline (4e)
3.2.6. 1-Fluoro-4-(4-fluorophenyl)benzene (2b) and 4-Amino-2,3-bis(4-fluorophenyl)quinoline (4f)
3.2.7. 1-Fluoro-4-(4-fluorophenyl)benzene (2b) and 4-Amino-2-(4-chlorophenyl)-3-(4-fluorophenyl)quinoline (4g)
3.2.8. 1-Fluoro-4-(4-fluorophenyl)benzene (2b) and 4-Amino-3-(4-fluorophenyl)-2-(4-methoxyphenyl)quinoline (4h)
3.2.9. 1-Methoxy-4-(4-methoxyphenyl)benzene (2c) and 4-Amino-3-(4-methoxyphenyl)-2-phenylquinoline (4i)
3.2.10. 1-Methoxy-4-(4-methoxyphenyl)benzene (2c) and 4-Amino-2-(4-fluorophenyl)-3-(4-methoxyphenyl)-quinoline (4j)
3.2.11. 1-Methoxy-4-(4-methoxyphenyl)benzene (2c) and 4-Amino-2-(4-chlorophenyl)-3-(4-methoxyphenyl)-quinoline (4k)
3.2.12. 1-Methoxy-4-(4-methoxyphenyl)benzene (2c), 4-Azido-2,3-bis(4-methoxyphenyl)quinoline (3l) and 4-Amino-2,3-bis(4-methoxyphenyl)quinoline (4l)
3.3. Typical Procedure for the Pd(OAc)2-Catalyzed Cross-Coupling Reactions of 1c,d with PhB(OH)2
3.3.1. Biphenyl (2a), 4-Azido-2-(4'-chlorophenyl)-3-phenylquinoline (3c) and 4-Amino-2-(4'-chloro-phenyl)quinoline (4c)
3.3.2. Biphenyl (2a), 4-Azido-2-(4'-methoxyphenyl)-3-phenylquinoline (3d) and 4-Amino-2-(4'-methoxyphenyl)quinoline (4d)
4. Conclusions
Acknowledgements
Conflict of Interest
References and Notes
- Wang, M.; Liu, Y.; Huang, Z. Novel and convenient synthesis of polyfunctionalized quinolines, quinolones and their annulation reactions. Tetrahedron Lett. 2001, 42, 2553–2555. [Google Scholar] [CrossRef]
- Hadjeri, M.; Mariotte, A.; Boumendjel, A. Alkylation of 2-phenyl-4-quinolones: Synthetic and structural studies. Chem. Pharm. Bull. 2001, 49, 1352–1355. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Xia, P.; Hackl, T.; Hamel, E.; Mauger, A.; Wu, J.; Lee, K.J. Antitumor agents. 211. Fluorinated 2-phenyl-4-quinolone derivatives as antimitotic antitumor agents. J. Med. Chem. 2001, 44, 3932–3936. [Google Scholar]
- De, S.K.; Gibbs, R.A. A mild and efficient one-step synthesis of quinolines. Tetrahedron Lett. 2005, 46, 1647–1649. [Google Scholar] [CrossRef]
- Moyer, M.P.F.; Weber, H.; Gross, J.L. Structure-activity relationships of imidazo[4,5-f]quinoline partial structures and analogs. Discovery of pyrazolo[3,4-f]quinoline derivatives as potent immunostimulants. J. Med. Chem. 1992, 35, 4595–4601. [Google Scholar]
- Palacios, F.; de Retana, A.M.O.; Oyarzabal, J. A simple synthesis of 3-phosphonyl-4-aminoquinolines from β-enaminophosphonates. Tetrahedron 1999, 55, 5947–5964. [Google Scholar] [CrossRef]
- Maguire, M.P.; Sheets, K.R.; McVety, K.; Spada, A.P.; Zilberstein, A. A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J. Med. Chem. 1994, 37, 2129–2137. [Google Scholar] [CrossRef]
- Pinard, E.; Alanine, A.; Bourson, A.; Büttelmann, B.; Heitz, M.-P.; Mutel, V.; Gill, R.; Trube, G.; Wyler, R. 4-Aminoquinolines as a novel class of NR1/2B subtype selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2002, 12, 2615–2619. [Google Scholar] [CrossRef]
- Paliakov, E.; Strekowski, L. Boron tribromide mediated debenzylation of benzylamino and benzyloxy groups. Tetrahedron Lett. 2004, 45, 4093–4095. [Google Scholar] [CrossRef]
- Strekowski, L.; Janda, L.; Lee, H. Synthesis of bis(2-arylquinolin-4-yl)amines by lithium bis(trimethylsilyl)amide-mediated cyclization of ketimines derived from 2-(trifluoromethyl)anilines and aryl methyl ketones. J. Org. Chem. 1997, 62, 4193–4196. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Elgemeie, G.H.; Kappe, T. Synthesis of some novel azido- and tetrazoloquinoline-3-carbonitriles and their conversion into 2,4-diaminoquinoline-3-carbonitriles. J. Chem. Res. 2005, 82–85. [Google Scholar]
- Mphahlele, M.J.; Gheevargheese, O.; Makhubela, N.F.H. Unprecedented outcome of base-promoted Neber rearrangement of O-mesyl oxime of 2-aryl-1,2,3,4-tetrahydro-1-methylsulfonyl-4-quinolone- synthesis of 4-amino-2-arylquinolines. Phosphor. Sulfur Silicon 2000, 166, 303–314. [Google Scholar] [CrossRef]
- Aizikovich, A.; Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Byk, G.; Gellerman, G. A new application of diphenylphosphorylazide (DPPA) reagent: Convenient transformations of quinolin-4-one, pyridin-4-one and quinazolin-4-one derivatives into the 4-azido and 4-amino counterparts. Tetrahedron Lett. 2004, 45, 4241–4243. [Google Scholar]
- Paliakov, E.; Strekowski, L. Boron tribromide mediated debenzylation of benzylamino and benzyloxy groups. Tetrahedron Lett. 2004, 45, 4093–4095. [Google Scholar] [CrossRef]
- Jones, G. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, A.R., Eds.; Pergamon: Oxford, UK, 1984; Volume 2, p. 395. [Google Scholar]
- Yum, E.K.; Yang, O.-K.; Kang, S.K.; Cheon, H.G.; Kim, S.S.; Choi, J.-K. Synthesis of 4-phenylamino-3-vinylquinoline derivatives as gastric H+/K+-ATPase inhibitors. Bull. Korean Chem. Soc. 2004, 25, 1091–1094. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Mphahlele, M.M. One-pot palladium-catalyzed C–I and C–H bond activation and subsequent Suzuki-Miyaura cross-coupling of 2-aryl-3-iodo-4-(phenylamino)quinolines with arylboronic acids. Tetrahedron 2011, 67, 4689–4695. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Mtshemla, V. 2-Aryl-4-azido-3-(bromo/iodo)quinolines as substrates for the synthesis of primary 4-amino-2,3-disubstituted quinoline derivatives. J. Heterocycl. Chem. 2008, 45, 1343–1350. [Google Scholar] [CrossRef]
- Stockmann, V.; Fiksdahl, A. Preparation of new pyrido[3,4-b]thienopyrroles and pyrido[4,3-e]thienopyridazines. Tetrahedron 2008, 64, 7626–7632. [Google Scholar] [CrossRef]
- Pudlo, M.; Csányi, D.; Moreau, F.; Hajós, G.; Riedl, Z.; Sapi, J. First Suzuki-Miyaura type cross-coupling of ortho-azidobromobenzene with arylboronic acids and its application to the synthesis of fused aromatic indole-heterocycles. Tetrahedron 2007, 63, 10320–10329. [Google Scholar] [CrossRef]
- Joshangani, M.; Faramarzi, E.; Rafiee, E.; Daryanavard, M.; Xiao, J.; Baillie, C. Highly efficient Suzuki coupling using moderately bulky tolylphosphine ligands. J. Mol. Catal. 2007, 273, 310–315. [Google Scholar] [CrossRef]
- Kürti, L.; Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis; Elsevier Academic Press: London, UK, 2005; p. 448. [Google Scholar]
- Slagt, V.F.; de Vries, A.H.M.; de Vries, J.G.; Kellog, R.M. Practical aspects of carbon-carbon cross-coupling reactions using heteroarenes. Org. Process Res. Dev. 2010, 14, 30–47. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar]
- Ali, N.M.; Chattopadhyah, S.K.; McKillop, A.; Perret-Gentil, R.M.; Ozturk, T.; Rebelo, R.A. A short new route to the pyrido[2,3,4-kl]acridine subunit common to pyridoacridine alkaloids of marine origin. J. Chem. Soc. Chem. Commun. 1992, 1453–1454. [Google Scholar]
- Stadlbauer, W.; Laschober, R.; Kappe, T. Potential non-steroidal estrogens and antiestrogens. IV. Organic azides in heterocyclic synthesis. Part 13. Synthesis of aza- and diazacoumestrols via azido derivatives. Monatsh. Chem. 1991, 122, 853–861. [Google Scholar]
- Haman, B.C.; Hartwig, J.F. Tandem ring-closing metathesis transannular cyclization as a route to hydroxylated pyrrolizidines. Asymmetric synthesis of (+)-Australine. J. Am. Chem. Soc. 1998, 120, 7369–7370. [Google Scholar]
- Itoh, T.; Mase, T. Direct synthesis of hetero-biaryl compounds containing an unprotected NH2 group via Suzuki-Miyaura reaction. Tetrahedron Lett. 2005, 46, 3573–3577. [Google Scholar] [CrossRef]
- Wang, H.-S.; Wang, Y.-C.; Pan, Y.-M.; Zhao, S.-L.; Chen, Z.-F. Simultaneous reduction of nitro- to amino-group in the palladium-catalyzed Suzuki cross-coupling reaction. Tetrahedron Lett. 2008, 49, 2634–2637. [Google Scholar]
- Yu, J.Y.; Shreiner, S.; Vaska, L. Homogeneous catalytic production of hydrogen and other molecules from water-DMF solutions. Inorg. Chim. Acta 1990, 170, 145–147. [Google Scholar] [CrossRef]
- Foucher, N.; Ambroise, Y.; Cintrat, J.-C.; Doris, E.; Pillon, F.; Rousseau, B. Highly chemoselective hydrogenolysis of iodoarenes. J. Org. Chem. 2002, 67, 932–934. [Google Scholar] [CrossRef]
- Moreno-Maňas, M.; Pérez, M.; Pleixats, R. Palladium-catalyzed Suzuki-type self-coupling of arylboronic acids. A mechanistic study. J. Org. Chem. 1996, 61, 2346–2351. [Google Scholar] [CrossRef]
- Song, Z.Z.; Wong, H.N.C. Regiospecific synthesis of furan-3,4-diyl oligomers via palladium-catalyzed self-coupling of organoboroxines. J. Org. Chem. 1994, 59, 33–41. [Google Scholar] [CrossRef]
- Hills, I.D.; Fu, G.C. Elucidating reactivity differences in palladium-catalyzed coupling processes: The chemistry of palladium hydrides. J. Am. Chem. Soc. 2004, 126, 13178–13179. [Google Scholar] [CrossRef]
- Zeng, M.; Du, Y.; Qi, C.; Zuo, S.; Li, X.; Shao, L.; Zhang, X.-M. An efficient and recyclable heterogeneous palladium catalyst utilizing naturally abundant pearl shell waste. Green Chem. 2011, 13, 350–356. [Google Scholar] [CrossRef]
- Hajduk, P.J.; Bures, M.; Praestgaard, J.; Fesik, S.W. Privileged molecules for protein binding identified from NMR-based screening. J. Med. Chem. 2000, 43, 3443–3447. [Google Scholar] [CrossRef]
- Faghih, R.; Dwight, W.; Pan, J.B.; Fox, G.B.; Krueger, K.M.; Esbenshade, T.A.; McVey, J.M.; Marsh, K.; Bennani, Y.L.; Hancock, A.A. Synthesis and SAR of aminoalkoxy-biaryl-4-carboxamides: Novel and selective histamine H3 receptor antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 1325–1328. [Google Scholar]
- Look, G.C.; Vacin, C.; Dias, T.M.; Ho, S.; Tran, T.H.; Lee, L.L.; Wiesner, C.; Fang, F.; Marra, A.; Westmacott, D.; et al. The discovery of biaryl acids and amides exhibiting antibacterial activity against Gram-positive bacteria. Bioorg. Med. Chem. Lett. 2004, 14, 1423–1426. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mphahlele, M.J.; Mphahlele, M.M. Direct One-Pot Synthesis of Primary 4-Amino-2,3-diaryl-quinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-azido-3-iodoquinolines with Arylboronic Acids. Molecules 2011, 16, 8958-8972. https://doi.org/10.3390/molecules16118958
Mphahlele MJ, Mphahlele MM. Direct One-Pot Synthesis of Primary 4-Amino-2,3-diaryl-quinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-azido-3-iodoquinolines with Arylboronic Acids. Molecules. 2011; 16(11):8958-8972. https://doi.org/10.3390/molecules16118958
Chicago/Turabian StyleMphahlele, Malose Jack, and Mamasegare Mabel Mphahlele. 2011. "Direct One-Pot Synthesis of Primary 4-Amino-2,3-diaryl-quinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-azido-3-iodoquinolines with Arylboronic Acids" Molecules 16, no. 11: 8958-8972. https://doi.org/10.3390/molecules16118958
APA StyleMphahlele, M. J., & Mphahlele, M. M. (2011). Direct One-Pot Synthesis of Primary 4-Amino-2,3-diaryl-quinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-azido-3-iodoquinolines with Arylboronic Acids. Molecules, 16(11), 8958-8972. https://doi.org/10.3390/molecules16118958




