Two New Epoxysteroids from Helianthus tuberosus
Abstract
:1. Introduction
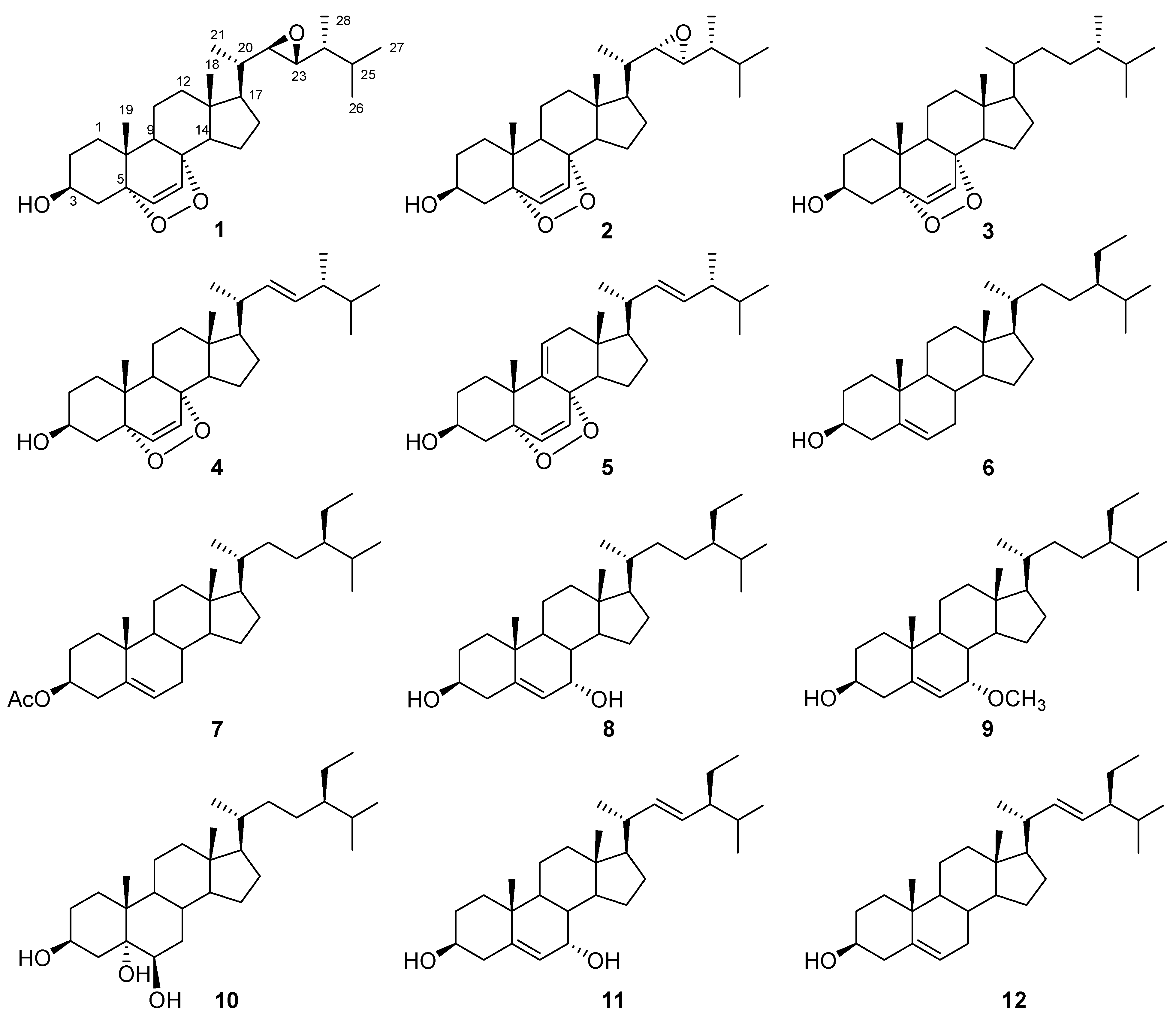
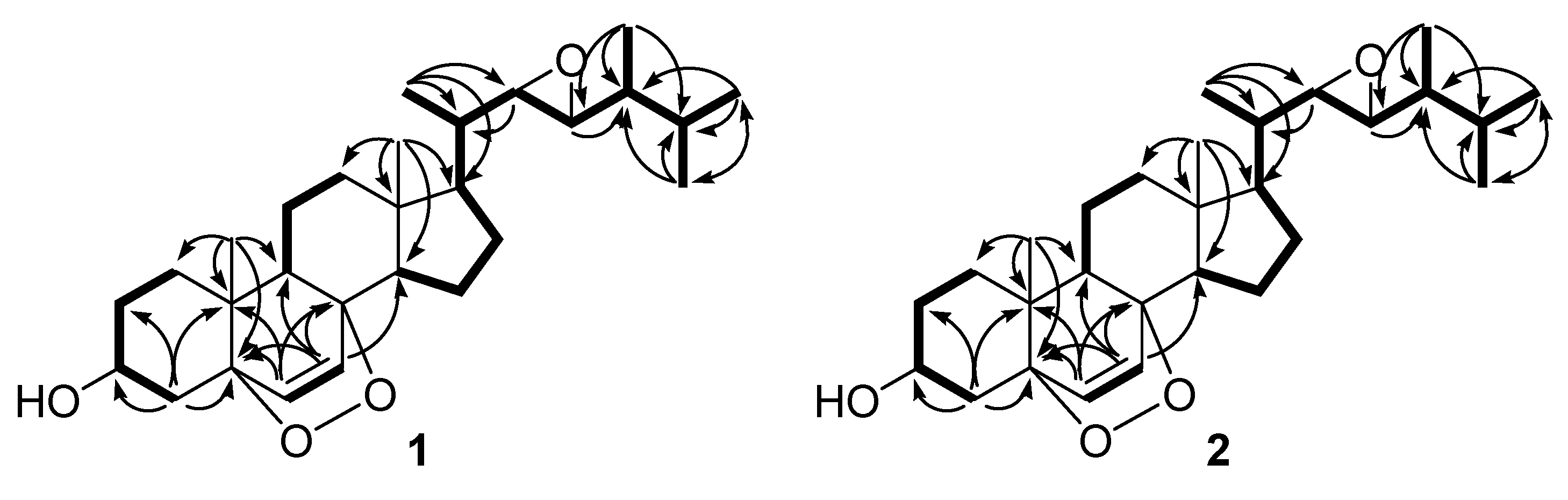
2. Results and Discussion
| No. | 1 | 2 | |||
| δH | δC | δH | δC | ||
| 1a | 1.70 (m) | 34.7 (CH2) | 1.69 (m) | 34.7 (CH2) | |
| 1b | 1.95 (m) | 1.95 (m) | |||
| 2a | 1.54 (m) | 30.1 (CH2) | 1.55 (m) | 30.1 (CH2) | |
| 2b | 1.85 (m) | 1.84 (m) | |||
| 3 | 3.97 (m) | 66.4 (CH) | 3.97 (m) | 66.4 (CH) | |
| 4a | 1.91 (m) | 36.9 (CH2) | 1.91 (m) | 36.9 (CH2) | |
| 4b | 2.12 (ddd, 13.8, 4.9, 1.4) | 2.12 (ddd, 13.8, 5.0, 1.8) | |||
| 5 | 82.2 (C) | 82.2 (C) | |||
| 6 | 6.26 (d, 8.5) | 135.6 (CH) | 6.25 (d, 8.5) | 135.5 (CH) | |
| 7 | 6.49 (d, 8.5) | 130.5 (CH) | 6.50 (d, 8.5) | 130.6 (CH) | |
| 8 | 79.3 (C) | 79.4 (C) | |||
| 9 | 1.51 (m) | 51.1 (CH) | 1.51 (m) | 51.2 (CH) | |
| 10 | 37.0 (C) | 37.0 (C) | |||
| 11a | 1.23 (m) | 23.4 (CH2) | 1.24 (m) | 23.4 (CH2) | |
| 11b | 1.53 (m) | 1.52 (m) | |||
| 12a | 1.28 (m) | 39.3 (CH2) | 1.25 (m) | 39.4 (CH2) | |
| 12b | 1.98 (m) | 1.93 (m) | |||
| 13 | 45.0 (C) | 45.0 (C) | |||
| 14 | 1.57 (m) | 51.3 (CH) | 1.57 (m) | 51.3 (CH) | |
| 15a | 1.47 (m) | 21.0 (CH2) | 1.48 (m) | 20.8 (CH2) | |
| 15b | 1.68 (m) | 1.68 (m) | |||
| 16a | 1.43 (m) | 28.0 (CH2) | 1.71 (m) | 27.1 (CH2) | |
| 16b | 1.96 (m) | 2.01 (m) | |||
| 17 | 1.37 (m) | 53.9 (CH) | 1.40 (m) | 56.3 (CH) | |
| 18 | 0.79 (s) | 12.7 (CH3) | 0.79 (s) | 12.8 (CH3) | |
| 19 | 0.89 (s) | 18.2 (CH3) | 0.88 (s) | 18.2 (CH3) | |
| 20 | 1.17 (m) | 39.2 (CH) | 1.31 (m) | 38.2 (CH) | |
| 21 | 1.08 (d, 6.4) | 16.9 (CH3) | 0.99 (d, 7.0) | 16.0 (CH3) | |
| 22 | 2.38 (dd, 8.2, 1.9) | 62.8 (CH) | 2.58 (dd, 7.1, 2.2) | 63.8 (CH) | |
| 23 | 2.66 (dd, 8.4, 1.9) | 63.9 (CH) | 2.45 (dd, 7.8, 2.2) | 60.3 (CH) | |
| 24 | 1.09 (m) | 42.4 (CH) | 1.05 (m) | 42.2 (CH) | |
| 25 | 1.78 (m) | 31.0 (CH) | 1.65 (m) | 31.1 (CH) | |
| 26 | 0.92 (d, 6.8) | 18.6 (CH3) | 0.92 (d, 6.8) | 19.5 (CH3) | |
| 27 | 0.96 (d, 6.8) | 20.2 (CH3) | 0.95 (d, 6.8) | 20.4 (CH3) | |
| 28 | 0.91 (d, 7.0) | 12.6 (CH3) | 0.97 (d, 6.9) | 13.6 (CH3) | |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Bioassays
4. Conclusions
Acknowledgments
References
- Baldini, M.; Danuso, F.; Turi, M.; Vannozzi, G.P. Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosus L.) for inulin and sugar yield from stalks and tubers. Ind. Crop. Prod. 2004, 19, 25–40. [Google Scholar] [CrossRef]
- Szambelan, K.; Nowak, J.; Chrapkowska, K.J. Comparison of bacterial and yeast ethanol fermentation yield from Jerusalem artichoke (Helianthus tuberosus L.) tubers pulp and juices. Acta Sci. Pol. Technol. Aliment. 2004, 3, 45–53. [Google Scholar]
- Pan, L.; Sinden, M.R.; Kennedy, A.H.; Chai, H.; Watson, L.E.; Graham, T.L.; Kinghorn, A.D. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke). Phytochem. Lett. 2009, 2, 15–18. [Google Scholar] [CrossRef]
- Spring, O. Sesquiterpene lactones from Helianthus tuberosus. Phytochemistry 1991, 30, 519–522. [Google Scholar]
- Chernenko, T.V.; Glushenkova, A.I.; Rakhimov, D.A. Lipids of Helianthus tuberosus tubers. Chem. Nat. Compd. 2008, 44, 1–2. [Google Scholar] [CrossRef]
- Uemura, M.; Yoshida, S. Studies on freezing injury in plant cells: II. protein and lipid changes in the plamsa membranes of Jerusalem artichoke tubers during a lethal freezing in vivo. Plant Physiol. 1986, 80, 187–195. [Google Scholar] [CrossRef]
- Xia, T.X.; Liu, Z.P.; Qi, C.H.; Chen, M.D. Effect of seawater irrigation on Helianthus tuberosus L. in Laizhou gulf. Agric. Res. Arid Areas 2004, 22, 60–63. [Google Scholar]
- Gauvin, A.; Smadja, J.; Aknin, M.; Faure, R.; Gaydou, E.M. Isolation of bioactive 5α,8α;-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem. 2000, 78, 986–992. [Google Scholar]
- Greca, M.D.; Mangoni, L.; Molinaro, A.; Monaco, P.; Previtera, L. 5β,8β-Epidioxyergosta-6,22-dien-3β-ol from Typha latifolia. Gazz. Chim. Ital. 1990, 120, 391–392. [Google Scholar]
- Wright, J.L.C.; McInnes, A.G.; Shimizu, S.; Smith, D.G.; Walter, J.A.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonance spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar] [CrossRef]
- Zhang, X.; Geoffroy, P.; Miesch, M.; Julien-David, D.; Raul, F.; Aoudé-Werner, D.; Marchioni, D. Gram-scale chromatographic purification of β-sitosterol, synthesis and characterization of β-sitosterol oxides. Steroids 2005, 70, 886–895. [Google Scholar] [CrossRef]
- Pettit, G.R.; Numata, A.; Cragg, G.M.; Herald, D.L.; Takada, T.; Iwamoto, C.; Riesen, R.; Schmidt, J.M.; Doubek, D.L.; Goswami, A. Isolation and structures of schleicherastatins 1–7 and schleicheols 1 and 2 from the teak forest medicinal tree Schleichera oleosa. J. Nat. Prod. 2000, 63, 72–78. [Google Scholar] [CrossRef]
- Notaro, G.; Piccialli, V.; Sica, D. 3β,5α,8β-Trihydroxylated sterols with a saturated nucleus from two populations of the marine sponge Cliona copiosa. J. Nat. Prod. 1991, 54, 1570–1575. [Google Scholar] [CrossRef]
- Lu, W.G.; Zhang, J.; Su, J.Y.; Zeng, L.M. Stereo-synthesis and cholinesterase inhibitory activities of Δ5-3β,7β-dihydroxy sterols and Δ5-3β,7α-dihydroxy sterols. Chin. J. Med. Chem. 2005, 15, 202–206. [Google Scholar]
- Rivera, A.; Benavides, O.L.; Rios-Motta, J. (22E)-Ergosta-6,22-diene-3β,5α,8α-triol: A new polyhydroxysterol isolated from Lentinus edodes (Shiitake). Nat. Prod. Res. 2009, 23, 293–300. [Google Scholar]
- Riccio, R.; Iorizzi, M.; Greco, O.S.; Minale, L.; Debray, M.; Menou, J.L. Starfish saponins, part 22. asterosaponins from the starfish Halityle regularis: a novel 22,23-epoxysteroidal glycoside sulfate. J. Nat. Prod. 1985, 48, 756–765. [Google Scholar] [CrossRef]
- Schulz, B.; Sucker, J.; Aust, H.J.; Krohn, K.; Ludewig, K.; Jones, P.G.; Döring, D. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res. 1995, 99, 1007–1015. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (Brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–12 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.-D.; Miao, F.-P.; Ji, N.-Y. Two New Epoxysteroids from Helianthus tuberosus. Molecules 2011, 16, 8646-8653. https://doi.org/10.3390/molecules16108646
Li X-D, Miao F-P, Ji N-Y. Two New Epoxysteroids from Helianthus tuberosus. Molecules. 2011; 16(10):8646-8653. https://doi.org/10.3390/molecules16108646
Chicago/Turabian StyleLi, Xiao-Dong, Feng-Ping Miao, and Nai-Yun Ji. 2011. "Two New Epoxysteroids from Helianthus tuberosus" Molecules 16, no. 10: 8646-8653. https://doi.org/10.3390/molecules16108646
APA StyleLi, X.-D., Miao, F.-P., & Ji, N.-Y. (2011). Two New Epoxysteroids from Helianthus tuberosus. Molecules, 16(10), 8646-8653. https://doi.org/10.3390/molecules16108646





