Application of Cycloaddition Reactions to the Syntheses of Novel Boron Compounds
Abstract
:1. Introduction
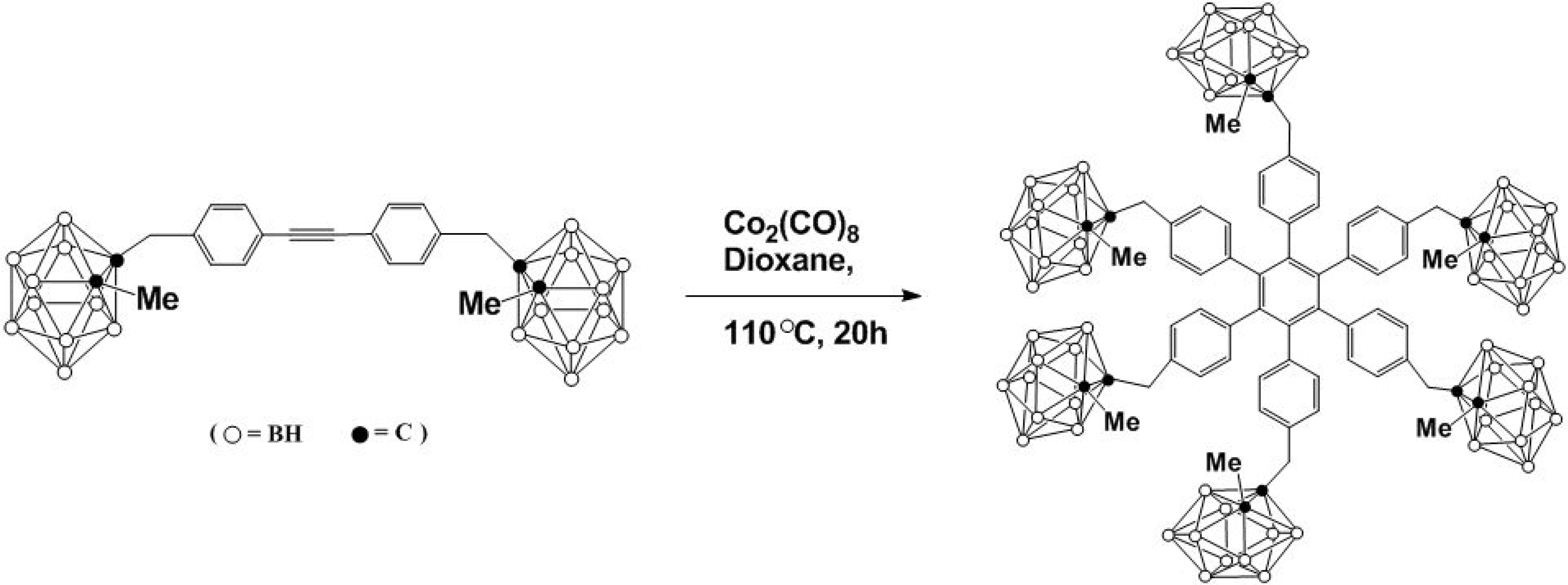
2. [2+2+2] and the [2+2] Cycloadditions
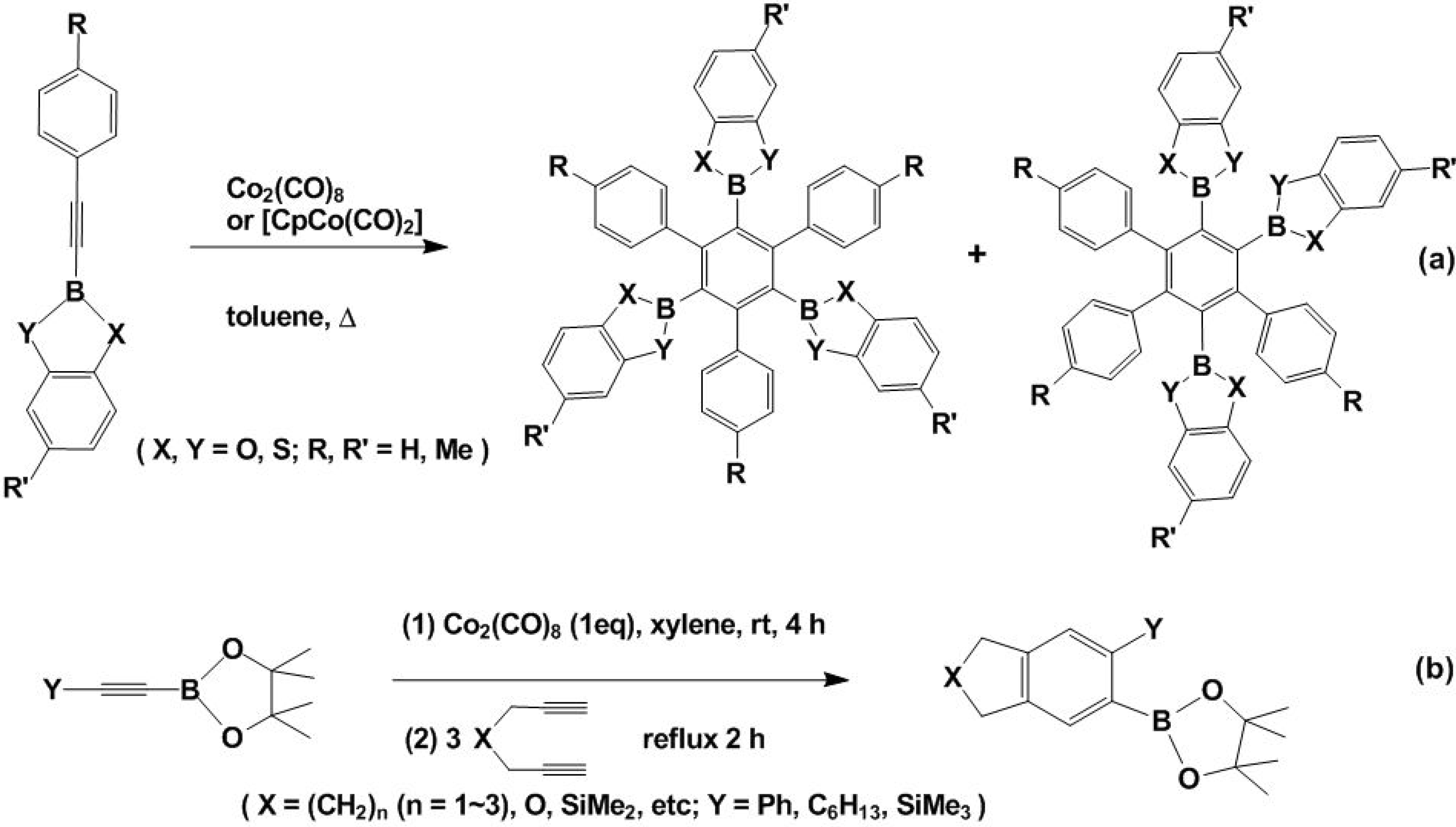
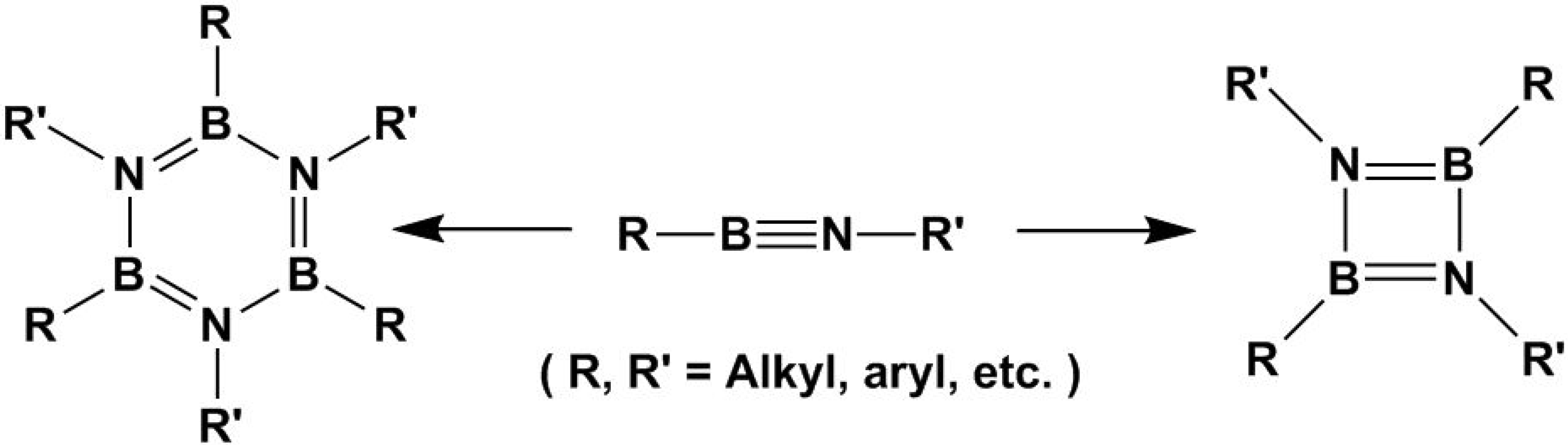
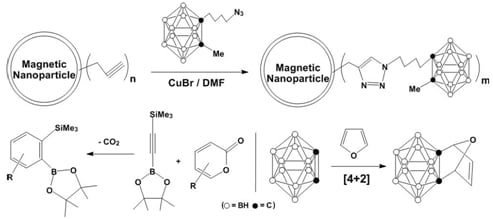
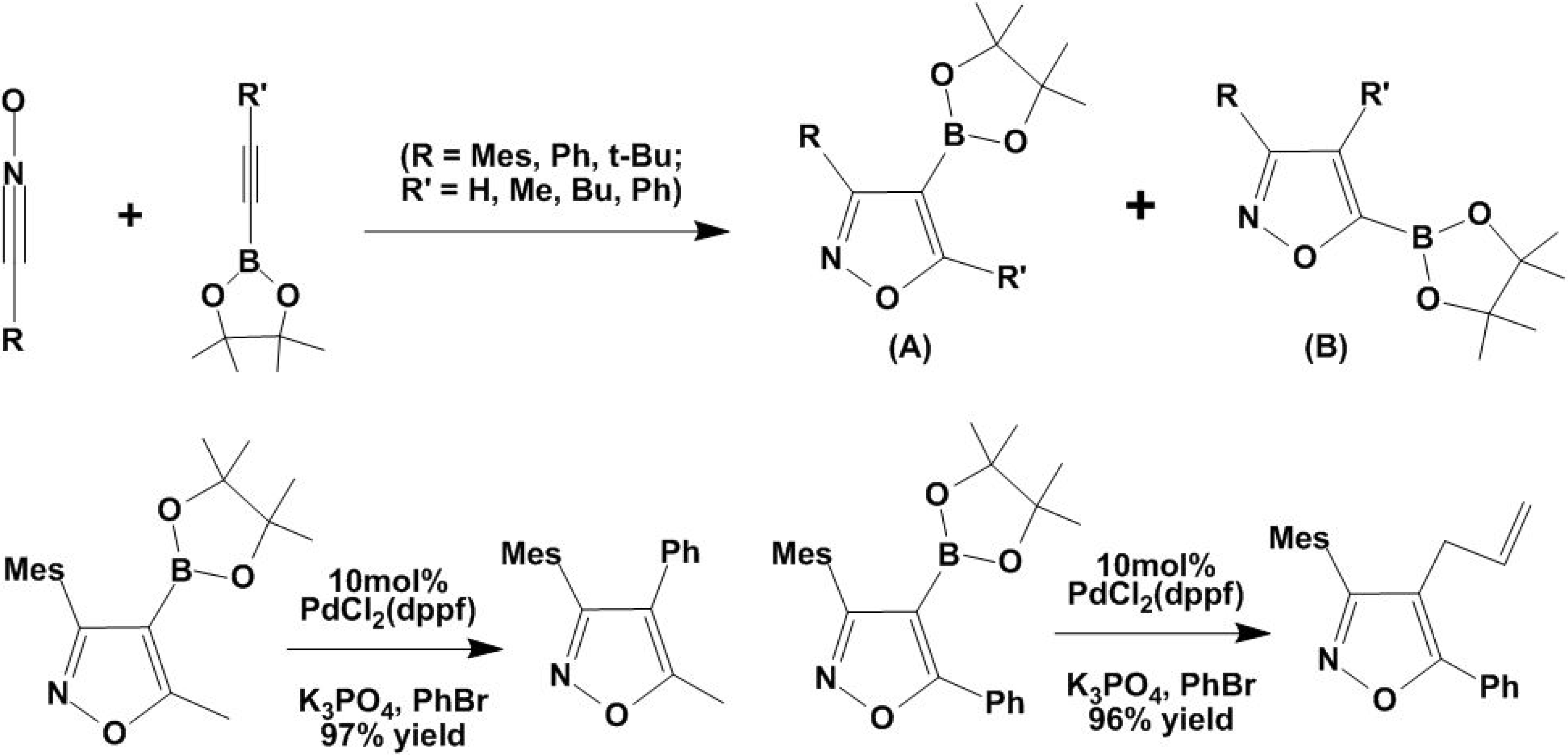
3. [2+3] Cycloadditions
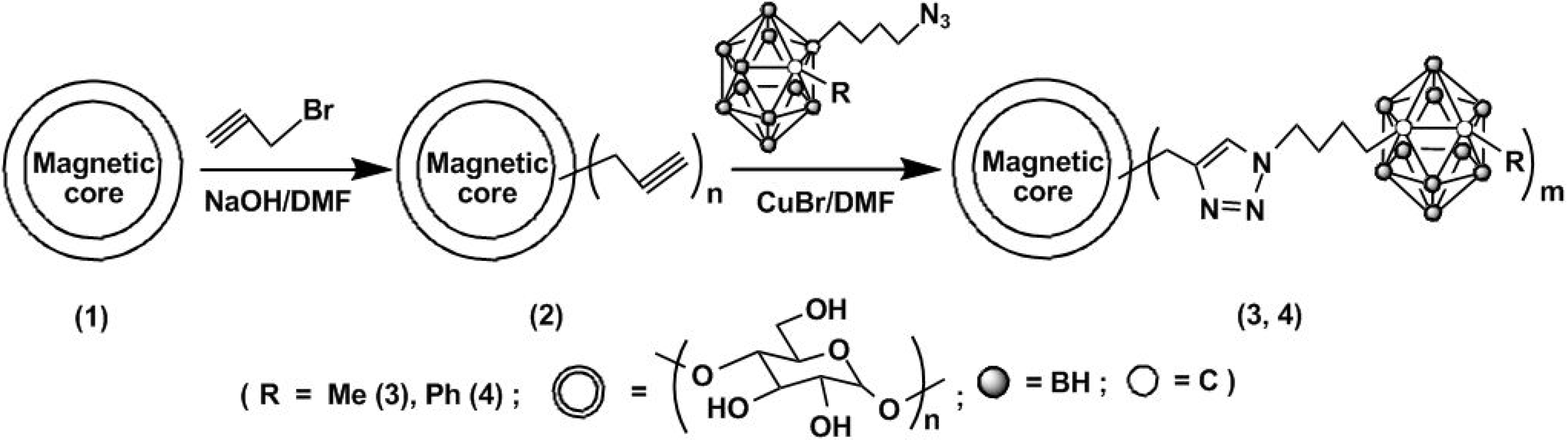
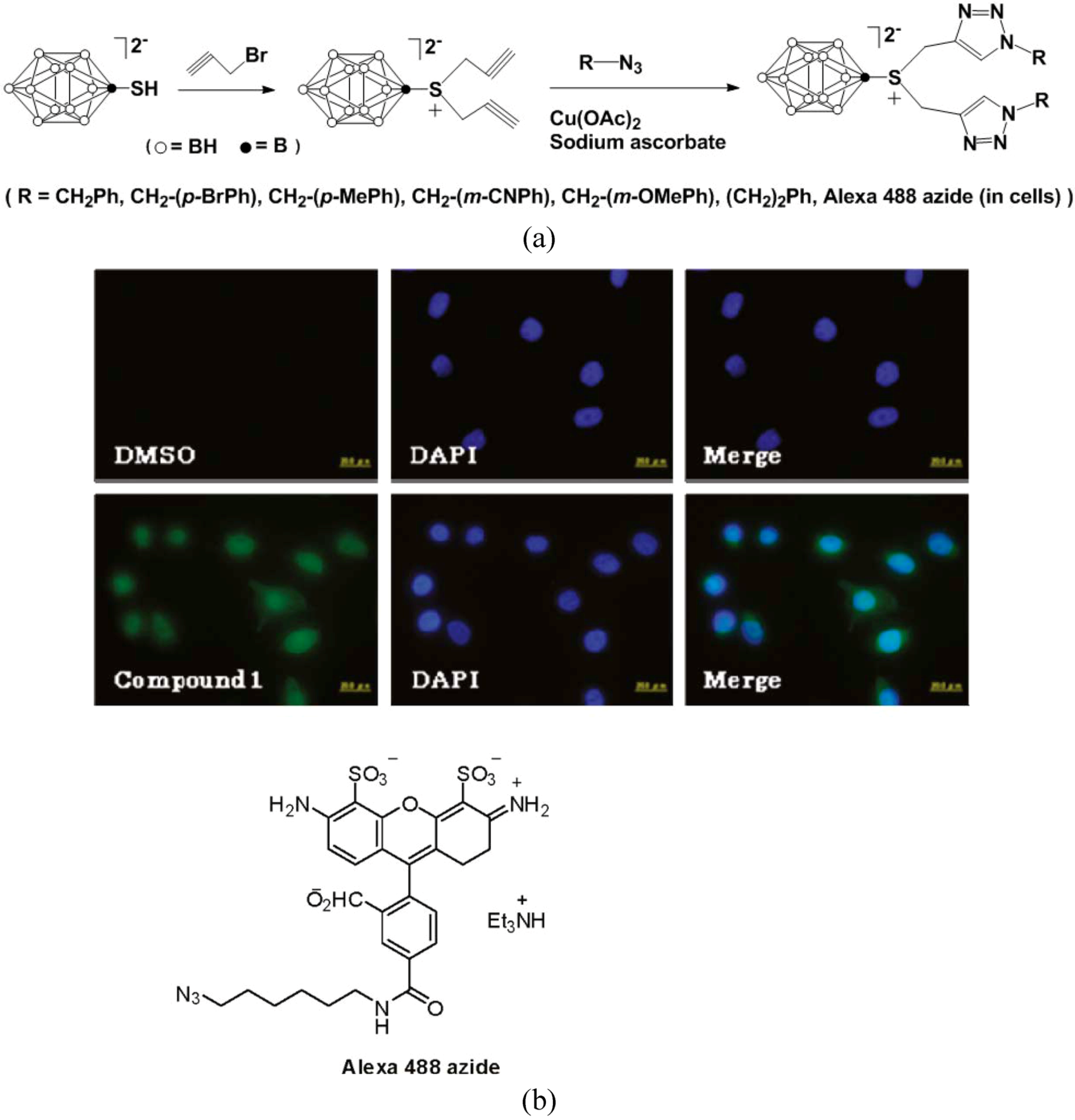
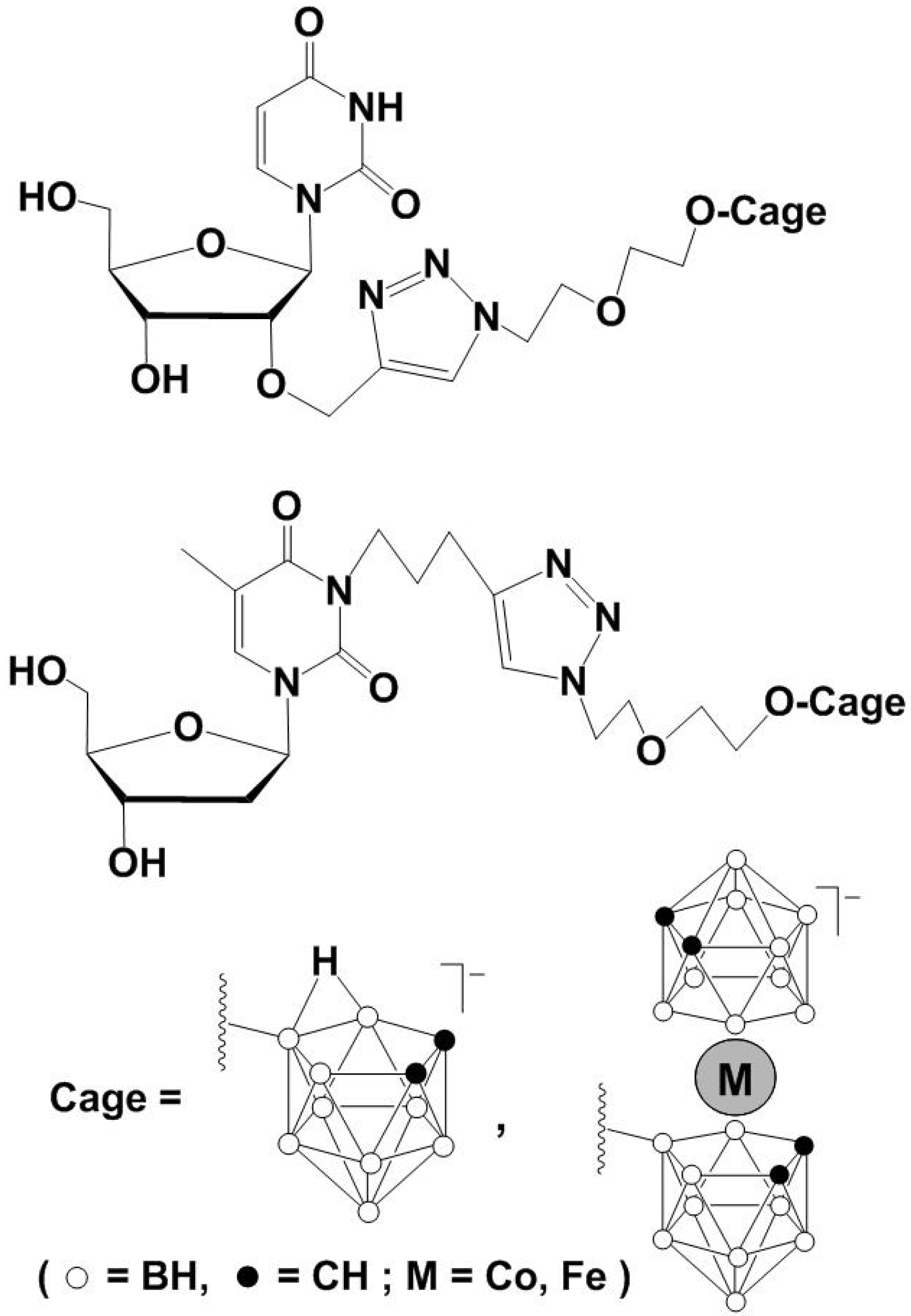
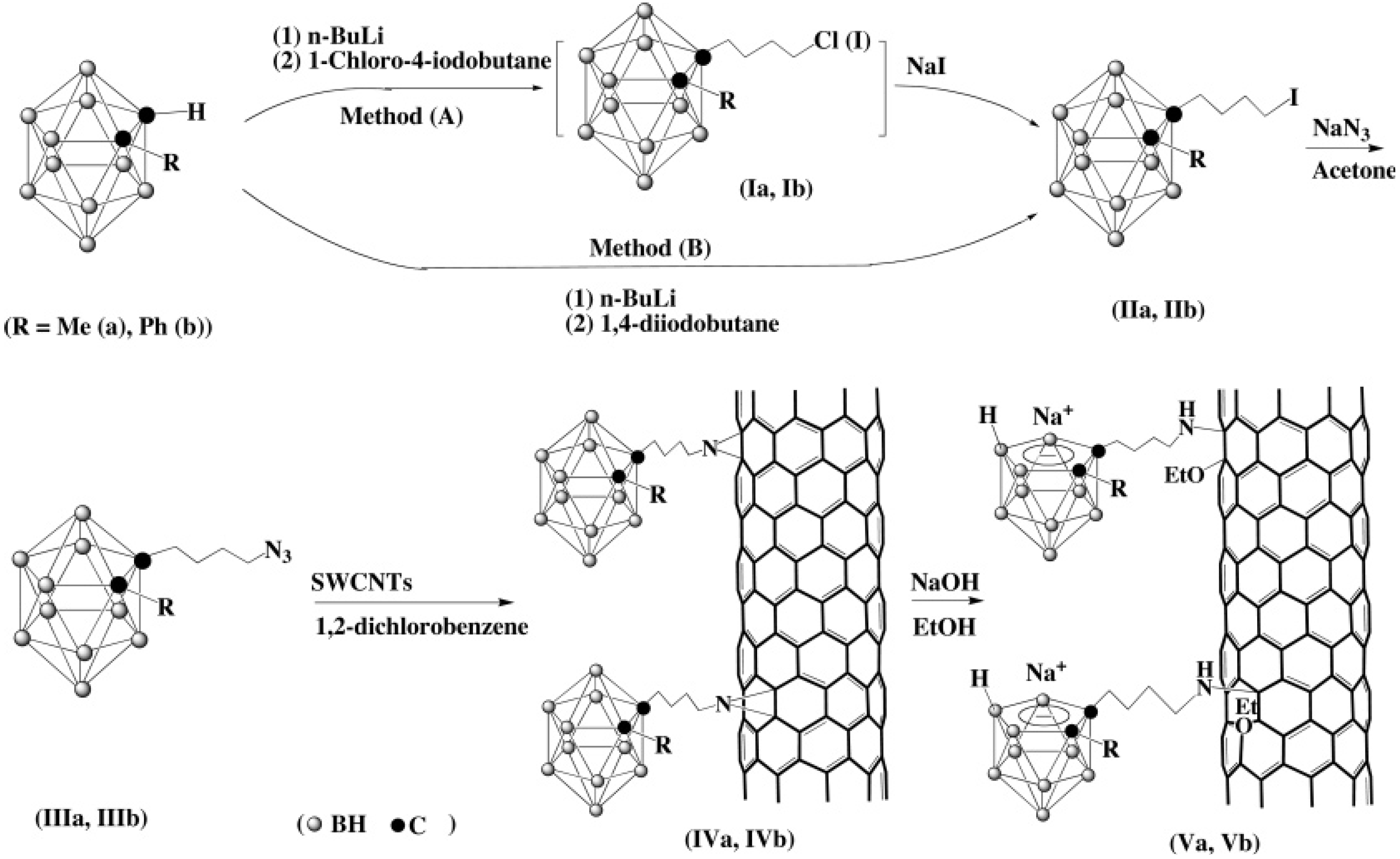
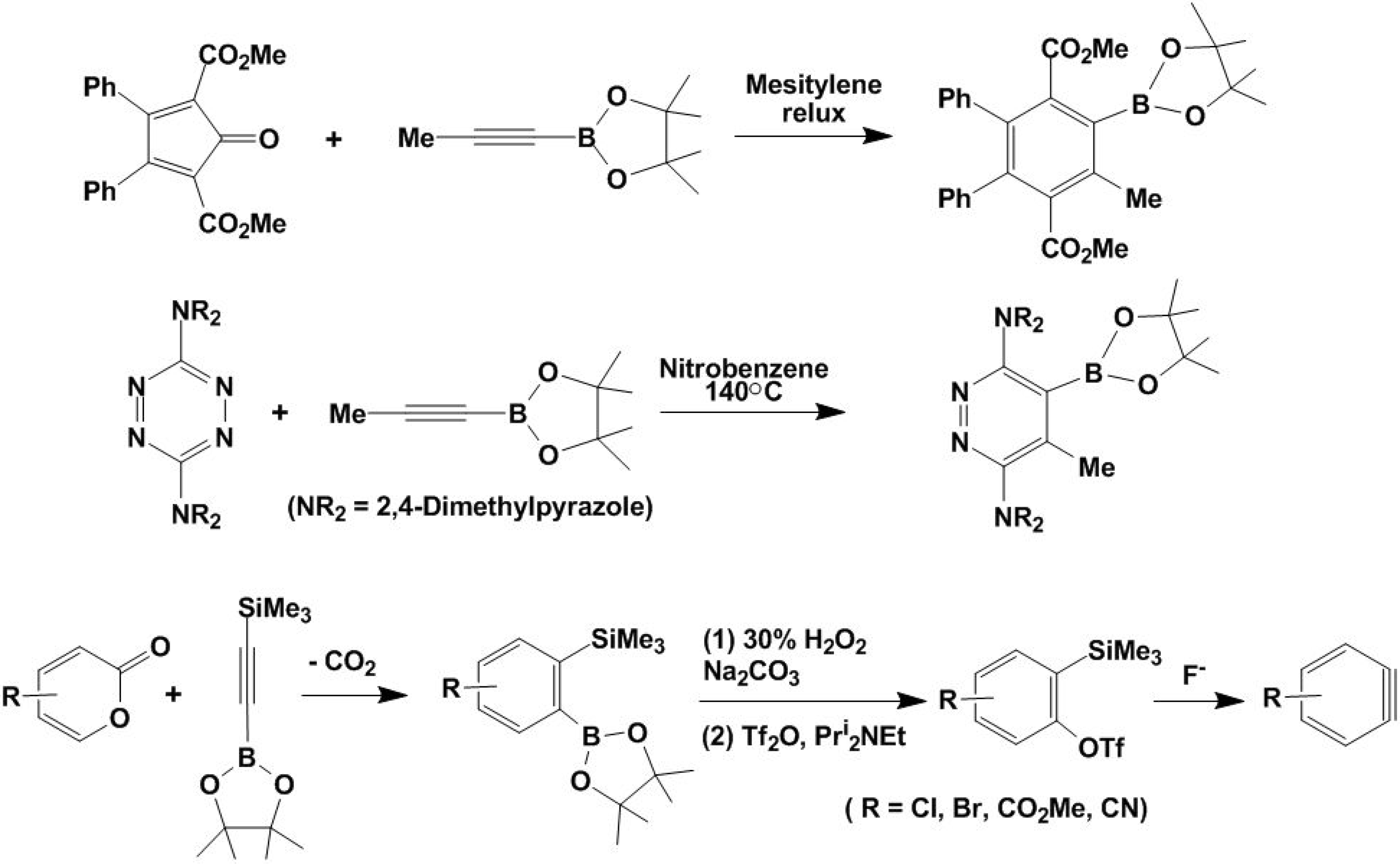
4. [2+4] Cycloaddition
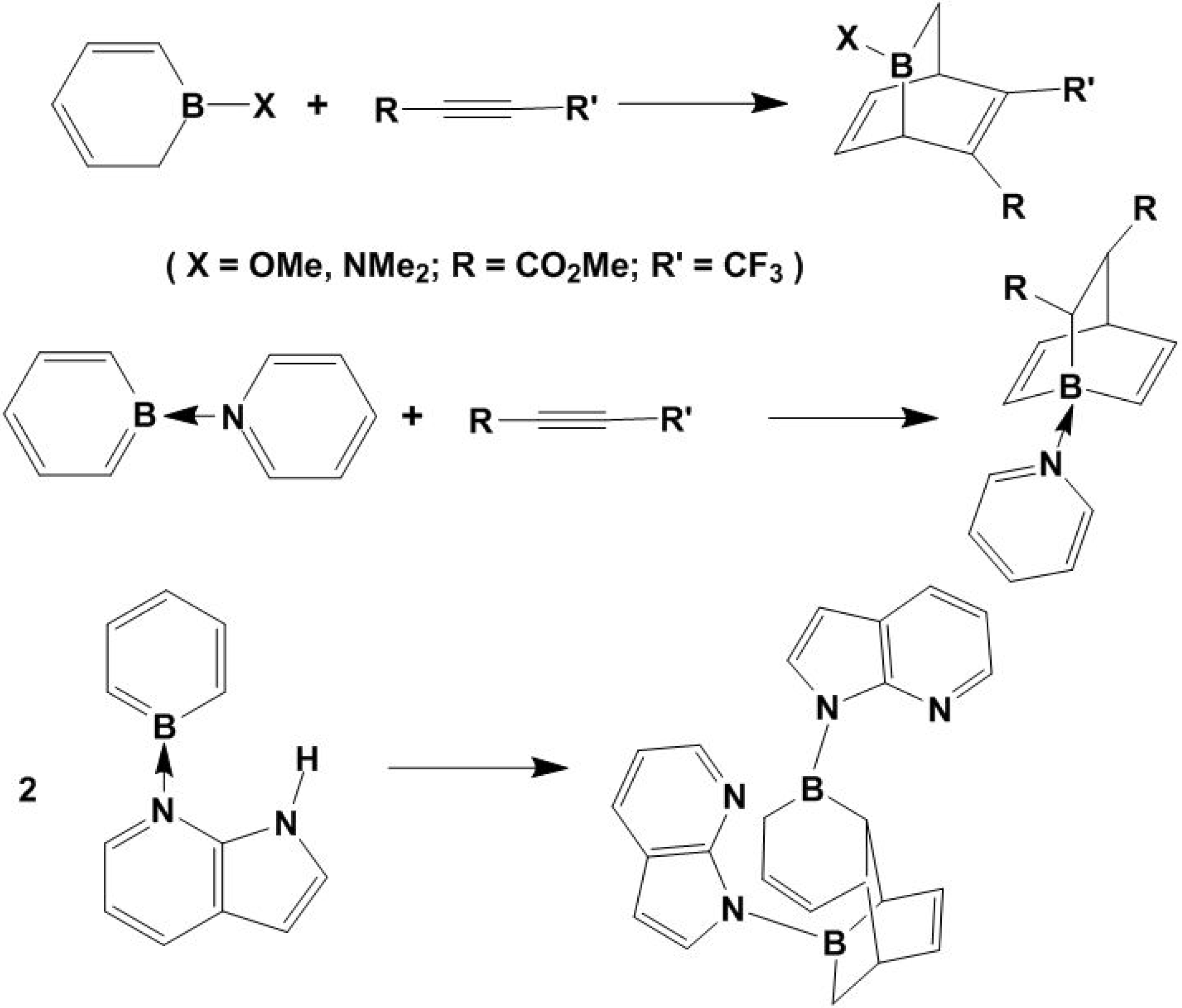

5. Concluding Remarks
Acknowledgements
- Sample Availability: Not available.
References and Notes
- Kobayashi, S.; Jorgensen, K.A. Cycloaddition Reactions in Organic Synthesis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002; pp. 5–326. [Google Scholar]
- Sankararaman, S. Pericyclic Reactions-A Textbook: Reactions, Applications and Theory; Wiley-VCH Verlag GmbH & Co. KgAa: Weinheim, Germany, 2005; pp. 19–354. [Google Scholar]
- Jia, C.; Kitamura, T.; Fujiwara, Y. Catalytic Functionalization of Arenes and Alkanes via C-H Bond Activation. Acc. Chem. Res. 2001, 34, 633–639. [Google Scholar] [CrossRef]
- Zapf, A.; Beller, M. Fine Chemical Synthesis with Homogeneous Palladium Catalysts: Examples, Status and Trends. Top. Catal. 2002, 19, 101–109. [Google Scholar]
- Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N.R.; Hartwig, J.F. Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate. J. Am. Chem. Soc. 2002, 124, 390–391. [Google Scholar] [CrossRef]
- Blaser, H.; Indoless, A.; Naud, F.; Nettekoven, U.; Schnyder, A. Industrial R&D on Catalytic C-C and C-N Coupling Reactions: A Personal Account on Goals, Approaches and Results. Adv. Synth. Catal. 2004, 346, 1583–1598. [Google Scholar] [CrossRef]
- Burkhardt, E.R.; Matos, K. Boron Reagents in Process Chemistry: Excellent Tools for Selective Reductions. Chem. Rev. 2006, 106, 2617–2650. [Google Scholar] [CrossRef]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C-H activation for the construction of C-B bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Davidson, M.G.; Hughes, A.K.; Marder, T.B.; Wade, K. Contemporary Boron Chemistry; Royal Society of Chemistry: Cambridge, UK, 2000. [Google Scholar]
- Grimes, R.N. Metallacarboranes in the new millennium. Coord. Chem. Rev. 2000, 200-202, 773–811. [Google Scholar] [CrossRef]
- Zhu, Y.; Koh, C.Y.; Maguire, J.A.; Hosmane, N.S. Recent developments in the Boron Neutron Capture therapy (BNCT) driven by nanotechnology. Curr. Chem. Biol. 2007, 1, 141–149, and references therein. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford, 1997, 2nd ed. Available online: http://goldbook.iupac.org/C01496.html accessed on 21 December 2010.
- Pisula, W.; Kastler, M.; Wasserfallen, D.; Pakula, T.; Mullen, K. Exceptionally long-range self-assembly of hexa-peri-hexabenzocorenenwith dove-tailed alkyl substituents. J. Am. Chem. Soc. 2004, 126, 8074–8075. [Google Scholar]
- Hilt, G.; Vogler, T.; Hess, W.; Galbiati, F. A simple cobalt catalyst system for the efficient and regioselective cyclotrimerisation of alkynes. Chem. Commun. 2005, 1474–1475. [Google Scholar]
- Fletcher, A.J.; Christie, S.D.R. Cobalt mediated cyclisations. J. Chem. Soc. Perkin Trans. 2000, 165–1668. [Google Scholar]
- Chebny, V.C.; Dhar, D.; Lindeman, S.V.; Rathore, R. Simultaneous ejection of six electrons at a constant potential by hexakis(4-ferrocenylphenyl)benzene. Org. Lett. 2006, 8, 5041–5044. [Google Scholar] [CrossRef]
- Gandon, V.; Aubert, C.; Malacria, M. Recent progress in cobalt-mediated [2 + 2 + 2] cycloaddition reactions. Chem. Commun. 2006, 2209–2217. [Google Scholar]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Synthesis of a new class of carborane-containing star-shaped molecules via silicon tetrachloride promoted cyclotrimerization reactions. Org. Lett. 2008, 10, 2247–2250. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Boron-enriched star-shaped molecule via cycloaddition reaction. Chem. Commun. 2009, 3267–3269. [Google Scholar]
- Dash, B.P.; Satapathy, R.; Zheng, C.; Gaillard, E.R.; Maguire, J.A.; Hosmane, N.S. Carborane-appended C3-symmetrical extended pi (π)-s ystems: Synthesis and properties. J. Am. Chem. Soc. 2010, 132, 6578–6587. [Google Scholar]
- Suzuki, A. New synthetic transformations via organoboron compounds. Pure Appl. Chem. 1994, 66, 213–222. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Valtier, M.; Carboni, B. Comprehensive Organometallic Chemistry II; Abel, E.W., Stone, F.G.A., Wilkinson, G., Eds.; Pergamon: Oxford, UK, 1995; Volume 11, p. 191. [Google Scholar]
- Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Synthesis of arylboronates via the palladium(0)- catalyzed cross-coupling reaction of tetra(alkoxo)diborons with aryl triflates. Tetrahedron Lett. 1997, 38, 3447–3450. [Google Scholar] [CrossRef]
- Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. Palladium-catalyzed borylation of aryl halides or triflates with dialkoxyborane: A novel and facile synthetic route to arylboronates. J. Org. Chem. 2000, 65, 164–168. [Google Scholar] [CrossRef]
- Goswami, A.; Maier, C.-J.; Pritzkow, H.; Siebert, W. Cobalt-mediated cyclooligomerization reactions of borylacetylenes. Eur. J. Inorg. Chem. 2004, 2635–2645. [Google Scholar]
- Gandon, V.; Leca, D.; Aechtner, T.; Vollhardt, K.P.C.; Malacria, M.; Aubert, C. Synthesis of fused arylboronic esters via cobalt(0)-mediated cycloaddition of alkynylboronates with α,ω-diynes. Org. Lett. 2004, 6, 3405–3407. [Google Scholar] [CrossRef]
- Paetzold, I.P. New perspectives in boron-nitrogen chemistry–I. Pure Appl. Chern. 1991, 63, 345–350. [Google Scholar] [CrossRef]
- Luckert, S.; Englert, U.; Paetzold, P. Clusters N2B4R 6 from the fusion of tings NB2R3: Mechanism. Inorg. Chimi. Acta 1998, 269, 43–46. [Google Scholar] [CrossRef]
- Sneddon, L.G.; Mirabelli, M.G.L.; Lynch, A.T.; Fazen, P.J.; Su, K.; Beck, J.S. olymeric Precursors to Boron-Based Ceramics. Pure Appl. Chem. 1991, 63, 407–410. [Google Scholar] [CrossRef]
- Inoue, M.; Sekiyama, S.; Nakamura, K.; Shishiguchi, S.; Matsuura, A.; Fukuda, T.; Yanazawa, H.; Uchimaru, Y. Borazine-siloxane Organic/Inorganic Hybrid Polymer. Rev. Adv. Mater. Sci. 2003, 5, 392–397. [Google Scholar]
- Iona, H.T.S.; Kwok, C.-C.; Che, C.-M.; Zhu, N. Borazine materials for organic optoelectronic applications. Chem. Commun. 2005, 3547–3549. [Google Scholar]
- Rolf, H. 1,3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M. G.; Sharpless, K. B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Link, A.J.; Tirrell, D. A. Cell surface labeling of escherichia coli via copper(I)-catalyzed [3+2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 11164–11165. [Google Scholar] [CrossRef]
- Gupta, S.S.; Raja, K.S.; Kaltgrad, E.; Strable, E.; Finn, M. G. Virus–glycopolymer conjugates by copper(I) catalysis of atom transfer radical polymerization and azide–alkyne cycloaddition. Chem. Commun. 2005, 4315–4317. [Google Scholar]
- Opsteen, J.A.; van Hest, J.C.M. Modular synthesis of block copolymers via cycloaddition of terminal azide and alkyne functionalized polymers. Chem. Commun. 2005, 57–59. [Google Scholar]
- Zhu, Y.; Lin, Y.; Zhu, Y.Z.; Lu, J.; Maguire, J.A.; Hosmane, N.S. Boron drug delivery via encapsulated magnetic nanocomposites: A new approach for BNCT in cancer treatment. J. Nanomater. 2010, 2010. [Google Scholar] [PubMed]
- El-Zaria, M.E.; Nakamura, H. New strategy for synthesis of mercaptoundecahydrododecaborate derivatives via click chemistry: Possible boron carriers and visualization in cells for Neutron Capture Therapy. Inorg. Chem. 2009, 48, 11896–11902. [Google Scholar] [CrossRef]
- Wojtczak, B.A.; Olejniczak, A.B.; Lesnikowski, Z.J. Nucleoside modification with boron clusters and their metal complexes. Curr. Protoc. Nucleic Acid Chem. 2009, 38, 4.37.1–4.37.26. [Google Scholar]
- Doyle, M.P. Reactive Intermediate Chemistry; Moss, R.A., Platz, M.S., Jones, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 561–592, Chapter 12. [Google Scholar]
- Zhu, Y.; Ang, T.P.; Keith, C.; Maguire, J.A.; Hosmane, N.S.; Takagaki, M. Substituted carborane-appended water-soluble single-wall carbon nanotubes: New approach to Boron Neutron Capture Therapy drug delivery. J. Am. Chem. Soc. 2005, 127, 9875–9880. [Google Scholar]
- Davies, M.W.; Wybrow, R.A.J.; Johnson, C.N.; Harrity, J.P.A. A regioselective cycloaddition route to isoxazoleboronic esters. Chem. Commun. 2001, 1558–1559. [Google Scholar]
- Diels, O.; Alder, K. Synthesis in the hydroaromatic series, IV. Announcement: The rearrangement of malein acid anhydride on arylated diene, triene and fulvene. Berichte 1929, 62B, 2081–2087. [Google Scholar]
- Kagan, H.B.; Riant, O. Catalytic asymmetric Diels Alder reactions. Chem. Rev. 1992, 92, 1007–1019. [Google Scholar]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T. Vassilikogiannakis, the Diels-Alder Reaction in Total Synthesis. G. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Kirkham, J.D.; Delaney, P.M.; Ellames, G.J.; Row, E.C.; Harrity, J.P.A. An alkynylboronate cycloaddition strategy to functionalised benzyne derivatives. Chem. Commun. 2010, 46, 5154–5156. [Google Scholar]
- Gomez-Bengoa, E.; Helm, M.D.; Plant, A.; Harrity, J.P.A. The Participation of alkynylboronates in inverse electron demand [2+4] cycloadditions: A mechanistic study. J. Am. Chem. Soc. 2007, 129, 2691–2699. [Google Scholar]
- Wood, T.K.; Piers, W.E.; Keay, B.A.; Prarvez, M. 1-Borabarrelene derivatives via Diels-Alder additions to borabenzenes. Org. Lett. 2006, 8, 2875–2878. [Google Scholar] [CrossRef]
- Maier, G.; Henkelmann, J.; Reisenauer, H.P. Simply substituted boraethenes. Angew. Chem. Int. Ed. 1985, 24, 1065–1066. [Google Scholar] [CrossRef]
- Cade, I.A.; Hill, A.F.; Wagler, J. Diels-Alder dimerization of a boracyclohexadiene. Organometallics 2008, 27, 4844–4846. [Google Scholar] [CrossRef]
- Bosdet, M.J.D.; Jaska, C.A.; Piers, W.E.; Sorensen, T.S.; Parvez, M. Blue Fluorescent 4a-aza-4b-bora-Phenanthrenes. Org. Lett. 2007, 9, 1395–1398. [Google Scholar] [CrossRef]
- Kutal, C.R.; Owen, D.A.; Todd, L.J. Closo-1,2-dicarbadodecaborane(12). Inorg. Synth. 1968, 11, 19–23. [Google Scholar]
- Henry, L.G.; Tirthankar, G.; Huang, Q.; Maitland, J. 1,2-Dehydro-o-carborane. J. Am. Chem. Soc. 1990, 112, 4082–4083. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Kiran, B. Structure and bonding in B10X2H10 (X = C and Si). The kinky surface of 1,2-dehydro-o-disilaborane. J. Am. Chem. Soc. 1997, 119, 4076–4077. [Google Scholar] [CrossRef]
- Kiran, B.; Anoop, A.; Jemmis, E.D. Control of stability through overlap matching: Closo-carboranes and closo-silaboranes. J. Am. Chem. Soc. 2002, 124, 4402–4407. [Google Scholar] [CrossRef]
- Deng, L.; Chan, H.-S.; Xie, Z. Nickel-Mediated regioselective [2+2+2] cycloaddition of carboryne with alkynes. J. Am. Chem. Soc. 2006, 128, 7728–7729. [Google Scholar] [CrossRef]
- Wang, S.R.; Qiu, Z.; Xie, Z. Insertion of carboryne into aromatic rings: Formation of cyclooctatetraenocarboranes. J. Am. Chem. Soc. 2010, 132, 9988–9989. [Google Scholar]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, Y.; Siwei, X.; Maguire, J.A.; Hosmane, N.S. Application of Cycloaddition Reactions to the Syntheses of Novel Boron Compounds. Molecules 2010, 15, 9437-9449. https://doi.org/10.3390/molecules15129437
Zhu Y, Siwei X, Maguire JA, Hosmane NS. Application of Cycloaddition Reactions to the Syntheses of Novel Boron Compounds. Molecules. 2010; 15(12):9437-9449. https://doi.org/10.3390/molecules15129437
Chicago/Turabian StyleZhu, Yinghuai, Xiao Siwei, John A. Maguire, and Narayan S. Hosmane. 2010. "Application of Cycloaddition Reactions to the Syntheses of Novel Boron Compounds" Molecules 15, no. 12: 9437-9449. https://doi.org/10.3390/molecules15129437
APA StyleZhu, Y., Siwei, X., Maguire, J. A., & Hosmane, N. S. (2010). Application of Cycloaddition Reactions to the Syntheses of Novel Boron Compounds. Molecules, 15(12), 9437-9449. https://doi.org/10.3390/molecules15129437