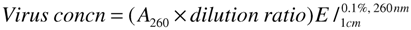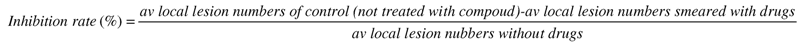Abstract
A series of novel chiral thioureas 3a-n bearing leucine and phosphonate moieties were synthesized in excellent yields. The structures of the compounds were completely characterized by elemental analysis, IR, 1H-, 13C-, 31P- and 19F-NMR spectral data. A half-leaf method was used to determine the in vivo protective and curative efficacies of the title products against tobacco mosaic virus (TMV). The compounds 3l and 3n displayed good in vivo protection and curative effects against TMV with inhibitory rates of 60.1, 62.8% (protection) and 56.7, 53.6% (curative) at 0.5 mg/mL, respectively. To the best of our knowledge, this is the first report on the antiviral activity of chiral thioureas containing leucine and phosphonate moieties.
1. Introduction
Chiral thioureas and their derivatives constitute an important class of compounds which exhibit a wide range of antibacterial, fungicidal, herbicidal, antiviral and plant growth regulatory activities. Some of them can serve not only as chiral organocatalysts in the synthesis of asymmetric compounds [1], but also as potential anticancer and anti-HIV drugs [2,3,4]. Amongst the noteworthy contributions reported in this field, Venkatachalam and his group [4] obtained chiral naphthyl thioureas (CNT) as potent non-nucleoside inhibitors (NNI) of the reverse transcriptase (RT) enzyme of HIV-1. Interestingly, the R-enantiomers of these derivatives could inhibit the recombinant RT in vitro with lower IC50 values compared to their corresponding S-enantiomers. Unfortunately, chiral thiourea derivatives have scarcely been evaluated for their plant antiviral activities for agricultural applications. It is well known that certain bioisosteres of natural amino acids and a-aminophosphonic acid analogues are associated with significant bioactivities and are often employed as enzyme inhibitors, antimicrobial, antitumor and antiviral agents [5,6,7,8,9]. Further, suitably substituted phosphonic analogues of L-leucine can play prominent role in leucine aminopeptidase inhibition and in the early stages of HIV infection [10,11]. In this context, we have previously discovered some novel α-aminophosphonate derivatives with appreciable antiviral activity against the tobacco mosaic virus (TMV) [12,13,14]. Based on these findings, we turned our attention to the incorporation of the thiourea and α-aminophosphonate pharmacophores into a single structure through a chiral L-leucine linker. The idea was to generate new potentially antiviral chiral thiourea derivatives 3a-n with high activity, low toxicity and minimal residues [15]. The synthetic route is shown in Scheme 1. The structures of the target compounds were firmly established by IR, 1H-, 13C-, 31P- and 19F-NMR spectra and elemental analysis. Preliminary bioassay tests showed that some of these compounds possessed good in vivo anti-TMV activities.
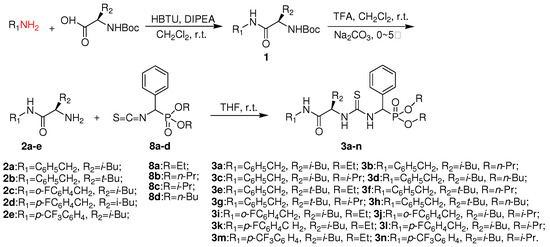
Scheme 1.
Synthetic route to chiral thiourea derivatives 3a-n.
2. Results and Discussion
2.1. Chemistry
The synthetic route designed for the chiral thiourea analogues 3a-n containing L-leucine and an α-aminophosphonate moiety is summarized in Scheme 1. Boc-protected intermediate amide 1 was first generated from the reaction of L-N-Boc-leucine and a substituted arylamine in the presence of O-benzotriazol-1-yl-N,N,N’,N’-tetramethyluronium hexafluorophosphate (HBTU) at room temperature. The α-amino carboxamide derivatives 2a-e were obtained through Boc deprotection using trifluoroacetic acid, as shown in Scheme 1. The desired chiral thiourea analogues 3a-n were then prepared by the addition of O,O′-dialkylisothiocyanato(phenyl)methylphosphonates 8a-d to 2a-e in THF at room temperature. The intermediates 8 were in turn obtained from benzaldehyde in five steps by following a literature procedure [13], as shown in Scheme 2.
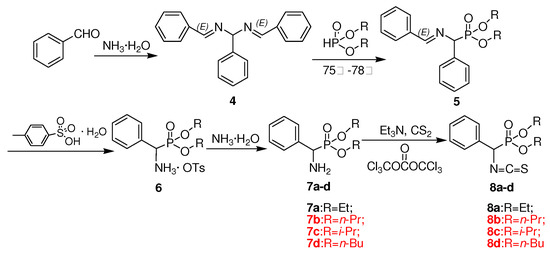
Scheme 2.
Synthetic route to intermediates 8a-d.
The existing method for the conversion of product 7 into 8 by the use of phosphorus oxychloride (POCl3) was however not satisfactory. This step of the reaction was further optimized by employing different reagents, such as bis(trichloromethyl) carbonate (BTC), thiophosgene (CSCl2) and 1,1-thiocarbonyldiimidazole (TCDI). The results of these experiments were compared with those obtained with the phosphorus oxychloride method (Table 1). As could be observed from the data, the best result was obtained with BTC, which afforded 67-79% isolated yield of 8a-8d against the reported yield [13] of 54-62%. Therefore, BTC was selected as the ideal reagent for this key conversion.

Table 1.
Comparison of the yield for intermediates 8a-d using different reagents.
| Compd. | R | Yield (%) | |||
|---|---|---|---|---|---|
| CSCl2 | TCDI | BTC | POCla | ||
| 8a | Et | 24 | trace | 79 | 60 |
| 8b | n-Pr | 71 | 62 | ||
| 8c | i-Pr | 67 | 54 | ||
| 8d | n-Bu | 75 | 58 | ||
a Reported yields in the reference [13].
With regard to the best solvent that could be used for the preparation of title compounds 3 from 2 and 8 (Scheme 1), the model compound 3e was synthesized in different solvents, e.g. tetrahydrofuran (THF), acetonitrile (CH3CN), acetone (CH3COCH3), dichloromethane (CH2Cl2) and toluene and the results are provided in Table 2. Under optimized conditions, a maximum yield of 98% could be obtained when the reaction mixture was stirred at room temperature in the solvent THF for 0.5 h.

Table 2.
Role of different solvents for the synthesis of 3e.
| No. | Solvent | Vol. (mL) | Reaction time (h) | Temperature (ºC) | Yield (%) |
|---|---|---|---|---|---|
| 1 | THF | 5 | 0.5 | room | 98 |
| 2 | CH3CN | 5 | 0.5 | room | 76 |
| 3 | CH3COCH3 | 5 | 0.5 | room | 89 |
| 4 | CH2Cl2 | 5 | 0.5 | room | 73 |
| 5 | toluene | 5 | 0.5 | room | 55.7 |
All the products were unequivocally characterized by IR and NMR spectral data and elemental analyses. The characteristic IR absorption bands at 3269-3467 cm-1, 1645-1678 cm-1, 1546-1564 cm-1, 1212-1227 cm-1, and 1008-1028 cm-1 confirmed the presence of NH, C=O, C=S, P=O and P-O-C functional groups, respectively. In the 1H-NMR spectra of title compounds 3a-n, all aromatic protons displyed the expected multiplet near 6.78-7.79 ppm. While the amidic NH protons appeared downfield in the 7.74-10.12 ppm range as broad singlets, the NH protons of thioureido groups revealed weak peaks due to the existence of hydrogen bonds. The typical phosphorus resonance at 19.7-22.8 ppm in the 31P-NMR spectra of all target compounds confirmed the presence of a phosphorus center coupled to an adjacent CH. In the 19F-NMR spectra, the fluorine resonance due to Ar-F and CF3 appeared at ‑115.1 to -118.8 ppm and -63.2 ppm, respectively. The typical carbon resonances at 183.1-184.6 and 170.6-174.4 ppm in the 13C-NMR spectra of the title compounds were indicative of the existence of C=S and C=O double bonds, respectively. All the nonequivalent carbon atoms were identified in the 13C-NMR and the total number of protons calculated from the 1H-NMR integration curves was in complete agreement with the assigned structures.
3. Experimental
3.1. General
The melting points of the products were determined on a XT-4 binocular microscope (Beijing Tech Instrument Co., China) and were not corrected. The IR spectra were recorded on a Bruker VECTOR22 spectrometer in KBr disks. The1H, 13C,19 F and 31P NMR spectra (solvent DMSO-d6 or CDCl3) were recorded at room temperature on a JEOL-ECX 500 NMR spectrometer operating at 500, 125, 470 and 200 MHz, respectively, using TMS as an internal standard. Data are reported as follows: chemical shifts in ppm (δ), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, br = broad and m = multiplet, coupling constant (Hz) and integration. Elemental analyses were performed on an Elementar Vario-III CHN analyzer. The reagents were all of analytical grade or chemically pure. Analytical TLC was performed on silica gel GF254 plates.
3.2. Preparation of intermediates 2a-e
L-N-Boc-leucine (2.31 g, 0.01 mol) and O-benzotriazol-1-yl-N,N,N’,N’-tetramethyluronium hexa- fluorophosphate (HBTU, 3.80 g, 0.01 mol) were loaded into an oven-dried round bottomed flask equipped with a magnetic stir bar, rubber septum, and argon inlet. Anhydrous dichloromethane (50 mL) was then added. After 3 minutes, anhydrous DIPEA (2.58 g, 0.02 mol) and arylamine (0.011 mol) were sequentially added and the reaction mixture was stirred at room temperature for 2-4 h. During the process, the state of the solution was seen to change from slightly heterogeneous to homogeneous confirming the consumption of HBTU. The reaction mixture was poured into a separatory funnel containing 1N HCl (50 mL), and was then partitioned between dichloromethane and aqueous hydrochloric acid solution. The organic layer was washed with 1N HCl (3 × 30 mL), dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The resulting light yellow oil was transferred to a 100-mL flask and redissolved in dichloromethane (30 mL). Trifluoroacetic acid (7 mL) was then added in one portion. After 2-4 h, the mixture was slowly partitioned with dichloromethane and chilled, saturated aqueous sodium carbonate solution (50 mL) was added. The aqueous layer was extracted with dichloromethane (3 × 30 mL), and the combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The crude product was purified by thin layer chromatography (TLC) on a silica gel (developing solvent: 3-4% MeOH/CH2Cl2, V/V) to give the intermediates 2a-e.
L-2-Amino-N-benzyl-4-methylpentanamide (2a). White crystals, yield 77%, m.p. 60-62 ºC; 1H-NMR (CDC13): δ 0.95 (d, 6H, J = 3.2 Hz, 2CH3), 1.73-1.75 (m, 2H, CH2), 1.96 (br s, 2H, NH2), 2.80-2.82 (m, 1H, CH), 3.45-3.48 (m, H, CH), 4.43 (d, J = 6.3 Hz, 2H, NCH2), 7.26-7.35 (m, 5H, ArH), 7.72 (br s, 1H, NH); 13C-NMR (CDC13): δ 175.6, 138.6, 128.7, 127.8, 127.4, 53.6, 44.1, 43.2, 24.9, 21.4; IR (KBr, cm-1): v 3304, 2958, 2868, 1647; Anal. Calcd. for C13H20N2O: C 70.87, H 9.15, N 12.72; Found: C 70.62, H 9.34, N 12.86.
L-2-Aamino-N-benzyl-3,3-dimethylbutanamide (2b). White crystals, yield 83%, m.p. 53-54 ºC, 1H-NMR (CDC13, 500 MHz): δ 0.99 (s, 9H, 3CH3), 1.56 (br s, 2H, NH2), 3.11 (s, 1H, CH), 4.42 (d, J = 3.45 Hz, 2H, NCH2), 7.25-7.31 (m, 5H, ArH), 7.78 (br s, 1H, NH); 13C-NMR (CDC13, 125 MHz): δ 173.6, 138.6, 128.7, 128.0, 127.5, 64.5, 43.2, 34.3, 26.9; IR (KBr, cm-1): v 3306, 2946, 1650; Anal. Calcd. for C13H20N2O: C 70.87, H 9.15, N 12.72; Found: C 70.71, H 9.23, N 12.36.
L-N-(2-Fluorobenzyl)-2-amino-4-methylpentanamide (2c). Colorless viscous liquid, yield 68%; 1H-NMR (CDC13): δ 0.99 (d, 6H, J = 4.6 Hz, 2CH3), 1.23 (t, 2H, J = 7.2 Hz, CH2), 1.65 (br s, 2H, NH2), 1.76-1.84 (m, 1H, CH), 3.52-3.55 (m, H, CH), 4.23 (d, J = 5.3 Hz, 2H, NCH2), 7.05-7.33(m, 4H, ArH), 8.43 (br s, 1H, NH); 13C-NMR (CDC13): δ 174.2, 159.7, 131.4, 128.8, 115.3, 124.1, 53.2, 43.5, 34.6, 24.6, 21.3; 19F-NMR (CDC13): δ -115.5; IR (KBr, cm-1) v: 3308, 2960, 2873, 1649; Anal. Calcd. for C13H19FN2O: C 65.52, H 8.04, N 11.76; Found: C 65.36, H 8.32, N 11.65.
L-N-(4-Fluorobenzyl)-2-amino-4-methylpentanamide (2d). Colorless liquid, yield 73%, n25D 1.5055; 1H-NMR (CDC13): δ 0.90 (d, 6H, J = 3.4 Hz, 2CH3), 1.63-1.72 (m, 2H, CH2), 2.25 (br s, 2H, NH2), 2.76-2.79 (m, 1H, CH), 3.35-3.38 (m, 2H, CH), 4.34 (d, J = 5.15 Hz, 2H, NCH2), 6.96-7.22 (m, 4H, ArH), 7.98 (br s, 1H, NH); 13C-NMR (CDC13): δ 174.7, 161.5, 137.4, 128.6, 115.2, 52.9, 44.5, 42.8, 24.7, 21.4; 19F-NMR (CDC13): δ -115.3; IR (KBr, cm-1): v 3309, 2954, 2870, 1645, 1504, 1223; Anal. Calcd. for C13H19FN2O: C 65.52, H 8.04, N 11.76; Found: C 65.33, H 8.26, N 11.51.
L-2-Amino-4-methyl-N-(4-(trifluoromethyl)phenyl)pentanamide (2e). Colorless liquid, yield 56%, n25D 1.4750; 1H-NMR (CDC13): δ 0.95 (d, 6H, J = 6.3 Hz, 2CH3), 1.43-1.47 (m, 1H, CH), 1.75-1.79 (m, 1H, CH2), 2.51 (br s, 2H, NH2), 3.53 (d, 1H,J = 4.0 Hz, , NCH), 7.54-7.78(d, 4H, J = 8.6 Hz, ArH), 9.99 (br s, 1H, NH); 13C-NMR (CDC13): δ 174.7, 141.3, 125.9, 125.6, 123.3, 119.1, 54.0, 43.7, 24.8, 21.4; 19F-NMR (CDC13): δ -62.0; IR (KBr, cm-1): v 3462, 3269, 2953, 1628, 1506, 1323, 1115, 1067; Anal. Calcd. for C13H17F3N2O: C 56.93, H 6.25, N 10.21; Found: C 57.18, H 6.47, N 10.39.
3.3. Preparation of O,O′-dialkylisothiocyanato(phenyl)methylphosphonate intermediates 8a-d
To a solution of α-aminophosphonate (6 mmol) in ether (15 mL), triethylamine (18 mmol) was added with constant stirring at room temperature and cooled to 0 ºC. Then, carbon disulfide (6 mmol) was added dropwise and the mixture stirred for 1 h at 0 ºC, the temperature was raised to 23 ºC, and stirring was continued for an additional 2 h. BTC (2 mmol) dissolved in ether (10 mL) was then added dropwise into the reaction mixture and stirred for 3 h at 23 ºC. The solid was filtered off, and the liquid was extracted with ether, treated with saturated sodium bicarbonate, and dried on anhydrous sodium sulfate, filtered. Removal of the solvent followed by chromatography of the crude product on silica using a mixture of petroleum ether and ethyl acetate as the developing solvent gave the intermediates 8 in 67-79% yields; data for 8a-d can be found in reference [13].
3.4. Preparation of title chiral thioureas 3a-n
A solution of O,O’-dialkylisothiocyanato(phenyl)methylphosphonate 8 (1 mmol) in tetrahydrofuran (5 mL) was stirred, followed by dropwise addition of chiral amine 2a-e (1.1 mmol). The stirring was continued for 0.5 h at 23 ºC, the solvent was evaporated and the crude product was purified by preparative TLC using a mixture of ethyl acetate and n-hexane (V:V = 1:1) as developing solvent to give title compound 3a-n.
Diethyl (3-(L-1-benzylamino-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methylphosphonate (3a). White solid, yield 94%; m.p. 43-45 ºC; [α]20D-17.9º (c 0.1, acetone); 1H-NMR (CDC13, 500 MHz): δ 0.79 (d, 6H, J = 6.3 Hz, 2CH3), 1.06 (t, 6H, J = 5.75 Hz, 2CH3), 1.24-1.46(m, 2H, CH2), 1.65-1.72 (m, 1H, CH), 3.68-4.07 (m, 2H, NCH2), 4.12-4.49 (m, 4H, 2OCH2) , 5.07 (d, 1H, J = 6.7 Hz, CH), 6.28 (s, 1H, PCH), 6.45 (s, 1H, NH), 6.95-7.48 (m, 10H, ArH), 7.91 (s, 1H, NH), 8.82 (br s, 1H, NH); 13C-NMR (CDCl3, 125 MHz): δ 183.1, 172.5, 139.2, 136.1, 128.5, 127.6, 126.9, 64.3, 58.1, 54.3, 44.5, 41.4, 26.4, 23.7, 16.3; 31P- NMR (CDCl3, 200 MHz):δ 21.8; IR (KBr, cm-1): v 3284, 3065, 2938, 1659, 1560, 1216, 1012; Anal. Calcd for C25H36N3O4PS: C 59.39, H 7.18, N 8.31; Found C 59.57, H 7.33, N 8.15.
Dipropyl (3-(L-1-benzylamino-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methylphosphonate (3b). Colorless viscous liquid, yield 89%; [α]20D-15.8º (c 0.1, acetone); 1H-NMR (CDC13): δ 0.81-1.04 (m, 12H, 4CH3), 1.27-1.45 (m, 6H, 3CH2), 1.67-1.70 (m, 1H, CH), 3.71-4.09 (m, 2H, NCH2), 4.12-4.53 (m, 4H, 2OCH2), 5.10 (d, 1H, J = 6.4 Hz, CH), 6.31 (s, 1H, PCH), 6.48 (s, 1H, NH), 6.94-7.49(m, 10H, ArH), 7.95 (s, 1H, NH), 9.07 (br s, 1H, NH); 13C-NMR (CDC13): δ 183.5, 172.3, 138.4, 135.6, 128.8, 127.1, 126.7, 68.2, 62.5, 54.4, 43.8, 41.3, 26.5, 24.2, 22.7, 10.1; 31P-NMR (CDCl3): δ 21.5; IR (KBr, cm-1): 3276, 3081, 2927, 1663, 1564, 1219, 1009 cm-1; Anal. Calcd for C27H40N3O4PS: C 60.77, H 7.55, N 7.87; Found: C 60.52, H 7.81, N 7.69.
Diisopropyl (3-(L-1-benzylamino-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methylphosphonate (3c). White solid, yield 95%; m.p. 51-53 ºC; [α]20D-14.2º (c 0.1, acetone); 1H-NMR (CDC13): δ 0.82 (d, 18H, J = 6.3 Hz, 6CH3), 1.42-1.59 (m, 2H, CH2), 1.76-1.89 (m, H, CH), 4.26-4.48 (m, 2H, NCH2), 4.69-4.78 (m, 2H, 2OCH), 5.09 (d, 1H, J = 5.75 Hz, CH), 6.27 (s, 1H, PCH), 7.06-7.46 (m, 10H, ArH), 7.81 (s, 1H, NH), 8.73 (br s, 1H, NH); 13C-NMR (CDC13): δ 183.9, 172.0, 138.2, 135.5, 128.7, 128.5, 127.6, 127.2, 73.2, 56.4, 55.9, 43.5, 40.2, 38.7, 24.3, 23.1; 31P-NMR (CDCl3): δ 22.8; IR (KBr, cm-1): v 3285, 3063, 2929, 1674, 1558, 1215, 1014; Anal. Calcd for C27H40N3O4PS: C 60.77, H 7.55, N 7.87; Found: C 60.63, H 7.24, N 7.66.
Dibutyl (3-(L-1-benzylamino-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methylphosphonate (3d). Colorless viscous liquid, yield 86%; [α]20D-16.4º (c 0.1, acetone); 1H-NMR (CDC13): δ 0.80-0.95 (m, 12H, 4CH3), 1.20-1.43 (m, 10H, 5CH2), 1.55-1.72 (m, 1H, CH), 3.66-4.05 (m, 2H, NCH2), 4.11-4.55 (m, 4H, 2OCH2), 5.04 (d, 1H, J = 6.3 Hz, CH), 6.27 (s, 1H, PCH), 6.43(s, 1H, NH), 6.92-7.44 (m, 10H, ArH), 7.83 (s, 1H, NH), 8.70 (br s, 1H, NH); 13C-NMR (CDC13): δ 184.5, 170.6, 138.2, 135.6, 128.9, 128.6, 127.8, 67.7, 65.8, 55.5, 43.4, 34.8, 32.2, 26.9, 18.8, 13.7; 31P-NMR (CDCl3): δ 22.1; IR (KBr, cm-1): v 3269, 3082, 2953, 1670, 1550, 1220, 1024; Anal. Calcd for C29H44N3O4PS: C, 62.01; H, 7.90; N, 7.48; Found: C 62.28, H 7.67, N 7.72.
Diethyl (3-(L-1-benzylamino-3,3-dimethyl-1-oxobutan-2-yl)thioureido)(phenyl)methylphosphonate (3e). White crystals, yield 98%; m.p. 157-159 ºC; [α]20D+15.5º (c 0.1, acetone); 1H-NMR (CDC13): δ 0.98 (s, 9H, C(CH3)3), 1.07 (t, 6H, J = 6.9 Hz, 2CH3), 3.75-4.06 (m, 2H, NCH2), 4.10-4.46 (m, 4H, 2OCH2), 4.93 (d, 1H, J = 8.6 Hz, CH(t-Bu)), 6.26 (s, 1H, PCH), 6.47 (s, 1H, NH), 7.15-7.47 (m, 10H, ArH), 7.92 (s, 1H, NH), 9.03 (br s, 1H, NH); 13C-NMR (CDCl3): δ 184.3, 170.7, 138.2, 135.4, 128.7, 128.0, 127.4, 66.3, 64.1, 55.4, 43.4, 34.8, 26.9; 16.5; 31P-NMR (CDCl3): δ 21.7; IR (KBr, cm-1): v 3292, 3068, 2965, 2867, 1669, 1562, 1221, 1013; Anal. Calcd for C25H36N3O4PS: C 59.39, H 7.18, N 8.31; Found: C 59.52, H 7.34, N 8.12.
Dipropyl (3-(L-1-benzylamino-3,3-dimethyl-1-oxobutan-2-yl)thioureido)(phenyl)methylphosphonate (3f). White crystals, yield 93%; m.p. 171-172 ºC; [α]20D+9.6º (c 0.1, acetone); 1H-NMR (CDC13): δ 0.76 (t, 6H, J = 6.85 Hz, 2CH3), 1.03 (s, 9H, 3CH3), 1.42-1.74 (m, 4H, 2CH2), 3.70-3.95 (m, 2H, NCH2), 4.09-4.47 (m, 4H, 2OCH2), 4.94 (d, 1H, J = 8.6 Hz, CH(t-Bu)), 6.25 (s, 1H, PCH), 6.53 (s, 1H, NH), 7.12-7.47 (m, 10H, ArH), 7.94 (s, 1H, NH), 9.11 (br s, 1H, NH); 13C-NMR (CDC13): δ 184.4, 170.6, 138.0, 135.2, 128.7, 128.4, 127.5, 69.4, 65.8, 55.3, 43.3, 34.7, 26.8, 24.0, 9.9; 31P-NMR (CDCl3): δ 21.5; IR (KBr, cm-1): v 3288, 3056, 2952, 2873, 1677, 1543, 1218, 1012; Anal. Calcd for C27H40N3O4PS: C 60.77, H 7.55, N 7.87; Found: C 60.52, H 7.71, N 7.59.
Diisopropyl (3-(L-1-benzylamino-3,3-dimethyl-1-oxobutan-2-yl)thioureido)(phenyl)methylphos- phonate (3g). White crystals, yield 96%; m.p. 176-177 ºC; [α]20D+7.2º (c 0.1, acetone); 1H-NMR (CDC13, 500 MHz): δ 1.01 (s, 9H, 3CH3), 1.19 (d, 12H, J = 6.3 Hz, 4CH3), 4.22-4.46 (m, 2H, NCH2), 4.63-4.84 (m, 2H, 2OCH), 4.96 (d, 1H, J = 7.45 Hz, CH(t-Bu)), 6.32 (s, 1H, PCH), 6.38 (s, 1H, NH), 7.11-7.52 (m, 10H, ArH), 7.80 (s, 1H, NH), 9.05 (br s, 1H, NH); 13C-NMR (CDC13, 125 MHz): δ 184.6, 170.7, 138.3, 135.8, 128.9, 128.6, 127.4, 73.1, 66.4, 55.9, 43.2, 34.6, 27.0, 24.3; 31P-NMR(CDCl3, 200 MHz): δ 19.7; IR (KBr, cm-1): v 3286, 3057, 2963, 2861, 1672, 1548, 1224, 1011; Anal. Calcd for C27H40N3O4PS: C 60.77, H 7.55, N 7.87; Found: C 60.91, H 7.34, N 7.48.
Dibutyl (3-(L-1-benzylamino-3,3-dimethyl-1-oxobutan-2-yl)thioureido)(phenyl)methylphosphonate (3h). White crystals, yield 91%; m.p. 122-124 ºC; [α]20D+12.1º (c 0.1, acetone); 1H-NMR (CDC13): δ 0.79 (t, 6H, J = 7.45 Hz, 2CH3), 0.96 (s, 9H, 3CH3), 1.16-1.71 (m, 8H, 2 CH2CH2), 3.71-4.01 (m, 2H, NCH2), 4.06-4.51 (m, 4H, 2OCH2), 4.92 (d, 1H, J = 8.6 Hz, CH(t-Bu)), 6.25 (s, 1H, PCH), 6.46 (s, 1H, NH), 7.13-7.46 (m, 10H, ArH), 7.92 (s, 1H, NH), 9.04 (br s, 1H, NH); 13C-NMR (CDC13): δ 184.5, 170.6, 138.2, 135.6, 128.9, 128.6, 127.8, 67.7, 65.8, 55.5, 43.4, 34.8, 32.2, 26.9, 18.8, 13.7; 31P-NMR (CDCl3): δ 21.5; IR (KBr, cm-1): v 3283, 3065, 2954, 1671, 1552, 1216, 1013; Anal. Calcd for C29H44N3O4PS: C, 62.01; H, 7.90; N, 7.48; Found: C 62.24, H 7.63, N 7.69.
Diethyl (3-(L-1-(2-fluorobenzylamino)-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methyl-phosphonate (3i). Colorless viscous liquid, yield 92%; [α]20D-22.3º (c 0.1, acetone); 1H-NMR (CDCl3): δ 0.85 (d, 6H, J = 6.8 Hz, 2CH3), 1.11 (t, 6H, J = 5.7 Hz, 2CH3), 1.31-1.53(m, 2H, CH2), 1.67-1.74 (m, 1H, CH), 3.67-3.96 (m, 2H, NCH2), 4.26-4.47 (m, 4H, 2OCH2) , 5.20 (d, 1H, J = 6.7 Hz, CH), 6.29 (s, 1H, PCH), 6.32 (s, 1H, NH), 6.79-7.45 (m, 9H, ArH), 7.86 (s, 1H, NH), 8.57 (br s, 1H, NH); 13C-NMR (CDCl3): δ 183.5, 171.4, 159.8, 136.3, 131.3, 128.6, 127.2, 126.5, 124.1, 115.2, 63.9, 58.2, 54.4, 41.6, 34.1, 26.1, 23.3, 16.4; 31P-NMR (CDCl3):δ 22.7; 19F-NMR (CDCl3):δ -118.6; IR (KBr, cm-1): v 3290, 3085, 2977, 1662, 1557, 1216, 1025; Anal. Calcd for C25H35FN3O4PS: C 57.35, H 6.74, N 8.03; Found: C 57.53, H 6.55, N 8.28.
Diisopropyl (3-(L-1-(2-fluorobenzylamino)-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methyl-phosphonate (3j). Colorless viscous liquid, yield 88%; [α]20D-17.2º (c 0.1, acetone); 1H-NMR (CDCl3): δ0.87 (d, 18H, J = 6.4 Hz, 6CH3), 1.36-1.43 (m, 2H, CH2), 1.51-1.62 (m, H, CH), 3.75-4.16 (m, 2H, NCH2), 4.53-4.71 (m, 2H, 2OCH), 5.23 (d, 1H, J = 6.8 Hz, CH), 6.27 (s, 1H, PCH), 6.30 (s, 1H, NH), 6.82-7.43 (m, 9H, ArH), 7.74 (s, 1H, NH), 8.42 (br s, 1H, NH); 13C-NMR (CDCl3):δ 183.7, 171.2, 159.5, 136.4, 131.5, 128.8, 127.0, 126.7, 124.2, 115.3, 73.0, 59.1, 55.4, 41.4, 34.3, 26.6, 23.5, 16.3; 31P-NMR (CDCl3):δ 20.3; 19F-NMR (CDCl3):δ -118.8; IR (KBr, cm-1): v 3275, 3067, 2961, 1668, 1554, 1227, 1015; Anal. Calcd for C27H39FN3O4PS: C 58.78, H 7.13, N 7.62; Found: C 58.53, H 7.32, N 7.34.
Diethyl (3-(L-1-(4-fluorobenzylamino)-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methylphos-phonate (3k). White solid, yield 94%; m.p. 31-32 ºC; [α]20D-16.7º (c 0.1, acetone); 1H-NMR (CDCl3): δ 0.82 (d, 6H, J = 6.5 Hz, 2CH3), 1.09 (t, 6H, J = 5.8Hz, 2CH3), 1.27-1.52(m, 2H, CH2), 1.66-1.71 (m, 1H, CH), 3.71-3.99 (m, 2H, NCH2), 4.14-4.43 (m, 4H, 2OCH2) , 5.20 (d, 1H, J = 6.7 Hz, CH), 6.29 (s, 1H, PCH), 6.37 (s, 1H, NH), 6.74-7.45 (m, 9H, ArH), 8.01 (s, 1H, NH), 8.74 (br s, 1H, NH); 13C-NMR (CDCl3): δ 183.6, 171.7, 160.9, 137.1, 135.8, 128.7, 127.1, 126.6, 115.4, 64.1, 58.5, 54.2, 44.2, 41.3, 26.3, 23.6, 16.5; 31P-NMR (CDCl3):δ 22.2; 19F-NMR (CDCl3):δ -115.1 ppm; IR (KBr, cm-1): v 3285, 3080, 2963, 1676, 1551, 1223, 1020 cm-1; Anal. Calcd for C25H35FN3O4PS: C 57.35, H 6.74, N 8.03; Found: C 57.59, H 6.48, N 7.96
Diisopropyl (3-(L-1-(4-fluorobenzylamino)-4-methyl-1-oxopentan-2-yl)thioureido)(phenyl)methyl-phosphonate (3l). White solid, yield 90%; m.p. 43-44 ºC; [α]20D-13.3º (c 0.1, acetone); 1H-NMR (CDCl3): δ 0.84 (d, 18H, J = 6.3 Hz, 6CH3), 1.39-1.57 (m, 2H, CH2), 1.64-1.69 (m, H, CH), 4.21-4.43 (m, 2H, NCH2), 4.63-4.72 (m, 2H, 2OCH), 5.19 (d, 1H, J = 5.8 Hz, CH), 6.25 (s, 1H, PCH), 6.35 (s, 1H, NH), 6.95-7.40 (m, 9H, ArH), 7.98 (s, 1H, NH), 8.71 (br s, 1H, NH); 13C-NMR (CDC13): δ 183.7, 172.1, 161.2, 137.3, 135.6, 128.8, 127.4, 126.8, 115.3, 73.3, 59.6, 55.7, 43.4, 40.8, 26.4, 24.5, 23.2; 31P-NMR (CDCl3): δ 21.9; 19F-NMR (CDCl3):δ -115.4; IR (KBr, cm-1): v 3287, 3072, 2968, 1678, 1548, 1220, 1008; Anal. Calcd for C27H39FN3O4PS: C 58.78, H 7.13, N 7.62; Found: C 58.64, H 7.35, N 7.41.
Diethyl (3-(L-4-methyl-1-oxo-1-(4-(trifluoromethyl)phenylamino)pentan-2-yl)thioureido)(phenyl)-methylphosphonate (3m). Colorless viscous liquid, yield 84%; [α]20D-8.9º (c 0.1, acetone); 1H-NMR (CDCl3): δ0.94 (d, 6H, J = 5.7 Hz, 2CH3), 1.13 (t, 6H, J = 6.9 Hz, 2CH3), 0.99-1.02(m, H, CH), 1.66-1.72 (m, 2H, CH2), 3.92-4.13 (m, 4H, 2OCH2), 5.03-5.11 (m, 1H, CH), 6.31 (s, 1H, PCH), 7.31-7.79 (m, 9H, ArH), 10.07 (s, 1H, NH); 13C-NMR (CDCl3): δ 183.8, 174.2, 141.7, 136.9, 128.4, 127.0, 126.5, 126.1, 125.7, 123.3, 119.8, 73.4, 58.2, 54.1, 41.4, 26.2, 23.4, 21.8; 31P NMR (CDCl3): δ 22.3; 19F-NMR (CDCl3): δ -63.4; IR (KBr, cm-1): v 3467, 3280, 2968, 1648, 1552, 1218, 1026; Anal. Calcd for C25H33F3N3O4PS: C 53.66, H 5.94, N 7.51; Found: C 53.74, H 6.17, N 7.22.
Diisopropyl(3-(L-4-methyl-1-oxo-1-(4-(trifluoromethyl)phenylamino)pentan-2-yl)thioureido) (phenyl -methylphosphonate (3n). Colorless viscous liquid, yield 79%; [α]20D-10.5º (c 0.1, acetone); 1H-NMR (CDCl3): δ 0.98 (d, 18H, J = 6.4 Hz, 6CH3), 1.36-1.42 (m, 1H, CH), 1.63-1.68 (m, H, CH2), 4.17-4.43 (m, 2H, 2OCH), 5.06-5.16 (m, 1H, CH), 6.33 (s, 1H, PCH), 7.29-7.74 (m, 9H, ArH), 10.12 (br s, 1H, NH); 13C-NMR (CDC13): δ 183.9, 174.4, 141.5, 137.2, 128.5, 127.1, 126.8, 126.2, 125.8, 123.5, 119.4, 73.6, 58.5, 54.2, 41.3, 26.1, 23.2, 21.7; 31P-NMR (CDCl3): δ 22.5; 19F-NMR (CDCl3): δ -63.2; IR (KBr, cm-1): v 3465, 3276, 2962, 1645, 1546, 1212, 1028; Anal. Calcd for C27H37F3N3O4PS: C 55.19, H 6.35, N 7.15; Found: C 55.26, H 6.71, N 6.97.
4. Conclusions
A series of novel chiral thioureas containing α-aminophosphonate moieties were synthesized in high yield under mild conditions by the addition in THF of O, O′-dialkyl isothiocyanato(phenyl)-methylphosphonates 8 to L-leucine derivatives. The bioassay results showed that title compounds exhibited moderate to good anti-TMV bioactivity. In particular, compounds 3l (R1= p-FC6H4CH2, R2= i-Bu, R= i-Pr) and 3n (R1= p-CF3C6H4, R2= i-Bu, R= i-Pr) showed better biological activity than other structurally related analogues. The L-leucyl and phosphonate moiety derived from branched alkyl chains are preferred over straight chain alkylated ones in the design of the title thioureas, moreover, the compounds bearing an electron withdrawing group (R1= p-F, p-CF3) at the 4-position of the aromatic ring showed better anti-TMV activities. At for the absolute configuration, compound L-3l (derived from L-leucine) showed more significant activity against TMV than its enantiomer D-3l and the racemate (±)-3l. The present work demonstrates that the antiviral activity of chiral thioureas was significantly improved through introduction of suitably substituted derivatives with L-configuration. The influence of different substituents, minor structural modifications and steric parameters on structure activity relationships for identifying lead bioactive compounds will be studied in our future investigations.
Acknowledgements
The authors wish to thank the National Key Project for Basic Research (2010CB126105) and the National Natural Science Foundation of China (20872021) for the financial support.
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Jiang, L.; Zheng, H.T.; Liu, T.Y.; Yue, L.; Chen, Y.C. Asymmetric direct vinylogous carbon-carbon bond formation catalyzed by bifunctional organocatalysts. Tetrahedron 2007, 63, 5123–5128. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Vassilev, A.O.; Benyunov, A.; Grigoriants, O.O.; Tibbles, H.E.; Uckun, F.M. Stereochemistry as a determinant of the anti-leukemic potency of halopyridyl and thiazolyl thiourea compounds. Lett. Drug Des. Discov. 2007, 4, 318–326. [Google Scholar]
- Manjula, S.N.; Malleshappa Noolvi, N.; Vipan Parihar, K. Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar]
- Venkatachalam, T.K.; Mao, C.; Uckun, F.M. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 2004, 12, 4275–4284. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Biological activity of aminophosphonic acids. Phosphor. Sulfur 1991, 63, 193–215. [Google Scholar]
- Kuhkar, V.P.; Hudson, H.R. Synthesis of α-Aminoalkanephosphonic and α-Aminophosphonic Acids; Wiley: Chichester, UK, 2000. [Google Scholar]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef]
- Song, B.A.; Wu, Y.L.; Yang, S.; Hu, D.Y. Synthesis and bioactivity of a-aminophosphonates containing fluorine. Molecules 2003, 8, 186–192. [Google Scholar] [CrossRef]
- Jin, L.H.; Song, B.A.; Zhang, G.P.; Xu, R.Q.; Zhang, S.M.; Gao, X.W.; Hu, D.Y.; Yang, S. Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O,O-dialkyl-α-aminophosphonates. Bioorg. Med. Chem. Lett. 2006, 16, 1537–1543. [Google Scholar]
- Bird, J.; De Mello, R.C.; Harper, G.P.; Hunter, D.J. Synthesis of novel N-phosphonoalkyl dipeptide inhibitors of human collagenase. J. Med. Chem. 1994, 37, 158–169. [Google Scholar]
- Grembecka, J.; Mucha, A.; Cierpicki, T.; Kafarski, P. The most potent organophosphorus inhibitors of leucine aminopeptidase. structure-based design, chemistry, and activity. J. Med. Chem. 2003, 46, 2641–2655. [Google Scholar]
- Hu, D.Y.; Wan, Q.Q.; Yang, S.; Song, B.A.; Bhadury, P.S.; Jin, L.H. Synthesis and antiviral activities of amide derivatives containing the aminophosphonate moiety. J. Agric. Food Chem. 2008, 56, 998–1001. [Google Scholar]
- Chen, M.H.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Cai, X.J.; Hu, D.Y. Synthesis and antiviral activities of chiral thiourea derivatives containing an α-aminophosphonate moiety. J. Agric. Food Chem. 2009, 57, 1383–1388. [Google Scholar] [CrossRef]
- Yang, J.Q.; Song, B.A.; Bhadury, P.S.; Chen, Z.; Yang, S.; Cai, X.J.; Hu, D.Y. Synthesis and antiviral bioactivities of 2-cyano-3-substituted-amino(phenyl)methylphosphonylacrylates-(acrylamides) containing alkoxyethyl moieties. J. Agric. Food Chem. 2010, 58, 2730–2735. [Google Scholar]
- Song, B.A.; Yang, S.; Jin, L.H.; Bhadury, P.S. Environment-friendly Antiviral Agents for Plants; Springer: Berlin, Germany, 2009; pp. 1–10. [Google Scholar]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
- Song, B.A.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y.; Pang, L.L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).