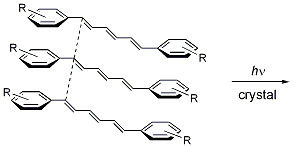
Solid-State [2+2] Photodimerization and Photopolymerization of α,ω-Diarylpolyene Monomers: Effective Utilization of Noncovalent Intermolecular Interactions in Crystals
Abstract
:1. Introduction
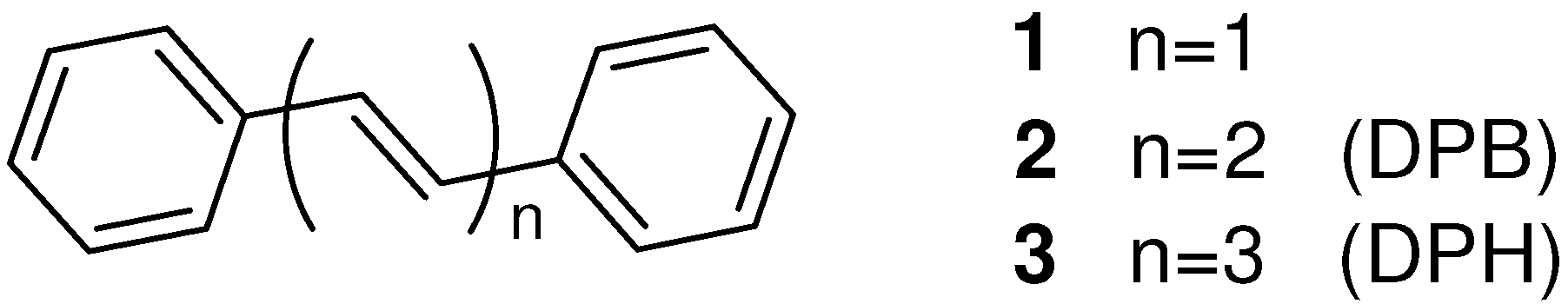
2. [2+2] Photodimerization and Photopolymerization of α,ω-Diarylpolyenes
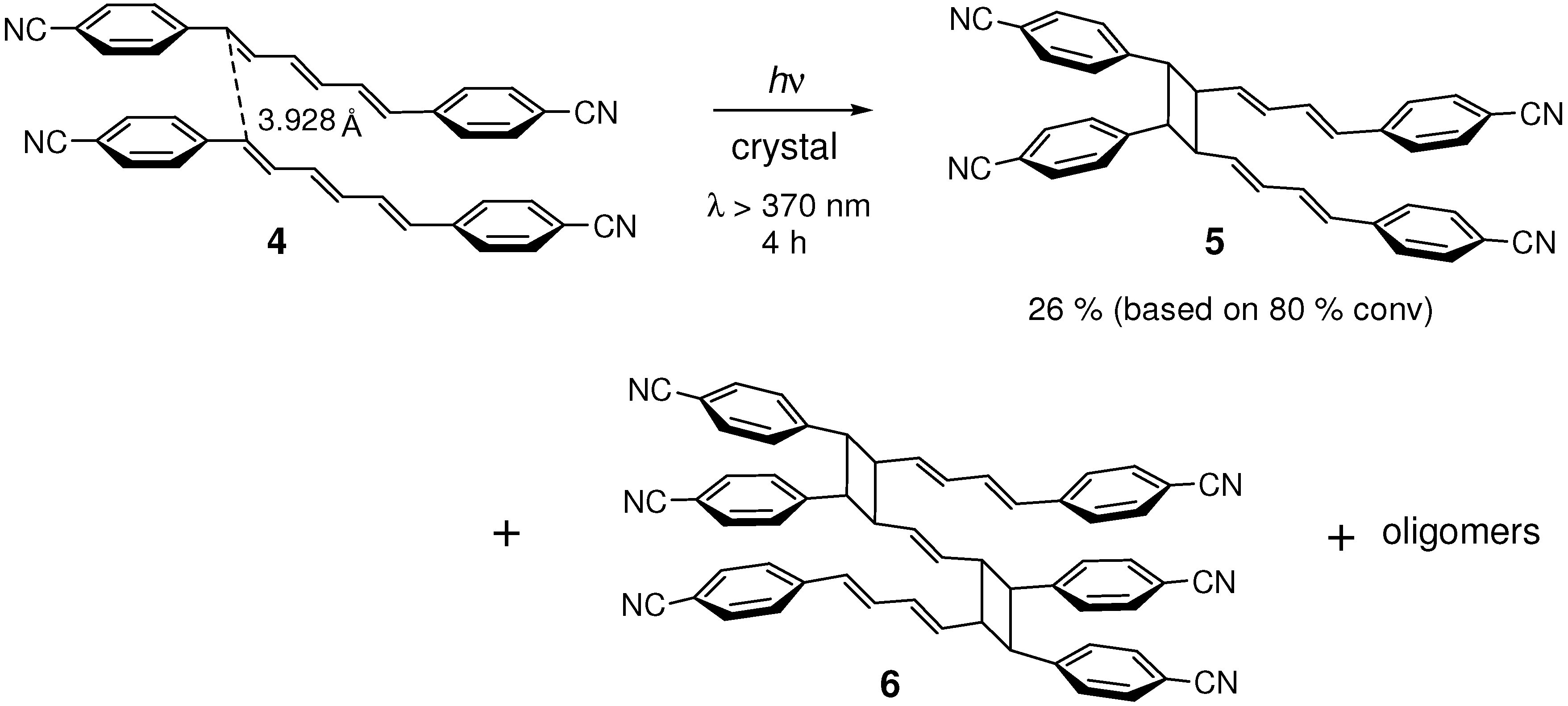
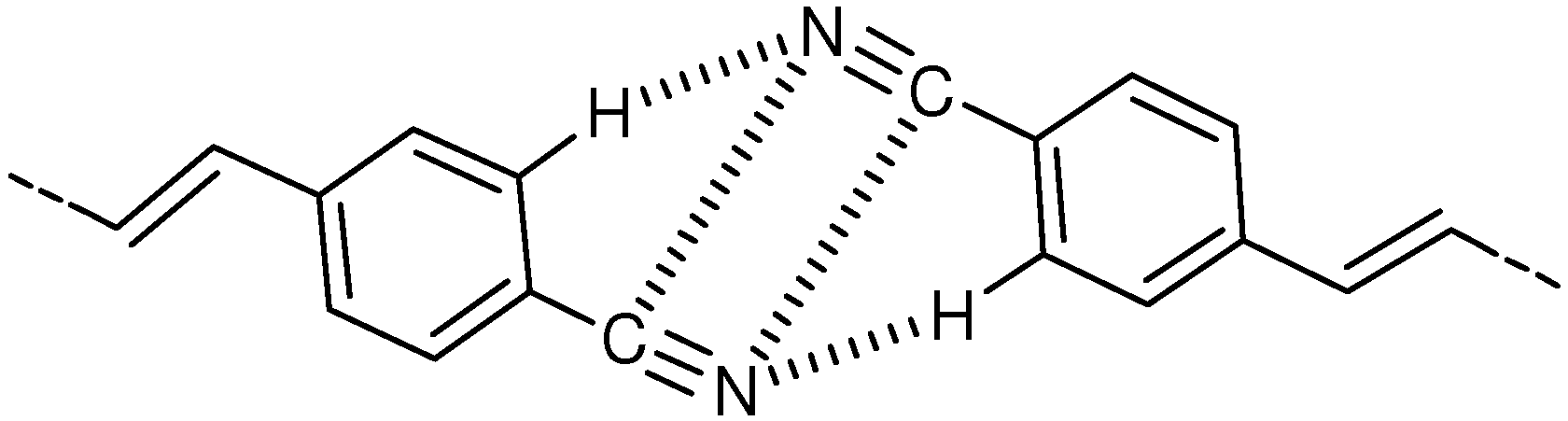
2.1. Cyano Substitution
2.2. Formyl Substitution

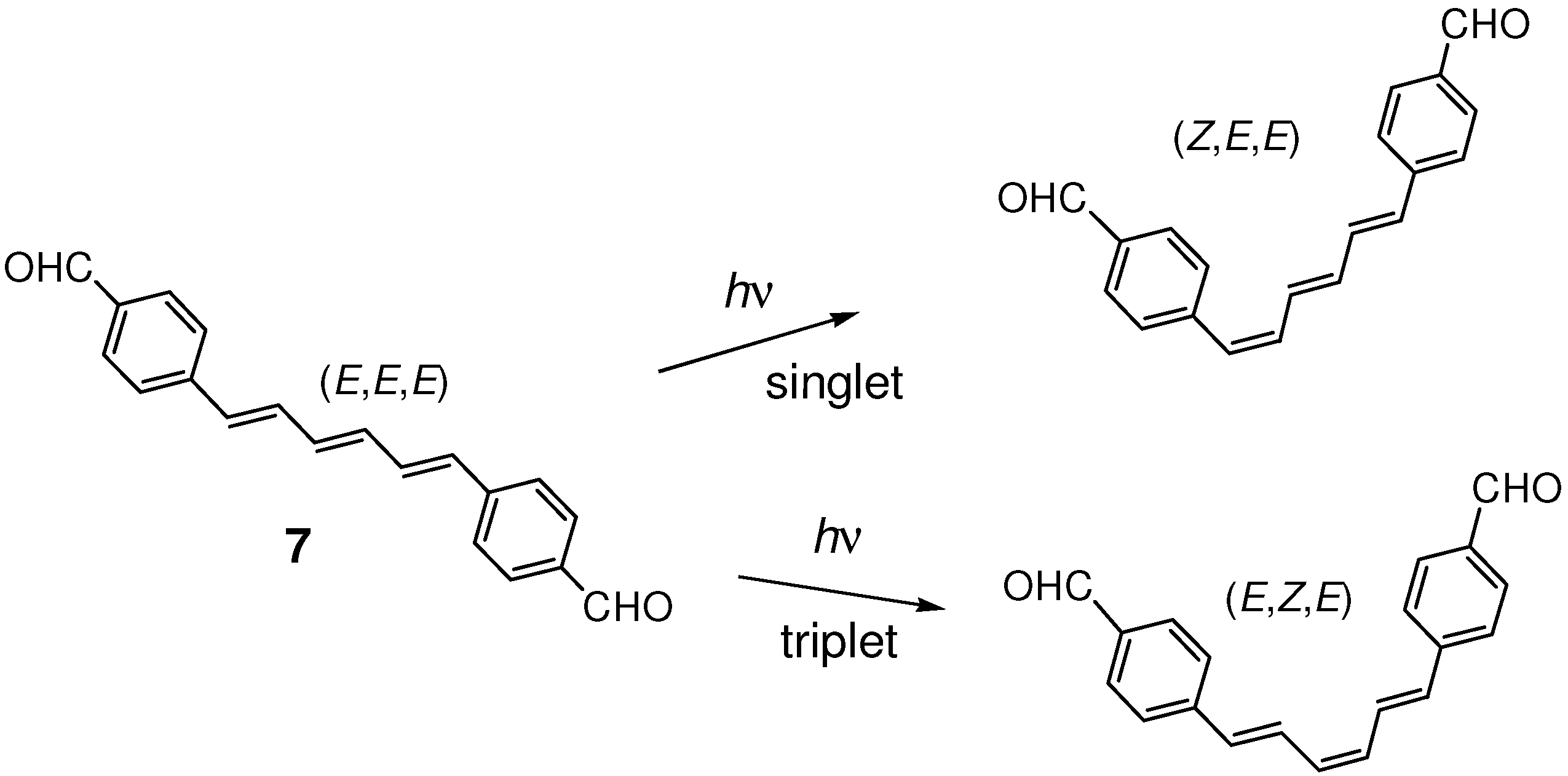
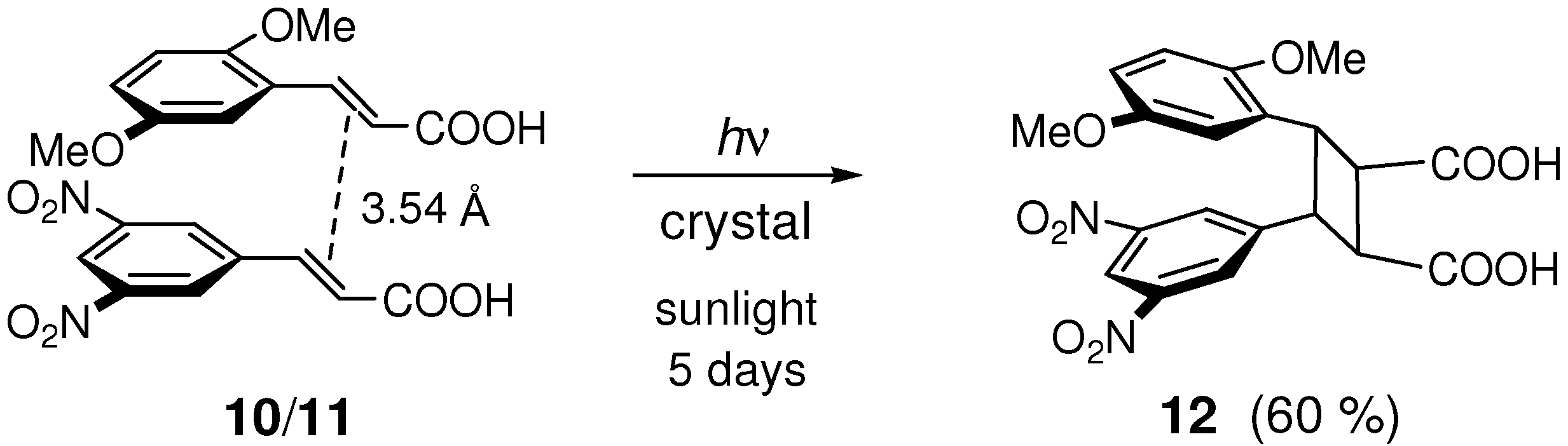
2.3. Nitro Substitution

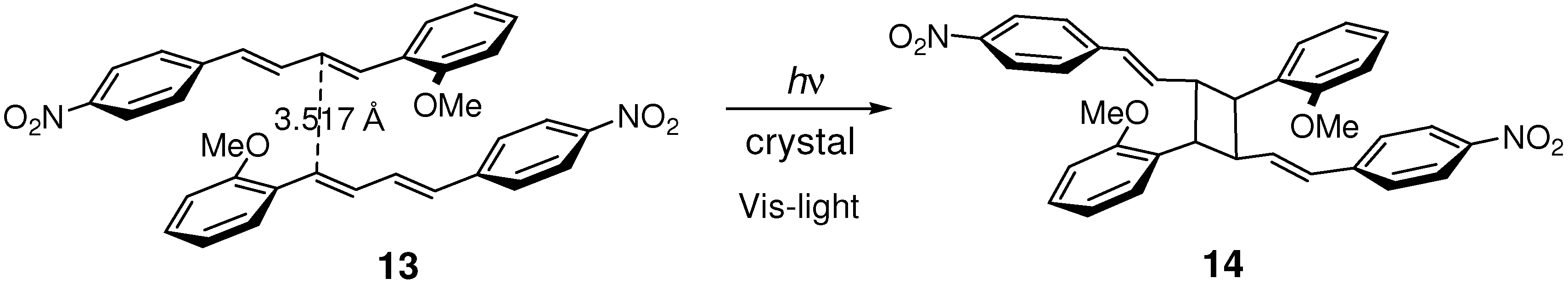
2.4. Alkoxy-Nitro (Donor-Acceptor) Substitution
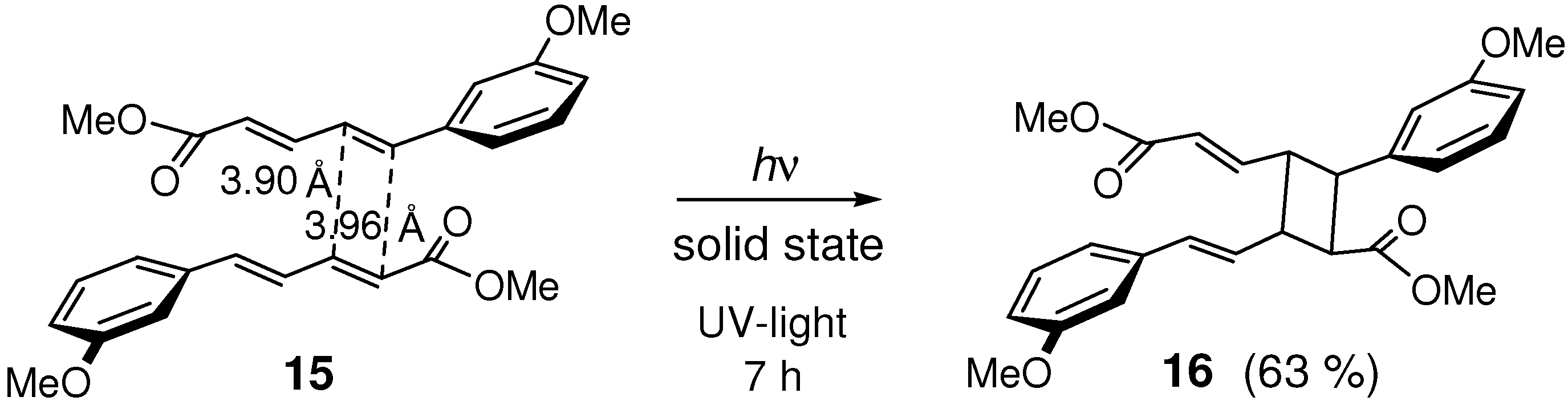

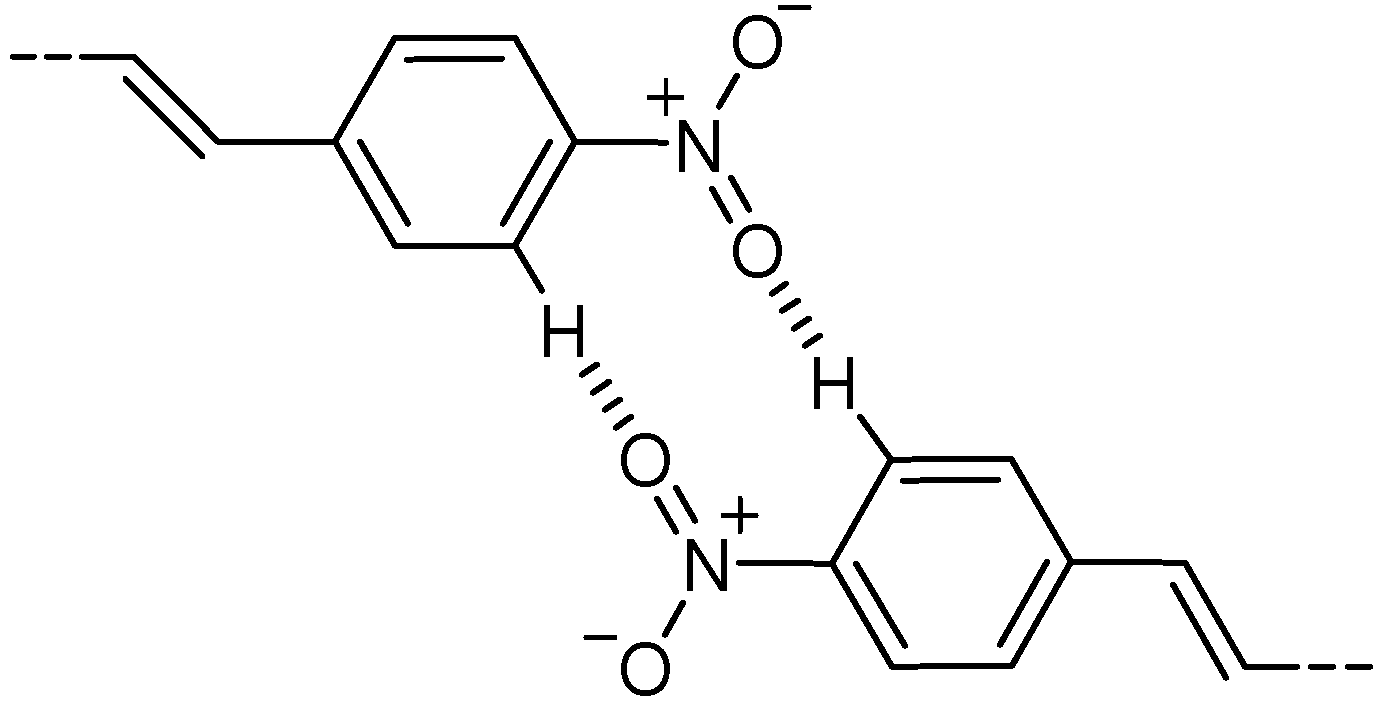
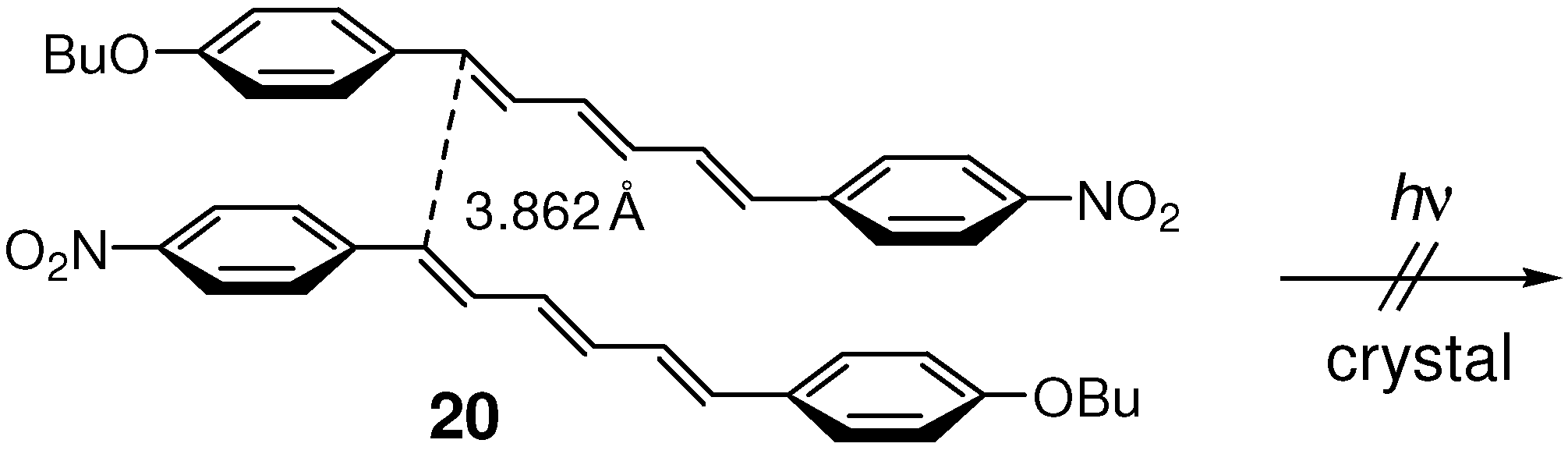
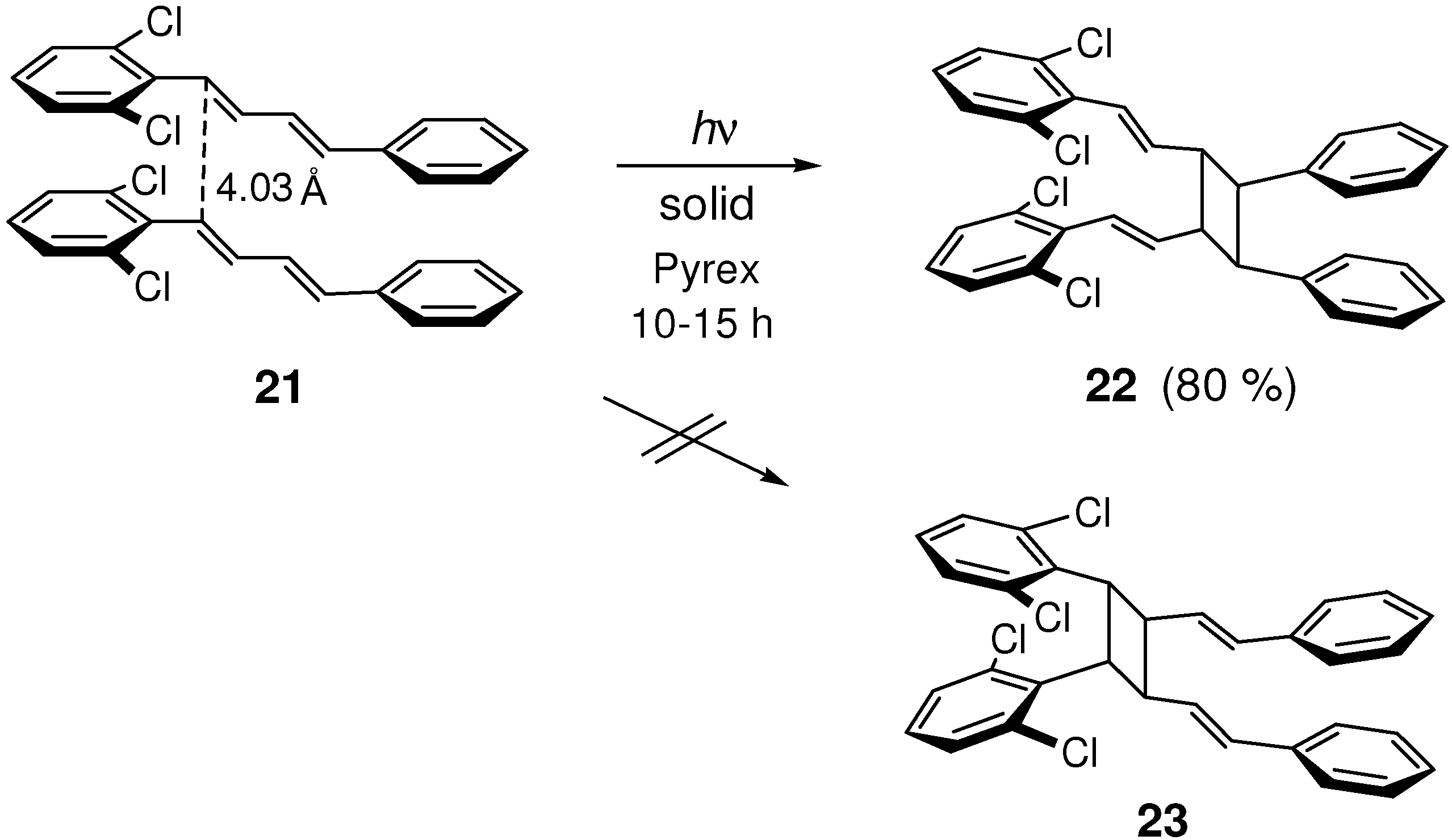
2.5. Halogen Substitution
2.5.1. Chlorine substitution
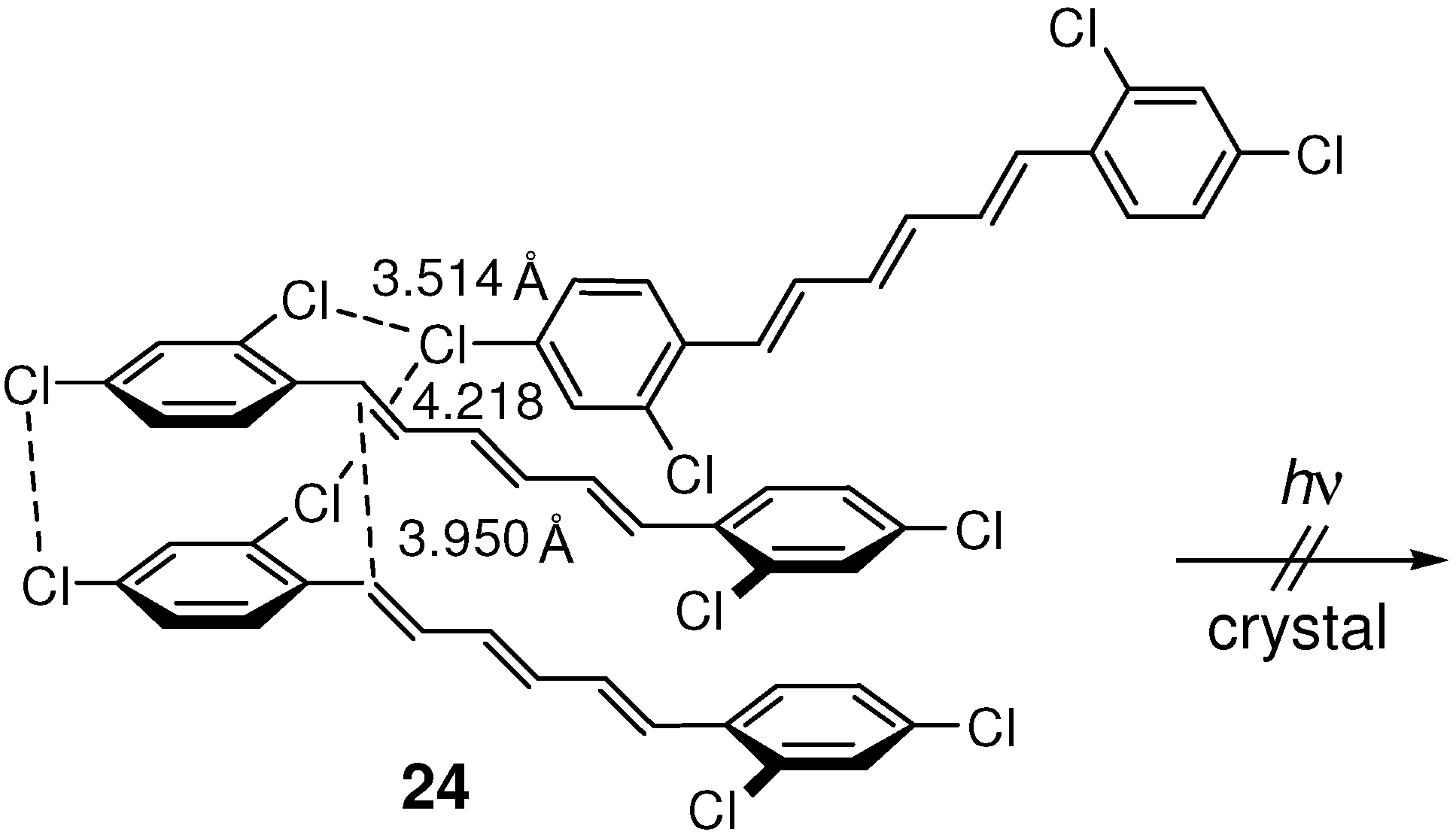
2.5.2. Fluorine substitution
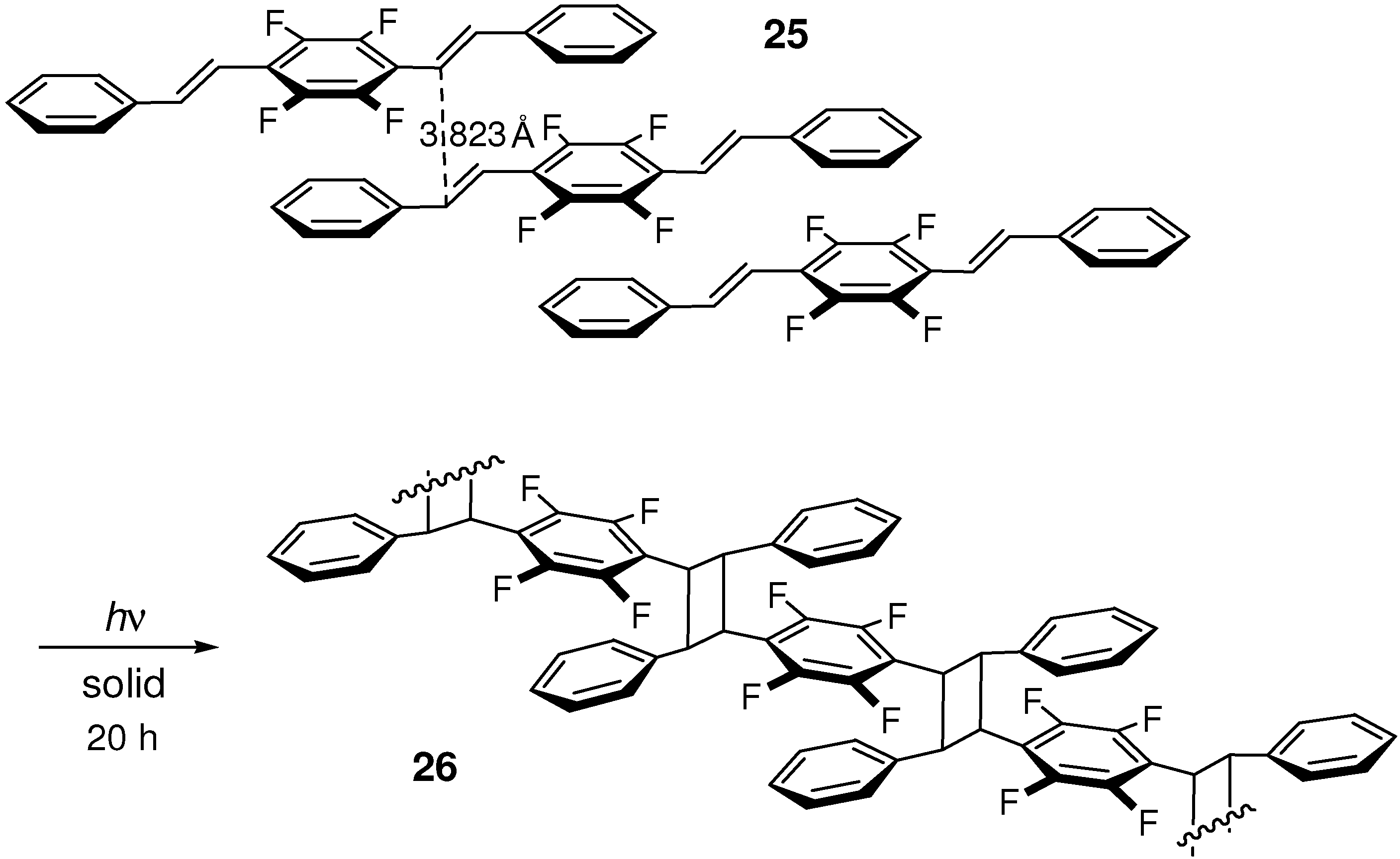
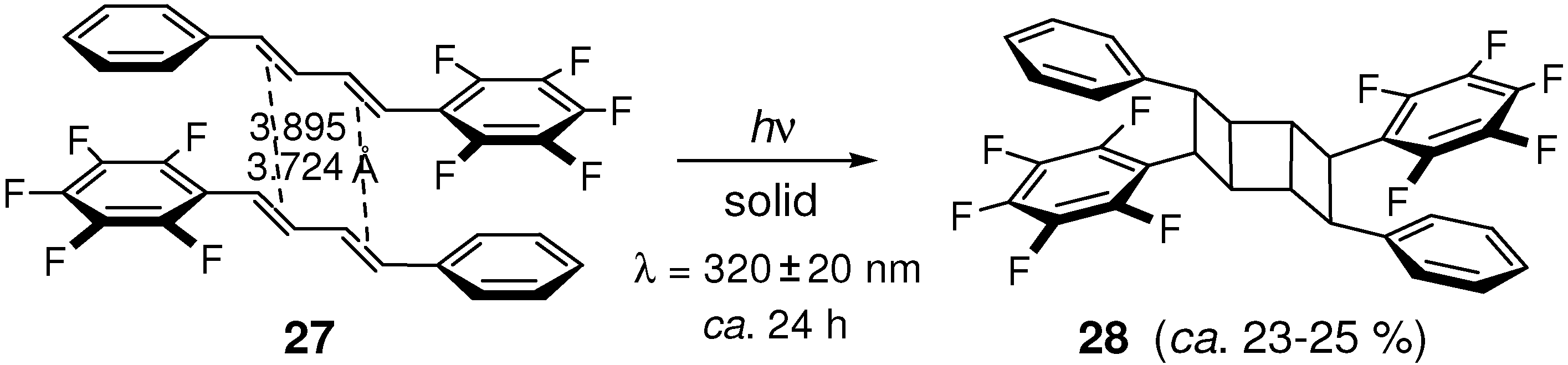
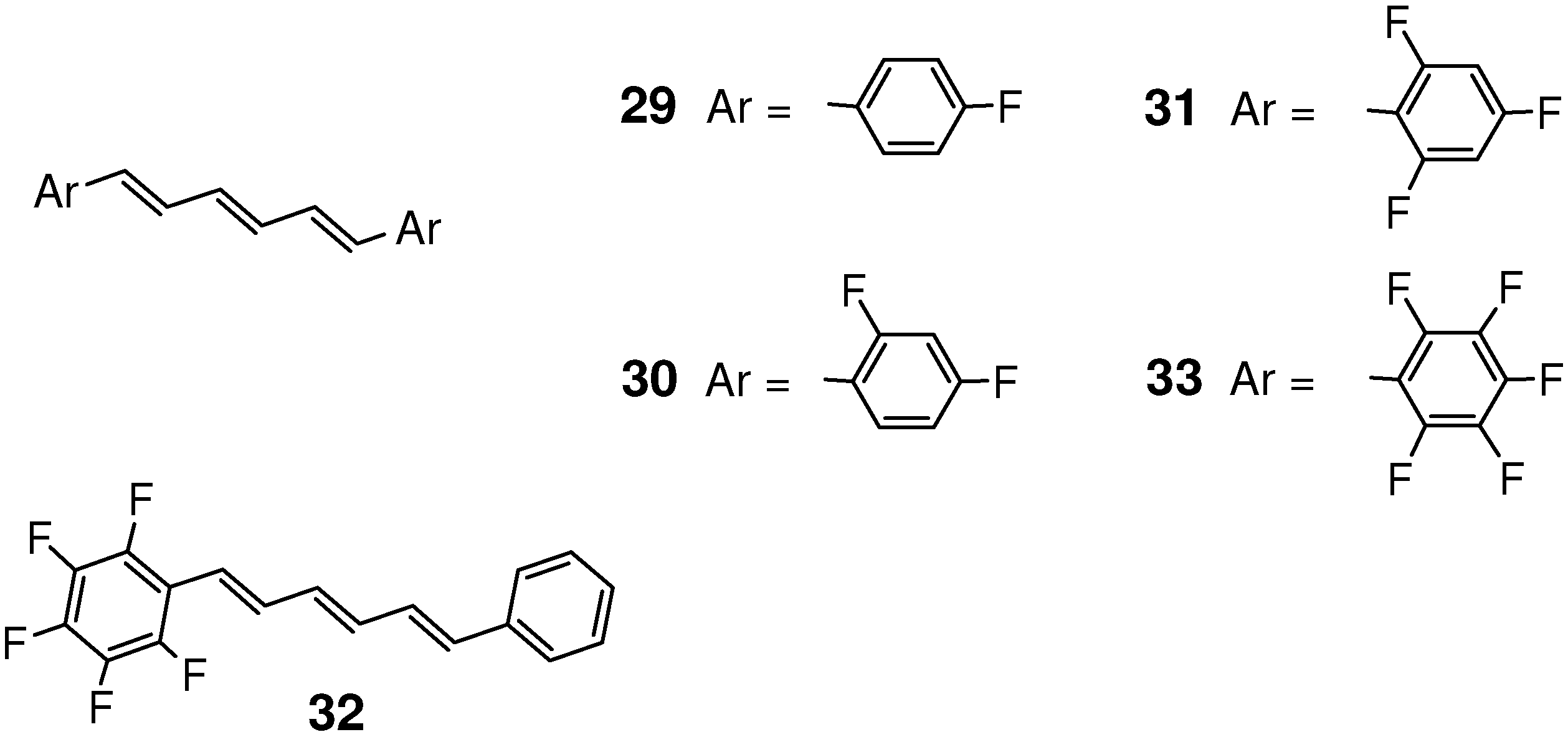
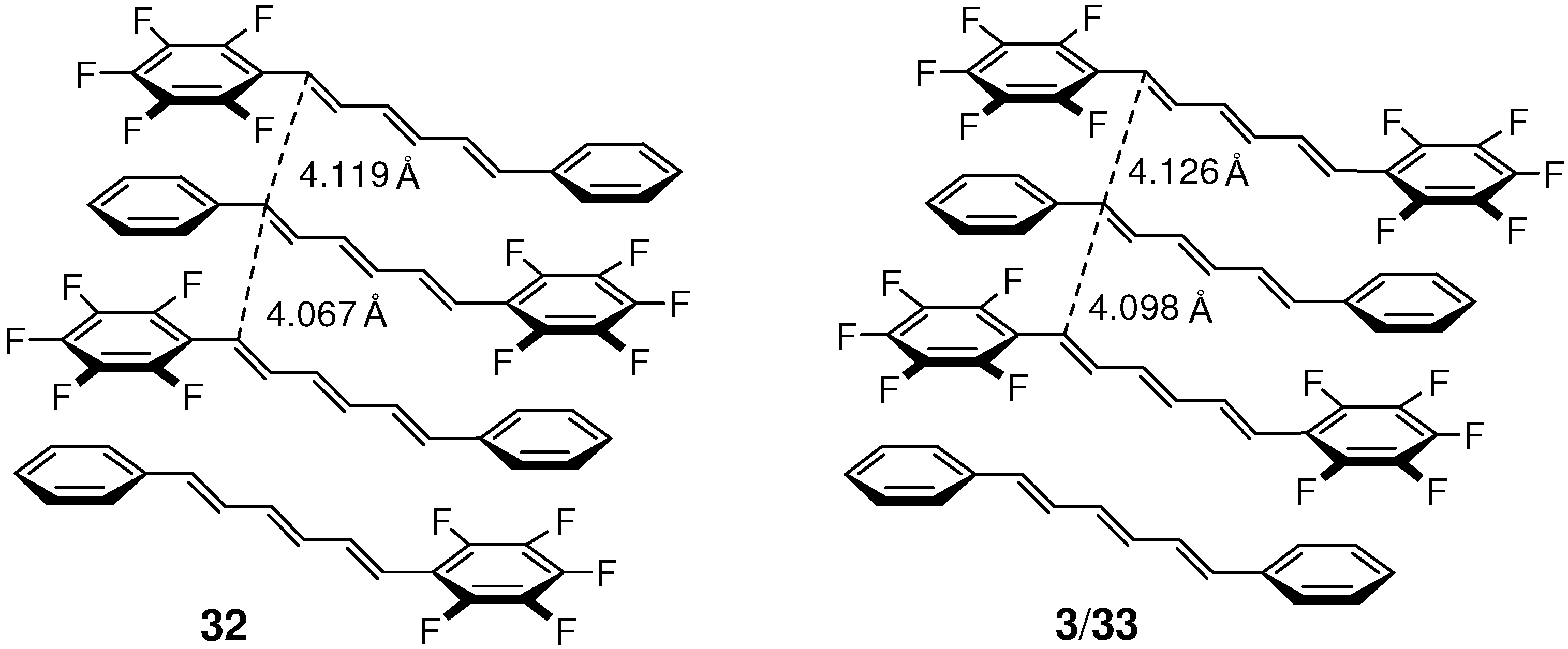
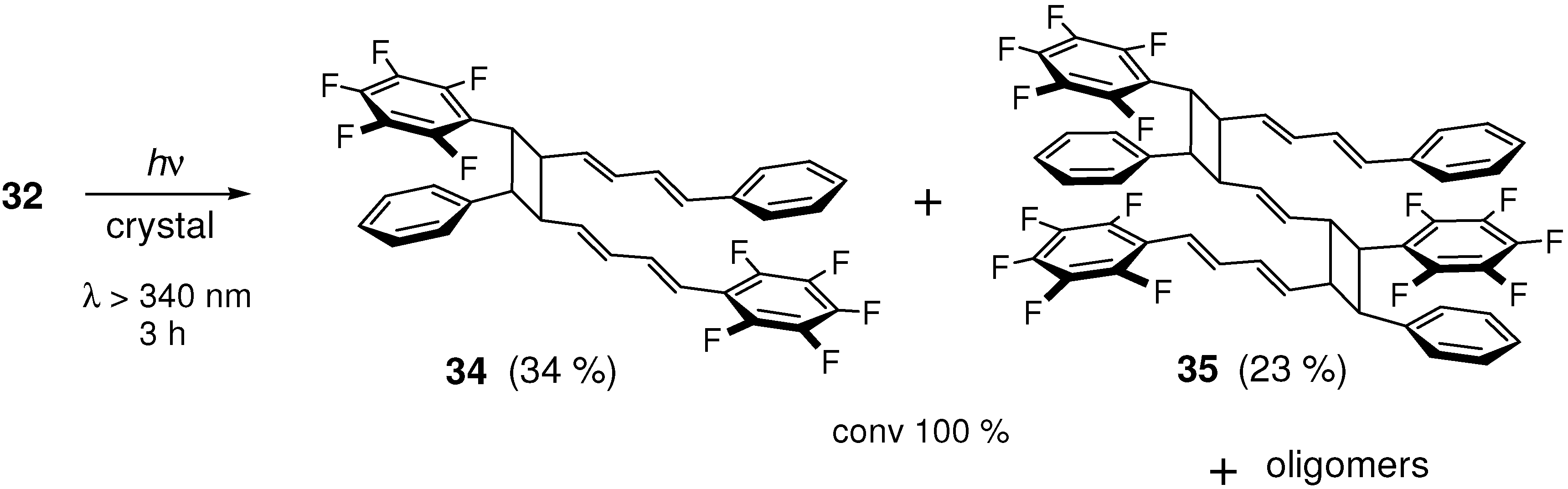
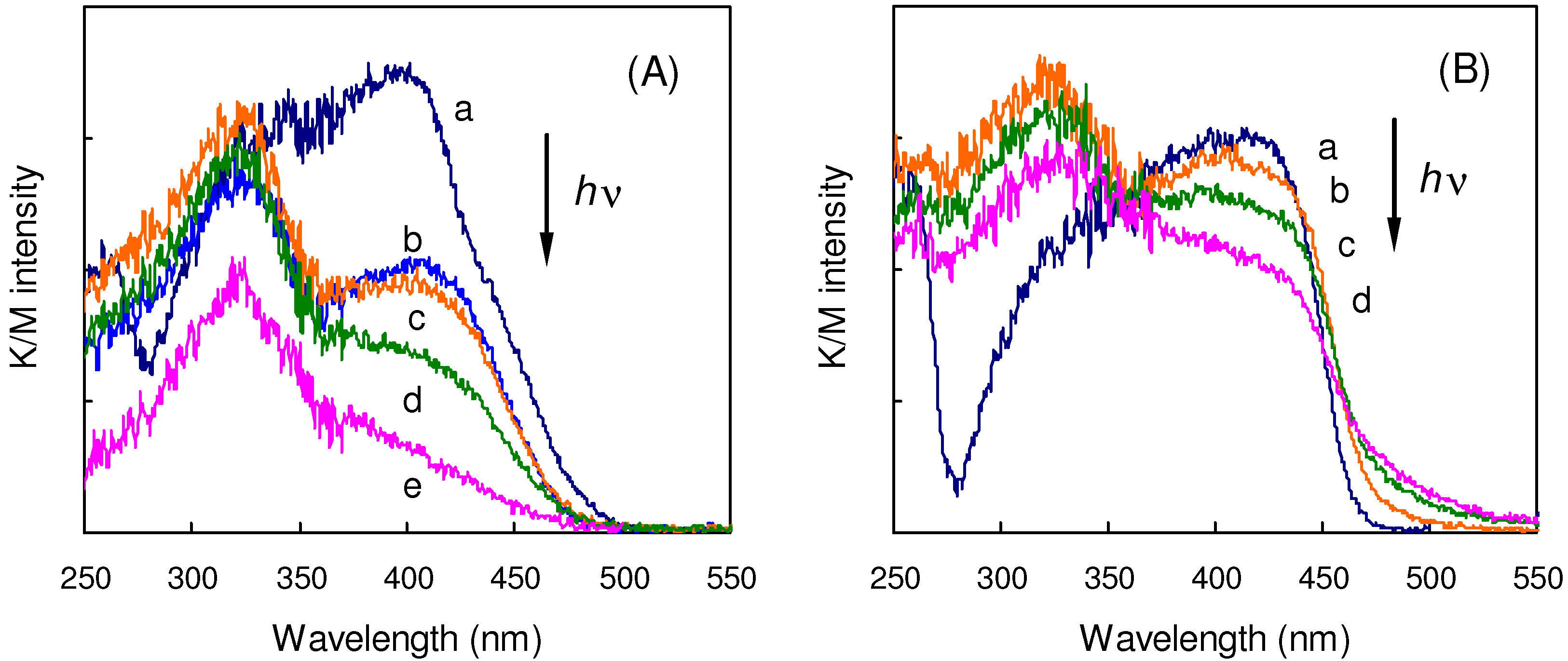
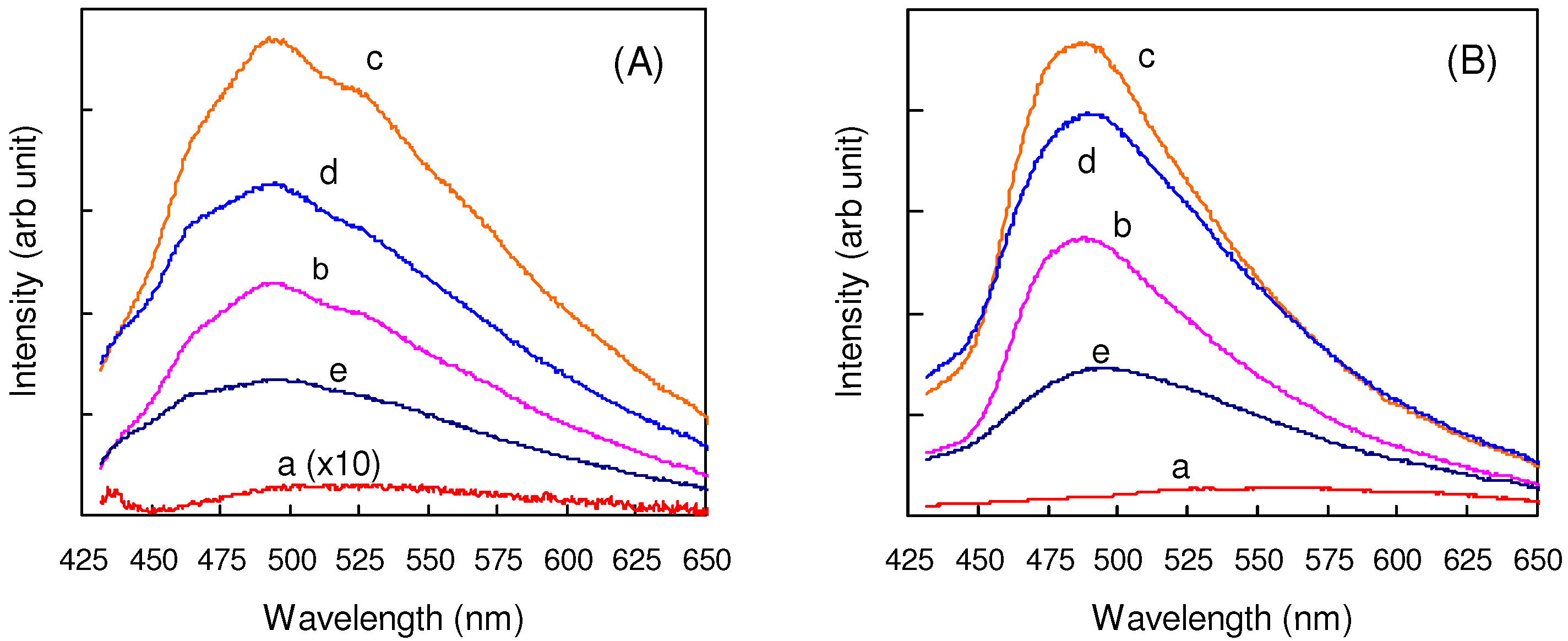
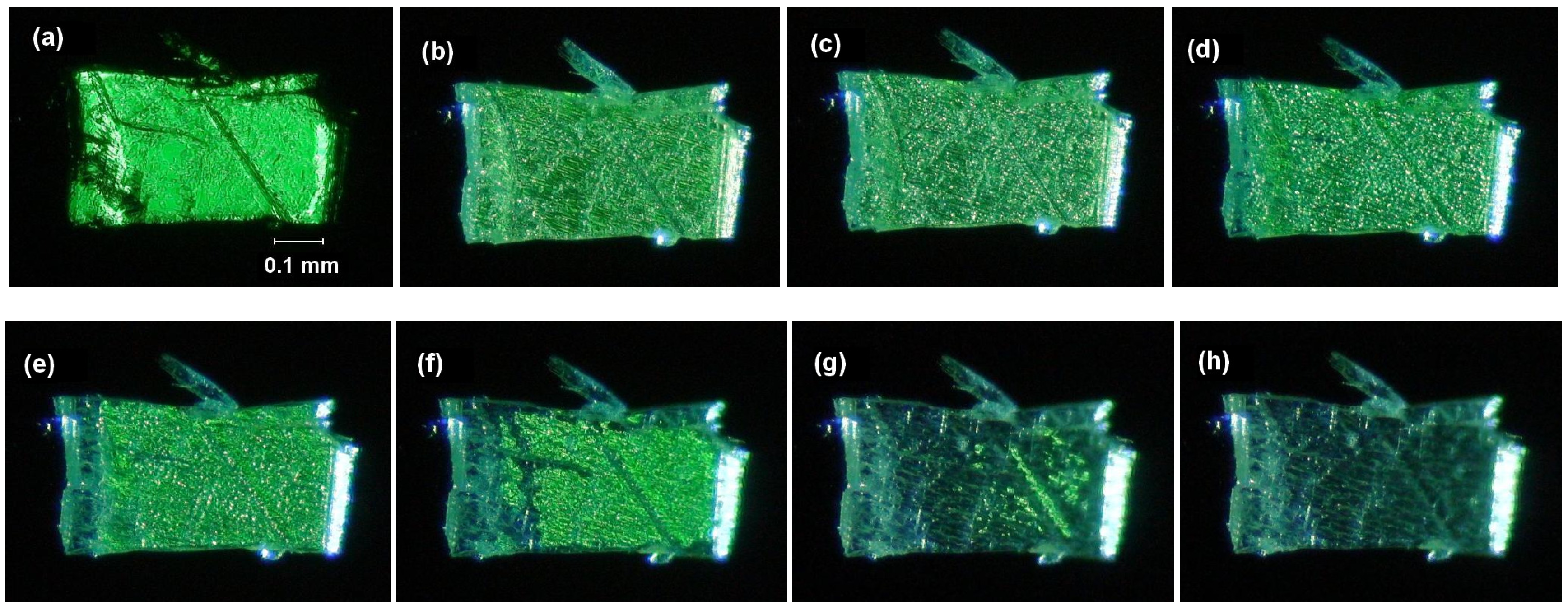
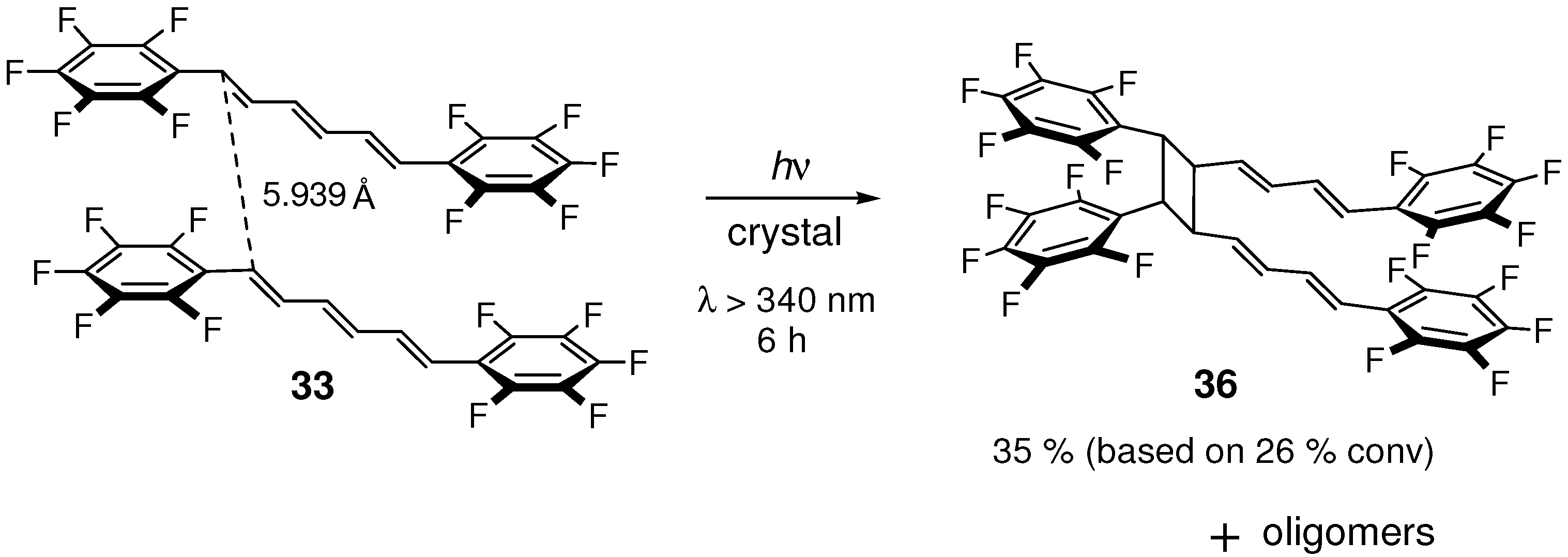
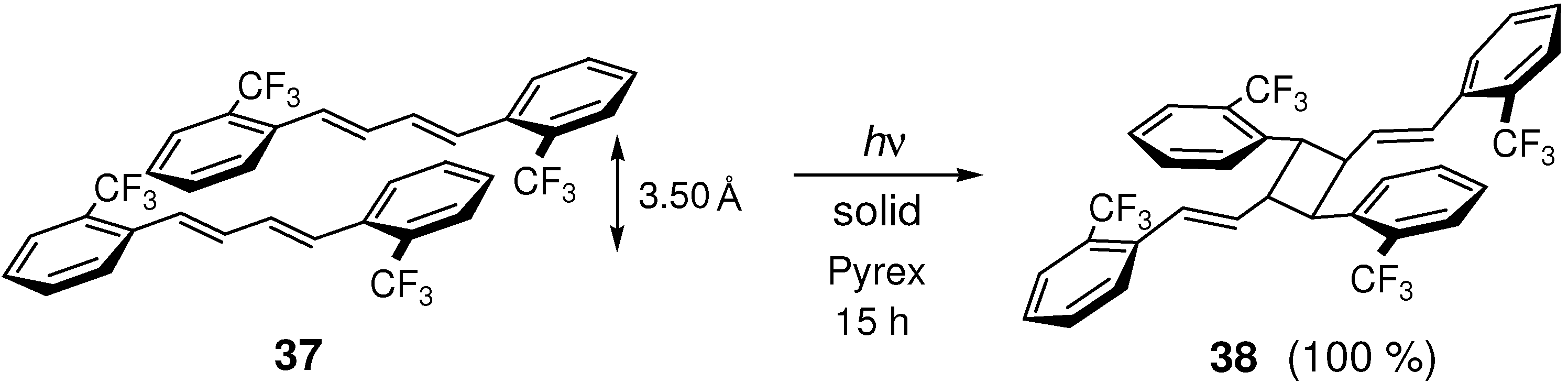
2.6. Substitution with Electron-Donating Groups
3. Atomic and Molecular Movements in Crystals during [2+2] Photodimerization and Photopolymerization
| Crystal | Double bond distance a (Å) | [2+2] Photoreactivity |
|---|---|---|
| 3 | 7.730 | no |
| 4 | 3.928 | yes |
| 7 | 3.926 | yes |
| 9 | 3.871 | no |
| 20 | 3.862 | no |
| 24 | 3.950 | no |
| 32 | 4.067, 4.119 | yes |
| 3/33 | 4.098, 4.126 | yes |
| 33 | 5.939 | yes |
4. Industrial Applications
4.1. Amorphous Materials
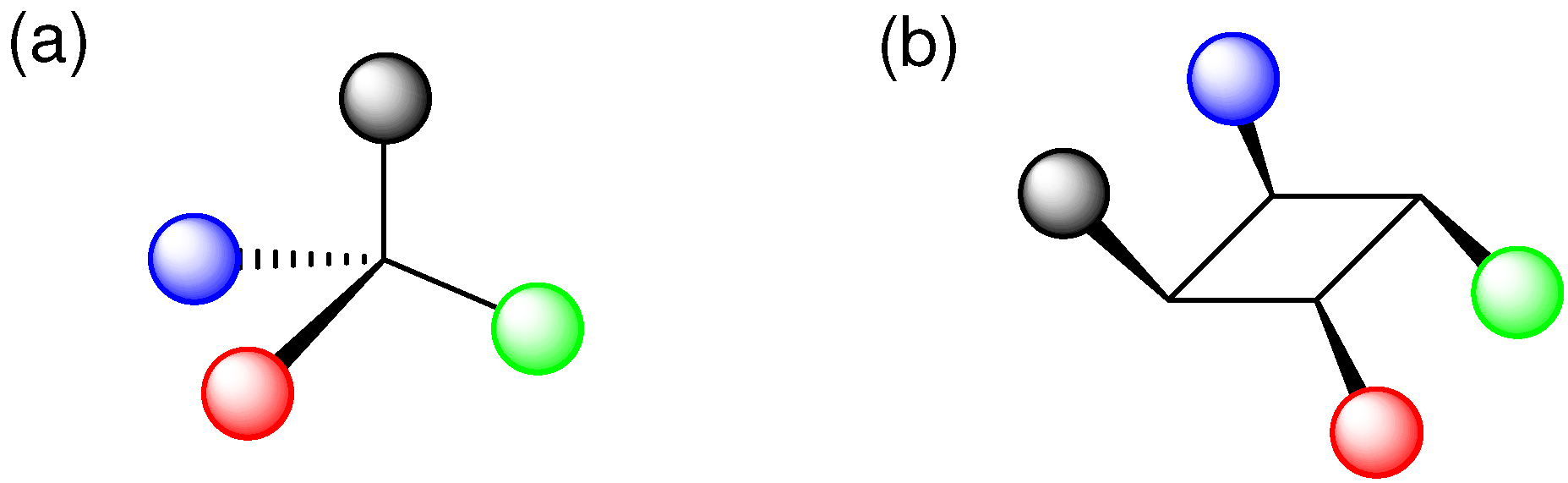
4.2. Photocrosslinking Materials
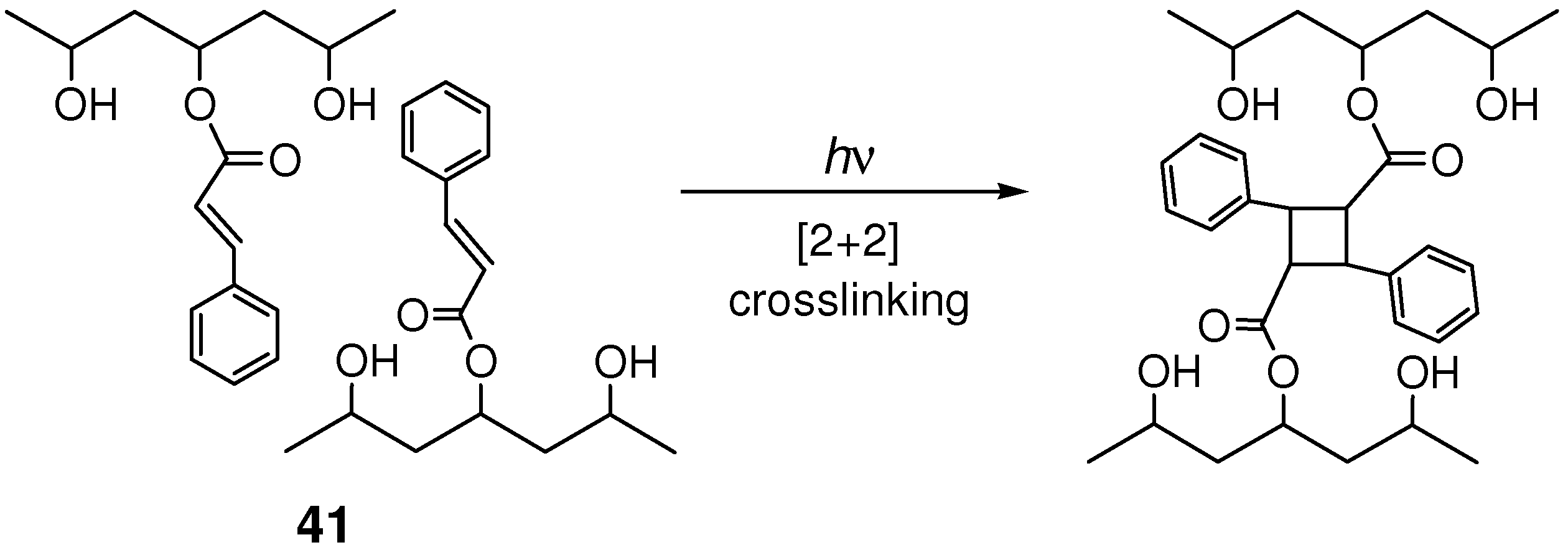
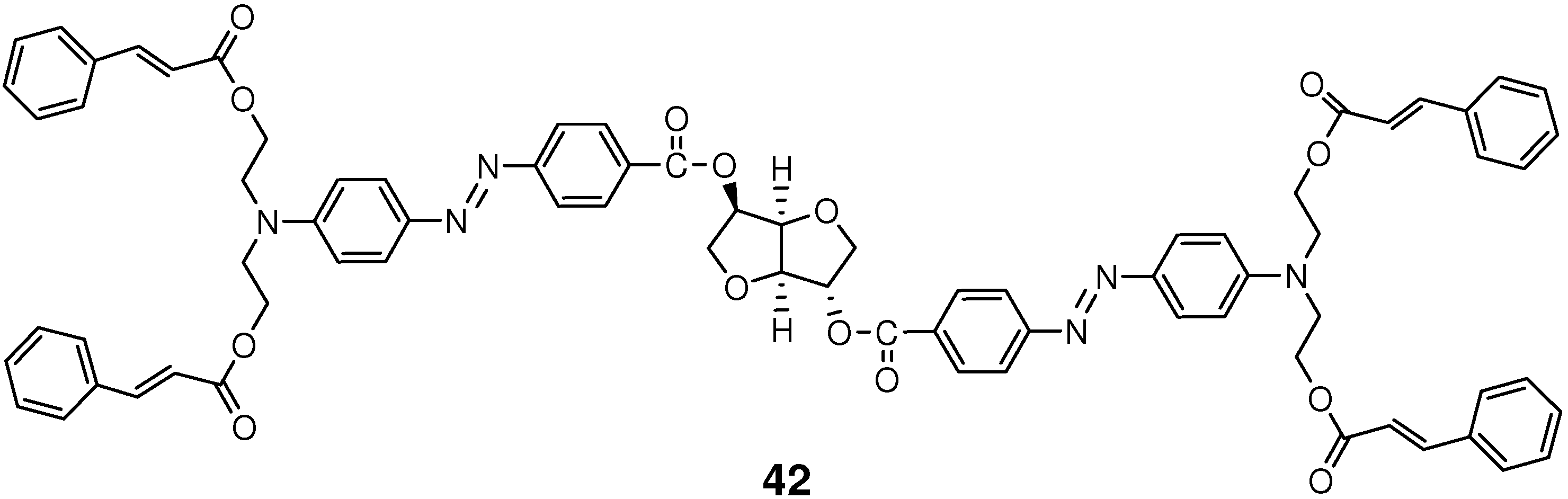
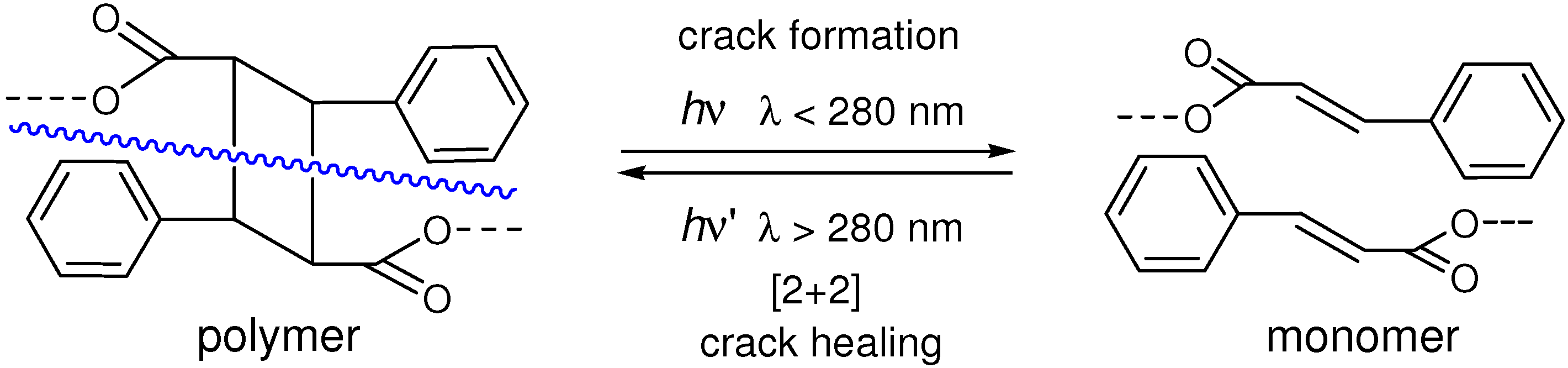
4.3. Photochemical Crack Healing in Polymeric Materials
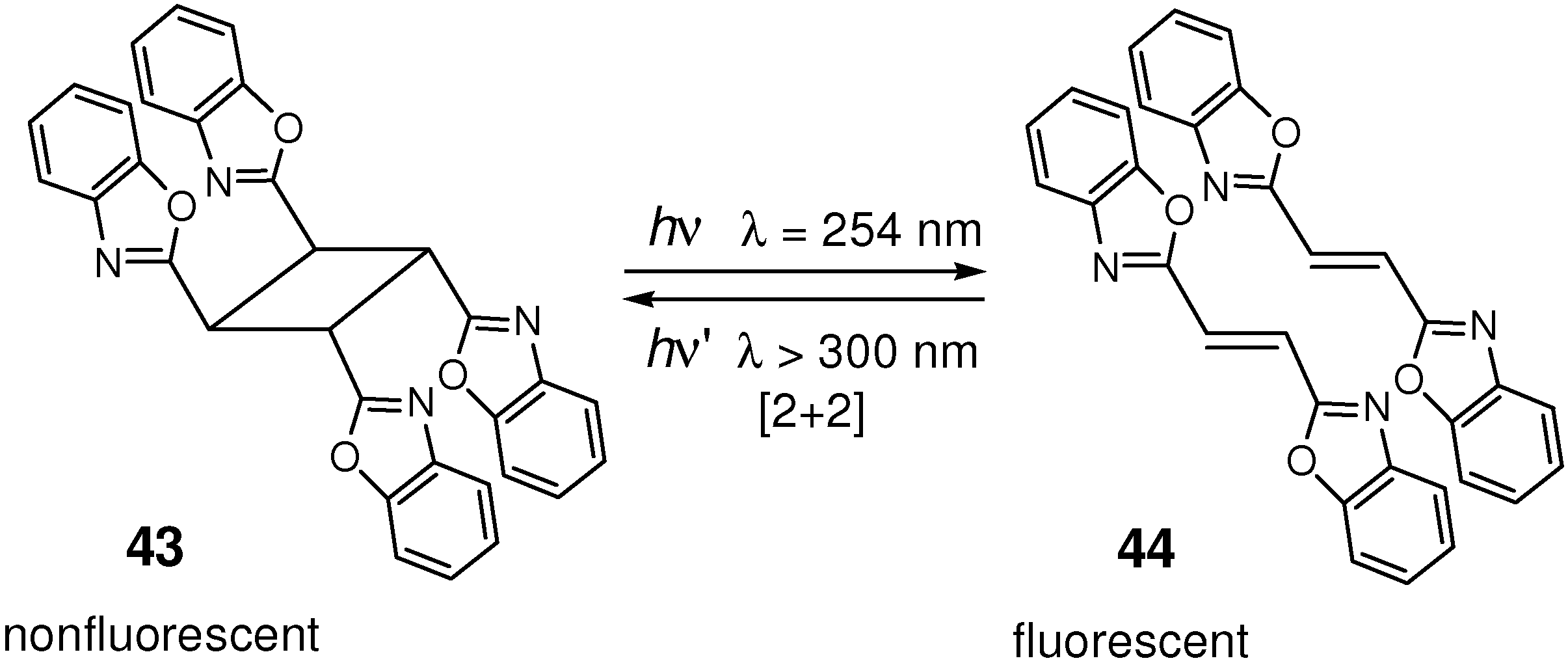
4.4. Optical Memories and Fluorescence Switches
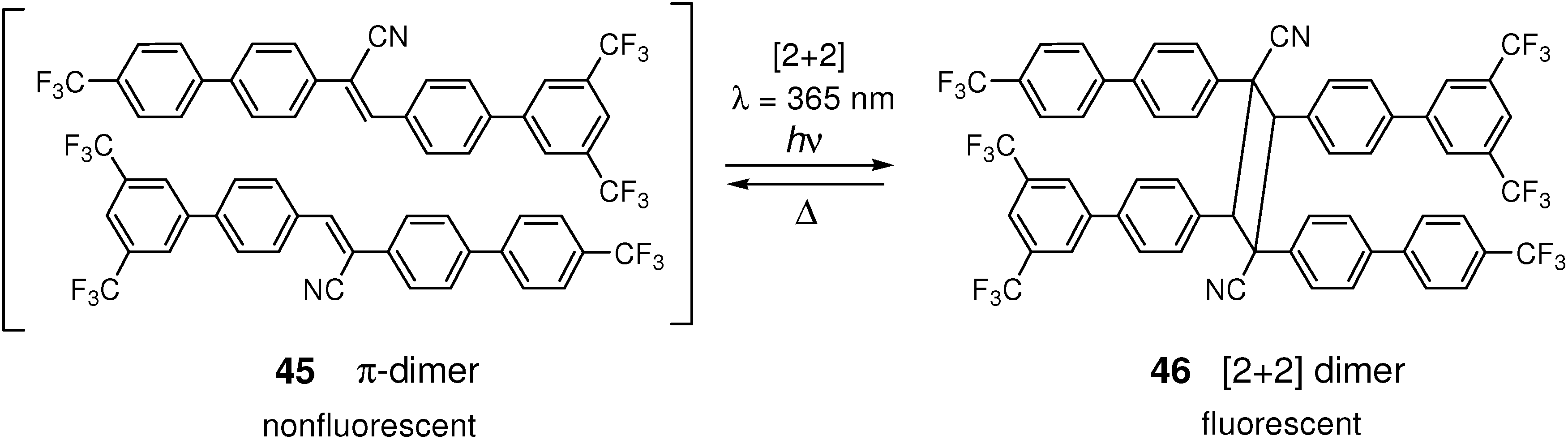
5. Conclusions
Acknowledgements
References and Notes
- Schmidt, G.M.J. Photodimerization in the solid state. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef]
- Cohen, M.D. The photochemistry of organic solids. Angew. Chem. Int. Ed. Engl. 1975, 14, 386–393. [Google Scholar] [CrossRef]
- Green, B.S.; Lahav, M.; Rabinovich, D. Asymmetric synthesis via reactions in chiral crystals. Acc. Chem. Res. 1979, 12, 191–197. [Google Scholar] [CrossRef]
- Gavezzotti, A.; Simonetta, M. Crystal chemistry in organic solids. Chem. Rev. 1982, 82, 1–13. [Google Scholar] [CrossRef]
- Ramamurthy, V. Organic photochemistry in organized media. Tetrahedron 1986, 42, 5753–5839. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Venkatesan, K. Photochemical reactions of organic crystals. Chem. Rev. 1987, 87, 433–481. [Google Scholar] [CrossRef]
- Murthy, G.S.; Arjunan, P.; Venkatesan, K.; Ramamurthy, V. Consequences of lattice relaxability in solid state photodimerizations. Tetrahedron 1987, 43, 1225–1240. [Google Scholar] [CrossRef]
- Ohashi, Y. Dynamical structure analysis of crystalline-state racemization. Acc. Chem. Res. 1988, 21, 268–274. [Google Scholar] [CrossRef]
- Photochemistry in Organized and Constrained Media; Ramamurthy, V. (Ed.) VCH: New York, NY, USA, 1991.
- Koshima, H.; Matsuura, T. Solid state photoreactions occurring at the interface between crystallites of two different organic compounds. J. Photochem. Photobiol. A Chem. 1996, 100, 85–91. [Google Scholar] [CrossRef]
- Ito, Y. Solid-state photoreactions in two-component crystals. Synthesis 1998, 1, 1–32. [Google Scholar] [CrossRef]
- Sonoda, Y. [2+2]-Photocycloadditions in the solid state. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd; Horspool, W., Lenci, F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; Chapter 73. [Google Scholar]
- Friščić, T.; MacGillivray, L.R. Single-crystal-to-single-crystal [2+2] photodimerizations: From discovery to design. Z. Kristallogr. 2005, 220, 351–363. [Google Scholar] [CrossRef]
- Toda, F. Solid state organic chemistry: Efficient reactions, remarkable yields, and stereoselectivity. Acc. Chem. Res. 1995, 28, 480–486. [Google Scholar] [CrossRef]
- Tanaka, K.; Toda, F. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef]
- Toda, F. Thermal and photochemical reactions in the solid state. Top. Curr. Chem. 2005, 254, 1–40. [Google Scholar]
- Kaupp, G. Waste-free synthesis and production all across chemistry with the benefit of self-assembled crystal packings. J. Phys. Org. Chem. 2008, 21, 630–643. [Google Scholar] [CrossRef]
- Maekawa, Y.; Lim, P.-J.; Saigo, K.; Hasegawa, M. Preparation of a crystalline linear high copolymer by topochemical photopolymerization of diolefin mixed crystals. Macromolecules 1991, 24, 5752–5755. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Reactions between or within molecular crystals. Angew. Chem. Int. Ed. 2004, 43, 4002–4011. [Google Scholar] [CrossRef]
- Avendaño, C.; Briceño, A. Concomitant [2+2] cycloaddition solid state reactions from co-crystals self-assembled via mechanochemistry. CrystEngCommunity 2009, 11, 408–411. [Google Scholar] [CrossRef]
- Nagarathinam, M.; Vittal, J.J. Solid-state synthesis of coordination polymers for [2+2] photoreactions by grinding. Aust. J. Chem. 2010, 63, 589–595. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.P.; Vittal, J.J. Solid-state photochemical behavior of a triple-stranded ladder coordination polymer. Inorg. Chem. 2010, 49, 10–12. [Google Scholar]
- Cohen, M.D.; Schmidt, G.M.J. Topochemistry. Part I. A survey. J. Chem. Soc. 1964, 1996–2000. [Google Scholar]
- Cohen, M.D.; Schmidt, G.M.J.; Sonntag, F.I. Topochemistry. Part II. The photochemistry of trans-cinnamic acids. J. Chem. Soc. 1964, 2000–2013. [Google Scholar]
- Schmidt, G.M.J. Topochemistry. Part III. The crystal chemistry of some trans-cinnamic acids. J. Chem. Soc. 1964, 2014–2021. [Google Scholar] [CrossRef]
- Quina, F.H.; Whitten, D.G. Photochemical reactions in organized monolayer assemblies. 4. Photodimerization, photoisomerization, and excimer formation with surfactant olefins and dienes in monolayer assemblies, crystals, and micelles. J. Am. Chem. Soc. 1977, 99, 877–883. [Google Scholar] [CrossRef]
- Novak, K.; Enkelmann, V.; Wegner, G.; Wagener, K.B. Crystallographic study of a single crystal to single crystal photodimerization and its thermal reverse reaction. Angew. Chem. Int. Ed. Engl. 1993, 32, 1614–1616. [Google Scholar] [CrossRef]
- Kole, G.K.; Tan, G.K.; Vittal, J.J. Anion-controlled stereoselective synthesis of cyclobutane derivatives by solid-state [2+2] cycloaddition reaction of the salts of trans-3-(4-pyridyl) acrylic acid. Org. Lett. 2010, 12, 128–131. [Google Scholar] [CrossRef]
- Vedernikov, A.I.; Kuz´mina, L.G.; Sazonov, S.K.; Lobova, N.A.; Loginov, P.S.; Churakov, A.V.; Strelenko, Y.A.; Howard, J.A.K.; Alfimov, M.V.; Gromov, S.P. Styryl dyes. Synthesis and study of the solid-state [2+2] autophotocycloaddition by NMR spectroscopy and X-ray diffraction. Russ. Chem. Bull. Int. Ed. 2007, 56, 1860–1883. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Vedernikov, A.I.; Sazonov, S.K.; Lobova, N.A.; Loginov, P.S.; Howard, J.A.K.; Alfimov, M.V.; Gromov, S.P. Specific features of the crystal packing that enable styryl dyes of the pyridine series to undergo the solid-phase [2+2] photocycloaddition including the process with single crystal retention. Crystallogr. Rep. 2008, 53, 428–450. [Google Scholar] [CrossRef]
- Tulyakova, E.V.; Fedorova, O.A.; Fedorov, Y.V.; Anisimov, A.V. [2+2]-Photocycloaddition reaction of self-assembled crown-containing 2-styrylpyridinium perchlorate in a solid state. J. Photochem. Photobiol. A Chem. 2008, 200, 90–95. [Google Scholar] [CrossRef]
- Kuz´mina, L.G.; Vedernikov, A.I.; Lobova, N.A.; Sazonov, S.K.; Basok, S.S.; Howard, J.A.K.; Gromov, S.P. Design of crystal packings of styrylheterocycles and [2+2] photocycloaddition reactions in their single crystals 6. Synthesis and crystal packings of neutral crown-containing and model styrylheterocycles. Russ. Chem. Bull. Int. Ed. 2009, 58, 1192–1210. [Google Scholar] [CrossRef]
- Friščić, T.; MacGillivray, L.R. Cyclophanes and ladderanes: Molecular targets for supramolecular chemists. Supramol. Chem. 2005, 17, 47–51. [Google Scholar]
- MacGillivray, L.R.; Papaefstathiou, G.S.; Friščić, T.; Hamilton, T.D.; Bučar, D.-K.; Chu, Q.; Varshney, D.B.; Georgiev, I.G. Supramolecular control of reactivity in the solid state: From templates to ladderanes to metal-organic frameworks. Acc. Chem. Res. 2008, 41, 280–291. [Google Scholar] [CrossRef]
- MacGillivray, L.R. Organic synthesis in the solid state via hydrogen-bond-driven self-assembly. J. Org. Chem. 2008, 73, 3311–3317. [Google Scholar] [CrossRef]
- Nagarathinam, M.; Vittal, J.J. A rational approach to crosslinking of coordination polymers using the photochemical [2+2] cycloaddition reaction. Macromol. Rapid Commun. 2006, 27, 1091–1099. [Google Scholar] [CrossRef]
- Nagarathinam, M.; Peedikakkal, A.M.P.; Vittal, J.J. Stacking of double bonds for photochemical [2+2] cycloaddition reactions in the solid state. Chem. Commun. 2008, 5277–5288. [Google Scholar]
- Han, Y.-F.; Lin, Y.-J.; Jia, W.-G.; Wang, G.-L.; Jin, G.-X. Template-controlled topochemical photodimerization based on ''organometallic macrocycles'' through single-crystal to single-crystal transformation. Chem. Commun. 2008, 1807–1809. [Google Scholar]
- Han, Y.-F.; Jia, W.-G.; Yu, W.-B.; Jin, G.-X. Stepwise formation of organometallic macrocycles, prisms and boxes from Ir, Rh and Ru-based half-sandwich units. Chem. Soc. Rev. 2009, 38, 3419–3434. [Google Scholar] [CrossRef]
- Zhang, W.-Z.; Han, Y.-F.; Lin, Y.-J.; Jin, G.-X. [2+2] Photodimerization in the solid state aided by molecular templates of rectangular macrocycles bearing oxamidato ligands. Organometallics 2010, 29, 2842–2849. [Google Scholar] [CrossRef]
- Hasegawa, M. Photopolymerization of diolefin crystals. Chem. Rev. 1983, 83, 507–518. [Google Scholar] [CrossRef]
- Hasegawa, M. Photodimerization and photopolymerization of diolefin crystals. Adv. Phys. Org. Chem. 1995, 30, 117–171. [Google Scholar] [CrossRef]
- Cohen, M.D.; Elgavi, A.; Green, B.S.; Ludmer, Z.; Schmidt, G.M.J. Photodimerization and excimer emission in a crystalline 1,4-diphenylbutadiene. J. Am. Chem. Soc. 1972, 94, 6776–6779. [Google Scholar] [CrossRef]
- Singh, A.K.; Krishna, T.S.R. Fluorescence and photodimerization studies of cyano-substituted diphenylbutadienes. J. Phys. Chem. A 1997, 101, 3066–3069. [Google Scholar] [CrossRef]
- Sonoda, Y.; Miyazawa, A.; Hayashi, S.; Sakuragi, M. Intermolecular [2+2] photocycloaddition of formyl- and cyano-substituted diphenylhexatrienes in the solid state. Chem. Lett. 2001, 30, 410–411. [Google Scholar]
- Drenth, W.; Wiebenga, E.H. Structure of α,ω-diphenylpolyenes. 1. Crystal data of 1,4-diphenyl-1,3-butadiene, 1,6-diphenyl-1,3,5-hexatriene and 1,8-diphenyl-1,3,5,7-octatetraene. Recl. Trav. Chim. Pays-Bas 1953, 72, 39–43. [Google Scholar]
- Glaser, R.; Dendi, L.R.; Knotts, N.; Barnes, C.L. Ab initio and crystal structures of (E,E)-1,4-diphenylbutadiene: A new type ofarene-arene double T-contact and an interesting interlayer cooperation involving diastereoisomeric contacts. Cryst. Growth Des. 2003, 3, 291–300. [Google Scholar] [CrossRef]
- Gavezzotti, A.; Desiraju, G.R. A systematic analysis of packing energies and other packing parameters for fused-ring aromatic hydrocarbons. Acta Crystallogr. 1988, B44, 427–434. [Google Scholar]
- Desiraju, G.R.; Gavezzotti, A. Crystal structures of polynuclear aromatic hydrocarbons. Classification, rationalization and prediction from molecular structure. Acta Crystallogr. 1989, B45, 473–482. [Google Scholar]
- Hall, T.; Bachrach, S.M.; Spangler, C.W.; Sapochak, L.S.; Lin, C.T.; Guan, H.W.; Rogers, R.D. Structure of all-trans-1,6-diphenyl- (A) and all-trans-1,6-bis(o-methoxyphenyl)-1,3,5-hexatriene (B). Acta Crystallogr. 1989, C45, 1541–1543. [Google Scholar]
- Sonoda, Y.; Morii, H.; Sakuragi, M.; Suzuki, Y. Substituent effect on the cis-trans photoisomerization of trans,trans,trans-1,6-diphenyl-1,3,5-hexatrienes. Chem. Lett. 1998, 27, 349–350. [Google Scholar]
- Sonoda, Y.; Kwok, W.M.; Petrasek, Z.; Ostler, R.; Matousek, P.; Towrie, M.; Parker, A.W.; Phillips, D. Solvent effects on the photophysical and photochemical properties of (E,E,E)-1,6-bis(4-nitrophenyl)hexa-1,3,5-triene. J. Chem. Soc. Perkin Trans. 2 2001, 308–314. [Google Scholar]
- The crystal structures of 4 and 7 are to be published elsewhere
- Reddy, D.S.; Goud, B.S.; Panneerselvam, K.; Desiraju, G.R. C–H···N mediated hexagonal network in the crystal structure of the 1:1 molecular complex 1,3,5-tricyanobenzene-hexamethylbenzene. J. Chem. Soc. Chem. Commun. 1993, 663–664. [Google Scholar]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Desiraju, G.R. Designer crystals: Intermolecular interactions, network structures and supramolecular synthons. Chem. Commun. 1997, 1475–1482. [Google Scholar] [CrossRef]
- Nangia, A.; Desiraju, G.R. Supramolecular structures - reason and imagination. Acta Crystallogr. 1998, A54, 934–944. [Google Scholar]
- Dhurjati, M.S.K.; Sarma, J.A.R.P.; Desiraju, G.R. Unusual [2+2] topochemical cycloadditions of 3-cyano- and 4-cyano-cinnamic acids: Temperature dependent solid state photochemical reactions. J. Chem. Soc. Chem. Commun. 1991, 1702–1703. [Google Scholar]
- Sonoda, Y.; Kawanishi, Y.; Ikeda, T.; Goto, M.; Hayashi, S.; Yoshida, Y.; Tanigaki, N.; Yase, K. Fluorescence spectra for the microcrystals and thin films of trans,trans,trans-1,6-diphenyl-1,3,5-hexatrienes. J. Phys. Chem. B 2003, 107, 3376–3383. [Google Scholar]
- Sonoda, Y.; Suzuki, Y. Stereoselective Z,E-photoisomerization of formyl-substituted (E,E,E)-1,6-diphenylhexa-1,3,5-triene in solution. J. Chem. Soc. Perkin Trans. 2 1996, 401–404. [Google Scholar] [CrossRef]
- Sonoda, Y.; Suzuki, Y. Solvent-dependent cis-trans one-way photoisomerization of bisformyl-substituted 1,6-diphenyl-1,3,5-hexatriene. Chem. Lett. 1996, 25, 659–660. [Google Scholar] [CrossRef]
- Sonoda, Y.; Kawanishi, Y.; Sakuragi, M. A heavy-atom effect on the cis-trans photoisomerization of bisforlmyl-substituted trans,trans,trans-1,6-diphenyl-1,3,5-hexatriene. Chem. Lett. 1999, 28, 587–588. [Google Scholar]
- Sarma, J.A.R.P.; Desiraju, G.R. The role of Cl···Cl and C–H···O interactions in the crystal engineering of 4-A short-axis structures. Acc. Chem. Res. 1986, 19, 222–228. [Google Scholar] [CrossRef]
- Desiraju, G.R. The C–H···O hydrogen bond in crystals: What is it? Acc. Chem. Res. 1991, 24, 290–296. [Google Scholar] [CrossRef]
- Desiraju, G.R. The C–H···O hydrogen bond: Structural implications and supramolecular design. Acc. Chem. Res. 1996, 29, 441–449. [Google Scholar] [CrossRef]
- Steiner, T.; Desiraju, G.R. Distinction between the weak hydrogen bond and the van der Waals interaction. Chem. Commun. 1998, 891–892. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Desiraju, G.R. C–H···O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem. Commun. 2005, 2995–3001. [Google Scholar] [CrossRef]
- Nakanishi, F.; Nakanishi, H.; Tasai, T.; Suzuki, Y.; Hasegawa, M. Water participation in the crystalline state photoreaction photodimerization of p-formylcinnamic acid. Chem. Lett. 1974, 3, 525–528. [Google Scholar]
- Nakanishi, F.; Nakanishi, H.; Tsuchiya, M.; Hasegawa, M. Water-participation in the crystalline-state photodimerization of cinnamic acid derivatives. A new type of organic photoreaction. Bull. Chem. Soc. Jpn. 1976, 49, 3096–3099. [Google Scholar] [CrossRef]
- Ghosh, U.; Misra, T.N. Spectroscopic studies of photochemical reactions in organic solids. Photodimerization of p-formylcinnamic acid. Bull. Chem. Soc. Jpn. 1985, 58, 2403–2406. [Google Scholar] [CrossRef]
- Nakanishi, H.; Hasegawa, M.; Mori, T. Structure of the β-form of p-formylcinnamic acid, C10H8O3, a photodimerizable crysta. Acta Crystallogr. 1985, C41, 70–71. [Google Scholar]
- Sharma, C.V.K.; Desiraju, G.R. C–H···O hydrogen bond patterns in crystalline nitro compounds: Studies in solid-state molecular recognition. J. Chem. Soc. Perkin Trans. 2 1994, 2345–2352. [Google Scholar]
- Thaimattam, R.; Xue, F.; Sarma, J.A.R.P.; Mak, T.C.W.; Desiraju, G.R. Inclusion compounds of tetrakis(4-nitrophenyl)methane: C–H···O networks, pseudopolymorphism, and structural transformations. J. Am. Chem. Soc. 2001, 123, 4432–4445. [Google Scholar]
- Sonoda, Y.; Kawanishi, Y.; Goto, M. (E,E,E)-1,6-Bis(4-nitrophenyl)hexa-1,3,5-triene. Acta Crystallogr. 2005, E61, o1200–o1202. [Google Scholar]
- Sonoda, Y.; Tsuzuki, S.; Goto, M.; Tohnai, N.; Yoshida, M. Fluorescence spectroscopic properties of nitro-substituted diphenylpolyenes: Effects of intramolecular planarization and intermolecular interactions in crystals. J. Phys. Chem. A 2010, 114, 172–182. [Google Scholar]
- Desiraju, G.R.; Sharma, C.V.K.M. C–H···O hydrogen bonding and topochemistry in crystalline 3,5-dinitrocinnamic acid and its 1:1 donor–acceptor complex with 2,5-dimethoxycinnamic acid. J. Chem. Soc. Chem. Commun. 1991, 1239–1241. [Google Scholar]
- Sharma, C.V.K.; Panneerselvam, K.; Shimoni, L.; Katz, H.; Carrell, H.L.; Desiraju, G.R. 3-(3',5'-Dinitrophenyl)-4-(2',5'-dimethoxyphenyl)cyclobutane-1,2-dicarboxylic acid: Engineered topochemical synthesis and molecular and supramolecular properties. Chem. Mater. 1994, 6, 1282–1292. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Codding, P.W. Photodimerization of α,ω-diarylbutadienes. I. Crystal and molecular structures of trans,trans-l-(2'-methoxyphenyl)-4-(4'-nitrophenyl)-l,3-butadiene and its photodimer. Can. J. Chem. 1993, 71, 942–950. [Google Scholar] [CrossRef]
- Mascitti, V.; Corey, E.J. Photochemical studies on ladderane formation from conjugated esters in solution or solid phase. Tetrahedron Lett. 2006, 47, 5879–5882. [Google Scholar] [CrossRef]
- Sonoda, Y.; Goto, M.; Tsuzuki, S.; Tamaoki, N. Fluorescence spectroscopic properties and crystal structure of a series of donor-acceptor diphenylpolyenes. J. Phys. Chem. A 2006, 110, 13379–13387. [Google Scholar] [CrossRef]
- Sonoda, Y.; Kawanishi, Y. Solvent-dependent cis-trans photoisomerization of p-methoxy-p′-nitro-substituted trans,trans,trans-1,6-diphenyl-1,3,5-hexatriene. Chem. Lett. 2003, 32, 978–979. [Google Scholar] [CrossRef]
- Sarma, J.A.R.P.; Desiraju, G.R. The chloro-substituent as a steering group: A comparative study of non-bonded interactions and hydrogen bonding in crystalline chloro-aromatics. Chem. Phys. Lett. 1985, 117, 160–164. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The nature of halogen···halogen interactions: Are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Pedireddi, V.R.; Reddy, D.S.; Goud, B.S.; Craig, D.C.; Rae, A.D.; Desiraju, G.R. The nature of halogen···halogen interactions and the crystal structure of 1,3,5,7-tetraiodoadamantane. J. Chem. Soc. Perkin Trans. 2 1994, 2353–2360. [Google Scholar]
- Reddy, C.M.; Kirchner, M.T.; Gundakaram, R.C.; Padmanabhan, K.A.; Desiraju, G.R. Isostructurality, polymorphism and mechanical properties of some hexahalogenated benzenes: The nature of halogen···halogen interactions. Chem. Eur. J. 2006, 12, 2222–2234. [Google Scholar] [CrossRef]
- Bui, T.T.T.; Dahaoui, S.; Lecomte, C.; Desiraju, G.R.; Espinosa, E. The nature ofhalogen···halogen interactions: A model derived from experimental charge-density analysis. Angew. Chem. Int. Ed. 2009, 48, 3838–3841. [Google Scholar]
- Sarma, J.A.R.P.; Desiraju, G.R. Crystal engineering via Cl···Cl non-bonded interactions. The novel 2:1 complex, 6-chloro-3,4-(methylenedioxy)cinnamic acid-2,4-dichlorocinnamic acid. Topochemical conversion into an unsymmetrical cyclobutane and kinetics of the reaction. J. Chem. Soc. Chem. Commun. 1984, 145–147. [Google Scholar]
- Cohen, M.D.; Green, B.S.; Ludmer, Z.; Schmidt, G.M.J. Excimer emission and photodimerization in a crystalline stilbene. Chem. Phys. Lett. 1970, 7, 486–490. [Google Scholar] [CrossRef]
- Cohen, R.; Ludmer, Z.; Yakhot, V. Structural influence on the excimer emission from a dimorphic crystalline stilbene. Chem. Phys. Lett. 1975, 34, 271–274. [Google Scholar] [CrossRef]
- Elgavi, A.; Green, B.S.; Schmidt, G.M.J. Reactions in chiral crystals. Optically active heterophotodimer formation from chiral single crystals. J. Am. Chem. Soc. 1973, 95, 2058–2059. [Google Scholar] [CrossRef]
- Green, B.S.; Heller, L. Solution and solid-state photodimerization of some styrylthiophenes. J. Org. Chem. 1974, 39, 196–201. [Google Scholar] [CrossRef]
- Warshel, A.; Shakked, Z. Theoretical study of excimers in crystals of flexible conjugated molecules. Excimer formation and photodimerization in crystalline 1,4-diphenylbutadiene. J. Am. Chem. Soc. 1975, 97, 5679–5684. [Google Scholar] [CrossRef]
- Sonoda, Y.; Kawanishi, Y.; Goto, M. (E,E,E)-1,6-Bis(2,4-di-chloro-phenyl)-hexa-1,3,5-triene. Acta Crystallogr. 2003, C59, o311–o313. [Google Scholar]
- Thalladi, V.R.; Weiss, H.-C.; Bläser, D.; Boese, R.; Nangia, A.; Desiraju, G.R. C–H···F interactions in the crystal structures of some fluorobenzenes. J. Am. Chem. Soc. 1998, 120, 8702–8710. [Google Scholar]
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef]
- Kumar, V.A.; Begum, N.S.; Venkatesan, K. Crystal engineering: Fluorine as a new steering group. J. Chem. Soc. Perkin Trans. 2 1993, 463–467. [Google Scholar]
- Vishnumurthy, K.; Guru Row, T.N.; Venkatesan, K. Studies in crystal engineering: Effect of fluorine substitution in crystal packing and topological photodimerization of styryl coumarins in the solid state. J. Chem. Soc. Perkin Trans. 2 1996, 1475–1478. [Google Scholar]
- Vishnumurthy, K.; Guru Row, T.N.; Venkatesan, K. Studies in crystal engineering: Crystal packing, topological photodimerization and structure–reactivity correlations in fluoro-substituted styrylcoumarins. J. Chem. Soc. Perkin Trans. 2 1997, 615–619. [Google Scholar]
- Vishnumurthy, K.; Guru Row, T.N.; Venkatesan, K. Unusual photodimerization of 7-fluoro-4-methylcoumarin and 6-fluoro-4-methylcoumarin in the solid state. Tetrahedron 1998, 54, 11235–11246. [Google Scholar] [CrossRef]
- Vishnumurthy, K.; Guru Row, T.N.; Venkatesan, K. Studies in crystal engineering: Steering ability of fluorine in 4-styrylcoumarins. Tetrahedron 1999, 55, 4095–4108. [Google Scholar] [CrossRef]
- Mori, Y.; Matsumoto, A. Photodimerization mechanism of bis(3,4,5-trifluorobenzyl) (E,E)-muconate in a columnar assembly in the crystalline state. Chem. Lett. 2007, 36, 510–511. [Google Scholar] [CrossRef]
- Mori, Y.; Matsumoto, A. Molecular stacking and photoreactions of fluorine-substituted benzyl muconates in the crystals. Cryst. Growth Des. 2007, 7, 377–385. [Google Scholar] [CrossRef]
- Cozzi, F.; Ponzini, F.; Annunziata, R.; Cinquini, M.; Siegel, J.S. Polar interactions between stacked π systems in fluorinated 1,8-diarylnaphthalenes: Importance of quadrupole moments in molecular recognition. Angew. Chem. Int. Ed. Engl. 1995, 34, 1019–1020. [Google Scholar] [CrossRef]
- Cozzi, F.; Siegel, J.S. Interaction between stacked aryl groups in 1,8-diarylnaphthalenes: Dominance of polar/π over charge-transfer effects. Pure Appl. Chem. 1995, 67, 683–689. [Google Scholar] [CrossRef]
- Adams, H.; Blanco, J.-L.J.; Chessari, G.; Hunter, C.A.; Low, C.M.R.; Sanderson, J.M.; Vinter, J.G. Quantitative determination of intermolecular interactions with fluorinated aromatic rings. Chem. Eur. J. 2001, 7, 3494–3503. [Google Scholar] [CrossRef]
- Gung, B.W.; Patel, M.; Xue, X. A threshold for charge transfer in aromatic interactions? A quantitative study of π-stacking interactions. J. Org. Chem. 2005, 70, 10532–10537. [Google Scholar] [CrossRef]
- Reichenbächer, K.; Süss, H.I.; Hulliger, J. Fluorine in crystal engineering—“the little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef]
- Gung, B.W.; Xue, X.; Zou, Y. Enthalpy (ΔH) and entropy (ΔS) for π-stacking interactions in near-sandwich configurations: Relative importance of electrostatic, dispersive, and charge-transfer effects. J. Org. Chem. 2007, 72, 2469–2475. [Google Scholar] [CrossRef]
- Brock, C.P.; Naae, D.G.; Goodhand, N.; Hamor, T.A. A statistical comparison of two determinations of the crystal structure of 2,3,4,5,6-pentafluorobiphenyl, a molecule forming mixed stacks in the solid state. Acta Crystallogr. 1978, B34, 3691–3696. [Google Scholar]
- Naae, D.G. Biphenyl–perfluorobiphenyl; 1:1 molecular complex. Acta Crystallogr. 1979, B35, 2765–2768. [Google Scholar]
- Bartholomew, G.P.; Bazan, G.C.; Bu, X.; Lachicotte, R.J. Packing modes of distyrylbenzene derivatives. Chem. Mater. 2000, 12, 1422–1430. [Google Scholar] [CrossRef]
- Bartholomew, G.P.; Bu, X.; Bazan, G.C. Preferential cocrystallization among distyrylbenzene derivatives. Chem. Mater. 2000, 12, 2311–2318. [Google Scholar] [CrossRef]
- Feast, W.J.; Lövenich, P.W.; Puschmann, H.; Taliani, C. Synthesis and structure of 4,4′-bis(2,3,4,5,6-pentafluorostyryl)stilbene, a self-assembling J aggregate based on aryl–fluoroaryl interactions. Chem. Commun. 2001, 505–506. [Google Scholar]
- Bunz, U.H.F.; Enkelmann, V. Structure elucidation, packing, and solid-state behavior of the eglinton - Galbraith dimer. Chem. Eur. J. 1999, 5, 263–266. [Google Scholar] [CrossRef]
- Nishinaga, T.; Nodera, N.; Miyata, Y.; Komatsu, K. Dehydro[12]- and -[18]annulenes fused with tetrafluorobenzene: Synthesis, electronic properties, packing structures, and reactivity in the solid state. J. Org. Chem. 2002, 67, 6091–6096. [Google Scholar] [CrossRef]
- Ponzini, F.; Zagha, R.; Hardcastle, K.; Siegel, J.S. Phenyl/pentafluorophenyl interactions and the generation of ordered mixed crystals: sym-Triphenethynylbenzene and sym-tris(perfluorophenethynyl)benzene. Angew. Chem. Int. Ed. 2000, 39, 2323–2325. [Google Scholar] [CrossRef]
- Watt, S.W.; Dai, C.; Scott, A.J.; Burke, J.M.; Thomas, R.L.; Collings, J.C.; Viney, C.; Clegg, W.; Marder, T.B. Structure and phase behavior of a 2:1 complex between arene- and fluoroarene-based conjugated rigid rods. Angew. Chem. Int. Ed. 2004, 43, 3061–3063. [Google Scholar]
- Smith, C.E.; Smith, P.S.; Thomas, R.L.; Robins, E.G.; Collings, J.C.; Dai, C.; Scott, A.J.; Borwick, S.; Batsanov, A.S.; Watt, S.W.; Clark, S.J.; Viney, C.; Howard, J.A.K.; Clegg, W.; Marder, T.B. Arene-perfluoroarene interactions in crystal engineering: Structural preferences in polyfluorinated tolans. J. Mater. Chem. 2004, 14, 413–420. [Google Scholar]
- Coates, G.W.; Dunn, A.R.; Henling, L.M.; Ziller, J.W.; Lobkovsky, E.B.; Grubbs, R.H. Phenyl-perfluorophenyl stacking interactions: Topochemical [2+2] photodimerization and photopolymerization of olefinic compounds. J. Am. Chem. Soc. 1998, 120, 3641–3649. [Google Scholar]
- Vishnumurthy, K.; Guru Row, T.N.; Venkatesan, K. Fluorine in crystal engineering: Photodimerization of (1E,3E)-1-phenyl-4-pentafluorophenylbuta-1,3-dienes in the crystalline state. Photochem. Photobiol. Sci. 2002, 1, 427–430. [Google Scholar] [CrossRef]
- Sonoda, Y.; Goto, M.; Tsuzuki, S.; Tamaoki, N. Fluorinated diphenylpolyenes:Crystal structures and emission properties. J. Phys. Chem. A 2007, 111, 13441–13451. [Google Scholar] [CrossRef]
- Sonoda, Y.; Goto, M.; Tsuzuki, S.; Akiyama, H.; Tamaoki, N. [2+2] Photodimerization and photopolymerization of diphenylhexatrienecrystals utilizing perfluorophenyl-phenyl stacking interactions. J. Fluorine Chem. 2009, 130, 151–157. [Google Scholar] [CrossRef]
- Coates, G.W.; Dunn, A.R.; Henling, L.M.; Dougherty, D.A.; Grubbs, R.H. Phenyl-perfluorophenyl stacking interactions: A new strategy for supermolecule construction. Angew. Chem. Int. Ed. Engl. 1997, 36, 248–251. [Google Scholar]
- Xu, R.; Gramlich, V.; Frauenrath, H. Alternating diacetylene copolymer utilizing perfluorophenyl-phenyl interactions. J. Am. Chem. Soc. 2006, 128, 5541–5547. [Google Scholar] [CrossRef]
- Liu, J.; Murray, E.M.; Young, V.G., Jr. π-Stacking interactions in some crystalline cisoid E,E-1,4-diaryl-1,3-butadienes. Chem. Commun. 2003, 1904–1905. [Google Scholar]
- Caronna, T.; Liantonio, R.; Logothetis, T.A.; Metrangolo, P.; Pilati, T.; Resnati, G. Halogen bonding and π···π stacking control reactivity in the solid state. J. Am. Chem. Soc. 2004, 126, 4500–4501. [Google Scholar] [CrossRef]
- Frontera, A.; Quiñonero, D.; Costa, A.; Ballester, P.; Deyà, P.M. MP2 study of cooperative effects between cation–π, anion–π and π–π interactions. New J. Chem. 2007, 31, 556–560. [Google Scholar] [CrossRef]
- Liu, J.; Boarman, K.J. Regiospecific topochemical reactions controlled by trifluoromethyl directing groups. Chem. Commun. 2005, 340–341. [Google Scholar]
- Liu, J.; Wendt, N.L.; Boarman, K.J. Trifluoromethyl groups in crystal design of 1,4-diphenyl-1,3-butadienes for topochemical [2 + 2] photodimerization. Org. Lett. 2005, 7, 1007–1010. [Google Scholar] [CrossRef]
- Jeannin, O.; Fourmigué, M. Fluorine segregation in crystalline materials: Structural control and solid-state [2+2] cycloaddition in CF3-substituted tetrathiafulvalene derivatives. Chem. Eur. J. 2006, 12, 2994–3005. [Google Scholar] [CrossRef]
- Hayashi, S. wo-dimensional 1H spin-exchange NMR study of molecular arrangements in diphenylhexatrienes. Bull. Chem. Soc. Jpn. 2004, 77, 2159–2164. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I.; Grześniak, K.; Trzop, E.; Zych, T. Monitoring structural transformations in crystals. Part 4. Monitoring structural changes in crystals of pyridine analogs of chalcone during [2+2]-photodimerization and possibilities of the reaction in hydroxy derivatives. J. Solid State Chem. 2003, 174, 459–465. [Google Scholar] [CrossRef]
- Mahon, M.F.; Raithby, P.R.; Sparkes, H.A. nvestigation of the factors favouring solid state [2 + 2] cycloaddition reactions; the [2 + 2] cycloaddition reaction of coumarin-3-carboxylic acid. CrystEngCommunity 2008, 10, 573–576. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Naumov, P.; Fukuzumi, S. Topochemical limits for solid-state photoreactivity by fine tuning of the π-π interactions. J. Am. Chem. Soc. 2009, 131, 7247–7249. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.S.; De Feyter, S.; Gesquière, A.; Sieffert, M.; Klapper, M.; Müllen, K.; De Schryver, F.C. Photodimerization of cinnamate derivatives studied by STM. Nano Lett. 2001, 1, 353–359. [Google Scholar] [CrossRef]
- Marubayashi, N.; Ogawa, T.; Hamasaki, T.; Hirayama, N. A buffer zone in the crystal structure that governs the solid-state photodimerization of bulky olefins with the 1,4-dihydropyridine skeleton. J. Chem. Soc. Perkin Trans. 2 1997, 1309–1314. [Google Scholar]
- Gnanaguru, K.; Ramasubbu, N.; Venkatesan, K.; Ramamurthy, V. Topochemical solid state photodimerization of non-ideally oriented monomers: 7-Chlorocoumarin and 7-methoxy-coumarin. J. Photochem. 1984, 27, 355–362. [Google Scholar]
- Bhadbhade, M.M.; Murthy, G.S.; Venkatesan, K.; Ramamurthy, V. Topochemical dimerization of non-parallel double bonds: 7-Methoxycoumarin. Chem. Phys. Lett. 1984, 109, 259–263. [Google Scholar] [CrossRef]
- Gnanaguru, K.; Ramasubbu, N.; Venkatesan, K.; Ramamurthy, V. A Study on the photochemical dimerization of coumarins in the solid state. J. Org. Chem. 1985, 50, 2337–2346. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Fang, J.-M.; Chow, T.; Ho, T.-I.; Lee, C.-R.; Wang, Y. Solution and solid-state photochemistry of 2-anilino-5-phenyl-2,4-pentadienenitriles. J. Org. Chem. 1994, 59, 5742–5747. [Google Scholar] [CrossRef]
- Fonseca, I.; Hayes, S.E.; Bertmer, M. Size effects of aromatic substitution in the ortho position on the photodimerization kinetics of α-trans cinnamic acid derivatives. A solid-state NMR study. Phys. Chem. Chem. Phys. 2009, 11, 10211–10218. [Google Scholar]
- Xu, L.-P.; Yan, C.-J.; Wan, L.-J.; Jiang, S.-G.; Liu, M.-H. Light-induced structural transformation in self-assembled monolayer of 4-(amyloxy)cinnamic acid investigated with scanning tunneling microscopy. J. Phys. Chem. B 2005, 109, 14773–14778. [Google Scholar] [CrossRef]
- Swiatkiewicz, J.; Eisenhardt, G.; Prasad, P.N.; Thomas, J.M.; Jones, W.; Theocharis, C.R. Phonon spectroscopy of photochemical reactions in organic solids: Photodimerization of 2-benzyl-5-benzylidenecyclopentanone and photopolymerization of 2,5-distyrylpyrazine. J. Phys. Chem. 1982, 86, 1764–1767. [Google Scholar] [CrossRef]
- Ghosh, M.; Mandal, T.K.; Chakrabarti, S.; Misra, T.N. Spectroscopic study of solid-state photoreaction in organic crystals: Photopolymerization of the dimethyl ester of p-phenylenediacrylic acid. J. Raman Spectrosc. 1998, 29, 807–811. [Google Scholar] [CrossRef]
- Eckhardt, C.J.; Luty, T.; Peachey, N.M. Collective interactions and solid state reactivity. Mol. Cryst. Liq. Cryst. 1998, 313, 25–38. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I. Structural transformations in a crystal during the photochemical reaction of 2-benzyl-5-benzylidenecyclopentanone. Chem. Eur. J. 2001, 7, 3401–3405. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I. Monitoring structural transformations in crystals. 5. A topotactic [2+2]-photodimerization reaction. Acta Crystallogr. 2003, B59, 670–675. [Google Scholar]
- Turowska-Tyrk, I. Structural transformations in organic crystals during photochemical reactions. J. Phys. Org. Chem. 2004, 17, 837–847. [Google Scholar] [CrossRef]
- Kim, J.H.; Matsuoka, M.; Fukunishi, K. Selective topochemical photoreaction of crystallized 2,3-bis(2-phenylethenyl)-4,5-dicyanopyrazines. Chem. Lett. 1999, 28, 143–144. [Google Scholar]
- Natarajan, A.; Mague, J.T.; Venkatesan, K.; Ramamurthy, V. Large molecular motions are tolerated in crystals of diamine double salt of trans-chlorocinnamic acids with trans-1,2-diaminocyclohexane. Org. Lett. 2005, 7, 1895–1898. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Pham, O.; Vande Velde, C.M.L.; Gembicky, M.; Coppens, P. Competitive isomerization and dimerization in co-crystals of1,1,6,6-tetraphenyl-2,4-hexadiyne-1,6-diol and sorbic acid: A new look at stereochemical requirements for [2+2] dimerization. Chem. Commun. 2008, 2538–2540. [Google Scholar]
- Robinson, M.R.; Wang, S.; Heeger, A.J.; Bazan, G.C. A tetrahedral oligo(phenylenevinylene) molecule of intermediate dimensions: Effect of molecular shape on the morphology and electroluminescence of organic glasses. Adv. Func. Mater. 2001, 11, 413–419. [Google Scholar] [CrossRef]
- Shirota, Y. Photo- and electroactive amorphous molecular materials—molecular design, syntheses, reactions, properties, and applications. J. Mater. Chem. 2005, 15, 75–93. [Google Scholar] [CrossRef]
- Nakanishi, F.; Nakanishi, H.; Hasegawa, M.; Yamada, Y. Four-center type photopolymerization in the solid state. VII. Photochemical reaction of m-phenylene diacrylic acid dimethyl ester. J. Polym. Sci. Part A 1975, 13, 2499–2506. [Google Scholar]
- Nakanishi, H.; Sasada, Y. The crystal and molecular structure of dimethyl m-phenylenediacrylate. Bull. Chem. Soc. Jpn. 1977, 50, 3182–3185. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Maity, A.K.; Misra, T.N. Spectroscopic study of solid-state photoreaction in organic crystal: Photopolymerization of dimethyl ester of α,α′-dicyano-p-phenylenediacrylic acid and diethyl ester of p-phenylenediacrylic acid. J. Polym. Sci. Part A 1992, 30, 1625–1631. [Google Scholar] [CrossRef]
- Minsk, L.M.; Smith, J.G.; Van Deusen, W.P.; Wright, J.F. Photosensitive polymers. I. Cinnamate esters of poly(vinyl alcohol) and cellulose. J. Appl. Polym. Sci. 1959, 2, 302–307. [Google Scholar] [CrossRef]
- Reiser, A.; Egerton, P.L. The mechanism of crosslink formation in solid polyvinylcinnamate and related photopolymers. Photogr. Sci. Eng. 1979, 23, 144–150. [Google Scholar]
- Ichimura, K. Preparation of water-soluble photoresist derived from poly(vinyl alcohol). J. Polym. Sci. Part A 1982, 20, 1411–1417. [Google Scholar]
- Ichimura, K.; Watanabe, S. Preparation and characteristics of photocrosslinkable poly(vinyl alcohol). J. Polym. Sci. Part A 1982, 20, 1419–1432. [Google Scholar]
- Ichimura, K.; Oohara, N. Photosensitive poly(methacrylates) having styrylpyridinium and styrylquinolinium groups. J. Polym. Sci. Part A 1987, 25, 3063–3077. [Google Scholar]
- Skloss, T.W.; Haw, J.F. Detection of cross-link formation by [2+2] photocycloaddition in poly(viny1 cinnamate) by 13C solid-state NMR. Macromolecules 1994, 27, 6998–6999. [Google Scholar] [CrossRef]
- Cockburn, E.S.; Davidson, R.S.; Pratt, J.E. The photocrosslinking of styrylpyridinium salts via a [2 + 2]-cycloaddition reaction. J. Photochem. Photobiol. A Chem. 1996, 94, 83–88. [Google Scholar] [CrossRef]
- Guo, M.; Xu, Z.; Wang, X. Photofabrication of two-dimensional quasi-crystal patterns on UV-curable molecular azo glass films. Langmuir 2008, 24, 2740–2745. [Google Scholar] [CrossRef]
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack healing in polymeric materials via photochemical [2+2] cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Li, F.; Zhuang, J.; Jiang, G.; Tang, H.; Xia, A.; Jiang, L.; Song, Y.; Li, Y.; Zhu, D. A rewritable optical data storage material system by [2+2] photocycloreversion-photocycloaddition. Chem. Mater. 2008, 20, 1194–1196. [Google Scholar] [CrossRef]
- Chung, J.W.; You, Y.; Huh, H.S.; An, B.-K.; Yoon, S.-J.; Kim, S.H.; Lee, S.W.; Park, S.Y. Shear- and UV-induced fluorescence switching in stilbenic π-dimer crystals powered by reversible [2+2] cycloaddition. J. Am. Chem. Soc. 2009, 131, 8163–8172. [Google Scholar]
- Enkelmann, V.; Wegner, G. Novak, K.; Wagener, K.B. Single-crystal-to-single-crystal photodimerization of cinnamic acid. J. Am. Chem. Soc. 1993, 115, 10390–10391. [Google Scholar]
- Enkelmann, V.; Wegner, G.; Novak, K.; Wagener, K.B. Crystal-to-crystal photodimerizations. Mol. Cryst. Liq. Cryst. 1994, 240, 121–126. [Google Scholar] [CrossRef]
- Takahashi, S.; Miura, H.; Kasai, H.; Okada, S.; Oikawa, H.; Nakanishi, H. Single-crystal-to-single-crystal transformation of diolefin derivatives in nanocrystals. J. Am. Chem. Soc. 2002, 124, 10944–10945. [Google Scholar] [CrossRef]
- Bučar, D.-K.; MacGillivray, L.R. Preparation and reactivity of nanocrystalline cocrystals formed via sonocrystallization. J. Am. Chem. Soc. 2007, 129, 32–33. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sonoda, Y. Solid-State [2+2] Photodimerization and Photopolymerization of α,ω-Diarylpolyene Monomers: Effective Utilization of Noncovalent Intermolecular Interactions in Crystals. Molecules 2011, 16, 119-148. https://doi.org/10.3390/molecules16010119
Sonoda Y. Solid-State [2+2] Photodimerization and Photopolymerization of α,ω-Diarylpolyene Monomers: Effective Utilization of Noncovalent Intermolecular Interactions in Crystals. Molecules. 2011; 16(1):119-148. https://doi.org/10.3390/molecules16010119
Chicago/Turabian StyleSonoda, Yoriko. 2011. "Solid-State [2+2] Photodimerization and Photopolymerization of α,ω-Diarylpolyene Monomers: Effective Utilization of Noncovalent Intermolecular Interactions in Crystals" Molecules 16, no. 1: 119-148. https://doi.org/10.3390/molecules16010119
APA StyleSonoda, Y. (2011). Solid-State [2+2] Photodimerization and Photopolymerization of α,ω-Diarylpolyene Monomers: Effective Utilization of Noncovalent Intermolecular Interactions in Crystals. Molecules, 16(1), 119-148. https://doi.org/10.3390/molecules16010119