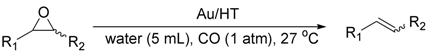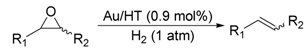Gold Nanoparticle-Catalyzed Environmentally Benign Deoxygenation of Epoxides to Alkenes
Abstract
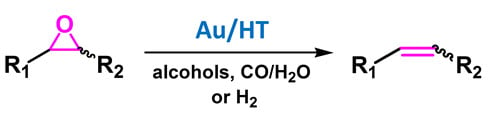
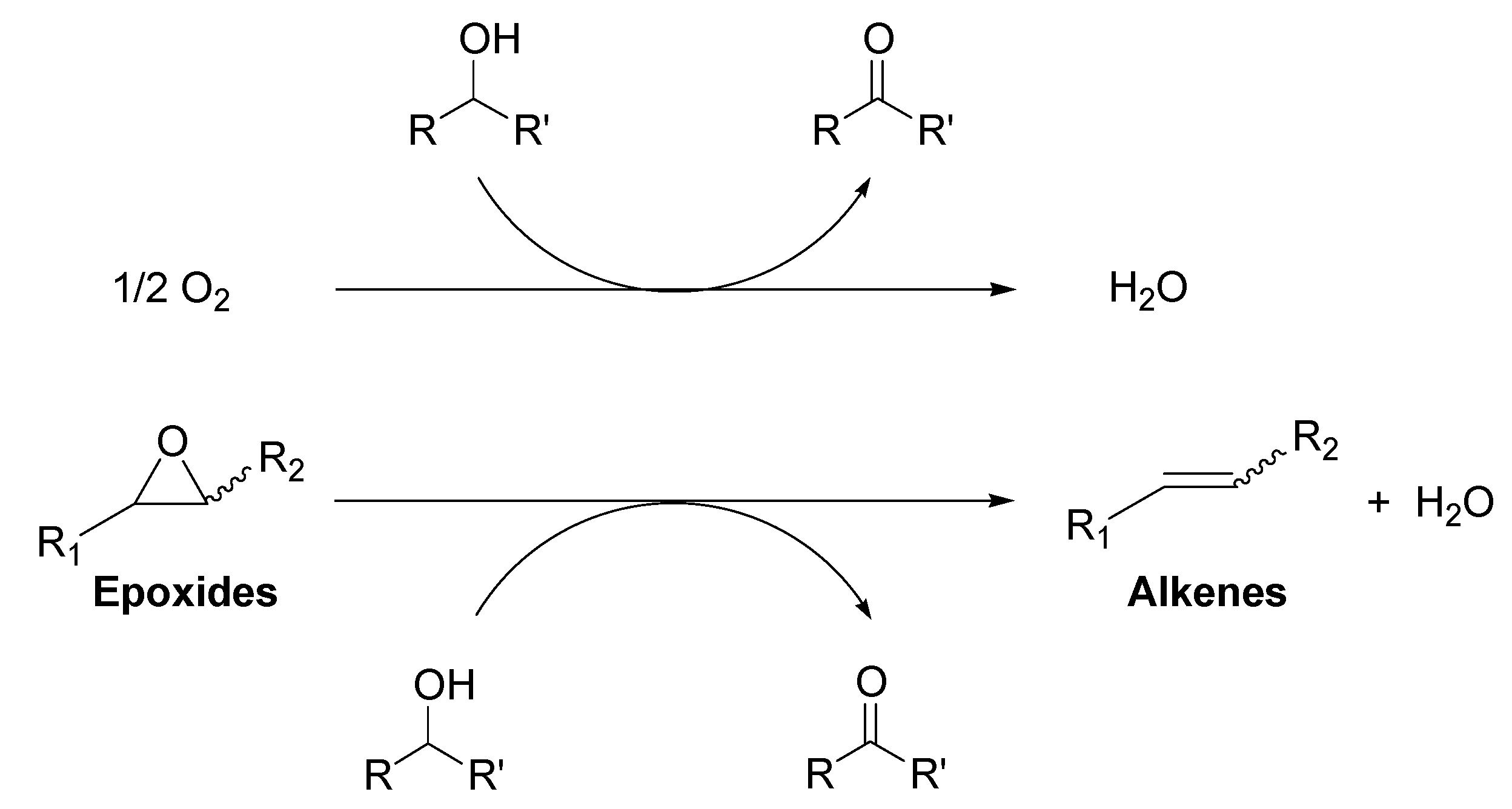
:1. Introduction

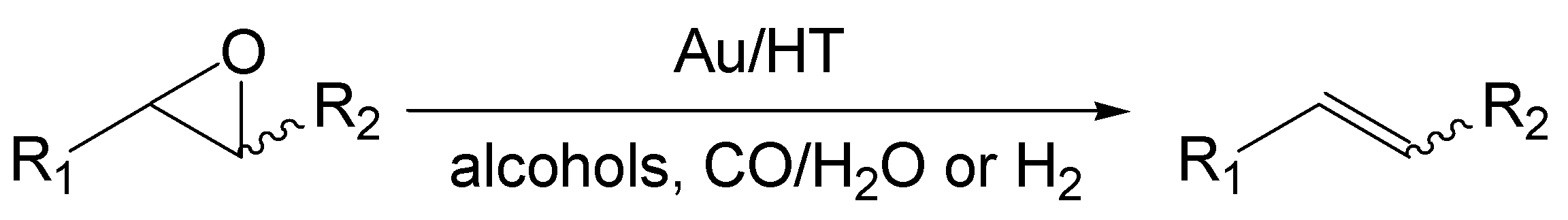
2. Results and Discussion
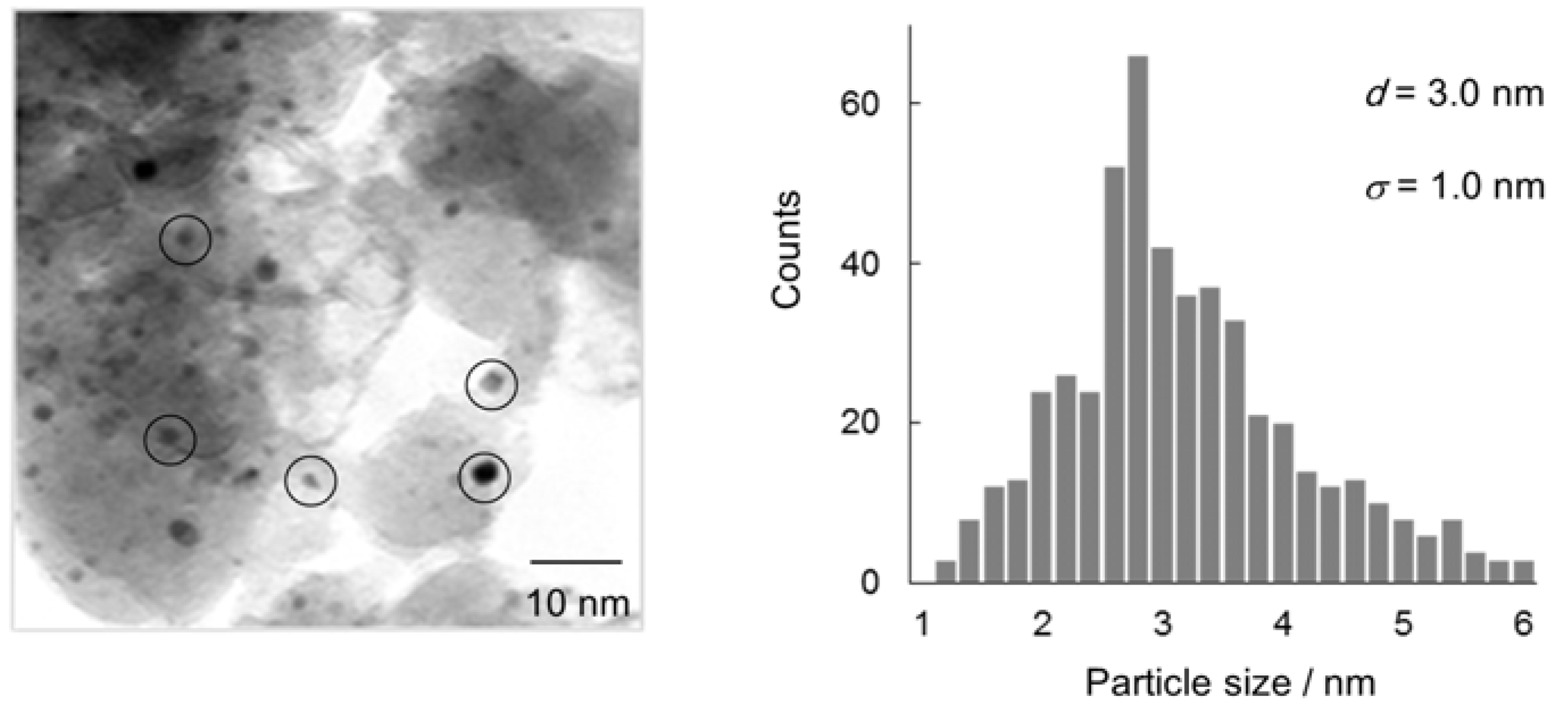
2.1. Characterization of Au/HT
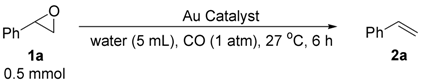
2.2. Deoxygenation of Epoxides to Alkenes
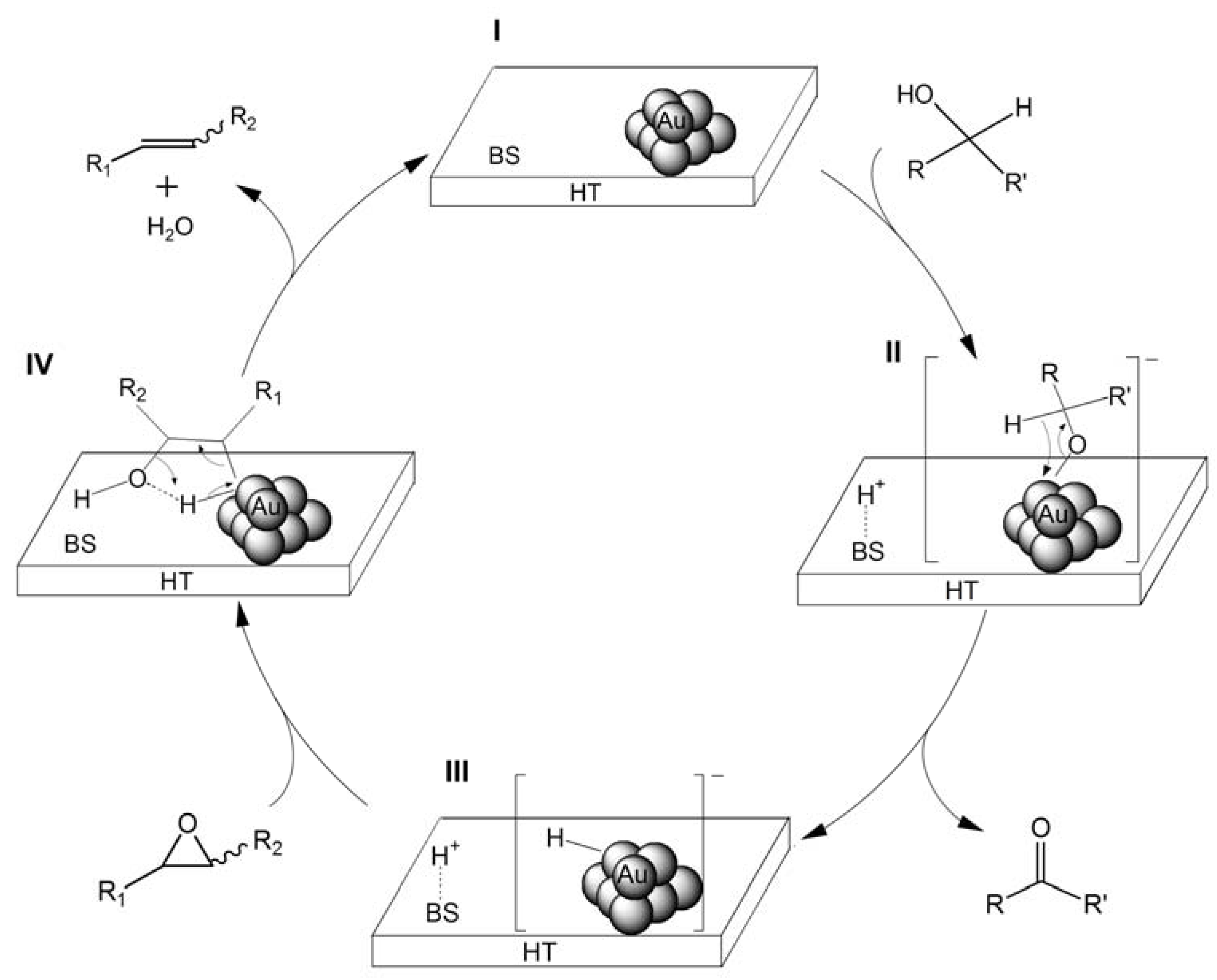
2.2.1. Au/HT-Catalyzed Deoxygenation of Epoxides Using Alcohols as a Reductant
| Entry | Catalyst | Alcohol | Conv. b (%) | Sel. b (%) | |
|---|---|---|---|---|---|
| 1 | Au/HT | 2-propanol | 99 | >99 | |
| 2 | Au/HT | 1-phenylethanol | 99 | >99 | |
| 3 | Au/HT | benzyl alcohol | 91 | >99 | |
| 4 | Au/HT | 1-octanol | 37 | >99 | |
| 5 | Au/Al2O3 | 2-propanol | 60 | >99 | |
| 6 | Au/MgO | 2-propanol | 43 | >99 | |
| 7 | Au/TiO2 | 2-propanol | 19 | >99 | |
| 8 | Au/SiO2 | 2-propanol | 5 | >99 | |
| 9 | HAuCl4 | 2-propanol | <1 | - | |
| 10 | Au2O3 | 2-propanol | <1 | - | |
| 11 | bulk Au metal | 2-propanol | 0 | - | |
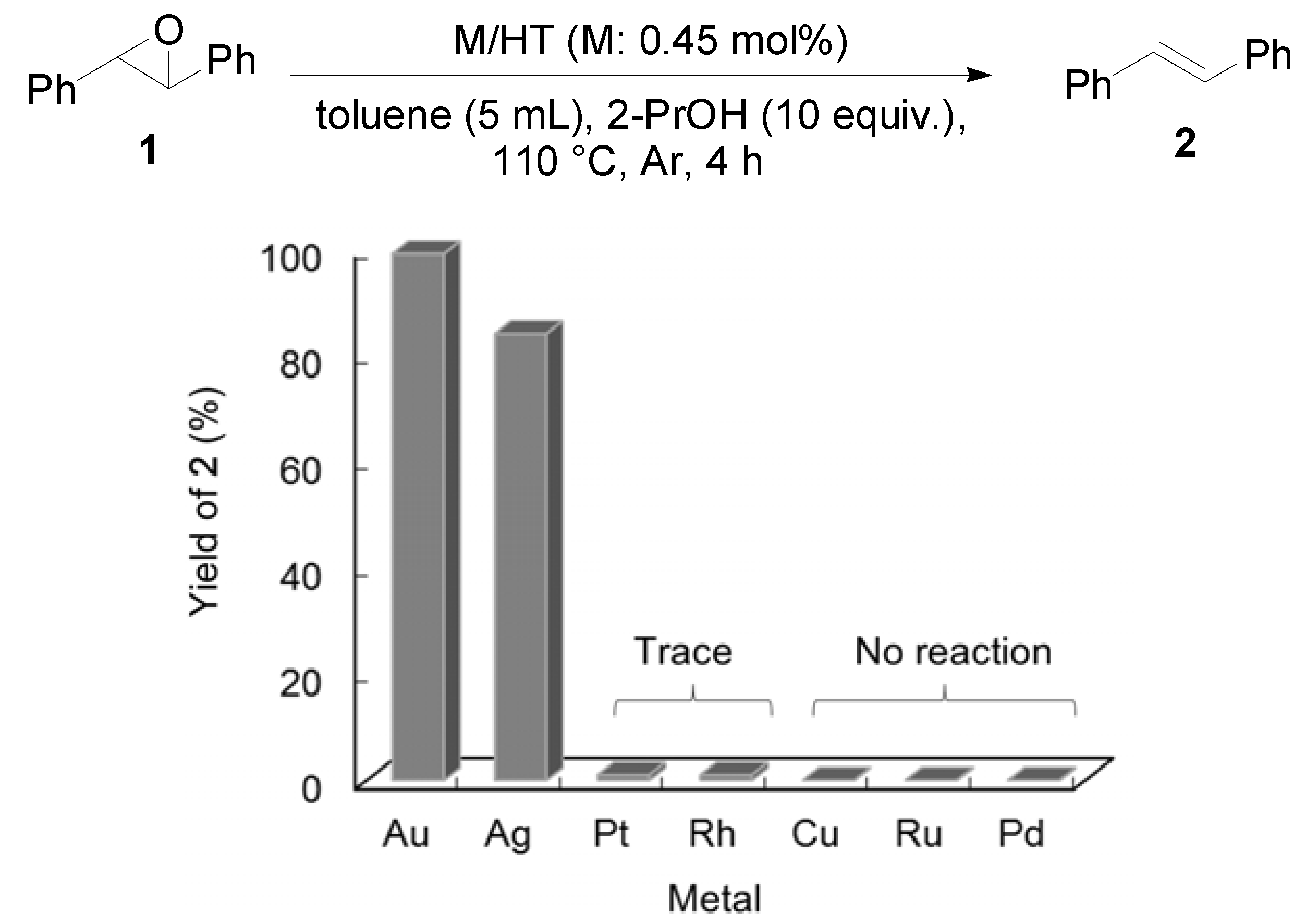
| Entry | Substrate | Product | Time (h) | Conv. b (%) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | 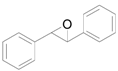 | 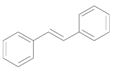 | 4 | 99 | 99 |
| 2 c | 4 | 99 | 99 | ||
| 3 d | 4 | 97 | 97 | ||
| 4 |  | 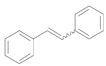 | 4 | 99 | 97 ( E/Z) = 2/3 |
| 5 | 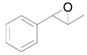 | 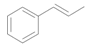 | 4 | 98 | 98 |
| 6 e |  | 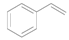 | 6 | >99 | 98 |
| 7 | 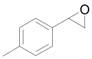 | 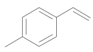 | 6 | 96 | 92 |
| 8 | 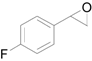 | 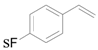 | 6 | 94 | 91 |
| 9 | 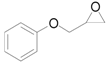 |  | 6 | 93 | 90 |
| 10 | 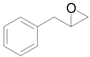 | 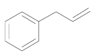 | 24 | 89 | 89 |
| 11 | 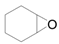 |  | 24 | 87 | 87 |
| 12 e |  |  | 4 | >99 | 97 |
| 13 f | 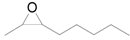 | 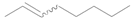 | 12 | 81 | 77 (E/Z) = 1/1 |
| 14 f | 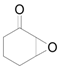 | 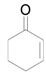 | 24 | 88 | 85 |
| 15 f | 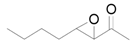 | 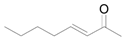 | 24 | 99 | 94 |
| 16 f | 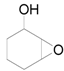 |  | 6 | 93 | 91 |
| 17 | 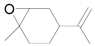 |  | 24 | 72 | 68 |
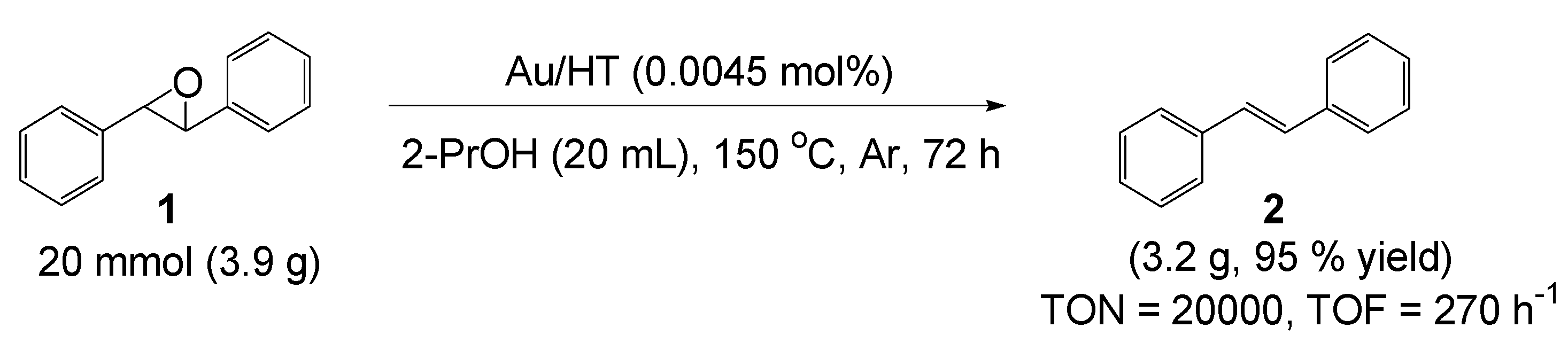
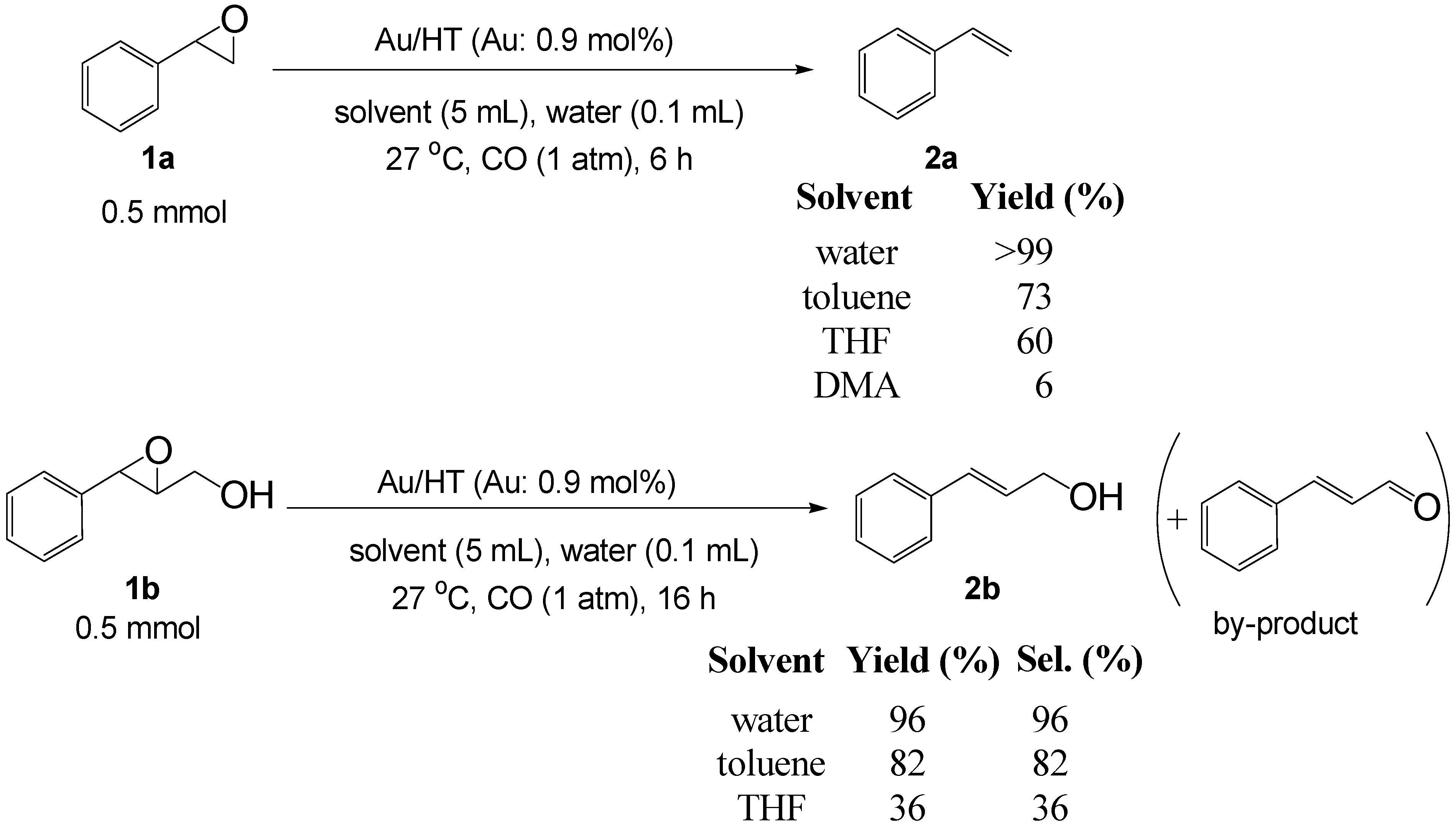
2.2.2. Deoxygenation of Epoxides with CO/H2O
| Entry | Catalyst | Yield b (%) | Sel. b (%) |
|---|---|---|---|
| 1 | Au/HT | >99 | >99 |
| 2 c | Au/HT | >99 | >99 |
| 3 d | Au/HT | 97 | >99 |
| 4 | Au/Al2O3 | 79 | >99 |
| 5 | Au/MgO | 36 | >99 |
| 6 | Au/TiO2 | 18 | >99 |
| 7 e | Au/TiO2 + Na2CO3 | 57 | >99 |
| 8 | Au/SiO2 | 3 | >99 |
| Entry | Substrate | Product | Time (h) | Yield b (%) | Sel. b (%) |
|---|---|---|---|---|---|
| 1 | 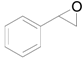 | 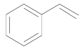 | 6 | >99 (94) | >99 |
| 2 | 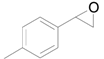 |  | 12 | 90 (85) | >99 |
| 3 |  |  | 12 | 99 (94) | >99 |
| 4 | 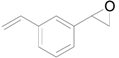 | 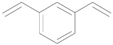 | 16 | 90 (85) | >99 |
| 5 |  | 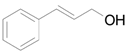 | 16 | 96 (90) | 96 |
| 6 | 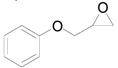 | 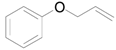 | 24 | 90 (83) | >99 |
| 7 |  |  | 24 | 75 (69) | >99 |
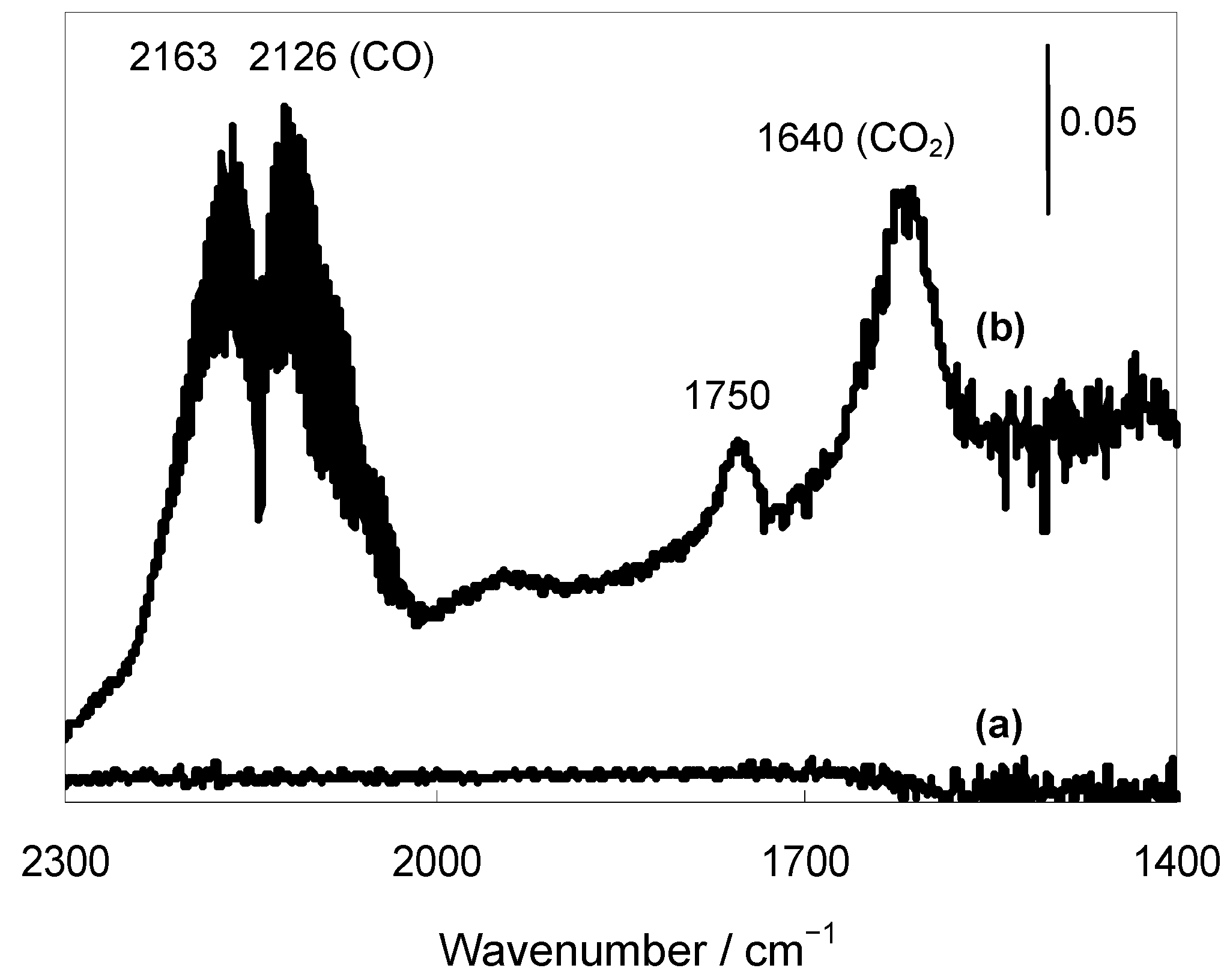
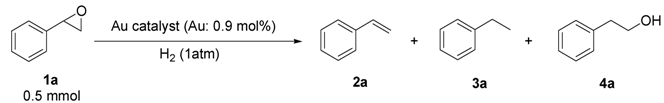
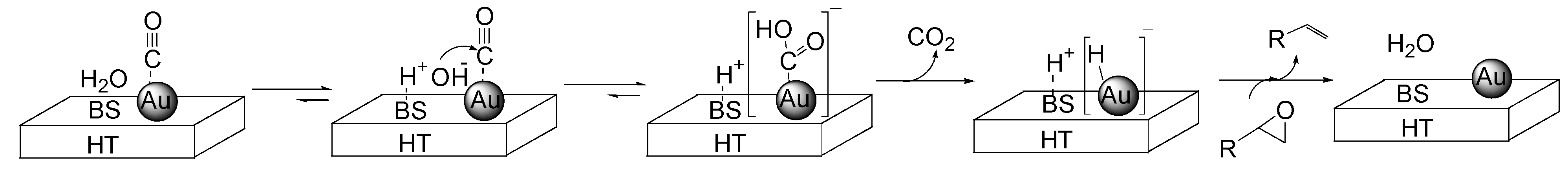
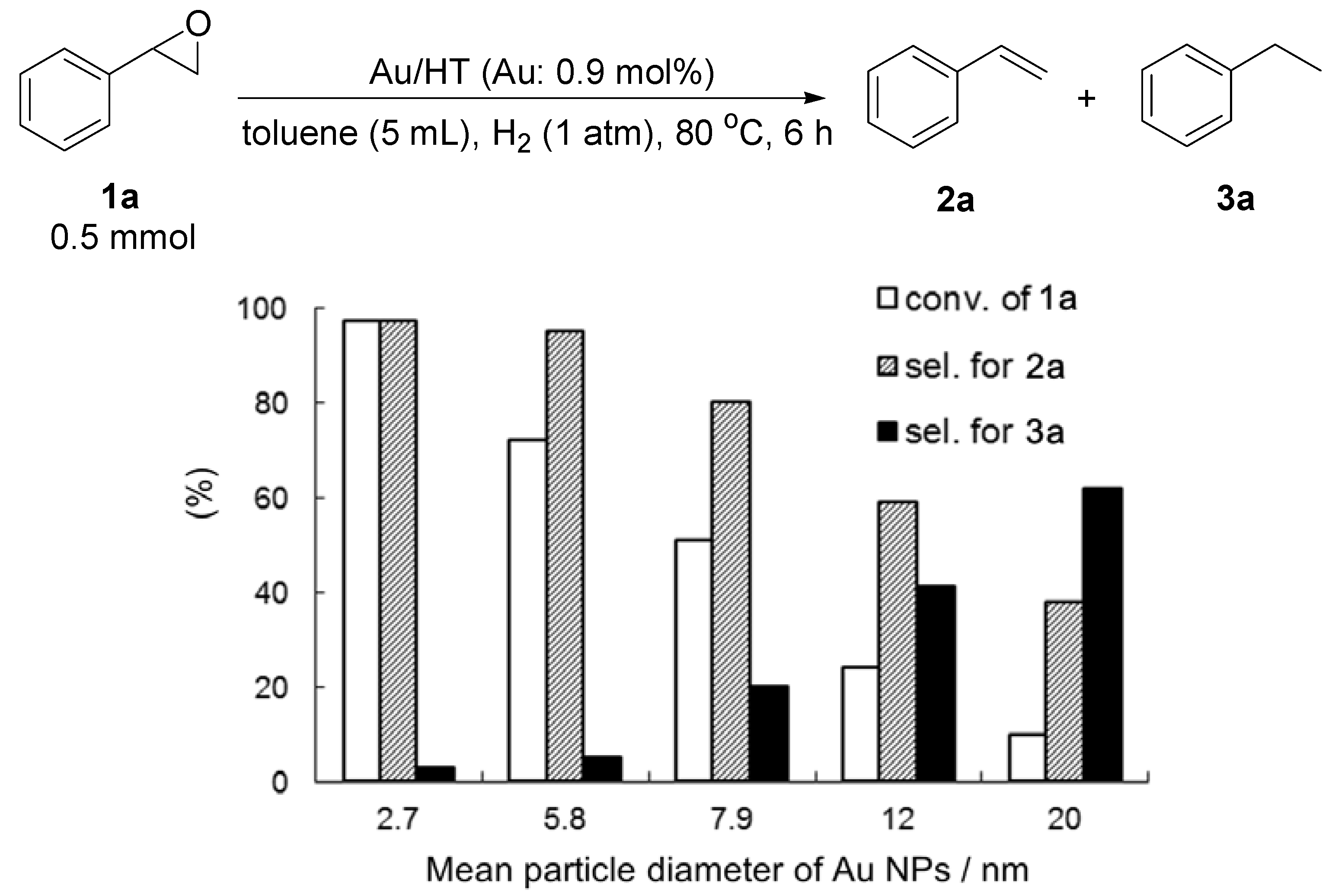
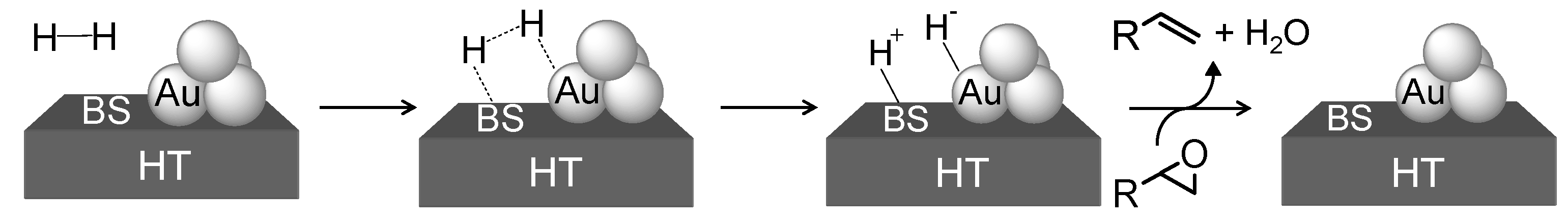
2.2.3. Selective Deoxygenation of Epoxides Using Molecular Hydrogen
| Entry | Catalyst | Temp. (°C) | Time (h) | Conv. b (%) | Sel. for 2a b (%) | Sel. for 3a b (%) | Sel. for 4a b (%) |
|---|---|---|---|---|---|---|---|
| 1 | Au/HT | 80 | 6 | 97 | 97 | 3 | 0 |
| 2 | Au/HT | 60 | 8 | >99 | >99 | 0 | 0 |
| 3 | Au/HT | 60 | 24 | >99 | >99 | 0 | 0 |
| 4 | Au/CeO2 | 80 | 6 | 64 | 81 | 19 | 0 |
| 5 | Au/Al2O3 | 80 | 6 | 82 | 36 | 64 | 0 |
| 6 | Au/TiO2 | 80 | 6 | 26 | >99 | <1 | 0 |
| 7 | Au/SiO2 | 80 | 6 | <1 | - | - | - |
| 8 | Pd/HT | 80 | 6 | >99 | 0 | 0 | >99 |
| 9 | Pt/HT | 80 | 6 | 87 | 0 | <1 | >99 |
| 10 | Ag/HT | 80 | 6 | <1 | - | - | - |
| 11 | Rh/HT | 80 | 6 | <1 | - | - | - |
| 12 | Ru/HT | 80 | 6 | <1 | - | - | - |
| 13 | Cu/HT | 80 | 6 | <1 | - | - | - |
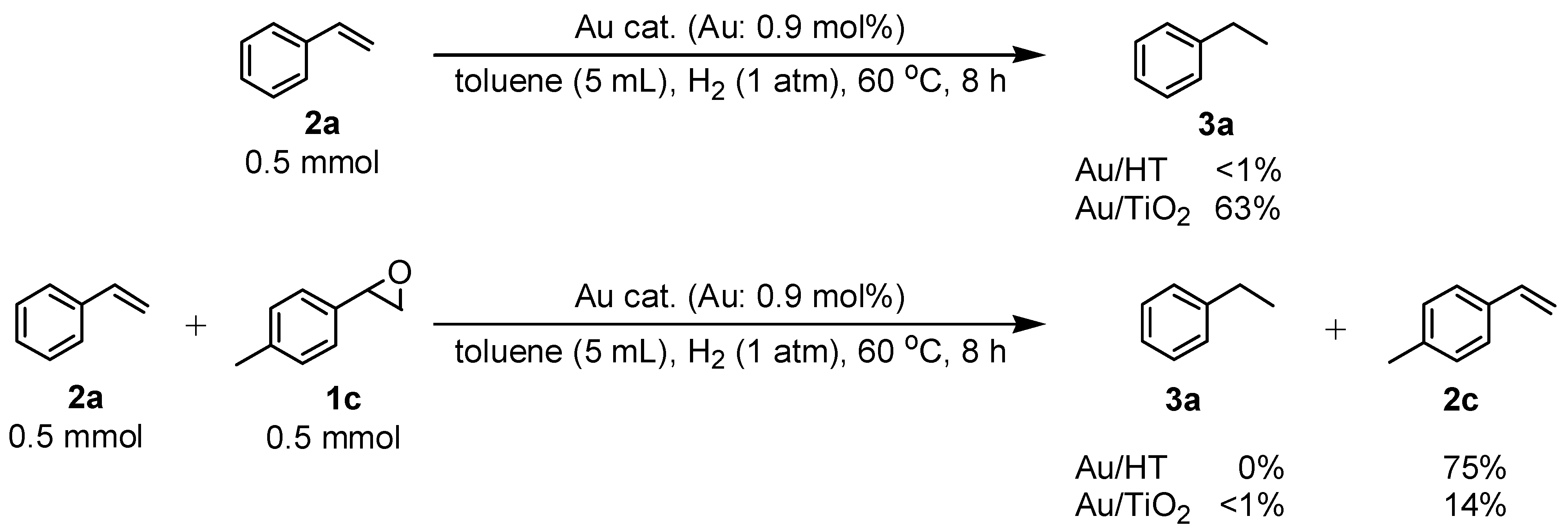
| Entry | Substrate | Product | Time (h) | Conv. b (%) | Sel. b(%) |
|---|---|---|---|---|---|
| 1 |  |  | 8 | >99 | >99 |
| 2 c | 8 | 97 | >99 | ||
| 3 d | 8 | 97 | >99 | ||
| 4 |  |  | 12 | 81 | >99 |
| 5 |  | 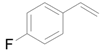 | 12 | 84 | >99 |
| 6 | 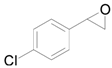 | 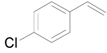 | 24 | 85 | >99 |
| 7 e | 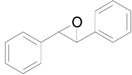 | 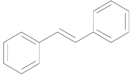 | 12 | 98 | >99 |
| 8 e |  | 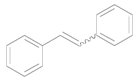 | 12 | 96 | >99 (E/Z = 2/3) |
| 9 | 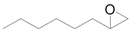 |  | 24 | 84 | >99 |
| 10 | 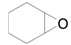 | 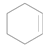 | 24 | 89 | >99 |
| 11 | 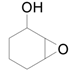 |  | 24 | 92 | >99 |
3. Experimental
3.1. General
3.2. Preparation of Au/HT
4. Conclusions
Acknowledgments
- Sample Availability: Not available.
References
- Corey, E.J.; Su, W.G. Total synthesis of a C15 ginkgolide, (±)-bilobalide. J. Am. Chem. Soc. 1987, 109, 7534–7536. [Google Scholar] [CrossRef]
- Kraus, G.A.; Thomas, P.J. Synthesis of 7,7,8-trideuteriated trichothecenes. J. Org. Chem. 1988, 53, 1395–1397. [Google Scholar] [CrossRef]
- Johnson, W.S.; Plummer, M.S.; Reddy, S.P.; Bartlett, W.R. The fluorine atom as a cationstabilizing auxiliary in biomimetic polyene cyclizations. Total synthesis of dl-β-amyrin. J. Am. Chem. Soc. 1993, 115, 515–521. [Google Scholar] [CrossRef]
- Silverman, R.B. Model studies for a molecular mechanism of action of oral anticoagulants. J. Am. Chem. Soc. 1981, 103, 3910–3915. [Google Scholar] [CrossRef]
- Preusch, P.C.; Suttie, J.W. A chemical model for the mechanism of vitamin K epoxide reductase. J. Org. Chem. 1983, 48, 3301–3305. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Umbreit, M.A.; Nieh, M.T.; Flood, T.C. Lower valent tungsten halides. New class of reagents for deoxygenation of organic molecules. J. Am. Chem. Soc. 1972, 94, 6538–6540. [Google Scholar] [CrossRef]
- McMurry, J.E.; Silvertri, M.G.; Fleming, M.P.; Hoz, T.; Grayston, M.W. Some deoxygenation reactions with low-valent titanium (TiCl3/LiAlH4). J. Org. Chem. 1978, 43, 3249–3255. [Google Scholar] [CrossRef]
- Kochi, J.K.; Singleton, D.M.; Andrews, L.J. Alkenes from halides and epoxides by reductive eliminations with CrII complexes. Tetrahedron 1968, 24, 3503–3515. [Google Scholar] [CrossRef]
- Sato, M.; Oshima, K. Reduction of organic compoumds with low-valent niobium (NbCl5/NaAlH4). Chem. Lett. 1982, 11, 157–160. [Google Scholar] [CrossRef]
- Mahesh, M.; Murphy, J.A.; Wessel, H.P.J. Novel deoxygenation reaction of epoxides by Indium. J. Org. Chem. 2005, 70, 4118–4123. [Google Scholar] [CrossRef]
- Girard, P.; Kagan, H.B. Divalent lanthanide derivatives in organic synthesis. 1. Mild preparation of samarium iodide and ytterbium iodide and their use as reducing or coupling agents. J. Am. Chem. Soc. 1980, 102, 2693–2698. [Google Scholar] [CrossRef]
- Schobert, R. Reduction of Epoxides, α,β-unsaturated aldehydes, and vicinal diketones with "Reactive Titanocene". Angew. Chem. Int. Ed. 1988, 27, 855–856. [Google Scholar] [CrossRef]
- RajanBabu, T.V.; Nguent, W.A.; Beattie, M.S. Free radical-mediated reduction and deoxygenation of epoxides. J. Am. Chem. Soc. 1990, 112, 6408–6409. [Google Scholar]
- Yamada, K.; Goto, S.; Nagase, H.; Kyotani, Y.; Hirata, Y. Convenient method for deoxygenation of epoxides to olefins. J. Org. Chem. 1978, 43, 2076–2077. [Google Scholar] [CrossRef]
- Yachi, K.; Maeda, K.; Shinokubo, H.; Oshima, K. Stereospecific conversion of iodohydrin derivertives into alkenes by means of an allylsilane-titanium tetrachloride system and its application to stereoretentive deoxygenation of epoxides. Tetrahedron Lett. 1997, 38, 5161–5164. [Google Scholar] [CrossRef]
- Antonioletti, R.; Bovicelli, P.; Fezzolari, E.; Righi, G. Stereo- and regiocontrolled transformations of vinyloxiranes with metal halides. Tetrahedron Lett. 2000, 41, 9315–9318. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Jafarpour, M. Rapid, highly efficient and stereoselective deoxygenation of epoxides by ZrCl4/NaI. Tetrahedron Lett. 2005, 46, 4107–4110. [Google Scholar]
- Speziale, A.J.; Bissing, D.E. The reactions of phosphorus compounds. VIII. Kinetics and mechanism of the Wittig reaction. J. Am. Chem. Soc. 1963, 85, 3878–3884. [Google Scholar] [CrossRef]
- Bissing, D.E.; Speziale, A.J. Reactions of phosphorus compounds. IX. The opening of epoxides with tertiary phosphines. J. Am. Chem. Soc. 1965, 87, 2683–2690. [Google Scholar] [CrossRef]
- Vedejs, E.; Fuchs, P.L. Inversion of acyclic olefins by the phosphorus betaine method. Scope and limitations. J. Am. Chem. Soc. 1973, 95, 822–825. [Google Scholar] [CrossRef]
- Dervan, P.B.; Shippey, M.A. Trimethylsilylpotassium. Deoxygenation of epoxides with inversion of stereochemistry. J. Am. Chem. Soc. 1976, 98, 1265–1267. [Google Scholar] [CrossRef]
- Mangette, J.E.; Powell, D.R.; Calabrese, J.C.; West, R. Oxadisilacyclopropane reactivity with stilbene oxides. Organometallics 1995, 14, 4064–4073. [Google Scholar] [CrossRef]
- Mangette, J.E.; Powell, D.R.; Firman, T.K.; West, R. The reactivity of tetramesityldisilene with epoxides: dependence of product distributions on steric and structural characteristics of the epoxide. J. Organomet. Chem. 1996, 521, 363–375. [Google Scholar]
- Zhu, Z.; Espenson, J.H. Methylrhenium trioxide as a catalyst for oxidations with molecular oxygen and for oxygen transfer. J. Mol. Catal. A 1995, 103, 87–94. [Google Scholar] [CrossRef]
- Gable, K.P.; Brown, E.C. Rhenium-catalyzed epoxide deoxygenation: Scope and limitations. Synlett 2003, 14, 2243–2245. [Google Scholar]
- Arterburn, J.B.; Liu, M.; Perry, M.C. Polystyrene-supported (catecholato)oxorhenium complexes: Catalysts for alcohol oxidation with DMSO and for deoxygenation of epoxides to alkenes with triphenylphosphine. Helv. Chim. Acta 2002, 85, 3225–3236. [Google Scholar] [CrossRef]
- Itoh, T.; Nagano, T.; Sato, M.; Hirobe, M. Deoxygenation of oxiran compounds to olefins by [Fe4S4(SC6H5)4]2− in the presence of NaBH4. Tetrahedron Lett. 1989, 46, 6387–6388. [Google Scholar]
- Isobe, H.; Branchaud, B.P. Epoxide deoxygenation mediated by Salen complexes. Tetrahedron Lett. 1999, 40, 8747–8749. [Google Scholar]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Espriu, B.S.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar]
- Abad, A.; Concepción, P.; Corma, A.; García, H. Collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2005, 44, 4066–4069. [Google Scholar]
- Huang, J.; Akita, T.; Faye, J.; Fujitani, T.; Takei, T.; Haruta, M. Propene epoxidation with dioxygen catalyzed by gold clusters. Angew. Chem. Int. Ed. 2009, 48, 7862–7865. [Google Scholar]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 2008, 454, 981–983. [Google Scholar]
- Corma, A.; Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 2006, 313, 332–334. [Google Scholar]
- He, L.; Wang, L.-C.; Sun, H.; Ni, J.; Cao, Y.; He, H.-Y.; Fan, K.-N. Efficient and selective room-temperature gold-catalyzed reduction of nitro compounds with CO and H2O as the hydrogen source. Angew. Chem. Int. Ed. 2009, 48, 9538–9541. [Google Scholar]
- Mitsudome, T.; Noujima, A.; Mikami, Y.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported gold and silver nanoparticles for green catalytic deoxygenation of epoxides into alkenes. Angew. Chem. Int. Ed. 2010, 49, 5545–5548. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mikami, Y.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Room temperature deoxygenation of epoxides with CO catalyzed by hydrotalcite-supported gold nanoparticles in water. Chem. Eur. J. 2010, 16, 11818–11821. [Google Scholar] [CrossRef]
- Noujima, A.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Selective deoxygenation of epoxides to alkenes with molecular hydrogen using a hydrotalcite-supported gold catalyst: A concerted effect between gold nanoparticles and basic sites on a support. Angew. Chem. Int. Ed. 2011, 50, 2986–2989. [Google Scholar]
- Mikami, Y.; Noujima, A.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Highly efficient gold nanoparticle catalyzed deoxygenation of amides, sulfoxides, and pyridine N-oxides. Chem. Eur. J. 2011, 17, 1768–1772. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Efficient aerobic oxidation of alcohols using hydrotalcite-supported gold nanoparticle catalyst. Adv. Synth. Catal. 2009, 351, 1890–1896. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported gold nanoparticles as a reusable catalyst for synthesis of lactones from diols using molecular oxygen as an oxidant under mild conditions. Green Chem. 2009, 11, 793–797. [Google Scholar] [CrossRef]
- A theoretical study supports our mechanism that a basic site assists the formation of an Au-alcoholate species via abstraction of the proton of an alcohol, which undergoes β-hydride elimination to give an Au-H species. See: Shang, C; Liu, Z.-P. Origin and activity of gold nanoparticles as aerobic oxidartion catalysts in aqueous solution. J. Am. Chem. Soc. 2011, 133, 9938–9947. [CrossRef]
- Kaneda, K.; Fujita, K.; Takemoto, T.; Imanaka, T. Selective deoxygenation of various N-O bonds catalyzed by rhodium carbonyl clusters in the presence of H2O and CO and their heterogenization using amino-substituted polystyrenes. Bull. Chem. Soc. Jpn. 1991, 64, 602–612. [Google Scholar] [CrossRef]
- Tabakova, T.; Manzoli, M.; Vindigni, F.; Idakiev, V.; Boccuzzi, F. CO-free hydrogen production for fuel cell applications over Au/CeO2 catalysts: FTIR insight into the role of dopant. J. Phys. Chem. A 2010, 114, 3909–3915. [Google Scholar]
- Pyykkö, P. Theoretical chemistry of gold. Angew. Chem. Int. Ed. 2004, 43, 4412–4456. [Google Scholar] [CrossRef]
- Ni, J.; He, L.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Mild and efficient CO-mediated eliminative deoxygenation of epoxides catalyzed by supported gold nanoparticles. Chem. Commun. 2011, 812–814. [Google Scholar]
- Ziegler, J.E.; Zdilla, M.J.; Evans, A.J.; Abu-Omar, M.M. H2-Driven deoxygenation of epoxides and diols to alkenes catalyzed by methyltrioxorhenium. Inorg. Chem. 2009, 48, 9998–10000. [Google Scholar] [CrossRef]
- The mean particle diameters of Au NPs on CeO2, Al2O3, TiO2 and SiO2 are 3.8, 3.6, 3.7 and 14 nm, respectively.
- Boronat, M.; Concepción, P.; Corma, A.; Gonzalez, S.; Illas, F.; Serna, P. A molecular mechanism for the chemoselective hydrogenation of substituted nitroaromatics with nanoparticles of gold on TiO2 catalysts: A cooperative effect between gold and the support. J. Am. Chem. Soc. 2007, 129, 16230–16237. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Noujima, A.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Gold Nanoparticle-Catalyzed Environmentally Benign Deoxygenation of Epoxides to Alkenes. Molecules 2011, 16, 8209-8227. https://doi.org/10.3390/molecules16108209
Noujima A, Mitsudome T, Mizugaki T, Jitsukawa K, Kaneda K. Gold Nanoparticle-Catalyzed Environmentally Benign Deoxygenation of Epoxides to Alkenes. Molecules. 2011; 16(10):8209-8227. https://doi.org/10.3390/molecules16108209
Chicago/Turabian StyleNoujima, Akifumi, Takato Mitsudome, Tomoo Mizugaki, Koichiro Jitsukawa, and Kiyotomi Kaneda. 2011. "Gold Nanoparticle-Catalyzed Environmentally Benign Deoxygenation of Epoxides to Alkenes" Molecules 16, no. 10: 8209-8227. https://doi.org/10.3390/molecules16108209
APA StyleNoujima, A., Mitsudome, T., Mizugaki, T., Jitsukawa, K., & Kaneda, K. (2011). Gold Nanoparticle-Catalyzed Environmentally Benign Deoxygenation of Epoxides to Alkenes. Molecules, 16(10), 8209-8227. https://doi.org/10.3390/molecules16108209