Abstract
6-[(4-Methoxy/4,9-dimethoxy)-7-methylfurochromen-5-ylideneamino]-2-thioxo-2,3-dihydropyrimidin-4-ones 1a,b were prepared by reaction of 6-amino-2-thiouracil with visnagen or khellin, respectively. Reaction of 1a,b with methyl iodide afforded furochromenylideneaminomethylsulfanylpyrimidin-4-ones 2a,b. Compounds 2a,b were reacted with secondary aliphatic amines to give the corresponding furochromen-ylideneamino-2-substituted pyrimidin-4-ones 3a-d. Reaction of 3a-d with phosphorus oxychloride yielded 6-chlorofurochromenylidenepyrimidinamines 4a-d, which were reacted with secondary amines to afford furochromenylideneamino-2,6-disubstituted pyrimidin-4-ones 5a-d. In addition, reaction of 5a-d with 3-chloropentane-2,4-dione gave 3-chloro-furochromenylpyrimidopyrimidines 6a-d. The latter were reacted with piperazine and morpholine to give 1-(furochromenyl)-pyrimidopyrimidine-3,6,8-triylpiperazines or-3,6,8-triylmorpholines 7a-d. The chemical structures of the newly synthesized compound ware characterized by IR, 1H-NMR, 13C-NMR and mass spectral analysis. These compounds were also screened for their analgesic and anti-inflammatory activities. Some of them, particularly 3-7, exhibited promising activities.
1. Introduction
Khellin and visnagen, 4,9-dimethoxy- or 4-methoxy-7-methyl-furo[3,2-g]chromen-5-one, respectively (Figure 1) are obtained from fruits and seeds of the plant Ammi visnaga. The khellin and visnagen extract has been widely employed as a herbal medicine in the treatment of angina [1]. Khellin is used as a spasmolytic agent and for kidney stone treatment [2]. Khellin and visnagen extract significantly prolongs the induction time of nucleation of calcium oxalate [3]. Khellin has been used for photochemotherapeutic treatment of vitiligo and psoriasis [4] and the photodynamic properties of khellin and visnagen in their photoreaction with DNA have been studied [5]. Recently, khellin was proved to have phototherapeutic properties that are similar to those of the psoralens, but with substantially lower phototoxic effects and DNA mutation effects. Its penetration into the hair follicles is enhanced by encapsulating it into liposomes. Subsequent activation of khellin with UV light stimulates the melanocytes in the hair follicles [6]. The fact that furochromone derivatives are known to have anti-inflammatory properties [7] and analgesic properties [8], prompted us to synthesize and investigate such properties in khellin and visnagen derivatives as typical furochromones.
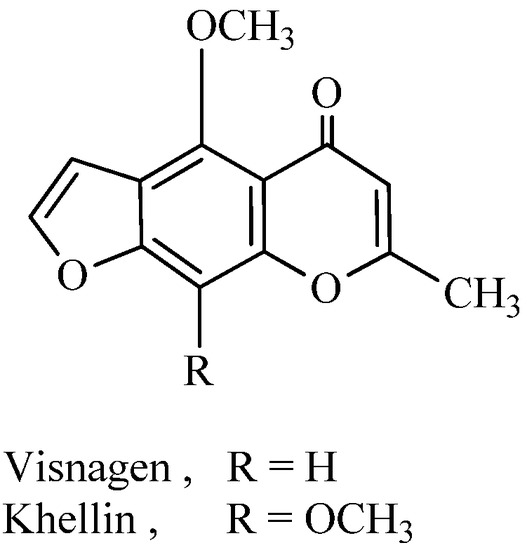
Figure 1.
Chemical structure of khellin and visnagen.
From a photobiological point of view, furochromones show valuable phototoxicity toward various kinds of microorganisms and also valuable genotoxic activity on various biological substrates [9,10]. In the present work, we planned the synthesis of various furochromenylidenylpyrimidine derivatives representing new heterocyclic compounds. These compounds were also screened for theiranti-inflammatory and analgesic activities.
2. Results and Discussion
Condensation of visnagen and khellin with 6-amino-2-thiouracil in dimethylformamide [11,12] yielded the 6-(4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-thioxo-2,3-dihydro-1H-pyrimidin-4-ones 1a,b. The IR spectra of compounds 1a,b revealed the presence of only one carbonyl band, in addition to the absence of the amino group band. The mass spectra of 1a and 1b showed molecular ion peaks at m/z 355 (96%) and 385 (90%), respectively. Compounds 1a,b underwent alkylation at the sulphur atom upon treatment with methyl iodide in aqueous ethanolic KOH solution, to afford 6-((4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylidene-amino)-2-methylsulfanyl-3H-pyrimidin-4-ones 2a,b. The 1H-NMR spectra of 2a and 2b showed singlets at δ 2.68 and 2.65 ppm, respectively, corresponding to a SCH3 group. Reaction of the2-methylsulfanyl derivatives 2a,b with secondary aliphatic amines, namely piperazine and morpholine, in methanol [13] produced the 6-(4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-(piperazin/morpholin)-1-yl-3H-pyrimidin-4-ones 3a-d (Scheme 1).
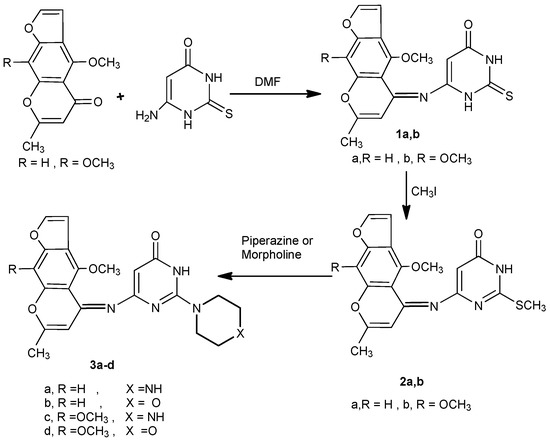
Scheme 1.
Condensation of khellin and visnagen with aminothiouracil, methylation and reaction with secondary amines.
Moreover, the reaction of 3a-d with phosphorus oxychloride in dry dioxane [14] afforded the (6-chloro-2-(piperazin/morpholin)-1-yl-pyrimidin-4-yl)-(4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]-chromen-5-ylidene)amines 4a-d. The IR spectra of 4a-d revealed the absence of any absorption bands in the NH and carbonyl regions. Compounds 4a-d, having an active chlorine substituent, reacted with either piperazine or morpholine in boiling methanol to produce 2,6-(dipiperazin/dimorpholin)-1-yl-pyrimidin-4-yl)-(4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]-chromen-5-ylidene)amines 5a-d. The structures of compounds 5a-d were confirmed by their correct elemental analyses values, as well as compatible spectral data (see Experimental). Compounds 5a-d reacted with 3-chloropentane-2,4-dione (a typical β-diketone) in acetic acid, in the presence of zinc dust, to afford the corresponding 3-chloro-1-(4-methoxy/4,9-dimethoxy)-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-6,8-dipiperazin/di-morpholin)-1-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidines 6a-d in good yield. Formation of 6a-d from the corresponding 5a-d may proceed by initial reduction of compounds 5a-d, followed by cyclocondensation of the intermediates produced with the diketone followed by a necessary final reduction step to produce 6a-d.
The steps of the suggested mechanism are shown in Scheme 2. The 1H-NMR spectrum of 6a, for example, showed a doublet at 1.15 ppm, a multiplet at 3.14 and a triplet at 3.92, which support the proposed reduced structure 6. Finally, the 3-chlorofurochromenepyrimido/pyrimidines reacted with either piperazine or morpholine in boiling methanol to give the corresponding 3,6,8-tripiperazin-1-yl or 3,6,8-trimorpholin-1-yl derivatives 7a-d (Scheme 3). Compatible analytical and spectral data were obtained for compounds 7a-d (see Experimental).
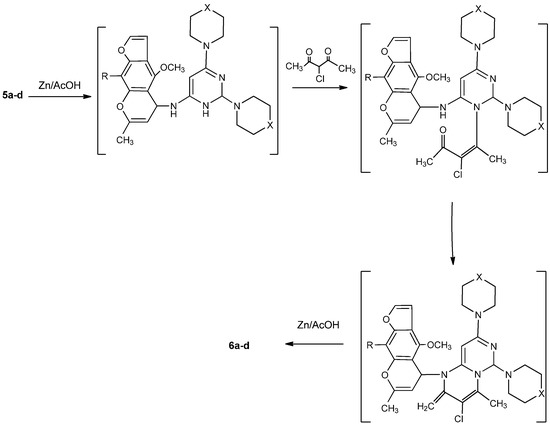
Scheme 2.
Suggested mechanism for the formation of 6a-d from 5a-d.
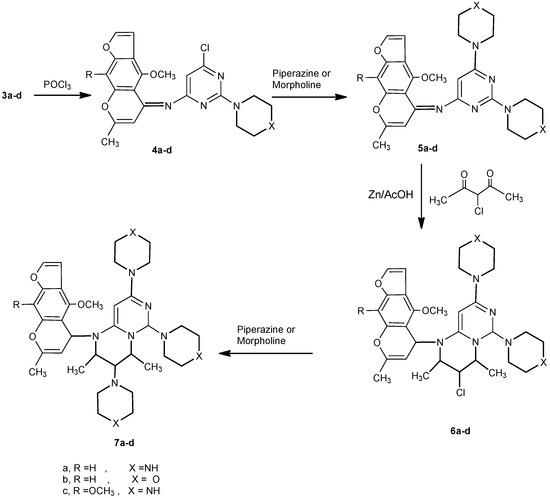
Scheme 3.
Chlorination of 3a-d, condensation with amines and cyclocondensation with β-diketone.
2.1. Anti-Inflammatory Activity
The anti-inflammatory activity was evaluated in rats by the carrageenan-induced paw edema test. The data (Table 1) indicated that all the tested compounds protected rats from carrageenan-induced inflammation, and that some of the tested compounds (6a-d and 7a-d) are more potent than our previously reported ones [15,16]. Compounds 2-5 showed similar and higher anti-inflammatory activity than diclofenac sodium.

Table 1.
Percent anti-inflammatory activity of the tested compounds (carrageenan-induced paw edema test in rats).
| Compd. No. | Percent protection | ||
|---|---|---|---|
| 1 hour | 2 hours | 3 hours | |
| Visnagen | 38.6 ± 1.38 * | 40.2 ± 1.39 * | 26.2 ± 1.29 * |
| Khellin | 40.3 ± 1.40 * | 41.5 ± 1.42 * | 30.4 ± 1.32 * |
| 1a | 41.5 ± 1.35 * | 40.4 ±1.36 * | 28.2 ± 1.28 * |
| 1b | 42.6 ± 1.45 * | 41.5 ±1.28 * | 30.1 ± 1.30 * |
| 2a | 44.5 ± 1.28 * | 43.6 ±1.24 * | 33.1 ± 1.01 * |
| 2b | 45.7 ± 1.30 * | 44.8 ±1.28 * | 34.2 ± 1.02 * |
| 3a | 52.6± 1.10 ** | 50.8 ± 1.30 * | 39.4± 1.25 * |
| 3b | 48.6 ± 1.40 * | 45.6 ±1.40 * | 35.1 ± 1.04 * |
| 3c | 52.8± 1.12 ** | 52.6 ± 1.10 * | 39.6± 1.23 * |
| 3d | 49.9 ± 1.50 ** | 48.8 ±1.42 * | 36.5 ± 1.20 * |
| 4a | 54.4± 1.23 ** | 53.6 ± 1.22 * | 40.4± 1.04 * |
| 4b | 53.8± 1.20 ** | 52.6 ± 1.10 * | 40.1± 1.02 * |
| 4c | 55.3 ± 1.25 ** | 54.8 ± 1.24 * | 40.6± 1.05 * |
| 4d | 54.1± 1.22 ** | 53.2 ± 1.20 * | 40.2± 1.03 * |
| 5a | 57.9 ± 1.39 ** | 58.9 ± 1.30 * | 41.5 ± 1.08 * |
| 5b | 57.3 ± 1.37 ** | 58.8 ± 1.29 * | 41.3± 1.06 * |
| 5c | 58.4 ± 1.40 ** | 59.1 ± 1.31 ** | 42.1 ± 1.23 * |
| 5d | 57.6 ± 1.38 ** | 60.0 ± 1.68 ** | 41.4 ± 1.07 * |
| 6a | 59.7 ± 1.45 ** | 59.5 ± 1.35 ** | 42.8 ± 1.26 * |
| 6b | 59.4 ± 1.05 ** | 59.1 ± 1.31 ** | 42.2 ± 1.24 * |
| 6c | 59.8 ± 1.52 ** | 59.7 ± 1.45 ** | 45.7 ± 1.52 * |
| 6d | 59.6 ± 1.41 ** | 59.2 ± 1.32 ** | 42.4 ± 1.25 * |
| 7a | 61.8± 1.88 ** | 61.4 ± 1.74 ** | 48.2 ± 1.65 * |
| 7b | 60.5± 1.80 ** | 60.2 ± 1.62 ** | 46.6 ± 1.60 * |
| 7c | 62.3± 1.92 ** | 61.6 ± 1.78 ** | 48.5 ± 1.68 * |
| 7d | 60.9± 1.85 ** | 60.4 ± 1.65 ** | 47.1 ± 1.63 * |
| Control | 6.3 ± 0.26 | 5.6 ± 0.40 | 3.4 ± 0.96 |
| Diclofenac Sodium | 52.6 ± 0.96 * | 60.5± 1.55 ** | 42.2 ± 1.39 * |
Each value represents the mean ± S.E (n = 6).Significance levels * p < 0.5, ** p < 0.001 as compared with respective control. Dose (20 mg/kg). For the selected tested compound.
2.2. Analgesic Activity
The analgesic activity was determined by the hot-plate test (central analgesic activity) and acetic acid induced writhing assay. The results (Table 2 and Table 3) revealed that all tested compounds exhibited significant activity. Most of the tested compounds have nearly the same activity as the reference drug and the remaining tested compound have good activities in central analgesic activity. Also compound 7c exhibited activities higher than the reference.

Table 2.
Central analgesic activity (Hot plate test).
| Group | Reaction time (min) | |||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | |
| Control | 8.25 ± 0.35 | 8.20 ± 0.36 b | 8.75 ± 0.55 b | 9.80 ± 0.48 b |
| Visnagen | 5.40 ± 0.56 | 6.30 ± 0.50 | 8.90 ± 0.60 | 9.80 ± 0.25 a |
| Khellin | 5.50 ± 0.58 | 6.50 ± 0.40 | 9.01 ± 0.70 | 10.01 ± 0.22 a |
| 1a | 5.60 ± 0.30 | 6.70± 0.43 | 9.25 ± 0.55 | 10.10 ± 0.20a |
| 1b | 6.10 ± 0.20 | 7.30 ± 0.10 b | 7.40 ± 0.10 a | 10.20 ± 0.25 a |
| 2a | 6.20 ± 0.10 | 7.45 ± 0.20 b | 8.10± 0.20 a | 10.50 ± 0.30 a |
| 2b | 6.40 ± 0.20 | 7.55 ± 0.25 b | 8.25± 0.30 a | 10.80 ± 0.35 a |
| 3a | 7.01 ± 0.30 | 8.10 ± 0.40 | 10.55 ± 0.50 a | 10.90 ± 0.60 a b |
| 3b | 6.65 ± 0.10 | 7.90 ± 0.20 b | 8.45 ± 0.50 a | 11.20 ± 0.30 a |
| 3c | 7.10 ± 0.10 | 8.20 ± 0.30 a | 9.50 ± 0.25 a | 10.55 ± 0.10 a b |
| 3d | 6.65 ± 0.10 | 7.90 ± 0.20 b | 8.45 ± 0.50 a | 11.20 ± 0.30 a |
| 4a | 7.85 ± 0.35 | 8.60 ± 0.40 a | 10.01 ± 0.20a | 11.05± 0.40 a b |
| 4b | 7.30 ± 0.20 | 8.30 ± 0.40 a | 9.80 ± 0.30 a | 10.80 ± 0.20 a b |
| 4c | 8.10 ± 0.20 | 8.80 ± 0.25 a | 10.10 ± 0.25 a | 11.10 ± 0.30 b |
| 4d | 7.40 ± 0.30 | 8.50 ± 0.30 a | 9.90 ± 0.35 a | 10.90 ± 0.30 a b |
| 5a | 8.75 ± 0.60 | 9.10 ± 0.50 a | 10.70 ± 0.40 a | 11.50 ± 0.40 b |
| 5b | 8.30 ± 0.30 | 8.90 ± 0.30 a | 10.30 ± 0.20 a | 11.20 ± 0.25 b |
| 5c | 8.95 ± 0.68 | 9.20 ± 0.60 a | 10.90 ± 0.50 a | 11.60 ± 0.30 b |
| 5d | 8.55 ± 0.50 | 9.01 ± 0.40 a | 10.50 ± 0.30 a | 11.40 ± 0.30 b |
| 6a | 9.30 ± 0.30 | 9.40 ± 0.30 a | 10.40 ± 0.25 a | 11.10± 0.30 a b |
| 6b | 9.10 ± 0.30 | 9.20 ± 0.40 a | 10.20 ± 0.20 a | 11.10 ± 0.40a b |
| 6c | 9.40 ± 0.40 | 9.50 ± 0.30 a | 10.50 ± 0.20 a | 11.20± 0.35 a b |
| 6d | 9.20 ± 0.20 | 9.30 ± 0.10 a | 10.30 ± 0.30 a | 11.01± 0.20 a b |
| 7a | 9.70 ± 0.40 | 9.80 ± 0.55 a | 10.75 ± 0.35 a | 11.44± 0.44 a b |
| 7b | 9.50 ± 0.20 | 9.60 ± 0.40 a | 10.60 ± 0.25 a | 11.30± 0.30 a b |
| 7c | 9.80 ± 0.50 | 9.95 ± 0.65 a | 10.85 ± 0.45 a | 11.98 ± 0.88 a b |
| 7d | 9.60 ± 0.30 | 9.70 ± 0.45 a | 10.65 ± 0.30 a | 11.40± 0.40 a b |
| Diclofanc sodium | 6.50 ± 0.45 | 10.05 ± 0.15 a | 11.40 ± 0.55 a | 13.18 ± 0.40 a |
Values represent the mean ± S.E. of six animals for each groups. a. P < 0.05: Statistically significant from Control. (Dunnett’s test). b. P < 0.05: Statistically significant from ASA. (Dunnett’s test).* Significant at P < 0.0.

Table 3.
Percent analgesic activity (peripheral, writhing test).
| Compd. No. | Percent protection | |||
|---|---|---|---|---|
| 30 min | 1 hours | 2 hour | 3 hours | |
| Visnagen | 39.20 ± 1.20 * | 44 ± 1.10 * | 48.1 ± 1.70 | 32.20 ± 1.25 * |
| Khellin | 40.7 ± 1.65 * | 46 ± 1.35 * | 49.4 ± 1.70 | 33.9 ± 1.10 * |
| 1a | 42.6 ± 1.40 * | 52 ± 1.25 * | 50.4 ± 1.60 | 35.8 ± 1.39 * |
| 1b | 45.0 ± 1.90 * | 53 ± 1.40 * | 55.6 ± 1.38 | 36.3 ± 1.20 * |
| 2a | 46.5 ± 1.50 * | 50 ± 1.10 ** | 52.2 ± 1.30 | 37.5 ± 1.30 * |
| 2b | 48.2 ± 1.55 * | 52 ± 1.20 * | 54.3 ± 1.25 | 38.7 ± 1.40 * |
| 3a | 54.6 ± 1.40 * | 58 ± 1.25 * | 62.6 ± 1.20 * | 46.4 ± 1.10 * |
| 3b | 50.3 ± 1.35 * | 54 ± 1.10 * | 56.4 ± 1.35 | 40.5 ± 1.35 * |
| 3c | 55.8 ± 1.50 * | 59 ± 1.20 * | 63.6 ± 1.30 * | 47.3 ± 1.30 * |
| 3d | 52.6 ± 1.35 * | 56 ± 1.05 * | 58.6 ± 1.35 | 42.1 ± 1.40 * |
| 4a | 60.4 ± 1.40 ** | 63 ± 1.50 ** | 67.7± 1.70 ** | 48.4 ± 1.55 * |
| 4b | 58.5 ± 1.20 * | 60 ± 1.30 * | 65.4 ± 1.55* | 46.5 ± 1.35 * |
| 4c | 61.5 ± 1.50 ** | 64 ± 1.55 ** | 68.8 ± 1.80 ** | 49.5 ± 1.60 * |
| 4d | 59.6 ± 1.30 * | 62 ± 1.40 * | 66.1 ± 1.60 * | 47.3 ± 1.50 * |
| 5a | 66.50 ± 1.20 ** | 68 ± 1.55 ** | 70.6 ± 1.30 ** | 54.5 ± 1.10 * |
| 5b | 62.50 ± 1.40 ** | 65 ± 1.35 ** | 69.10 ±1.25 ** | 52.1 ± 1.30 * |
| 5c | 68.10 ± 1.25 ** | 70 ± 1.85 ** | 71.8 ± 1.35 ** | 55.4 ± 1.05 * |
| 5d | 65.40 ± 1.10 ** | 67 ± 1.50 ** | 70.20 ±1.15 ** | 53.2 ± 1.20 * |
| 6a | 69.60 ± 1.55 ** | 72 ± 1.90 ** | 74.3 ± 1.50 ** | 58.8 ± 1.20 * |
| 6b | 69.1 ± 1.45 ** | 70 ± 1.80 ** | 72.6 ± 1.40 ** | 56.3 ± 1.10 * |
| 6c | 69.80 ± 1.60 ** | 73 ± 1.95 ** | 75.5 ± 1.55 ** | 59.8 ± 1.19 * |
| 6d | 69.4 ± 1.50 ** | 71 ± 1.85 ** | 73.5 ± 1.45 ** | 57.5 ± 1.15 * |
| 7a | 74.4 ± 1.10 ** | 77.5 ± 1.30 ** | 78.3 ± 1.20 | 64.4 ± 1.25 ** |
| 7b | 70.1 ± 1.01 ** | 75.3 ± 1.50 ** | 76.5 ± 1.25 | 62.4 ± 1.20 ** |
| 7c | 75.6 ± 1.20 ** | 78.4 ± 1.40 ** | 79.5 ± 1.35 | 65.6 ± 1.30 ** |
| 7d | 72.2 ± 1.05 ** | 76.2 ± 1.45 ** | 77.6 ± 1.30 | 63.5 ± 1.35 ** |
| Control | 02.0 ± 0.36 | 05.0 ± 0.50 | 04.0 ± 0.58 | 04.0 ± 0.90 |
| Diclfenac sodium | 45.0 ± 0.96 * | 54.3 ± 1.18 * | 61 ± 1.50 * | 38 ± 1.14 * |
Each value represents the mean ± S.E (n = 6).Significance levels * p < 0.5, ** p < 0.001 as compared with respective control. Dose (20 mg/kg). For the selected tested compound .Drug in peripheral analgesic activity testing. The remaining compounds have the same activity in Peripheral analgesic activity testing.
3. Conclusions
The new ring systems prepared seem to be interesting for biological activity studies. Furthermore, the present investigation offers rapid and effective new procedures for the synthesis of the poly-condensed new heterocyclic ring systems. Compounds 6a-d and 7a-d exhibited potent anti-inflammatory and analgesic activities. It is worth mentioning that the incorporation of methoxy, dimethoxy, -furo[3,2-g]chromen, di- and tri-(piperazine or morpholine) and tetrahydropyrimido[1,6-a]pyrimidine moieties resulted in significant anti-inflammatory and analgesic activities. In conclusion, we report herein a simple and convenient route for the synthesis of some new heterocyclic compounds based on furochromene pyrimidine derivatives for anti-inflammatory and analgesic evaluation.
4. Experimental
4.1. General
All melting points were taken on an Electrothermal IA 9100 series digital melting point apparatus (Shimadzu, Japan). Elemental analyses were performed at Vario EL (Elementar, Germany). Microanalytical data were processed in the microanalytical center, Faculty of Science, Cairo University and National Research Center. The IR spectra (KBr disc) were recorded using a Perkin-Elmer 1650 spectrometer (USA).1H-NMR spectra were determined using Jeol 270 MHz and Jeol JMS-AX 500 MHz (Jeol, Japan) spectrometers with Me4Si as an internal standard. Mass spectra were recorded on an EIMs-QP 1000 EX instrument (Shimadzu) at 70 eV. Pharmacological evaluations were done by the Pharmacology unit, Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Egypt.
4.2. General Procedure for the Synthesis of 6-((4-Methoxy/4,9-dimethoxy)7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-thioxo-2,3-dihydro-1H-pyrimidin-4-ones 1a,b
A mixture of visnagen (2.3 g, 10 mmol) or khellin (2.6 g, 10 mmol) and 6-amino-2-thiouracil (1.43 g, 10 mmol), was refluxed in dimethylformamide (50 mL) for 6-8 h. The reaction mixture was cooled; the deposited precipitate was filtered off, washed with ethanol, dried, and recrystallized to obtain 1a,b as crystalline products.
6-(4-Methoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one (1a). Obtained from visnagen as yellow crystals, m.p. 228-230 ºC, crystallized from methanol(80% yield); IR (KBr, cm−1): 3,390 (br, NHs), 3,030 (CH, aryl), 2,915 (CH, alkyl), 1,690 (CO), 1,630 (C=N); 1H-NMR: 1.75 (s, 3H, CH3), 3.75 (s, 3H, OCH3), 5.45 (s, 1H, Hpyran), 6.69 (s, 1H, Hfuran), 6.74 (s, 1H, Hbenzene), 6.90 (s, 1H, Hpyrimidine), 7.55 (s, 1H, Hfuran), 10.40, 11.60 (2 br s, 2NH, D2O exchangeable). 13C-NMR: 23.3, 56.4 (2C, CH3, OCH3), 93.5, 95.4, 99.7, 102.5, 108.4, 110.30, 147.1, 155.5, 157.4, 160.5, 162.3, 165.5, 166.3 (Ar-C), 169.2 (CO), 178.4 (CS). MS (70 eV, %) m/z, 355 (M+, 96%). Anal. Calc. for C17H13N3O4S (355.37); Requires (Found): C, 57.46 (57.52); H, 3.69 (3.62); N, 11.82 (11.88); S, 9.02 (9.15).
6-(4,9-Dimethoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one (1b). Obtained from khellin as yellowish crystals, m.p. 240-242 ºC, crystallized from dimethylformamide (82% yield); IR (KBr, cm−1): 3,391 (br, NHs), 3,031 (CH, aryl), 2,914 (CH, alkyl), 1,688 (CO), 1,631 (C=N). 1H-NMR: 1.74 (s, 3H, CH3), 3.76 (s, 6H, 2 OCH3), 5.46 (s, 1H, Hpyran), 6.68 (s, 1H, Hfuran), 6.91 (s, 1H, Hpyrimidine), 7.54 (s, 1H, Hfuran), 10.42, 11.62 (2 br s, 2NH, D2O exchangeable). MS (70 eV, %) m/z 385 (M+, 90%). Anal. Calc. (Found) for C18H15N3O5S (385.39): C, 56.10 (56.20); H, 3.92 (3.98); N, 10.90 (10.85); S, 8.32 (8.37).
4.3. General Procedure for the Synthesis of 6-((4-Methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-methylsulfanyl-3H-pyrimidin-4-ones 2a,b
To a warmed ethanolic KOH solution (prepared by dissolving 0.01 mol of KOH in 50 mL ethanol) was added each of 1a (3.55 g, 10 mmol), or 1b (3.85 g, 10 mmol), heating was continued for 30 min and the mixture was allowed to cool to room temperature, and methyl iodide (12 mmol) was added. The mixture was stirred under reflux for 6 hours, then cooled to room temperature and poured into cold water (100 mL). The solid product that precipitated was filtered off, washed with 100 mL water; the product was dried and crystallized to produce 2a,b.
6-(4-Methoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-methylsulfanyl-3H-pyrimidin-4-one (2a). Obtained from 1a as white crystals, m.p. 275-277 ºC, crystallized from dioxane (75% yield); IR (KBr, cm−1): 3,385 (br, NH), 3,032 (CH, aryl), 2,918 (CH, alkyl), 1,686 (CO), 1,629 (C=N); 1H-NMR: 1.73 (s, 3H, CH3), 2.68 (s, 3H, SCH3), 3.75 (s, 3H, OCH3), 5.50 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 6.72 (s, 1H, Hbenzene), 6.93 (s, 1H, Hpyrimidine), 7.53 (s, 1H, Hfuran), 10.20 (brs, NH, D2O exchangeable).MS (70 eV, %) m/z 369 (M+, 92%). Anal. Calc. (Found) for C18H15N3O4S (369.39): C, 58.53 (58.50); H, 4.09 (4.14); N, 11.38 (11.45); S, 8.68 (8.73).
6-(4,9-Dimethoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-methylsulfanyl-3H-pyrimidin-4-one (2b). Obtained from 1b as a white powder, m.p. 293-295 ºC, crystallized from methanol (70% yield); IR (KBr, cm−1): 3,384 (br, NH), 3,030 (CH, aryl), 2,919 (CH, alkyl), 1,689 (CO), 1,630 (C=N); 1H-NMR: 1.75 (s, 3H, CH3), 2.65 (s, 3H, SCH3), 3.77 (s, 6H, 2 OCH3), 5.49 (s, 1H, Hpyran), 6.68(s, 1H, Hfuran), 6.89 (s, 1H, Hpyrimidine), 7.52 (s, 1H, Hfuran), 10.15 (br s, NH, D2O exchangeable).13C-NMR: 19.5, 23.2, 56.6, (4C, CH3, S-CH3, 2OCH3), 99.7, 101.5, 107.4, 109.2, 112.50, 127.1, 140.3, 145.1, 146.1, 149.2, 156.5, 157.5, 161.5, 165.3 (Ar-C), 169.4 (CO). MS (70 eV, %) m/z 399(M+, 91%). Anal. Calc. (Found) for C19H17N3O5S (399.42): C, 57.13 (57.20); H, 4.29 (4.25); N, 10.52 (10.57); S, 8.03 (8.18).
4.4. General Procedure for the Synthesis of 6-((4-Methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-(piperazin/morpholin)-1-yl-3H-pyrimidin-4-ones 3a-d
To a warm solution of 2a (3.69 g, 10 mmol) or 2b (3.99 g, 10 mmol) in methanol (100 mL) was added the freshly distilled secondary aliphatic amines (piperazine and morpholine, 10 mmol). The reaction mixture was stirred under reflux for 8 h, and then allowed to cool to 0 ºC for 12 h. The solid obtained was filtered, washed with water (100 mL), dried, and crystallized from the appropriate solvent to produce 3a-d.
6-(4-Methoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-piperazin-1-yl-3H-pyrimidin-4-one (3a). Obtained from 2a and piperazine (0.86 g, 10 mmol) as a yellow powder, m.p. 215-217 ºC, crystallized from ethanol (74% yield); IR (KBr, cm−1): 3,390 (br, NH), 3,035 (CH, aryl), 2,922 (CH, alkyl), 1,690 (CO), 1,631(C=N); 1H-NMR: 1.76 (s, 3H, CH3), 2.67-2.73(m, 8H, Hpiperazine), 3.74 (s, 3H, OCH3), 5.51 (s, 1H, Hpyran), 6.66 (s, 1H, Hfuran), 6.70 (s, 1H, Hbenzene), 6.90 (s, 1H, Hpyrimidine), 7.54 (s, 1H, Hfuran), 9.75 (br s, NH, D2O exchangeable), 10.25 (br s, NH, D2O exchangeable). MS (70 eV, %) m/z 407 (M+, 88%). Anal. Calc. (Found) for C21H21N5O4 (407.42): C, 61.91 (61.88); H, 5.20 (5.10); N, 17.19 (17.22).
6-(4-Methoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-morpholin-4-yl-3H-pyrimidin-4-one (3b). Obtained from 2a and morpholine (0.87 g, 10 mmol) as yellowish crystals, m.p. 203-205 ºC, crystallized from dioxane (76% yield); IR (KBr, cm−1): 3,391 (br, NH), 3,034 (CH, aryl), 2,921 (CH, alkyl), 1,688 (CO), 1,629 (C=N); 1H-NMR: 1.75 (s, 3H, CH3), 3.10 (t, 4H, Hmorpholine) 3.57 (t, 4H, Hmorpholine), 3.72 (s, 3H, OCH3), 5.52 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 6.71 (s, 1H, Hbenzene), 6.92(s, 1H, Hpyrimidine), 7.53 (s, 1H, Hfuran), 10.20 (br s, NH, D2O exchangeable). 13C-NMR: 23.3, 48.4, 56.5, 71.8 (6C, CH3, 4CH2, OCH3), 99.9, 101.1, 105.3, 106.9, 108.9, 112.40, 142.3, 145.1, 150.3, 154.2, 155.1, 156.3, 158.5, 160.1 (Ar-C), 169.1 (CO). MS (70 eV, %) m/z 408 (M+, 89%). Anal. Calc. (Found) for C21H20N4O(408.14): C, 61.76 (61.70); H, 4.94 (4.90); N, 13.72 (13.63).
6-(4,9-Dimethoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-piperazin-1-yl-3H-pyrimidin-4-one (3c). Obtained from 2b and piperazine (0.86 g, 10 mmol) as yellow crystals, m.p. 265-267 ºC, crystallized from benzene (77% yield); IR (KBr, cm−1): 3,390 (br, NH), 3,033 (CH, aryl), 2,920 (CH, alkyl), 1,685 (CO), 1,627 (C=N); 1H-NMR: 1.73 (s, 3H, CH3), 2.65-2.71 (m, 8H, Hpiperazine), 3.76(s, 6H, 2 OCH3), 5.54 (s, 1H, Hpyran), 6.66 (s, 1H, Hfuran), 6.95 (s, 1H, Hpyrimidine), 7.55 (s, 1H, Hfuran), 9.80 (br s, NH, D2O exchangeable), 10.30 (br s, NH, D2O exchangeable). MS (70 eV, %) m/z 437(M+, 86%). Anal. Calc. (Found) for C22H23N5O5 (437.45): C, 60.40 (60.35); H, 5.30 (5.39);N, 16.01 (16.21).
6-(4,9-Dimethoxy-7-methylfuro[3,2-g]chromen-5-ylideneamino)-2-morpholin-4-yl-3H-pyrimidin-4-one (3d). Obtained from 2b and morpholine (0.87 g, 10 mmol) as a white powder, m.p. 254-256 ºC, crystallized from hexane (75% yield); IR (KBr, cm−1): 3,394 (br, NH), 3,031 (CH, aryl), 2,918 (CH, alkyl), 1,680 (CO), 1,625 (C=N); 1H-NMR: 1.74 (s, 3H, CH3), 3.12 (t, 4H, Hmorpholine), 3.59 (t, 4H, Hmorpholine), 3.75 (s, 6H, 2 OCH3), 5.56 (s, 1H, Hpyran), 6.66 (s, 1H, Hfuran), 6.97 (s, 1H, Hpyrimidine), 7.52 (s, 1H, Hfuran), 10.26 (br s, NH, D2O exchangeable), 13C-NMR: 23.24, 28.2, 56.6, 72.1 (7C, CH3, 4 CH2, 2 OCH3), 99.3, 100.8, 106.9, 108.9, 112.2, 126.5, 140.1, 144.6, 145.4, 148.8, 158.7, 160.2, 162.3, 164.8 (Ar-C), 169.6 (CO). MS (70 eV, %) m/z 438 (M+, 85%). Anal. Calc. (Found) for C22H22N4O6 (438.43): C, 60.27 (60.30); H, 5.06 (5.12); N, 12.78 (12.70).
4.5. General Procedure for the Synthesis of (6-Chloro-2-(piperazin/morpholin)-1-yl-pyrimidin-4-yl)-(4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylidene)amines 4a-d
A solution of 3a-d (10 mmol) in dry dioxane (40 mL) was treated with 10 mL of phosphorus oxychloride, and the mixture was stirred under reflux for 7 h. The reaction mixture was allowed to cool to room temperature, and poured into cold water (100 mL), whereby a solid was separated, filtered off, and crystallized from the appropriate solvent to produce (4a-d).
(6-Chloro-2-piperazin-1-ylpyrimidin-4-yl)-(4-methoxy-7-methylfuro[3,2-g]chromen-5-ylidene)amine (4a). Obtained from 3a (4.07 g, 10 mmol) as a brown powder, m.p. 281-283 ºC, crystallized from methanol (82% yield); IR (KBr, cm−1): 3,380 (br, NH), 3,025 (CH, aryl), 2,915 (CH, alkyl), 1,615 (C=N); 1H-NMR: 1.74 (s, 3H, CH3), 2.75-2.81 (m, 8H, Hpiperazine), 3.73 (s, 3H, OCH3), 5.52 (s, 1H, Hpyran), 6.65 (s, 1H, Hfuran), 6.71 (s, 1H, Hbenzene), 6.95 (s, 1H, Hpyrimidine), 7.53 (s, 1H, Hfuran), 9.82 (br s, NH, D2O exchangeable). MS (70 eV, %) m/z 425 (M+, 84%). Anal. Calc. (Found) for C21H20ClN5O3 (425.87): C, 59.23 (59.20); H, 4.73 (4.68); N, 16.44 (16.33).
(6-Chloro-2-morpholin-4-yl-pyrimidin-4-yl)-(4-methoxy-7-methyl-furo[3,2-g]chromen-5-ylidene)-amine (4b). Obtained from 3b (4.08 g, 10 mmol) as yellow crystals, m.p. 256-258 ºC, crystallized from isopropanol (80% yield); IR (KBr, cm−1): 3,020 (CH, aryl), 2,918 (CH, alkyl), 1,617 (C=N); 1H-NMR: 1.75 (s, 3H, CH3), 3.11 (t, 4H, Hmorpholine), 3.55 (t, 4H, Hmorpholine), 3.72 (s, 3H, OCH3), 5.54 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 6.72 (s, 1H, Hbenzene), 7.1 (s, 1H, Hpyrimidine), 7.55 (s, 1H, Hfuran). MS (70 eV, %) m/z 426 (M+, 97%). Anal. Calc. (Found) for C21H19ClN4O4 (426.85): C, 59.09 (59.15); H, 4.49 (4.45); N, 13.13 (13.22).
(6-Chloro-2-piperazin-1-yl-pyrimidin-4-yl)-(4,9-dimethoxy-7-methylfuro[3,2-g]chromen-5-ylidene)-amine (4c). Obtained from 3c (4.37 g, 10 mmol) as a yellowish powder, m.p. 355-357 ºC, crystallized from dimethylformamide (78% yield); IR (KBr, cm−1): 3,391 (br, NH), 3,031 (CH, aryl), 2,922 (CH, alkyl), 1,616 (C=N); 1H-NMR: 1.71 (s, 3H, CH3), 2.78-2.84 (m, 8H, Hpiperazine), 3.74 (s, 6H, 2 OCH3), 5.55 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 7.12 (s, 1H, Hpyrimidine), 7.56 (s, 1H, Hfuran), 9.85 (br s, NH, D2O exchangeable). 13C-NMR: 23.4, 50.3, 56.6, 62.1 (7C, CH3, 4 CH2, 2 OCH3), 99.8, 101.2, 105.2, 106.8, 108.8, 126.4, 140.1, 144.9, 145.2, 148.8, 156.5, 162.4, 164.7, 170.6, 179.8 (Ar-C). MS (70 eV, %) m/z 455 (M+, 78%). Anal. Calc. (Found) for C22H22ClN5O4 (455.89): C, 57.96 (57.90); H, 4.86 (4.81);N, 15.36 (15.32).
(6-Chloro-2-morpholin-4-yl-pyrimidin-4-yl)-(4,9-dimethoxy-7-methylfuro[3,2-g]chromen-5-ylidene)-amine (4d). Obtained from 3d (4.38 g, 10 mmol) as a white powder, m.p. 340-342 ºC, crystallized from hexane (76% yield); IR (KBr, cm−1): 3,030 (CH, aryl), 2,916 (CH, alkyl), 1,615 (C=N);1H-NMR: 1.72 (s, 3H, CH3), 3.18 (t, 4H, Hmorpholine), 3.63 (t, 4H, Hmorpholine), 3.74 (s, 6H, 2 OCH3), 5.54 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 7.1 (s, 1H, Hpyrimidine), 7.53 (s, 1H, Hfuran). MS (70 eV, %) m/z 456 (M+, 75%). Anal. Calc. (Found) for C22H21ClN4O5 (456.88): C, 57.83 (57.79); H, 4.63(4.58);N, 12.26 (12.20).
4.6. General Procedure for the Synthesis of ((2,6-di-(Piperazin/morpholin))-1-yl-pyrimidin-4-yl)-((4-methoxy/4,9-dimethoxy)-7-methylfuro[3,2-g]chromen-5-ylidene)amines 5a-d
To a warm solution of 4a-d (10 mmol) in methanol (100 mL) was added the freshly distilled piperazine (10 mmol) or morpholine (10 mmol). The reaction mixture was stirred under reflux for 10 h, then allowed to cool to 0 ºC for 12 h. The solid obtained was filtered, washed with water (100 mL), dried, and crystallized from appropriate solvent to produce 5a-d.
(2,6-Di-piperazin-1-yl-pyrimidin-4-yl)-(4-methoxy-7-methylfuro[3,2-g]chromen-5-ylidene)amine (5a). Obtained from 4a (4.25 g, 10 mmol) as yellow crystals, m.p. 261-263 ºC, crystallized from ethanol (85% yield); IR (KBr, cm−1): 3,390 (br, NH), 3,030 (CH, aryl), 2,931 (CH, alkyl), 1,620 (C=N); 1H-NMR: 1.72 (s, 3H, CH3), 2.74-2.80 (m, 8H, Hpiperazine) 3.13-3.19 (m, 8H, Hpiperazine), 3.72 (s, 3H, OCH3), 5.54 (s, 1H, Hpyran), 6.68 (s, 1H, Hfuran), 6.72 (s, 1H, Hbenzene), 6.98 (s, 1H, Hpyrimidine), 7.55(s, 1H, Hfuran), 9.80-9.95 (br s, 2 NH, D2O exchangeable). MS (70 eV, %) 475 (M+, 84%). Anal. Calc. (Found) for C25H29N7O3 (475.54): C, 63.14 (63.10); H, 6.15 (6.20); N, 20.62 (20.65).
(2,6-Di-morpholin-4-yl-pyrimidin-4-yl)-(4-methoxy-7-methylfuro[3,2-g]chromen-5-ylidene)amine (5b). Obtained from 4b (4.26 g, 10 mmol) as brown crystals, m.p. 246-248 ºC, crystallized from ethanol (68% yield); IR (KBr, cm−1): 3,031 (CH, aryl), 2,920 (CH, alkyl), 1,622 (C=N); 1H-NMR: 1.75 (s, 3H, CH3), 2.96-3.02 (m, 8H, Hmorpholine), 3.63-3.69 (m, 8H, Hmorpholine), 3.73 (s, 3H, OCH3), 5.56(s, 1H, Hpyran), 6.66 (s, 1H, Hfuran), 6.70 (s, 1H, Hbenzene), 7.2 (s, 1H, Hpyrimidine), 7.54 (s, 1H, Hfuran).MS (70 eV, %) m/z 477 (M+, 86%). Anal. Calc. (Found) for C25H27N5O5 (477.51): C, 62.88 (62.80);H, 5.70 (5.68); N, 14.67 (14.55).
(4,9-Dimethoxy-7-methylfuro[3,2-g]chromen-5-ylidene)-(2,6-di-piperazin-1-yl-pyrimidin-4-yl)-amine (5c). Obtained from 4c (4.55 g, 10 mmol) as yellow crystals, m.p. 308-310 ºC, crystallized from dioxane (73 % yield); IR (KBr, cm−1); 3,395 (br, 2NH), 3,033 (CH, aryl), 2,921 (CH, alkyl), 1,624 (C=N); 1H-NMR: 1.72 (s, 3H, CH3), 2.73-2.79 (m, 8H, Hpiperazine), 3.13-3.19 (m, 8H, Hpiperazine), 3.75 (s, 6H, 2 OCH3), 5.57 (s, 1H, Hpyran), 6.68 (s, 1H, Hfuran), 7.1 (s, 1H, Hpyrimidine), 7.53 (s, 1H, Hfuran), 9.83-9.98 (br s, 2NH, D2O exchangeable). MS (70 eV, %) m/z 505 (M+, 93%). Anal. Calc. (Found) for C26H31N7O4 (505.57): C, 61.77 (61.70); H, 6.18 (6.13); N, 19.39 (19.32).
(4,9-Dimethoxy-7-methylfuro[3,2-g]chromen-5-ylidene)-(2,6-di-morpholin-4-yl-pyrimidin-4-yl)-amine (5d). Obtained from 4d (4.56 g, 10 mmol) as a white powder, m.p. 332-334 ºC, crystallized from hexane (78% yield); IR (KBr, cm−1): 3,032 (CH, aryl), 2,920 (CH, alkyl), 1,630 (C=N); 1H-NMR (DMSO-d6, δ, ppm); 1.73 (s, 3H, CH3), 2.97-3.04 (m, 8H, Hmorpholine), 3.69-3.76 (m, 8H, Hmorpholine), 3.73 (s, 6H, 2 OCH3), 5.57 (s, 1H, Hpyran), 6.69 (s, 1H, Hfuran), 7.2 (s, 1H, Hpyrimidine), 7.52 (s, 1H, Hfuran). 13C-NMR: 23.2, 58.8, 56.6, 71.4 (11C, CH3, 8CH2, 2 OCH3), 99.5, 100.2, 103.2, 106.9, 108.9, 126.5, 140.4, 145.1, 146.3, 148.9, 157.5, 161.5, 165.7, 169.7, 177.6 (Ar-C). MS (70 eV, %) m/z 507 (M+, 82%). Anal. Calc. (Found) for C26H29N5O6 (507.54): C, 61.53 (61.57); H, 5.76 (5.70);N, 13.80 (13.85).
4.7. General Procedure for the Synthesis of 3-Chloro-1-((4-methoxy/4,9-dimethoxy)-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-6,8-di(piperazin/morpholin)-1-yl-1,3,4,6-tetrahydro-2H-pyrim-ido-[1,6-a]pyrimidines 6a-d
To a well stirred mixture of 5a-d (10 mmol) and 3-chloropentane-2,4-dione (1.35 g, 10 mmol) in glacial acetic acid (40 mL), activated zinc dust (10.00 g) was added portionwise at room temperature over a period of 2 h. Stirring was continued for an additional 3 h. Thereafter, the reaction mixture was heated on a water bath (80-90 ºC) for 3 h. The progress of reaction was monitored by TLC. After allowing the reaction mixture to cool to room temperature, it was poured into cold water (100 mL). The insoluble solid which separated was filtered, washed with water, dried and crystallized to produce 6a-d.
3-Chloro-1-(4-methoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-6,8-di-piperazin-1-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (6a). Obtained from 5a (4.75 g, 10 mmol) as a yellowish powder, m.p. 350-352 ºC, crystallized from dioxane (82% yield); IR (KBr, cm−1): 3,395 (br, 2NH), 3,034 (CH, aryl), 2,932 (CH, alkyl), 1,632 (C=N); 1H-NMR: 1.15 (d, 6H, 2CH3), 1.73 (s, 3H, CH3), 2.63-2.69 (m, 8H, Hpiperazine), 2.83-2.89 (m, 8H, Hpiperazine), 3.14 (m, 2H, Hdihydropyrimidine), 3.73 (s, 3H, OCH3), 3.92 (t, 1H, CH-Cl), 4.60 (s, 1H, Hpyran), 5.25 (s, 1H, Hpyran), 6.35 (s, 1H, Hbenzene), 6.67 (s, 1H, Hfuran), 6.83 (s, 1H, Hpyrimidine), 7.15 (s, 1H, Hpyrimidine), 7.51 (s, 1H, Hfuran), 9.85, 9.96 (br s, 2NH, D2O exchangeable). MS (70 eV, %) m/z 582 (M+, 80%). Anal. Calc. (Found) for C30H40ClN7O3 (582.14): C, 61.90 (61.85); H, 6.93 (6.90); N, 16.84 (16.75).
3-Chloro-1-(4-methoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-6,8-di-morpholin-4-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (6b). Obtained from 5b (4.77g, 10 mmol) as a yellow powder, m.p. 335-337 ºC, crystallized from methanol (74% yield); IR (KBr, cm−1): 3,028 (CH, aryl), 2,918 (CH, alkyl), 1,619 (C=N); 1H-NMR: 1.16 (t, 6H, 2CH3), 1.72 (s, 3H, CH3), 2.99-3.05(m, 8H, Hmorpholine), 3.14 (m, 2H, Hpyrimidine), 3.66-3.72 (m, 8H, Hmorpholine), 3.75 (s, 3H, OCH3), 3.94 (t, 1H, CH-Cl), 4.62 (s, 1H, Hpyran), 5.24 (s, 1H, Hpyran), 6.34 (s, 1H, Hbenzene), 6.68 (s, 1H, Hfuran), 6.75 (s, 1H, Hpyrimidine), 7.30 (s, 1H, Hpyrimidine), 7.52 (s, 1H, Hfuran). MS (70 eV, %) m/z 584 (M+, 77%). Anal. Calc. (Found) for C30H38ClN5O5 (584.11): C, 61.69 (61.60); H, 6.56 (6.48); N, 11.99 (11.88).
3-Chloro-1-(4,9-dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-6,8-di-piperazin-1-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (6c). Obtained from 5c (5.05g, 10 mmol) as a brown powder, m.p. 370-372 ºC, crystallized from benzene (72% yield); IR (KBr, cm−1): 3,390 (br, 2NH), 3,035 (CH, aryl), 2,924 (CH, alkyl), 1,620 (C=N); 1H-NMR: 1.17 (d, 6H, 2CH3), 1.76 (s, 3H, CH3), 2.70-2.76 (m, 8H, Hpiperazine), 3.15 (m, 2H, Hpyrimidine), 3.18-3.24 (m, 8H, Hpiperazine), 3.74 (s, 6H, 2 OCH3), 3.93 (t, 1H, CH-Cl), 4.62 (s, 1H, Hpyran), 5.27 (s, 1H, Hpyran), 6.69 (s, 1H, Hfuran), 6.85 (s, 1H, Hpyrimidine), 7.20 (s, 1H, Hpyrimidine), 7.52 (s, 1H, Hfuran), 9.80-9.91 (br s, 2 NH, D2O exchangeable). 13C-NMR: 22.1, 22.2, 23.1 (3C, 3 CH3), 36.5, 45.5, 49.5 (3C, CH), 50.8 (2C, CH2), 51.3 (4C,4 CH2), 52.3 (2C, 2 CH2), 56.7 (2C, 2 OCH3), 61.1, 65.2, 84.5 (3C, 3 CH), 101.1, 106.6, 107.1, 108.5, 125.9, 139.5, 142.3, 146.2, 148.7, 150.6, 165.4, 170.8 (Ar-C). MS (70 eV, %) m/z 612 (M+, 74%). Anal. Calc. for (Found) C31H42ClN7O4 (612.16): C, 60.82 (60.89); H, 6.92 (6.97); N, 16.02 (16.10).
3-Chloro-1-(4,9-dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-6,8-di-morpholin-4-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (6d). Obtained from 5d (5.07 g, 10 mmol) as white crystals, m.p. 385-387 ºC, crystallized from dioxane (69% yield); IR (KBr, cm−1): 3,029 (CH, aryl), 2,921 (CH, alkyl), 1,629 (C=N); 1H-NMR: 1.16 (d, 6H, 2CH3), 1.74 (s, 3H, CH3), 2.93-2.99(m, 8H, Hmorpholine), 3.13 (m, 2H, Hpyrimidine), 3.66-3.72 (m, 8H, Hmorpholine), 3.75 (s, 6H, 2 OCH3), 3.95 (t, 1H, CH-Cl), 4.61 (s, 1H, Hpyran), 5.28 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 6.88 (s, 1H, Hpyrimidine), 7.25 (s, 1H, Hpyrimidine), 7.55 (s, 1H, Hfuran). MS (70 eV, %) m/z 614 (M+, 78%). Anal. Calc. (Found) for C31H40ClN5O6 (614.13): C, 60.63 (60.57); H, 6.56 (6.50); N, 11.40 (11.35).
4.8. General Procedure for the Synthesis of 1-((4-Methoxy/4,9-dimethoxy)-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-3,6,8-tri-(piperazin/morpholin)-1-yl-1,3,4,6-tetrahydro-2H-pyrimido [1,6-a]pyrimidines 7a-d
To a warm solution of 6a-d (10 mmol) in methanol (100 mL) was added freshly distilled piperazine (10 mmol) or morpholine (10 mmol). The reaction mixture was stirred under reflux for 12 h, and then allowed to cool to 0 ºC for 12 h. The solid obtained was filtered, washed with water (100 mL), dried, and crystallized from the appropriate solvent to produce 7a-d.
1-(4-Methoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-3,6,8-tripiperazin-1-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (7a). Obtained from 6a (5.82 g, 10 mmol) as yellow crystals, m.p. 300-302 ºC, crystallized from ethanol (75% yield); IR (KBr, cm−1): 3,398 (br, 3NH), 3,035 (CH, aryl), 2,930 (CH, alkyl), 1,630 (C=N); 1H-NMR: 1.12 (s, 6H, 2CH3), 1.72 (s, 3H, CH3), 2.38-2.44 (m, 8H, Hpiperazine), 2.60-2.66 (m, 8H, Hpiperazine) 2.80-2.86 (m, 8H, Hpiperazine), 3.05 (s, 2H, Hpyrimidine), 3.1 (s, 1H, Hpyrimidine), 3.74 (s, 3H, OCH3), 4.55 (s,1H, Hpyran), 5.24 (s, 1H, Hpyran), 6.33 (s, 1H, Hbenzene), 6.66 (s, 1H, Hfuran), 6.80 (s,1H, Hpyrimidine), 7.10 (s, 1H, Hpyrimidine), 7.53 (s, 1H, Hfuran), 9.70, 9.80, 9.90 (3 br s, 3NH, D2O exchangeable). MS (70 eV, %) m/z 631 (M+, 80%). Anal. Calc. (Found) for C34H49N9O3 (631.81): C, 64.63 (64.69); H, 7.82 (7.89); N, 19.95 (19.85).
1-(4-Methoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-3,6,8-tri-morpholin-4-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (7b). Obtained from 6b (5.84 g,10 mmol) as pale yellow crystals, m.p. 296-298 ºC, crystallized from hexane (79% yield); IR (KBr, cm−1): 3,029 (CH, aryl), 2,920 (CH, alkyl), 1,623 (C=N) ; 1H-NMR: 1.10 (s, 6H, 2CH3), 1.73 (s, 3H, CH3), 2.36-2.42 (m, 8H, Hmorpholine), 2.93-2.99 (m, 8H, Hmorpholine), 3.04 (s, 2H, Hpyrimidine), 3.09 (s, 1H, Hpyrimidine), 3.64-3.70(m, 8H, Hmorpholine), 3.76 (s, 3H, OCH3), 4.60 (s, 1H, Hpyran), 5.25 (s, 1H, Hpyran), 6.35 (s, 1H, Hbenzene), 6.67 (s, 1H, Hfuran), 6.78 (s, 1H, Hpyrimidine), 7.20 (s, 1H, Hpyrimidine), 7.51 (s, 1H, Hfuran). 13C-NMR: 21.7, 21.8, 23.2 (3C, 3CH3), 36.8, 45.8, 48.5 (3C, CH), 50.4 (2C, 2CH2), 51.3 (2C, 2CH2), 54.7 (2C, 2CH2), 56.7 (1C, OCH3), 71.7 (2C, 2 CH2), 72.1 (2C, 2 CH2), 72.4 (2C, 2 CH2), 92.2, 95.8, 99.8, 102.1, 107.2, 107.8, 108.3, 125.5, 139.2, 143.5, 145.2, 148.5, 150.3, 164.4, 169.8 (Ar-C). MS (70 eV, %) m/z 634 (M+, 82%). Anal. Calc. (Found) for C34H46N6O6 (634.77): C, 64.33 (64.28); H, 7.30 (7.35);N, 13.24 (13.30).
1-(4,9-Dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-3,6,8-tripiperazin-1-yl-1,3,4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (7c). Obtained from 6c (6.12 g, 10 mmol) as a white powder, m.p. 340-342 ºC, crystallized from dimethylformamide (72% yield); IR (KBr, cm−1): 3,394 (br, NH), 3,032 (CH, aryl), 2,925 (CH, alkyl), 1,622 (C=N); 1H-NMR: 1.14 (s, 6H, 2CH3), 1.74 (s, 3H, CH3), 2.37-2.43 (m, 8H, Hpiperazine), 2.62-2.68 (m, 8H, Hpiperazine), 2.82-2.88 (m, 8H, Hpiperazine), 3.08 (s, 2H, Hpyrimidine), 3.15 (s, 1H, Hpyrimidine), 3.76 (s, 6H, OCH3), 4.58 (s, 1H, Hpyran), 5.22 (s, 1H, Hpyran), 6.67 (s, 1H, Hfuran), 6.78 (s, 1H, Hpyrimidine), 6.92 (s, 1H, Hpyrimidine), 7.56 (s, 1H, Hfuran), 9.75, 9.85, 9.95 (3 br s, 3 NH, D2O exchangeable). MS (70 eV, %) m/z 661 (M+, 78%). Anal. Calc. (Found) for C35H51N9O4 (661.84): C, 63.52 (63.58); H, 7.77 (7.83); N, 19.05 (19.15).
1-(4,9-Dimethoxy-7-methyl-5H-furo[3,2-g]chromen-5-yl)-2,4-dimethyl-3,6,8-trimorpholin-4-yl-1,3, 4,6-tetrahydro-2H-pyrimido[1,6-a]pyrimidine (7d). Obtained from 6d (6.14g, 10 mmol) as yellow crystals, m.p. 355-357 ºC, crystallized from dioxane (70% yield); IR (KBr, cm−1): 3,030 (CH, aryl), 2,920 (CH, alkyl), 1,627 (C=N); 1H-NMR: 1.14 (s, 6H, 2CH3), 1.72 (s, 3H, CH3), 2.35-2.42 (m, 8H, Hmorpholine), 2.85-2.92 (m, 8H, Hmorpholine), 3.06 (s, 2H, Hpyrimidine ), 3.10 (s, 1H, Hpyrimidine), 3.64-3.71 (m, 8H, Hmorpholine), 3.74 (s, 6H, 2 OCH3), 4.60 (s, 1H, Hpyran), 5.24 (s, 1H, Hpyran), 6.68(s, 1H, Hfuran), 6.83 (s, 1H, Hpyrimidine), 7.10 (s, 1H, Hpyrimidine), 7.54 (s, 1H, Hfuran). MS (70 eV, %) m/z 664 (M+, 88%). Anal. Calc. (Found) for C35H48N6O7 (664.79): C, 63.23 (63.28); H, 7.28 (7.20); N, 12.64 (12.58).
5. Biological Evaluation
5.1. Animals
Female Sprague-Dawley rats (150-200 g) were used in the anti-inflammatory activity study. Swiss mice of both sexes weighing 25-30 g were used in analgesic activity tests. International principles and local regulations concerning the care and use of laboratory animals were taken into account. The animals had access to standard commercial diet and water at libitum and were kept in rooms maintained at 22 ± 1 °C with a 12 h light-dark cycle.
5.2. Anti-Inflammatory Activity (Carrageenan-Induced Rat Hind Paw Edema Model)
The method adopted essentially resembles that described in the literature [17]. Distilled water was selected as vehicle to suspend the standard drugs and the test compounds. Sprague-Dawley rats were starved for 18 h prior to the experiment. The animals were weighed, marked for identification and divided into 28 groups each containing six animals. Edema was induced in the left hind paw of all rats by subcutaneous injection of 0.1 mL of 1% (w/v) carrageenan in distilled water into their footpads. The 1st group was kept as control and was given the respective volume of the solvent (0.5 mL distilled water). The 2nd to 16th groups were orally administered aqueous suspension of the synthesized compounds in dose of 20 mg/kg 1 h before carrageenan injection. The last group (standard) was orally administered diclofenac sodium at a dose of 20 mg/kg as an aqueous suspension [18]. The paw volume of each rat was measured immediately by a mercury plethysmometer, before carrageenan injection and then hourly for 3 h post administration of aqueous suspension of the synthesized compounds. The edema rate and inhibition rate of each group were calculated as follows: Edema rate (E)% = Vt − Vo/Vo, Inhibition rate (I)% =Ec − Et/Ec where Vo is the volume before carrageenan injection (mL), Vt is the volume at t h after carrageenan injection (mL), Ec and Et are the edema rates of the control and treated groups, respectively.
5.3. Analgesic Activity Using Hot-Plate Test
The experiment was carried out as described in the literature [19], using a hot-plate apparatus, maintained at 53 ± 0.5 °C. The mice were divided into 28 groups of six animals each. The reaction time of the mice to the thermal stimulus was the time interval between placing the animal in the hot plate and when it licked its hind paw or jumped. The reaction time was measured prior to aqueous suspension of synthesized compounds and drug treatment (0 min). Group 1 was kept as normal control. The aqueous suspension of synthesized compounds was orally administered to mice of groups 2-16 at doses of20 mg/kg. Mice of group 17 (reference) were orally treated with diclofenac sodium at a dose of20 mg/kg body wt. The reaction time was again measured at 15 min and repeated at, 30, 60 and 90 min after treatment. To avoid tissue damage to the mice paws, cut-off time for the response to the thermal stimulus was set at 60 s. The reaction time was calculated for each synthesized compound anddrug-treated group.
5.4. Analgesic Activity (Acetic Acid Induced Writhing Response Model)
The compounds were selected for investigating their analgesic activity in acetic acid induced writhing response in Swiss albino mice, following the method described in literature [20]. One hundred and two mice were divided into 28 groups (six in each group) starved for 16 h, pretreated as follows, the 1st group which served as control positive orally received distilled water in appropriate volumes. The 2nd to 16th groups received the aqueous suspension of synthesized compounds orally at a dose of 20 mg/kg. The last group orally received diclofenac sodium at a dose of 20 mg/kg. After 30 min, each mouse was administrated 0.7% of an aqueous solution of acetic acid (10 mL/kg) and the mice were then placed in transparent boxes for observation. The number of writhes was counted for 20 min after acetic acid injection. The number of writhes in each treated group was compared to that of a control group. The number of writhing was recorded and the percentage protection was calculated using the following ratio: % protection = (control mean − treated mean/control mean) × 100.
Acknowledgements
The authors wish to express their most sincere thanks to Farid Abd-Elraheem Badria (Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University 35516, Egypt) for carrying out the biological activity tests. The present work was supported by Department of Photochemistry (Heterocyclic unit); Chemical Industries Research Division, National Research Centre in Cairo, Egypt.
References
- Dewar, H.A.; Grimson, T.A. Khellin in the treatment of angina of effort. Br. Heart J. 1950, 12, 54–60. [Google Scholar] [CrossRef]
- Vanachayangkul, P.; Byer, K.; Khan, S.; Butterweck, V. An aqueous extract of Ammi visnaga fruits and its constituents Khellin and Visnagen prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine 2010, 17, 653–658. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Daosukho, S.; El-Shall, H. Effect of supersaturation ratio and Khella extract on nucleation and morphology of kidney stones. J. Crystal. Growth 2009, 311, 2673–2681. [Google Scholar] [CrossRef]
- Vedaldi, D.; Caflleri, S.; Dall’Acqua, F.; Andrea, L.; Bovalini, L.; Martelli, P. Khellin, a naturally occurring furochromone, used for the photochemotherapy of skin diseases: mechanism of action. Farmaco 1988, 4, 333–346. [Google Scholar]
- Trabalzini, L.; Martelli, P.; Bovalini, L.; Dall’Acqua, F.; Sage, E. Photosensitiza tion of DNA of defined sequence by furochromones, Khellin and Visnagen. J. Photochem. Photobiol. B: Bid. 1990, 7, 317–336. [Google Scholar] [CrossRef]
- Leeuw, J.D; Assen, Y.J.; van der Beek, N.; Bjerring, P.; Martino Neumann, H.A. Treatment of vitiligo with Khellin liposomes, ultraviolet light and blister roof transplantation. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 74–81. [Google Scholar] [CrossRef]
- Ghate, M.; Kulkarni, M.V. Synthesis and anti-inflammatory activity of 4-(5’-acetyl-6’-hydroxy-3’-methylbenzofuran-2’-yl)coumarin and 6-acetyl-3,7-dimethyl-2-coumarin-4’-yl)furo[3,2-g]chromen-5-one. Ind. J. Chem. 2005, 44B, 1674–1678. [Google Scholar]
- Frasinyuk, M.S.; Gorelov, S.V.; Bondarenko, S.P.; Khilya, V.P. Synthesis and properties of 4-(3-amino-2-benzofuranyl)-coumarins. Chem. Heterocycl. Comp. 2009, 45, 1261–1269. [Google Scholar]
- Kittler, L.; Hradečná, Z.; Sühnel, J. Cross-link formation of phage lambda DNA in situ photochemically induced by the furocoumarin derivative angelicin. Biochim. et Biophys. Acta 1980, 607, 215–220. [Google Scholar] [CrossRef]
- Abeysekera, B.F.; Abramovski, Z.; Towers, G.H.N. Genetoxicity of the natural furochromones, Khellin and Visnagen and the identification of Khellin-thymine photoadduct. Photochem. Photobiol. 1983, 38, 311–315. [Google Scholar] [CrossRef]
- Schönberg, A.; Badran, N.; Starkowsky, N.A. Furo-chromones and coumarins. XIII. The dicoumarol analogs of bergapten, isopimpinellin and pimpinellin. J. Am. Chem. Soc. 1955, 77, 5438–5439. [Google Scholar]
- Schönberg, A.; Badran, N.; Starkowsky, N.A. Furo-chromones and coumarins. XIV.2-(3’-Pyridyl) analogs of Khellin and Visnagen. J. Chem. Soc. 1955, 77, 5439–5440. [Google Scholar] [CrossRef]
- El-Gazzar, A.B.A.; Youssef, M.M.; Youssef, A.M.S.; Abu-Hashem, A.A.; Badria, F.A. Design and synthesis of azolopyrimidoquinolines, pyrimido quinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009, 44, 609. [Google Scholar] [CrossRef]
- Shishoo, C.J.; Jain, K.S. Synthesis of some novel azido/ tetrazolothieno pyrimidines and their reduction to 2,4-diaminothieno[2,3-d]pyrimidines. J. Heterocycl. Chem. 1992, 29, 883–893. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Synthesis of some new pyrimido[2’,1’:2,3]thiazolo[4,5-b]quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 1976. [Google Scholar] [CrossRef]
- El-Gazzar, A.B.A.; El-Enany, M.M.; Mahmoud, M.N. Synthesis, analgesic, anti-inflammatory and antimicrobial activity of some novel pyrimido[4,5-b]-quinolin-4-ones. Bioorg. Med. Chem. 2008, 16, 3261–3273. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenan-induced edema in hind paw of the rats as an assay anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544. [Google Scholar]
- Mino, J.; Moscatelli, V.; Hnatyszyn, O.; Gorzalczany, S.; Acevedo, C.; Ferraro, G. Antinociceptive and antiinflammatory activities of artemisia copa extracts. Pharmacol. Res. 2004, 50, 59–63. [Google Scholar] [CrossRef]
- Aiyelero, O.M.; Ibrahim, Z.G.; Yaro, A.H. An Aalgesic and anti-inflammatory properties of the methanol leaf extract of Ficus Ingens (Moraceae) in rodents. Nig. J. Pharm. Sci. 2009, 8, 79–86. [Google Scholar]
- Collier, D.J.; Dinnin, L.C.; Johnson, C.A.; Schneider, C. The abdominal response and its suppression by analgesic drugs in the mouse. J. Pharmacol. Chemother. 1968, 32, 295. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).