Regioselective Synthesis of New 2-(E)-Cyano(thiazolidin-2-ylidene)thiazoles
Abstract
:Introduction
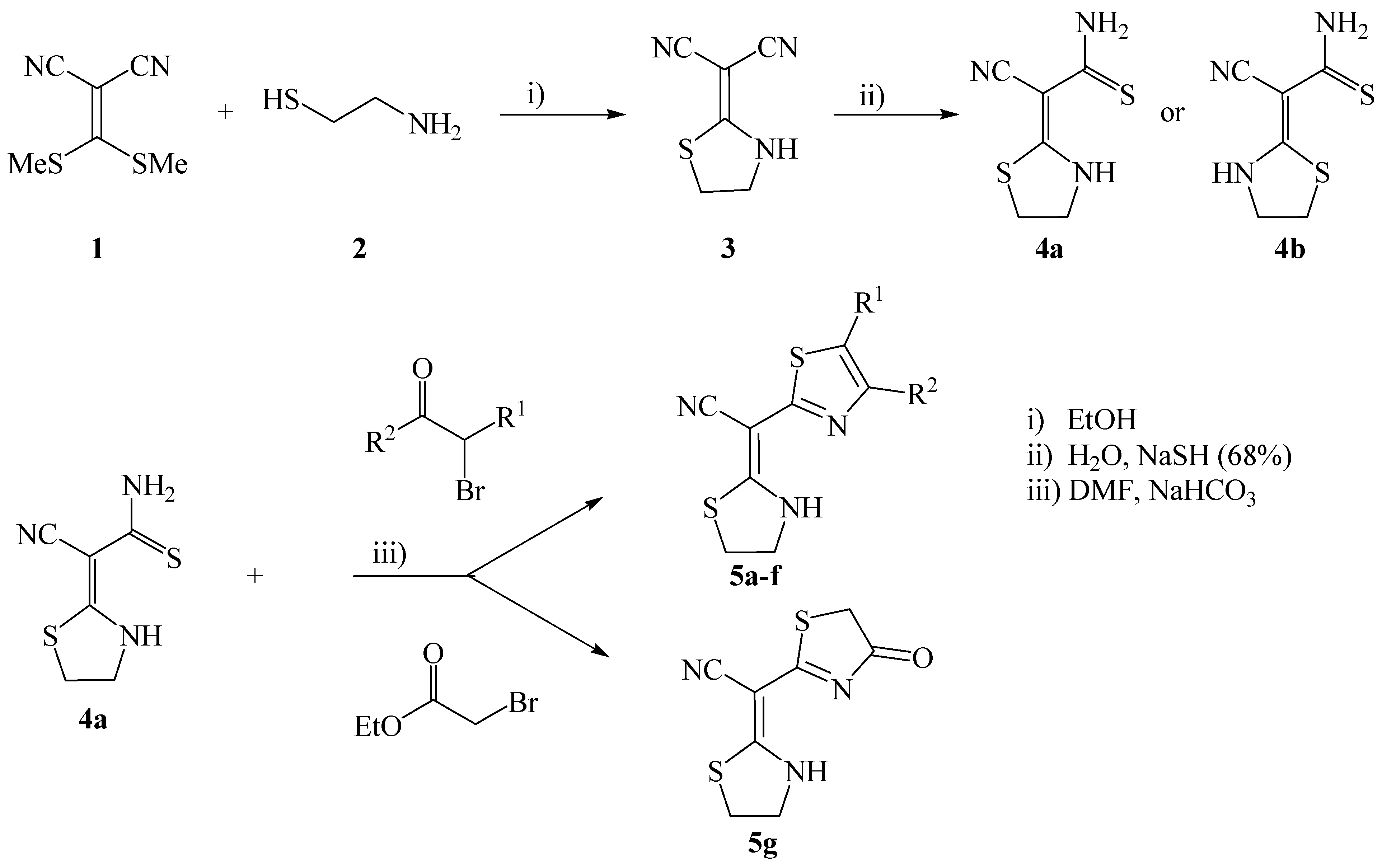
Results and Discussion
Experimental
General
Synthesis of 2-(thiazolidin-2-ylidene)malononitrile (3)
Synthesis of (E)-2-cyano-2-(thiazolidin-2-ylidene)ethanethioamide (4a)
General procedure for the synthesis of 2- (E)-cyano(thiazolidin-2-ylidene)thiazoles 5a-g
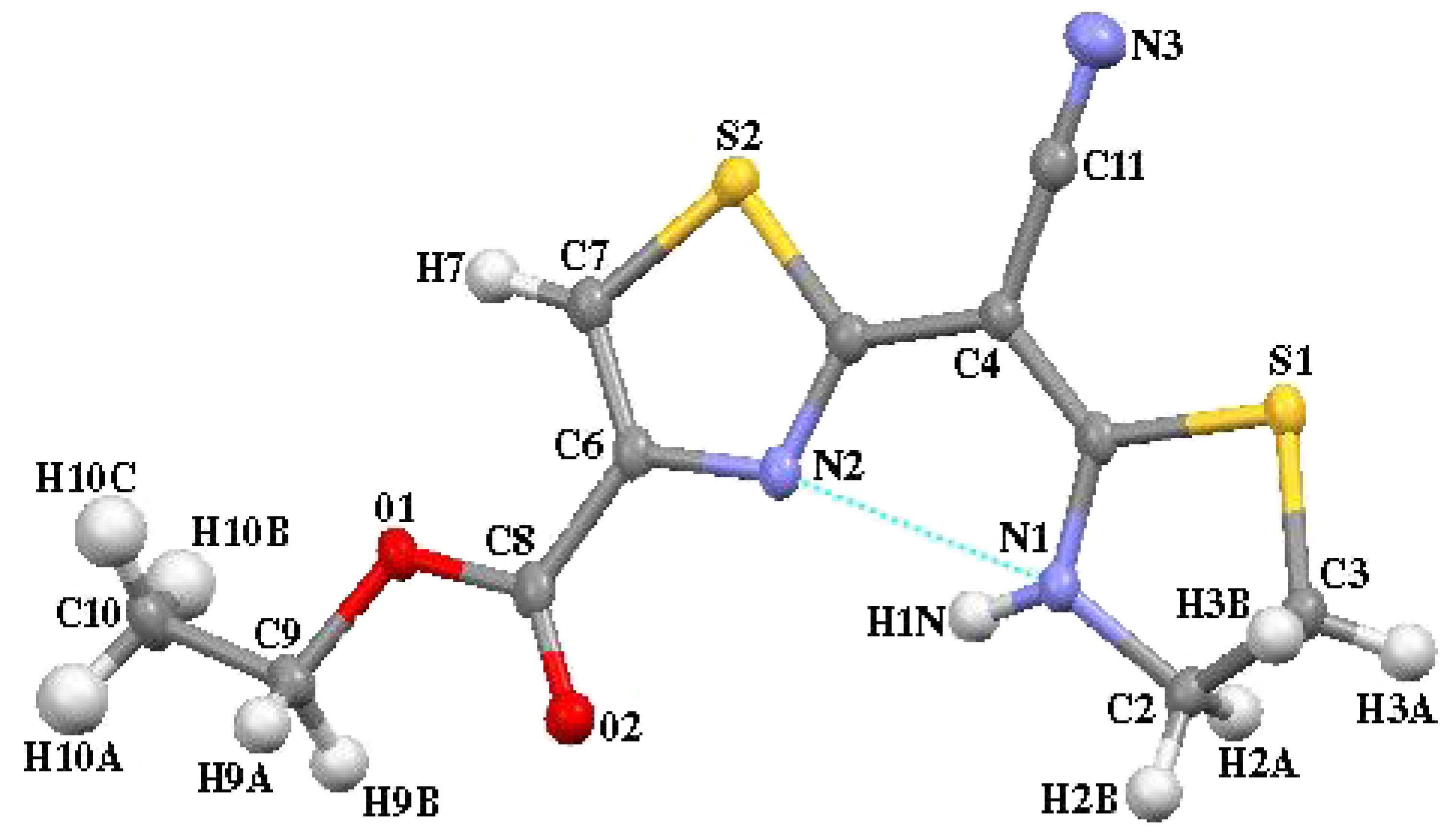
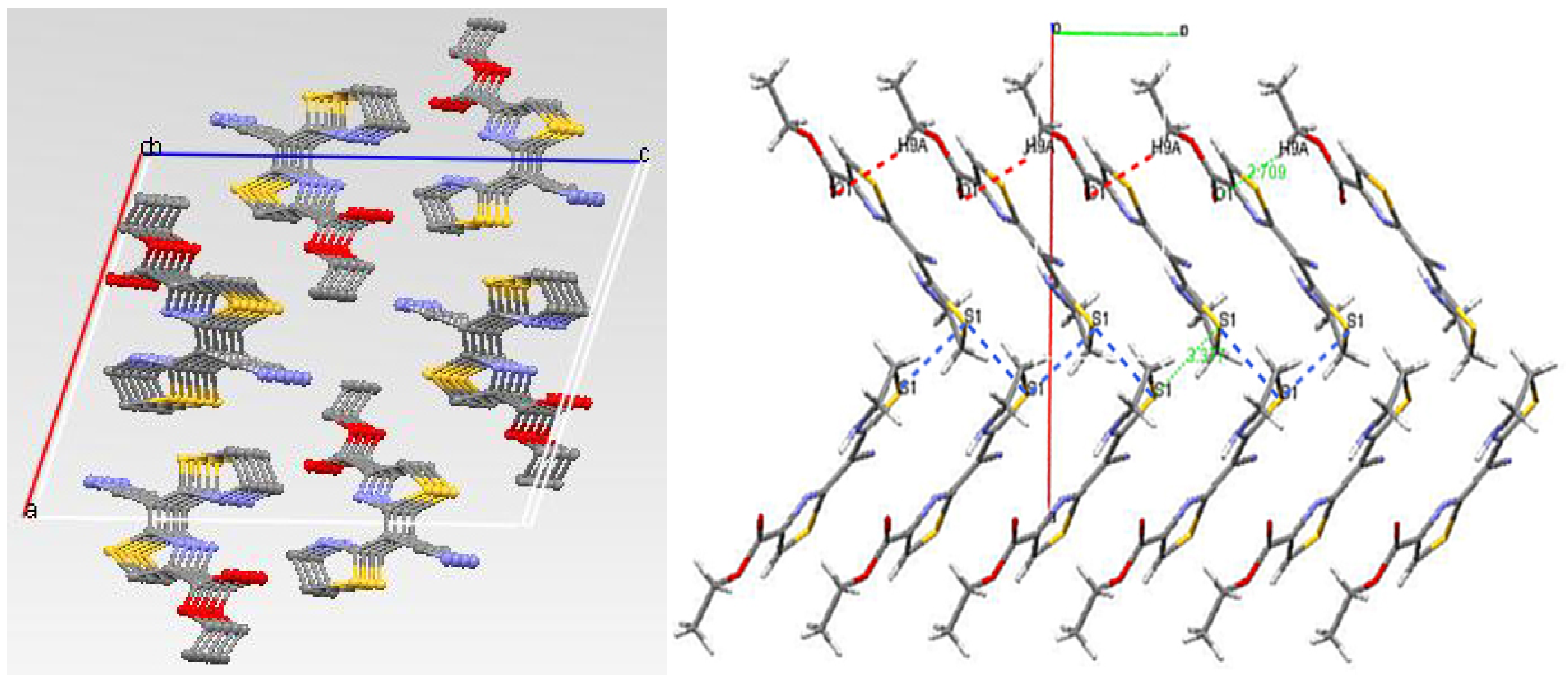
Crystal data for 5a
Conclusions
References and Notes
- Masquelin, T.; Obrecht, D. A new general three component solution-phase synthesis of 2-amino-1,3-thiazole and 2,4-diamino-1,3-thiazole combinatorial libraries. Tetrahedron 2001, 6, 153–156. [Google Scholar] [CrossRef]
- Hirai, K.; Sugimoto, H.; Ishiba, T. Heterocyclic cation systems. 14. Synthesis of thieno[3,2-e][1,4]diazepine, thiazolo[4,5-e][1,4]diazepine, and s-triazolo[3,4-c]thiazolo[4,5-e][1,4]diazepine. J. Org. Chem. 1980, 45, 253–260. [Google Scholar] [CrossRef]
- Walczynski, K.; Zuiderveld, O.P.; Timmerman, H. Non-imidazole histamine H3 ligands. Part III. New 4-n-propylpiperazines as non-imidazole histamine H3-antagonists. Eur. J. Med. Chem. 2005, 40, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Andreani, A.; Rambaldi, M.; Leoni, A.; Locatelli, A.; Andreani, F.; Gehret, J.C. Synthesis of imidazo[2,1-b]thiazoles as herbicides. Pharm. Acta Helv. 1996, 71, 247–252. [Google Scholar] [CrossRef]
- Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Calonghi, N.; Cappadone, C.; Farruggia, G.; Zini, M.; Stefanelli, C.; Masotti, L.; Radin, N.S.; Shoemaker, R.H. New antitumor imidazo[2,1-b]thiazole guanylhydrazones and analogues. J. Med. Chem. 2008, 51, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Budriesi, R.; Ioan, P.; Locatelli, A.; Cosconati, S.; Leoni, A.; Ugenti, M.P.; Andreani, A.; Di Toro, R.; Bedini, A.; Spampinato, S.; Marinelli, L.; Novellino, E.; Chiarini, A. Imidazo[2,1-b]thiazole system: A scaffold endowing dihydropyridines with selective cardiodepressant activity. J. Med. Chem. 2008, 51, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.K.; Ward, J.S.; Brokx, R.D.; Denny, T.; Bray, M.R.; Patel, D.V.; Xiao, X.Y. Design and synthesis of thiazole-5-hydroxamic acids as novel histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions: A practical approach towards the synthesis of Fanetizole. Tetrahedron 2007, 63, 11066–11069. [Google Scholar] [CrossRef]
- Zambon, A.; Borsato, G.; Brussolo, S.; Frascella, P.; Lucchini, V. Efficient access to 5-substituted thiazoles by a novel metallotropic rearrangement. Tetrahedron Lett. 2008, 49, 66–69. [Google Scholar] [CrossRef]
- Franklin, P.X.; Pillai, A.D.; Rathod, P.D.; Yerande, S.; Nivsarkar, M.; Padh, H.; Vasu, K.K.; Sudarsanam, V. 2-Amino-5-thiazolyl motif: A novel scaffold for designing anti-inflammatory agents of diverse structures. Eur. J. Med. Chem. 2008, 43, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Nishi, Y.; Hashimoto, S.; Tsuchimoto, Y.; Chen, D.W. Synthesis of 2,4-disubstituted thiazoles from (Z)-(2-acetoxyvinyl)phenyl-λ3-iodanes: Nucleophilic substitution of λ3-iodanyl ketones with thioureas and thioamides. J. Org. Chem. 2003, 68, 7887–7888. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, M.; Ishihara, H. Synthesis and applications of chalcogenoamide: Thio-, seleno- and telluroamides. Curr. Org. Synth. 2007, 4, 15–29. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Hojabri, L.; Dohendou, M. Microwave-assisted conversion of nitriles to thioamides in solvent-free condition. Syn. Commun. 2003, 33, 4279–4284. [Google Scholar] [CrossRef]
- Poupaert, J.; Duarte, S.; Colacino, E.; Depreux, P.; McCurdy, C.; Lambert, D. Willgerodt-kindler’s microwave-enhanced synthesis of thioamide derivatives. Phosphor. Sulfur Silicon 2004, 179, 1959–1973. [Google Scholar] [CrossRef]
- Liboska, R.; Zyka, D.; Bobek, M. Synthesis of primary thioamides from nitriles and hydrogen sulfide catalyzed by anion-exchange resin. Synthesis 2002, 1649–1651. [Google Scholar] [CrossRef]
- Manaka, A.; Sato, M. Synthesis of aromatic thioamide from nitrile without handling of gaseous hydrogen sulfide. Syn. Commun. 2005, 35, 761–764. [Google Scholar] [CrossRef]
- Begley, M.C.; Chapaneri, K.; Eleanor, G.; Merritt, A. Simple microwave-assisted method for the synthesis of primary thioamides from nitriles. Synlett 2004, 2615–2617. [Google Scholar] [CrossRef]
- Kaboudin, B.; Elhamifar, D. Phosphorus pentasulfide: A mild and versatile reagent for the preparation of thioamides from nitriles. Synthesis 2006, 224–226. [Google Scholar] [CrossRef]
- Wang, X.J.; Huang, Z.T. Synthesis and spectral and structural characteristics of cyano substituted heterocyclic ketene aminals. Acta. Chim. Sin. 1989, 47, 890–895. [Google Scholar]
- SAINTPlus v6.2—Data Reduction and Correction Program; Bruker AXS, Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SADABS v2.03—Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS, Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXTL v6.12—Structure Determination Software Suite; Bruker AXS, Inc.: Madison, WI, USA, 2001. [Google Scholar]
Sample Availability: Not available. |
| Entry | R1 | R2 | Substrate | α-Bromocarbonyl | Time, h | Yield, % |
|---|---|---|---|---|---|---|
| 1 | H | CO2Et | 5a | Ethyl bromopyruvate | 2 | 71 |
| 2 | H | CH3 | 5b | Bromoacetone | 2 | 73 |
| 3 | H | C6H5 | 5c | Phenacyl bromide | 2 | 79 |
| 4 | H | p-NO2C6H4 | 5d | p-Nitrobromoacetophenone | 2 | 82 |
| 5 | COCH3 | CH3 | 5e | 3-Bromo acetylacetone | 2 | 77 |
| 6 | CO2Et | CH3 | 5f | Ethyl 2-bromoacetoacetate | 2 | 77 |
| 7 | - | - | 5g | Ethyl bromoacetate | 8 | 75 |
| Empirical formula | C11H11N3O2S2 |
| Formula weight | 281.35 |
| Temperature | 100(2) K |
| Crystal system | Monoclinic |
| Crystal size | 0.30 × 0.08 × 0.04 mm3 |
| Space group | P21/n |
| a | 14.9127(16) Å |
| b c | 4.6129(5) Å 18.658(2) Å |
| β | 106.175(2)° |
| V | 1232.7(2) Å3 |
| Z | 4 |
| Dcalc | 1.516 Mgm-3 |
| µ | 0.429 mm-1 |
| F(000) | 584 |
| θ range | 1.55 to 30.66° |
| Index ranges | -21<=h<=21, -6<=k<=6, -26<=l<=26 |
| Reflections collected | 15302 |
| Data/restraints/parameters | 3802/0/164 |
| Goodness-of-fit on F2 | 1.006 |
| Radiation | Mo Kα 0.71073 (λ, Å) |
| Independent reflections | 3802 [R(int) = 0.0473] |
| Completeness to theta = 30.66° | 99.2 % |
| Absorption correction | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on F2 |
| Final R indices [for 4865 rfln. with I>2σ(I)] | R1 = 0.0419, wR2 = 0.0947 |
| R indices (all data) | R1 = 0.0588, wR2 = 0.1022 |
| Largest diff. peak and hole | 0.368 and -0.257 e.Å-3 |
| S(1)-C(1) | 1.7507(17) | C(1)-S(1)-C(3) | 90.58(8) |
| S(1)-C(3) | 1.8222(19) | C(7)-S(2)-C(5) | 89.28(8) |
| S(2)-C(7) | 1.7141(18) | C(8)-O(2)-C(9) | 115.60(14) |
| S(2)-C(5) | 1.7459(17) | C(1)-N(1)-C(2) | 115.26(14) |
| O(1)-C(8) | 1.207(2) | C(5)-N(2)-C(6) | 110.17(14) |
| O(2)-C(8) | 1.342(2) | N(1)-C(1)-C(4) | 125.56(15) |
| O(2)-C(9) | 1.462(2) | N(1)-C(1)-S(1) | 112.39(12) |
| N(1)-C(1) | 1.333(2) | C(4)-C(1)-S(1) | 122.04(13) |
| N(1)-C(2) | 1.463(2) | N(1)-C(2)-C(3) | 104.77(14) |
| N(2)-C(5) | 1.315(2) | C(2)-C(3)-S(1) | 104.89(12) |
| N(2)-C(6) | 1.382(2) | C(1)-C(4)-C(11) | 119.36(15) |
| N(3)-C(11) | 1.153(2) | C(1)-C(4)-C(5) | 121.88(15) |
| C(1)-C(4) | 1.385(2) | C(11)-C(4)-C(5) | 118.75(15) |
| C(2)-C(3) | 1.522(3) | N(2)-C(5)-C(4) | 125.14(15) |
| C(4)-C(11) | 1.418(2) | N(2)-C(5)-S(2) | 114.32(12) |
| C(4)-C(5) | 1.443(2) | C(4)-C(5)-S(2) | 120.53(13) |
| C(6)-C(7) | 1.358(2) | C(7)-C(6)-N(2) | 116.01(16) |
| C(6)-C(8) | 1.484(2) | C(7)-C(6)-C(8) | 125.49(16) |
| C(9)-C(10) | 1.506(3) | N(2)-C(6)-C(8) | 118.41(15) |
| C(6)-C(7)-S(2) | 110.22(13) | ||
| O(1)-C(8)-O(2) | 124.65(16) | ||
| O(1)-C(8)-C(6) | 124.04(16) | ||
| O(2)-C(8)-C(6) | 111.30(15) | ||
| O(2)-C(9)-C(10) | 107.40(15) | ||
| N(3)-C(11)-C(4) | 179.5(2) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bakavoli, M.; Beyzaie, H.; Rahimizadeh, M.; Eshghi, H.; Takjoo, R. Regioselective Synthesis of New 2-(E)-Cyano(thiazolidin-2-ylidene)thiazoles. Molecules 2009, 14, 4849-4857. https://doi.org/10.3390/molecules14114849
Bakavoli M, Beyzaie H, Rahimizadeh M, Eshghi H, Takjoo R. Regioselective Synthesis of New 2-(E)-Cyano(thiazolidin-2-ylidene)thiazoles. Molecules. 2009; 14(12):4849-4857. https://doi.org/10.3390/molecules14114849
Chicago/Turabian StyleBakavoli, Mehdi, Hamid Beyzaie, Mohammad Rahimizadeh, Hossein Eshghi, and Reza Takjoo. 2009. "Regioselective Synthesis of New 2-(E)-Cyano(thiazolidin-2-ylidene)thiazoles" Molecules 14, no. 12: 4849-4857. https://doi.org/10.3390/molecules14114849
APA StyleBakavoli, M., Beyzaie, H., Rahimizadeh, M., Eshghi, H., & Takjoo, R. (2009). Regioselective Synthesis of New 2-(E)-Cyano(thiazolidin-2-ylidene)thiazoles. Molecules, 14(12), 4849-4857. https://doi.org/10.3390/molecules14114849




