Sonogashira Reaction of Aryl and Heteroaryl Halides with Terminal Alkynes Catalyzed by a Highly Efficient and Recyclable Nanosized MCM-41 Anchored Palladium Bipyridyl Complex
Abstract
:1. Introduction
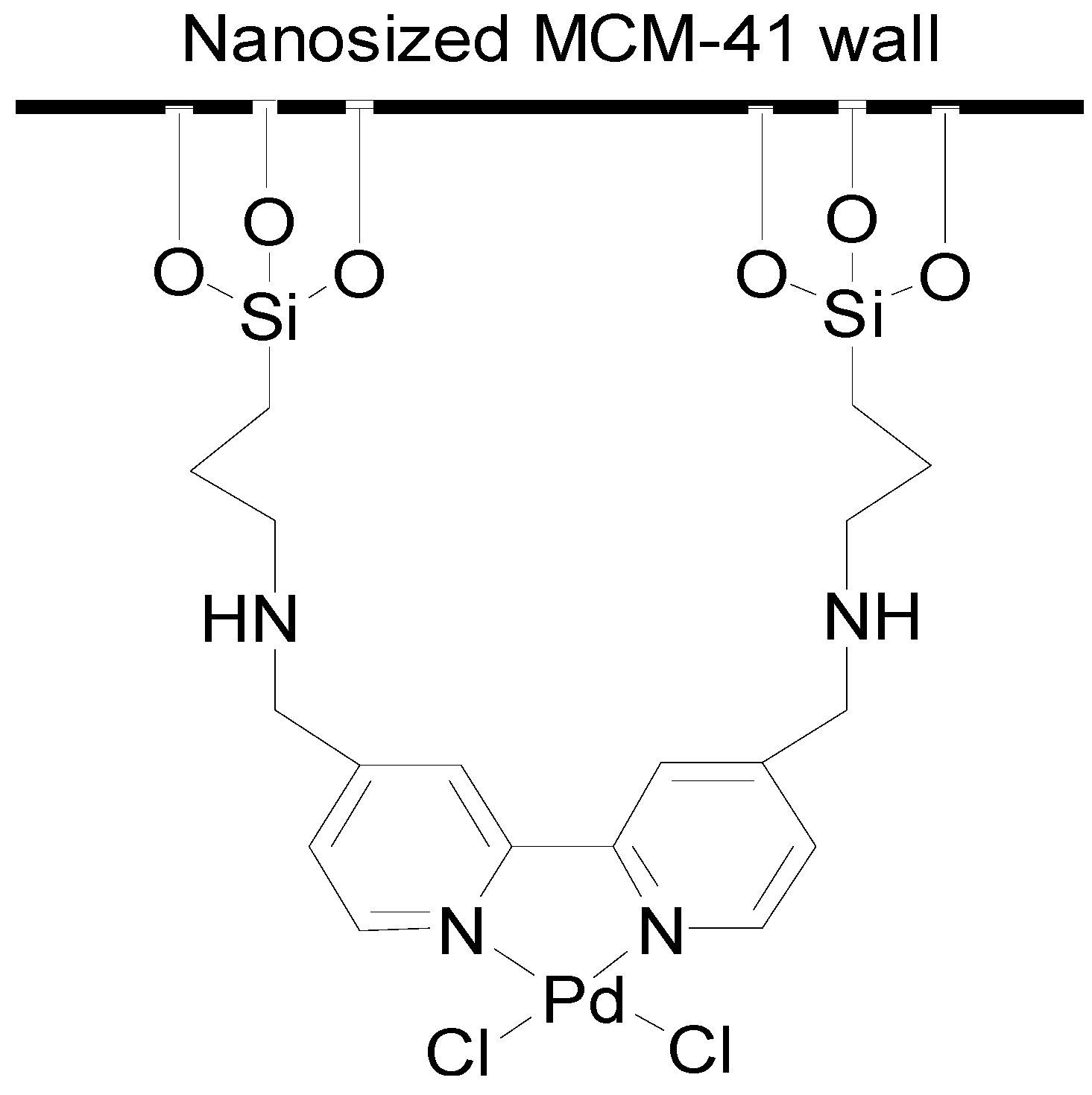
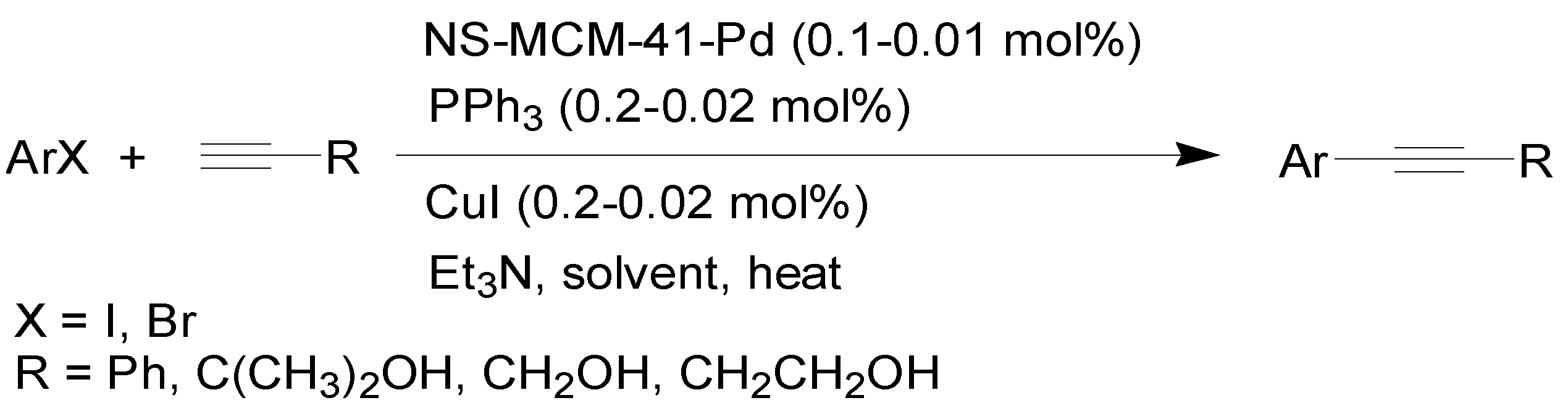
2. Results and Discussion
2.1. Optimization of reaction conditions for the Sonogashira reaction catalyzed by NS-MCM-41-Pd
| Entry | Pd (mol%) | CuI (mol%) | PPh3 (mol%) | Solvent | Base | Yield (%)b |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 0.2 | 0.2 | Et3N | Et3N | 97 |
| 2 | 0.1 | 0.2 | 0.2 | Toluene | Et3Nc | 60 |
| 3 | 0.1 | 0.2 | 0.2 | DMF | Et3Nc | 34 |
| 4 | 0.1 | 0.2 | 0.2 | DMSO | Et3Nc | 26 |
| 5 | 0.1 | 0.2 | 0.2 | NMP | Et3Nc | 5 |
| 6 | 0.1 | 0.2 | 0 | Et3N | Et3N | 38 |
| 7 | 0.1 | 0 | 0.2 | Et3N | Et3N | 0 |
| 8 | 0.1 | 0 | 0 | Et3N | Et3N | 0 |
| 9 | 0.1 | 0.2 | 0.2 | Toluene | KOHc | 0 |
| 10 | 0.1 | 0.2 | 0.2 | DMF | KOHc | 0 |
| 11 | 0.1 | 0.2 | 0.2 | Toluene | K2CO3c | 0 |
| 12 | 0.1 | 0.2 | 0.2 | DMF | K2CO3c | 0 |
2.2. Sonogashira reaction of aryl halides with phenylacetylene
| Entry | Aryl halide | Pd (mol%) | Solvent/Base | T (°C) | t (h) | Yield (%)b | TON | |
|---|---|---|---|---|---|---|---|---|
| 1 | C6H5I | 1a | 0.1 | Et3N/Et3N | 50 | 3 | 3a, 97 | 970 |
| 2 | C6H5I | 1a | 0.01 | Et3N/Et3N | 50 | 12 | 3a, 98 | 9800 |
| 3 | 4-IC6H4CN | 1b | 0.1 | Et3N/Et3N | 50 | 3 | 3b, 96 | 960 |
| 4 | 4-IC6H4CN | 1b | 0.01 | Et3N/Et3N | 50 | 9 | 3b, 96 | 9600 |
| 5 | 4-MeOC6H4I | 1c | 0.1 | Et3N/Et3N | 50 | 24 | 3c, 87 | 870 |
| 6 | C6H5Br | 1d | 0.1 | NMP/Bu3Nc | 140 | 24 | 3a, 30 | 300 |
| 7 | C6H5Br | 1d | 0.1 | Toluene/Bu3Nc | 100 | 24 | 3a, 56 | 560 |
| 8 | 4-BrC6H4CN | 1e | 0.1 | Et3N/Et3N | 90 | 3 | 3b, 93 | 930 |
| 9 | 4-MeCOC6H4Br | 1f | 0.1 | NMP/Et3Nc | 90 | 6 | 3d, 98 | 980 |
| 10 | 4-NO2C6H4Br | 1g | 0.01 | NMP/Et3Nc | 90 | 6 | 3e, 99 | 9900 |
| 11 | 4-ClC6H4Br | 1h | 0.1 | NMP/Et3Nc | 90 | 24 | 3f, 46 | 460 |
| 12 | 4-MeOC6H4Br | 1i | 0.1 | NMP/Et3Nc | 90 | 72 | 3c, 40 | 400 |
| 13 | 2-Bromothiophene | 1j | 0.1 | NMP/Et3Nc | 90 | 48 | 3g, 71 | 710 |
| 14 | 3-Bromothiophene | 1k | 0.1 | NMP/Et3Nc | 90 | 96 | 3h, 36 | 360 |
| 15 | 2-Bromopyridine | 1l | 0.1 | NMP/Et3Nc | 90 | 3 | 3i, 99 | 990 |
| 16 | 3-Bromopyridine | 1m | 0.1 | NMP/Et3Nc | 90 | 24 | 3j, 98 | 980 |
2.3. Sonogashira reaction of aryl halides with alkynols
| Entry | Aryl halide | Alkynyl alcohol | Pd (mol%) | t (h) | Yield (%)b | TON | ||
|---|---|---|---|---|---|---|---|---|
| 1 | C6H5I | 1a | HC≡CC(CH3)2OH | 4a | 0.1 | 3 | 5a, 94 | 940 |
| 2 | 4-IC6H4CN | 1b | HC≡CC(CH3)2OH | 4a | 0.1 | 3 | 5b, 98 | 980 |
| 3 | 4-MeOC6H4I | 1c | HC≡CC(CH3)2OH | 4a | 0.1 | 72 | 5c, 61 | 610 |
| 4 | C6H5Br | 1d | HC≡CC(CH3)2OH | 4a | 0.1 | 96 | 5a, 21 | 210 |
| 5 | 4-BrC6H4CN | 1e | HC≡CC(CH3)2OH | 4a | 0.1 | 3 | 5b, 98 | 980 |
| 6 | 4-BrC6H4CN | 1e | HC≡CC(CH3)2OH | 4a | 0.01 | 12 | 5b, 96 | 9600 |
| 7 | 4-MeCOC6H4Br | 1f | HC≡CC(CH3)2OH | 4a | 0.1 | 3 | 5d, 98 | 980 |
| 8 | 4-NO2C6H4Br | 1g | HC≡CC(CH3)2OH | 4a | 0.1 | 3 | 5e, 97 | 970 |
| 9 | 4-ClC6H4Br | 1h | HC≡CC(CH3)2OH | 4a | 0.1 | 24 | 5f, 69 | 690 |
| 10 | 4-MeOC6H4Br | 1i | HC≡CC(CH3)2OH | 4a | 0.1 | 96 | 5c, 20 | 200 |
| 11 | 2-Bromothiophene | 1j | HC≡CC(CH3)2OH | 4a | 0.1 | 48 | 5g, 99 | 990 |
| 12 | 3-Bromothiophene | 1k | HC≡CC(CH3)2OH | 4a | 0.1 | 96 | 5h, 59 | 590 |
| 13 | 2-Bromopyridine | 1l | HC≡CC(CH3)2OH | 4a | 0.1 | 3 | 5i, 99 | 990 |
| 14 | 3-Bromopyridine | 1m | HC≡CC(CH3)2OH | 4a | 0.1 | 6 | 5j, 98 | 980 |
| 15 | 3-Bromopyridine | 1m | HC≡CC(CH3)2OH | 4a | 0.01 | 24 | 5j, 34 | 3400 |
| 16 | C6H5I | 1a | HC≡CCH2OH | 4b | 0.1 | 12 | 5k, 85 | 850 |
| 17 | C6H5I | 1a | HC≡CCH2OH | 4b | 0.01 | 24 | 5k, 84 | 8400 |
| 18 | 4-IC6H4CN | 1b | HC≡CCH2OH | 4b | 0.1 | 3 | 5l, 83 | 830 |
| 19 | C6H5Br | 1d | HC≡CCH2OH | 4b | 0.1 | 96 | 5k, 10 | 100 |
| 20 | 4-BrC6H4CN | 1e | HC≡CCH2OH | 4b | 0.1 | 24 | 5l, 71 | 710 |
| 21 | 4-BrC6H4COMe | 1f | HC≡CCH2OH | 4b | 0.1 | 3 | 5m, 98 | 980 |
| 22 | 4-BrC6H4COMe | 1f | HC≡CCH2OH | 4b | 0.01 | 48 | 5m, 98 | 9800 |
| 23 | 4-BrC6H4NO2 | 1g | HC≡CCH2OH | 4b | 0.1 | 24 | 5n, 99 | 990 |
| 24 | 4-BrC6H4Cl | 1h | HC≡CCH2OH | 4b | 0.1 | 72 | 5o, 15 | 150 |
| 25 | 2-Bromothiophene | 1j | HC≡CCH2OH | 4b | 0.1 | 48 | 5p, 18 | 180 |
| 26 | 3-Bromothiophene | 1k | HC≡CCH2OH | 4b | 0.1 | 96 | 5q, 8 | 80 |
| 27 | 2-Bromopyridine | 1l | HC≡CCH2OH | 4b | 0.1 | 3 | 5r, 81 | 810 |
| 28 | 3-Bromopyridine | 1m | HC≡CCH2OH | 4b | 0.1 | 48 | 5s, 65 | 650 |
| 29 | C6H5I | 1a | HC≡CCH2CH2OH | 4c | 0.1 | 6 | 5t, 78 | 780 |
| 30 | 4-MeOC6H4I | 1c | HC≡CCH2CH2OH | 4c | 0.1 | 12 | 5u, 45 | 450 |
| 31 | 4-BrC6H4CN | 1e | HC≡CCH2CH2OH | 4c | 0.1 | 12 | 5v, 99 | 990 |
| 32 | 4-BrC6H4COMe | 1f | HC≡CCH2CH2OH | 4c | 0.1 | 12 | 5w, 89 | 890 |
| 33 | 2-Bromothiophene | 1j | HC≡CCH2CH2OH | 4c | 0.1 | 12 | 5x, 32 | 320 |
| 34 | 2-Bromopyridine | 1l | HC≡CCH2CH2OH | 4c | 0.1 | 12 | 5y, 64 | 640 |
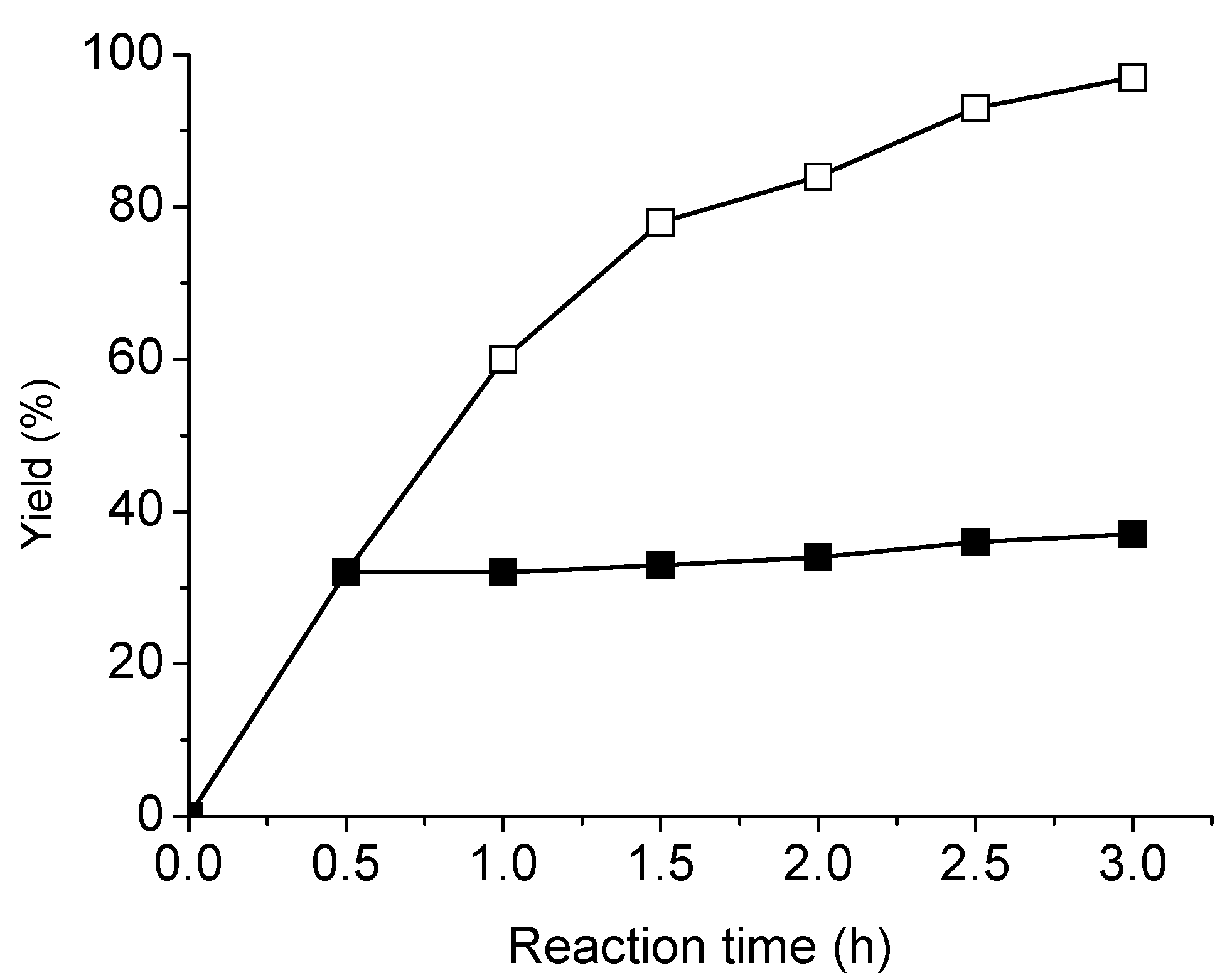
2.4. Recycling and leaching studies of NS-MCM-41-Pd in the Sonogashira reaction
| Entry | Aryl halide | Alkyne | Solvent/Base | T (°C) | t (h) | Yield, % b (TON) | ||
|---|---|---|---|---|---|---|---|---|
| Initial cycle | 1st recycle | 2nd recycle | ||||||
| 1 | 1a | 2a | Et3N/Et3N | 50 | 3 | 99 (990) | 99 (990) | 98 (980) |
| 2 | 1f | 2a | NMP/Et3Nc | 90 | 6 | 98 (980) | 93 (930) | 91 (910) |
| 3 | 1a | 4a | Et3N/Et3N | 90 | 3 | 94 (940) | 90 (900) | 88 (880) |
| 4 | 1f | 4a | Et3N/Et3N | 90 | 3 | 98 (980) | 99 (990) | 95 (950) |
| 5d | 1b | 4b | Et3N/Et3N | 90 | 3 | 83 (830) | 82 (820) | 80 (800) |
| 6d | 1f | 4b | Et3N/Et3N | 90 | 3 | 98 (980) | 88 (880) | 76 (760) |
3. Experimental
3.1. General
3.2. General procedure for the Sonogashira coupling
3.3. General procedure for recycling of nanosized MCM-41-Pd
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Moore, J.S. Shape-persistent molecular architectures of nanoscale dimension. Acc. Chem. Res. 1997, 30, 402–413. [Google Scholar] [CrossRef]
- Sonogashira, K. Metal-Catalyzed Cross-Coupling Reactions; Diederich, F., Stang, P.J., Eds.; Wiley-VCH: Weinheim, Germany, 1998; pp. 203–209. [Google Scholar]
- Brandsma, L.; Vasilevsky, S.F.; Verkruijsse, H.D. Application of Transition Metal Catalysts in Organic Synthesis; Springer: Berlin, Germany, 1998; pp. 179–225. [Google Scholar]
- Sonogashira, K. Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E., de Meijere, A., Eds.; Wiley-VCH: New York, NY, USA, 2002; p. 493. [Google Scholar]
- Sonogashira, K. Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Negishi, E.; Anastasia, L. Palladium-catalyzed alkynylation. Chem. Rev. 2003, 103, 1979–2017. [Google Scholar] [CrossRef]
- Taylor, E.C.; Dowling, J.E. Replacement of the 1′,4′-phenylene region in 5,10-dideaza-5,6,7,8-tetrahydrofolic acid (DDATHF) by 4,5,6,7-tetrahydrobenzo[c]thiophene and 4,5,6,7-tetrahydroisobenzofuran Nuclei. J. Org. Chem. 1997, 62, 1599–1603. [Google Scholar] [CrossRef]
- Nakamura, H.; Aizawa, M.; Takeuchi, D.; Murai, A.; Shimoura, O. Convergent and short-step syntheses of dl-Cypridina luciferin and its analogues based on Pd-mediated cross couplings. Tetrahedron Lett. 2000, 41, 2185–2188. [Google Scholar] [CrossRef]
- Liu, T.-Z.; Isobe, M. Synthesis of the H-I-J tricyclic fragment of ciguatoxin, a marine polyether toxin. Synlett 2000, 266–268. [Google Scholar]
- de Kort, M.; Correa, V.; Valentijn, A.R.P.M.; van der Marel, G.A.; Potter, B.V.L.; Taylor, C.W.; van Boom, J.H. Synthesis of potent agonists of the D-myo-inositol 1,4,5-trisphosphate receptor based on clustered disaccharide polyphosphate analogues of adenophostin A. J. Med. Chem. 2000, 43, 3295–3303. [Google Scholar] [CrossRef]
- Amiet, G.; Hügel, H.M.; Nurlawis, F. The synthesis of the kynurenamines K1 and K2, metabolites of melatonin. Synlett 2002, 495–497. [Google Scholar]
- Cosford, N.D.P.; Tehrani, L.; Roppe, J.; Schweiger, E.; Smith, N.D.; Anderson, J.; Bristow, L.; Brodkin, J.; Jiang, X.; McDonald, I.; Rao, S.; Washburn, M.; Varney, M.A. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: A potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003, 46, 204–206. [Google Scholar]
- Nicolaou, K.C.; Dai, W.-M. Chemistry and biology of the enediyne anticancer antibiotics. Angew. Chem., Int. Ed. Engl. 1991, 30, 1387–1416. [Google Scholar]
- Yoshimura, F.; Kawata, S.; Hirama, M. Synthetic study of kedarcidin chromophore: Atropselective construction of the ansamacrolide. Tetrahedron Lett. 1999, 40, 8281–8285. [Google Scholar] [CrossRef]
- Toyota, M.; Komori, C.; Ihara, M. A concise formal total synthesis of mappicine and nothapodytine B via an intramolecular hetero Diels-Alder reaction. J. Org. Chem. 2000, 65, 7110–7113. [Google Scholar] [CrossRef]
- Paterson, I.; Davies, R.D.M.; Marquez, R. Total synthesis of the callipeltoside Aglycon. Angew. Chem., Int. Ed. 2001, 40, 603–607. [Google Scholar]
- Kiehl, A.; Müllen, K. Catalysis in Precision Polymerization; Kobayashi, S., Ed.; Wiley-VCH: Chichester, England, 1997; pp. 162–169. [Google Scholar]
- Bunz, U.H.F. Poly(aryleneethynylene)s: Syntheses, properties, structures, and applications. Chem. Rev. 2000, 100, 1605–1644. [Google Scholar] [CrossRef]
- Höger, S.; Rosselli, S.; Ramminger, A.-D.; Enkelmann, V. A facile synthesis of large extraannular-functionalized phenyl-ethynyl macrocycles containing m-terphenyl units. Org. Lett. 2002, 4, 4269–4272. [Google Scholar] [CrossRef]
- Li, C.-J.; Slaven, W.T., IV; John, V.T.; Banerjee, S. Palladium catalysed polymerization of aryl diodides with acetylene gas in aqueous medium: A novel synthesis of areneethynylene polymers and oligomers. Chem. Commun. 1997, 1569–1570. [Google Scholar]
- Sonogashira, K. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: New York, NY, USA, 1991; Volume 3, pp. 521–549. [Google Scholar]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef]
- Heidenreich, R.G.; Köhler, K.; Krauter, J.G.E.; Pietsch, J. Pd/C as a highly active catalyst for Heck, Suzuki and Sonogashira reactions. Synlett 2002, 1118–1122. [Google Scholar]
- Novák, Z.; Szabó, A.; Répási, J.; Kotschy, A. Sonogashira coupling of aryl halides catalyzed by palladium on charcoal. J. Org. Chem. 2003, 68, 3327–3329. [Google Scholar] [CrossRef]
- Komáromi, A.; Novák, Z. Efficient copper-free Sonogashira coupling of aryl chlorides with palladium on charcoal. Chem. Commun. 2008, 4968–4970. [Google Scholar]
- Mori, S.; Yanase, T.; Aoyagi, S.; Monguchi, Y.; Maegawa, T.; Sajiki, H. Ligand-free sonogashira coupling reactions with heterogeneous Pd/C as the catalyst. Chem. Eur. J. 2008, 14, 6994–6999. [Google Scholar] [CrossRef]
- Reddy, E.A.; Barange, D.K.; Islam, A.; Mukkanti, K.; Pal, M. Synthesis of 2-alkynylquinolines from 2-chloro and 2,4-dichloroquinoline via Pd/C-catalyzed coupling reaction in water. Tetrahedron 2008, 64, 7143–7150. [Google Scholar] [CrossRef]
- Shang, H.; Hua, R.; Zheng, Q.; Zhang, J.; Liang, X.; Zhu, Q. An improved practical Pd/C-catalyzed Sonogashira cross-coupling reaction for the synthesis of liquid crystals of trans-cyclohexyltolans. Appl. Organomet. Chem. 2010, 24, 473–476. [Google Scholar]
- Duplais, C.; Forman, A.J.; Baker, B.A.; Lipshutz, B.H. UC Pd: A new form of PdVC for sonogashira couplings. Chem. Eur. J. 2010, 16, 3366–3371. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.; Li, H. Application of recoverable nanosized palladium(0) catalyst in Sonogashira reaction. Tetrahedron 2005, 61, 8633–8640. [Google Scholar] [CrossRef]
- Chouzier, S.; Gruber, M.; Djakovitch, L. New hetero-bimetallic Pd-Cu catalysts for the one-pot indole synthesis via the Sonogashira reaction. J. Mol. Catal. A: Chem. 2004, 212, 43–52. [Google Scholar]
- Djakovitch, L.; Rollet, P. Sonogashira cross-coupling reactions catalysed by copper-free palladium zeolites. Adv. Synth. Catal. 2004, 346, 1782–1792. [Google Scholar] [CrossRef]
- Djakovitch, L.; Rollet, P. Sonogashira cross-coupling reactions catalysed by heterogeneous copper-free Pd-zeolites. Tetrahedron Lett. 2004, 45, 1367–1370. [Google Scholar] [CrossRef]
- Tyrrell, E.; Whiteman, L.; Williams, N. Sonogashira cross-coupling reactions and construction of the indole ring system using a robust, silica-supported palladium catalyst. Synthesis 2009, 829–835. [Google Scholar]
- Anwander, R. SOMC@PMS. Surface organometallic chemistry at periodic mesoporous silica. Chem. Mater. 2001, 13, 4419–4438. [Google Scholar] [CrossRef]
- Biffis, A.; Zecca, M.; Basato, M. Palladium metal catalysts in Heck C-C coupling reactions. J. Mol. Catal. A: Chem. 2001, 173, 249–274. [Google Scholar]
- He, X.; Antonelli, D. Recent advances in synthesis and applications of transition metal containing mesoporous molecular sieves. Angew. Chem. Int. Ed. 2002, 41, 215–229. [Google Scholar]
- De Vos, D.E.; Dams, M.; Sels, B.F.; Jacobs, P.A. Ordered mesoporous and microporous molecular sieves functionalized with transition metal complexes as catalysts for selective organic transformations. Chem. Rev. 2002, 102, 3615–3640. [Google Scholar] [CrossRef]
- Trong On, D.; Desplantier-Giscard, D.; Danumah, C.; Kaliaguine, S. Perspectives in catalytic applications of mesostructured materials. Appl. Catal. A: Gen. 2003, 253, 545–602. [Google Scholar]
- Thomas, J.M.; Raja, R. Catalytic significance of organometallic compounds immobilized on mesoporous silica: Economically and environmentally important examples. J. Organomet. Chem. 2004, 689, 4110–4124. [Google Scholar] [CrossRef]
- Li, C. Chiral synthesis on catalysts immobilized in microporous and mesoporous materials. Catal. Rev. 2004, 46, 419–492. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Mprell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem., Int. Ed. 2006, 45, 3216–3251. [Google Scholar]
- Rollet, P.; Kleist, W.; Dufaud, V.; Djakovitch, L. Copper-free heterogeneous catalysts for the Sonogashira cross-coupling reaction: Preparation, characterisation, activity and applications for organic synthesis. J. Mol. Catal. A: Chem. 2005, 241, 39–51. [Google Scholar]
- Cai, M.; Xu, Q.; Wang, P. A novel MCM-41-supported sulfur palladium(0) complex catalyst for Sonogashira coupling reaction. J. Mol. Catal. A: Chem. 2006, 250, 199–202. [Google Scholar]
- Cai, M.; Sha, J.; Xu, Q. MCM-41-supported bidentate phosphine palladium(0) complex: a highly active and recyclable catalyst for the Sonogashira reaction of aryl iodides. Tetrahedron 2007, 63, 4642–4647. [Google Scholar] [CrossRef]
- Cai, M.; Xu, Q.; Sha, J. Copper-free Sonogashira coupling reaction catalyzed by MCM-41-supported thioether palladium(0) complex in water under aerobic conditions. J. Mol. Catal. A: Chem. 2007, 272, 293–297. [Google Scholar]
- Alonso, D.A.; Nájera, C.; Pacheco, M.C. A copper- and amine-free Sonogashira-type coupling procedure catalyzed by oxime palladacycles. Tetrahedron Lett. 2002, 43, 9365–9368. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Cavell, K.J. Donor-Functionalized Heterocyclic Carbene Complexes of Palladium(II): Efficient Catalysts for C-C Coupling Reactions. Organometallics 2000, 19, 741–746. [Google Scholar] [CrossRef]
- Buchmeiser, M.R.; Schareina, T.; Kempe, R.; Wurst, K. Bis(pyrimidine)-based palladium catalysts: Synthesis, X-ray structure and applications in Heck-, Suzuki-, Sonogashira-Hagihara couplings and amination reactions. J. Organomet. Chem. 2001, 643, 39–46. [Google Scholar]
- Feuerstein, M.; Berthiol, F.; Doucet, H.; Santelli, M. Palladium-tetraphosphine complex: An efficient catalyst for the coupling of aryl halides with alkynes. Org. Biomol. Chem. 2003, 1, 2235–2237. [Google Scholar] [CrossRef]
- Feuerstein, M.; Doucet, H.; Santelli, M. Coupling reactions of aryl bromides with 1-alkynols catalysed by a tetraphosphine/palladium catalyst. Tetrahedron Lett. 2004, 45, 1603–1606. [Google Scholar] [CrossRef]
- Feuerstein, M.; Doucet, H.; Santelli, M. Sonogashira cross-coupling reactions with heteroaryl halides in the presence of a tetraphosphine-palladium catalyst. Tetrahedron Lett. 2005, 46, 1717–1720. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Wu, C.-L.; Mou, C.-Y.; Chao, M.-C.; Lin, H.-P.; Liu, S.-T. Palladium bipyridyl complex anchored on nanosized MCM-41 as a highly efficient and recyclable catalyst for Heck reaction. Tetrahedron Lett. 2004, 45, 7503–7506. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Lin, B.-N.; Chen, M.-J.; Mou, C.-Y.; Liu, S.-T. Anchored palladium bipyridyl complex in nanosized MCM-41: a recyclable and efficient catalyst for the Kumada-Corriu reaction. Tetrahedron 2007, 63, 4304–4309. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Chen, S.-C.; Tang, Y.-J.; Mou, C.-Y.; Tsai, F.-Y. Coupling of acyl chlorides with triarylbismuths catalyzed by palladium bipyridyl complex anchored on nanosized MCM-41: A recyclable and atom-efficient catalytic process for the synthesis of diaryl and alkyl aryl ketones. J. Mol. Catal. A: Chem. 2009, 307, 88–92. [Google Scholar]
- Chen, J.-Y.; Lin, T.-C.; Chen, S.-C.; Chen, A.-J.; Mou, C.-Y.; Tsai, F.-Y. Highly-efficient and recyclable nanosized MCM-41 anchored palladium bipyridyl complex-catalyzed coupling of acyl chlorides and terminal alkynes for the formation of ynones. Tetrahedron 2009, 65, 10134–10141. [Google Scholar] [CrossRef]
- Oki, A.R.; Morgan, R.J. An efficient preparation of 4,4'-dicarboxy-2,2'-bipyridine. Synth. Commun. 1995, 25, 4093–4097. [Google Scholar] [CrossRef]
- Will, G.; Boschloo, G.; Rao, S.N.; Fitzmaurice, D. Potentiostatic modulation of the lifetime of light-induced charge separation in a heterosupermolecule. J. Phys. Chem. B 1999, 103, 8067–8079. [Google Scholar]
- Lin, H.-P.; Tsai, C.-P. Synthesis of Mesoporous Silica Nanoparticles from a Low-concentration CnTMAX-Sodium Silicate Components. Chem. Lett. 2003, 32, 1092–1093. [Google Scholar] [CrossRef]
- Elangovan, A.; Wang, Y.-H.; Ho, T.-I. Sonogashira coupling reaction with diminished homocoupling. Org. Lett. 2003, 5, 1841–1844. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Wang, L.; Pagni, R.M. Sonogashira coupling and cyclization reactions on alumina: A route to aryl alkynes, 2-substituted-benzo[b]furans and 2-substituted-indoles. Tetrahedron 2001, 57, 8017–8028. [Google Scholar] [CrossRef]
- Van den Hoven, B.G.; Alper, H. The first regioselective hydroformylation of acetylenic thiophenes catalyzed by a zwitterionic rhodium complex and triphenyl phosphate. J. Org. Chem. 1999, 64, 9640–9645. [Google Scholar] [CrossRef]
- Sørensen, U.S.; Pombo-Villar, E. Copper-free palladium-catalyzed sonogashira-type coupling of aryl halides and 1-aryl-2-(trimethylsilyl)acetylenes. Tetrahedron 2005, 61, 2697–2703. [Google Scholar] [CrossRef]
- Novák, Z.; Nemes, P.; Kotschy, A. Tandem Sonogashira coupling: An efficient tool for the synthesis of diarylalkynes. Org. Lett. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Pal, M.; Subramanian, V.; Parasuraman, K.; Yeleswarapu, K.R. Palladium catalyzed reaction in aqueous DMF: Synthesis of 3-alkynyl substituted flavones in the presence of prolinol. Tetrahedron 2003, 59, 9563–9570. [Google Scholar] [CrossRef]
- Kayaki, Y.; Yamamoto, M.; Ikariya, T. Stereoselective formation of α-alkylidene cyclic carbonates via carboxylative cyclization of propargyl alcohols in supercritical carbon dioxide. J. Org. Chem. 2007, 72, 647–649. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Marandi, G.B. Preparation of conjugated enynes and arylacetylenic compounds from arylalkynols using alumina in dry media. J. Chem. Res. 2002, 11, 552–555. [Google Scholar] [CrossRef]
- Adjabeng, G.; Brenstrum, T.; Frampton, C.S.; Robertson, A.J.; Hillhouse, J.; McNulty, J.; Capretta, A. Palladium complexes of 1,3,5,7-tetramethyl-2,4,8-trioxa-6-phenyl-6- phosphaadamantane: Synthesis, crystal structure and use in the Suzuki and Sonogashira reactions and the α-arylation of ketones. J. Org. Chem. 2004, 69, 5082–5086. [Google Scholar]
- Kondo, K.; Fujitani, T.; Ohnishi, N. Synthesis and non-linear properties of disubstituted diphenylacetylene and related compounds. J. Mater. Chem. 1997, 7, 429–433. [Google Scholar] [CrossRef]
- Alonso, D.A.; Najera, C.; Pacheco, M.C. C(sp2)-C(sp) and C(sp)-C(sp) coupling reactions catalyzed by oxime-derived palladacycles. Adv. Synth. Catal. 2003, 345, 1146–1158. [Google Scholar] [CrossRef]
- Sarkar, A.; Talwar, S.S. Heteroaryl functionalised diacetylenes: Preparation and solid-state reactivity. J. Chem. Soc., Perkin Trans. 1 1998, 4141–4146. [Google Scholar]
- Sarkar, A.; Manjunath, S.P.; Kamath, B.; Bhagwat, L.; Babu, K.N.; Rajalakshmi, K.; Talwar, S.S. A convenient method for the preparation of thienylacetylnes. Indian J. Chem. Sect. B 1991, 30, 360–362. [Google Scholar]
- Ishikawa, T.; Mizuta, T.; Hagiwara, K.; Aikawa, T.; Kudo, T.; Saito, S. Catalytic alkynylation of ketones and aldehydes using quaternary ammonium hydroxide base. J. Org. Chem. 2003, 68, 3702–3705. [Google Scholar] [CrossRef]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Forte, G.; Petrucci, F.; Prastaro, A.; Niembro, S.; Shafir, A.; Vallribera, A. Alkynylation of aryl halides with perfluoro-tagged palladium nanoparticles immobilized on silica gel under aerobic, copper- and phosphine-free conditions in water. Org. Biomol. Chem. 2009, 7, 2270–2273. [Google Scholar] [CrossRef]
- Bumagin, N.A.; Ponomaryov, A.B.; Beletskaya, I.P. A convenient synthesis of substituted propargyl alcohols and terminal acetylenes. Synthesis 1984, 9, 728–729. [Google Scholar]
- Batey, R.A.; Shen, M.; Lough, A.J. Carbamoyl-substituted N-heterocyclic carbene complexes of palladium(II): Application to Sonogashira cross-coupling reactions. Org. Lett. 2002, 4, 1411–1414. [Google Scholar] [CrossRef]
- Harris, M.A.; McMillan, I.; Nayler, J.H.C.; Osborne, N.F.; Pearson, M.J.; Southgate, R. Syntheses based on 1,2-secopenicillins. Part II. Preparation of 4-(3-substituted prop-2-ynylthio)azetidin-2-ones. J. Chem. Soc., Perkin Trans. 1 1976, 1612–1615. [Google Scholar]
- Al-Arnaout, A.; Courtois, G.; Miginiac, L. Synthèse régiosélective par voie organométallique de pyridines, 4-picolines et 3,5-lutidines substituées en 2 par un groupe insaturé et/ou fonctionnel. J. Organomet. Chem. 1987, 333, 139–154. [Google Scholar]
- Bleicher, L.S.; Cosford, N.D.P.; Herbaut, A.; McCallum, J.S.; McDonald, I.A. A practical and efficient synthesis of the selective neuronal acetylcholine-gated ion channel agonist (S)-(-)-5-ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine maleate (SIB-1508Y). J. Org. Chem. 1998, 63, 1109–1118. [Google Scholar] [CrossRef]
- Kim, I.S.; Dong, G.R.; Jung, Y.H. Palladium(II)-catalyzed isomerization of olefins with tributyltin hydride. J. Org. Chem. 2007, 72, 5424–5426. [Google Scholar] [CrossRef]
- Collins, C.J.; Hanack, M.; Stutz, H.; Auchter, G.; Schoberth, W. Vinyl cations. 41. Influence of 4-aryl and 4-alkyl substituents on the π-route solvolyses of homopropargyl esters. J. Org. Chem. 1983, 48, 5260–5268. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, B.-N.; Huang, S.-H.; Wu, W.-Y.; Mou, C.-Y.; Tsai, F.-Y. Sonogashira Reaction of Aryl and Heteroaryl Halides with Terminal Alkynes Catalyzed by a Highly Efficient and Recyclable Nanosized MCM-41 Anchored Palladium Bipyridyl Complex. Molecules 2010, 15, 9157-9173. https://doi.org/10.3390/molecules15129157
Lin B-N, Huang S-H, Wu W-Y, Mou C-Y, Tsai F-Y. Sonogashira Reaction of Aryl and Heteroaryl Halides with Terminal Alkynes Catalyzed by a Highly Efficient and Recyclable Nanosized MCM-41 Anchored Palladium Bipyridyl Complex. Molecules. 2010; 15(12):9157-9173. https://doi.org/10.3390/molecules15129157
Chicago/Turabian StyleLin, Bo-Nan, Shao-Hsien Huang, Wei-Yi Wu, Chung-Yuan Mou, and Fu-Yu Tsai. 2010. "Sonogashira Reaction of Aryl and Heteroaryl Halides with Terminal Alkynes Catalyzed by a Highly Efficient and Recyclable Nanosized MCM-41 Anchored Palladium Bipyridyl Complex" Molecules 15, no. 12: 9157-9173. https://doi.org/10.3390/molecules15129157
APA StyleLin, B.-N., Huang, S.-H., Wu, W.-Y., Mou, C.-Y., & Tsai, F.-Y. (2010). Sonogashira Reaction of Aryl and Heteroaryl Halides with Terminal Alkynes Catalyzed by a Highly Efficient and Recyclable Nanosized MCM-41 Anchored Palladium Bipyridyl Complex. Molecules, 15(12), 9157-9173. https://doi.org/10.3390/molecules15129157






