Molecular Iodine-Mediated Cyclization of Tethered Heteroatom-Containing Alkenyl or Alkynyl Systems
Abstract
:1. Introduction
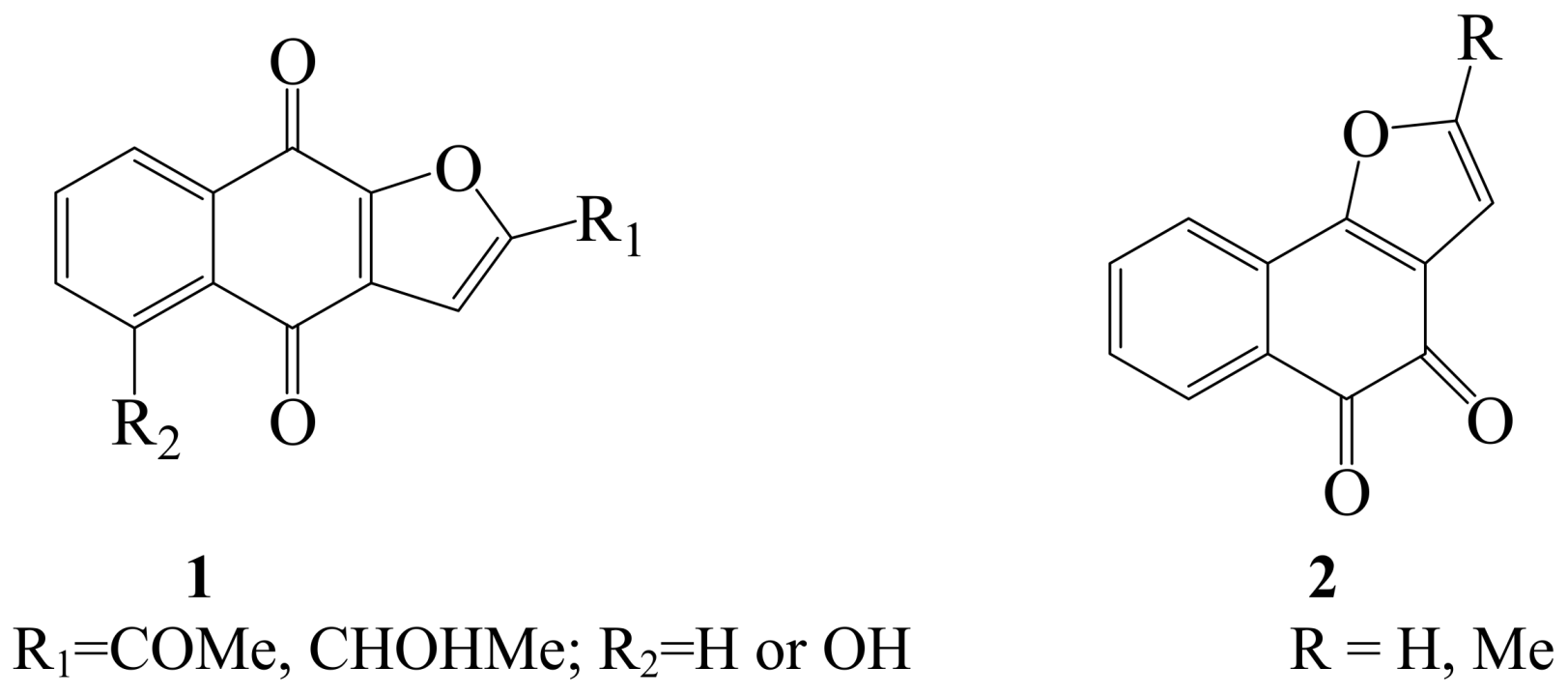
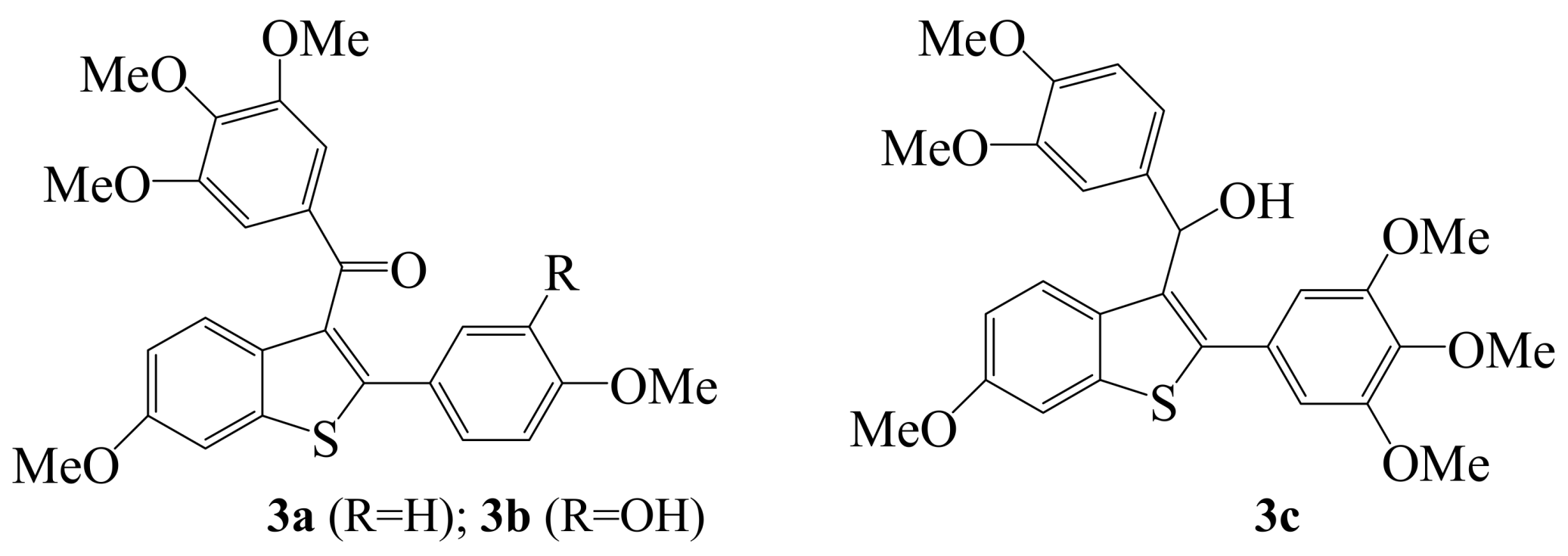
2. Iodine as an Electrophile in Cyclization Reactions
2.1. Iodine-promoted cyclization reactions
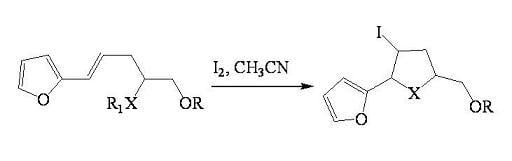
2.1.1. Iodocyclization of heteroatom-containing alkenyl derivatives
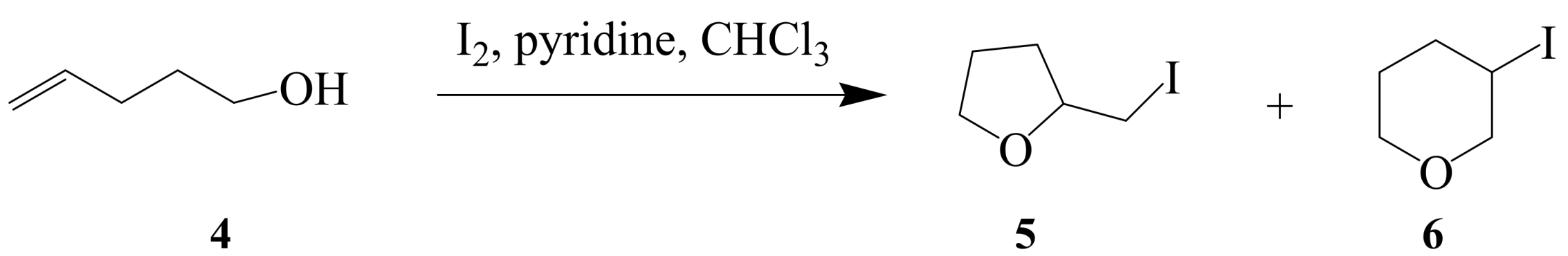
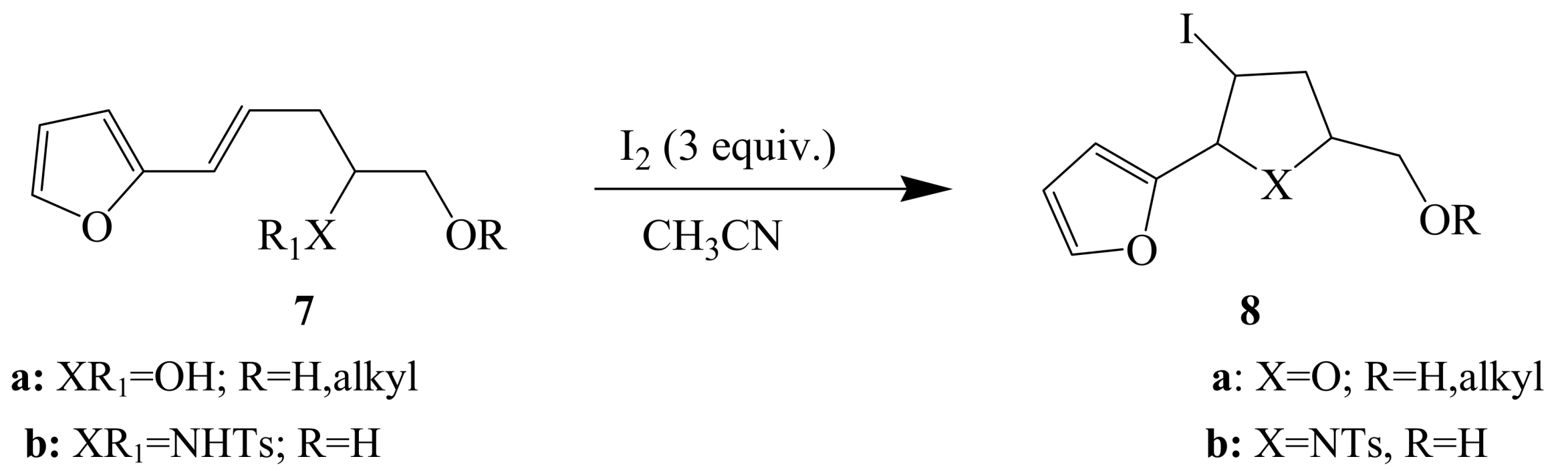
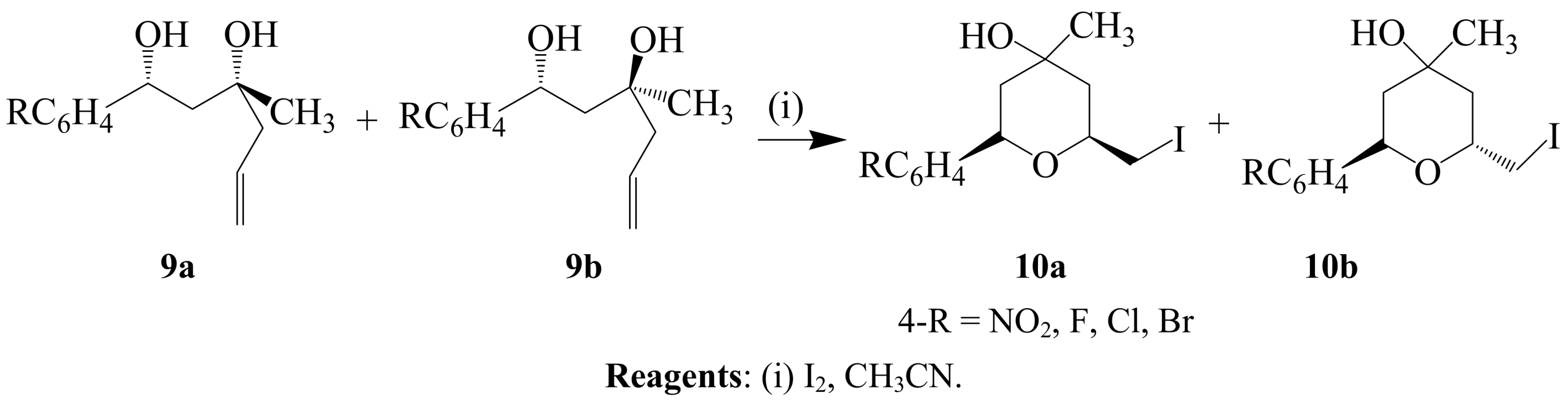
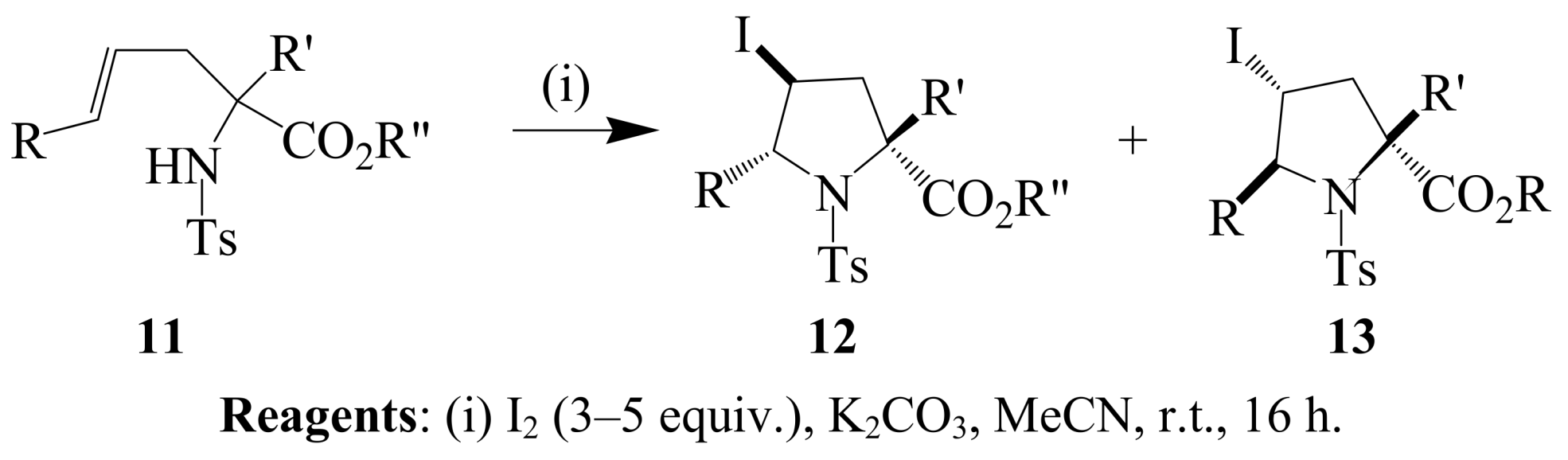
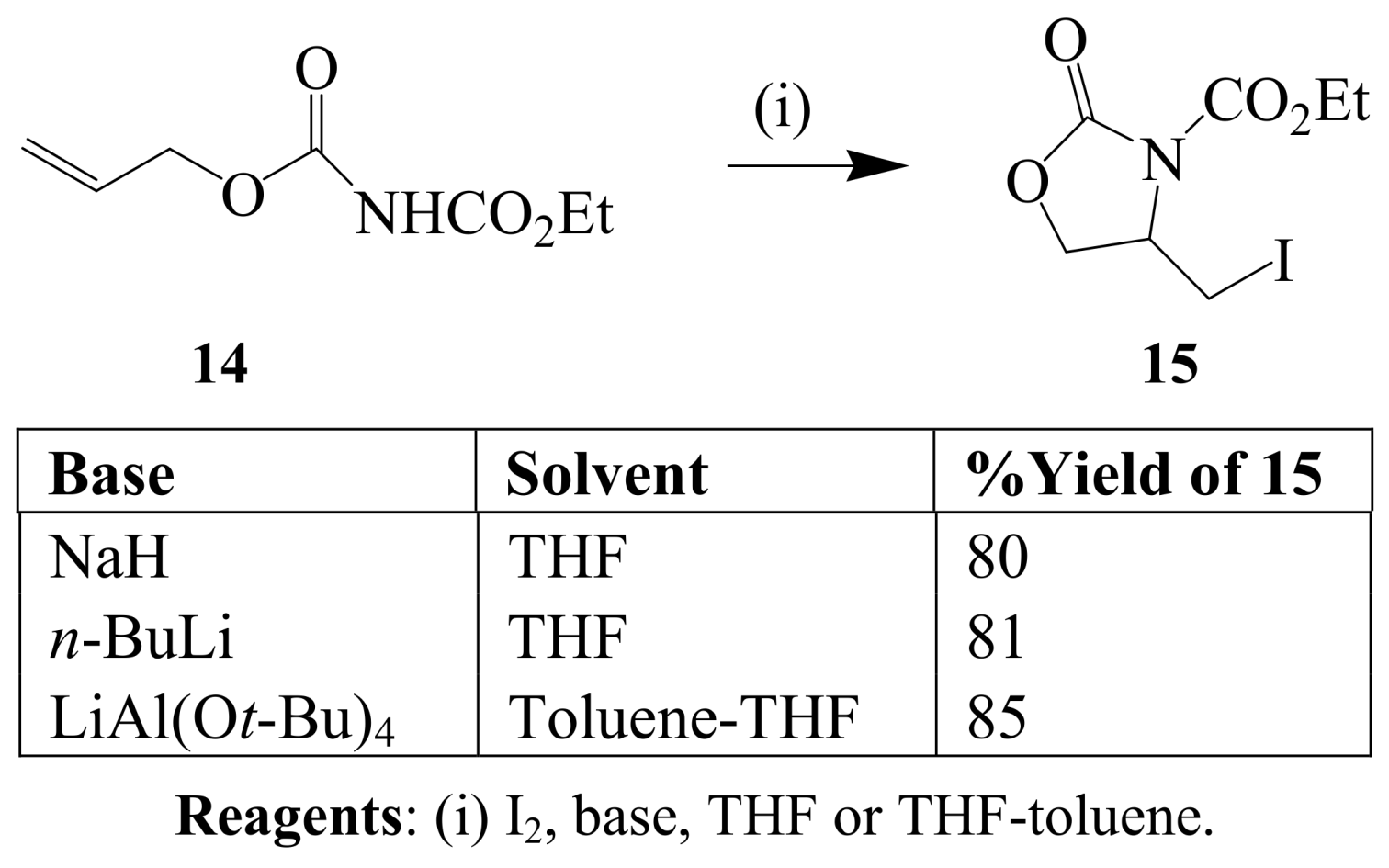
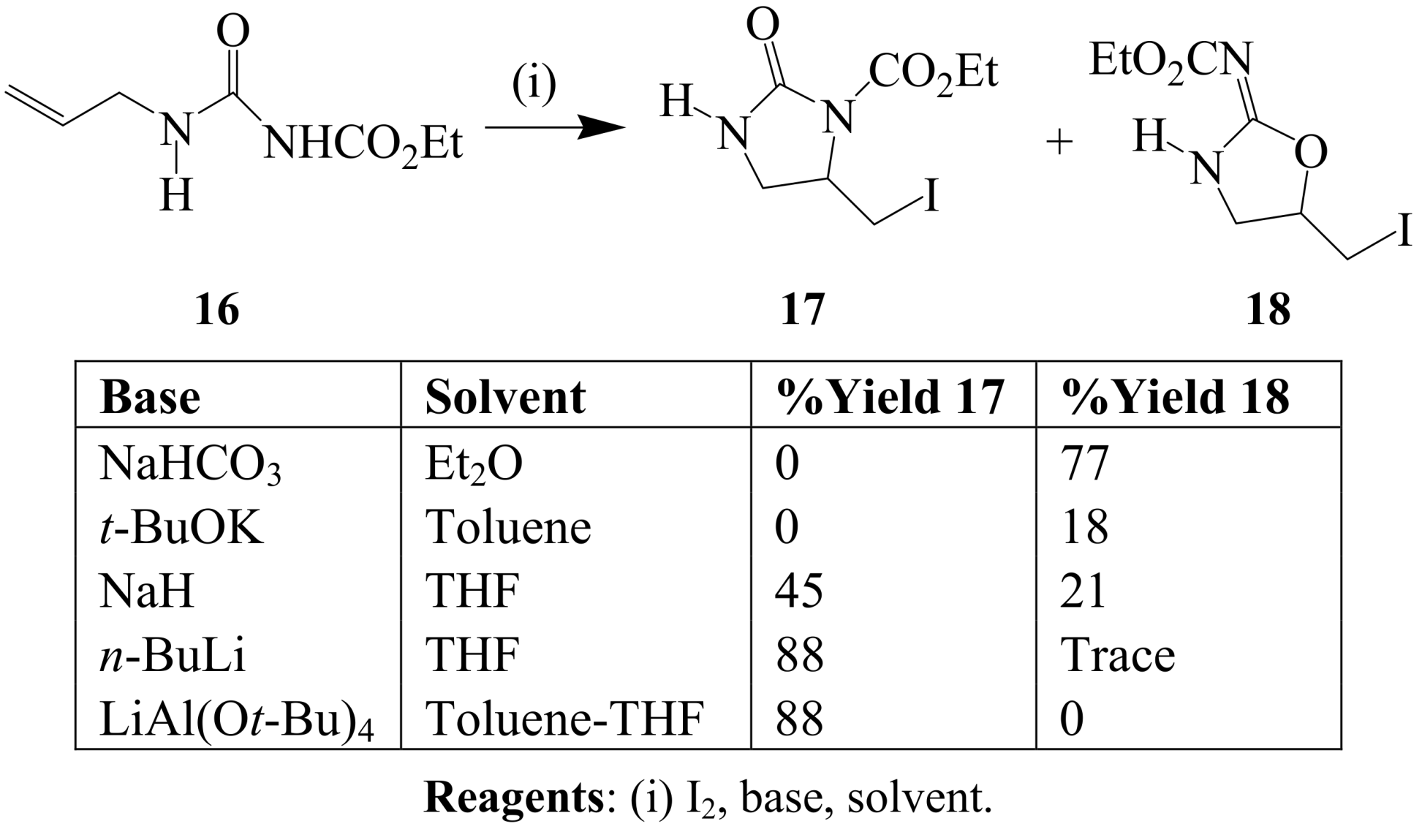
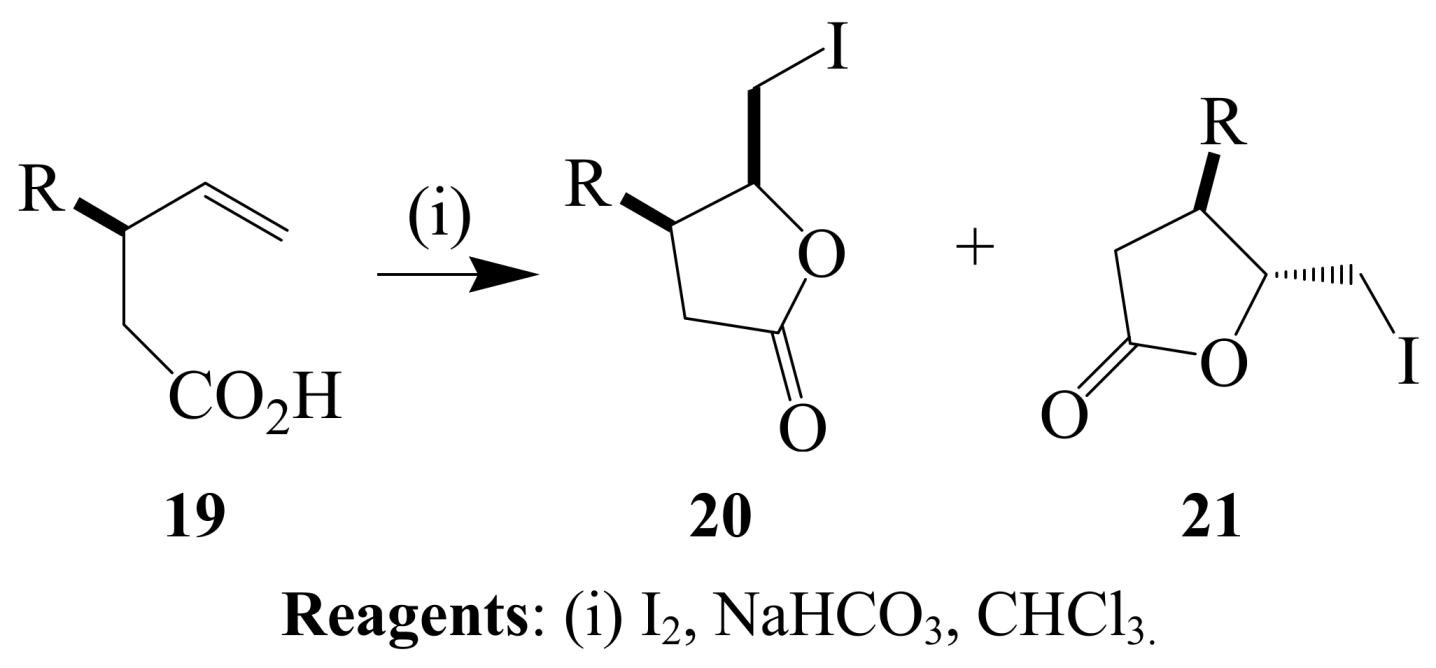
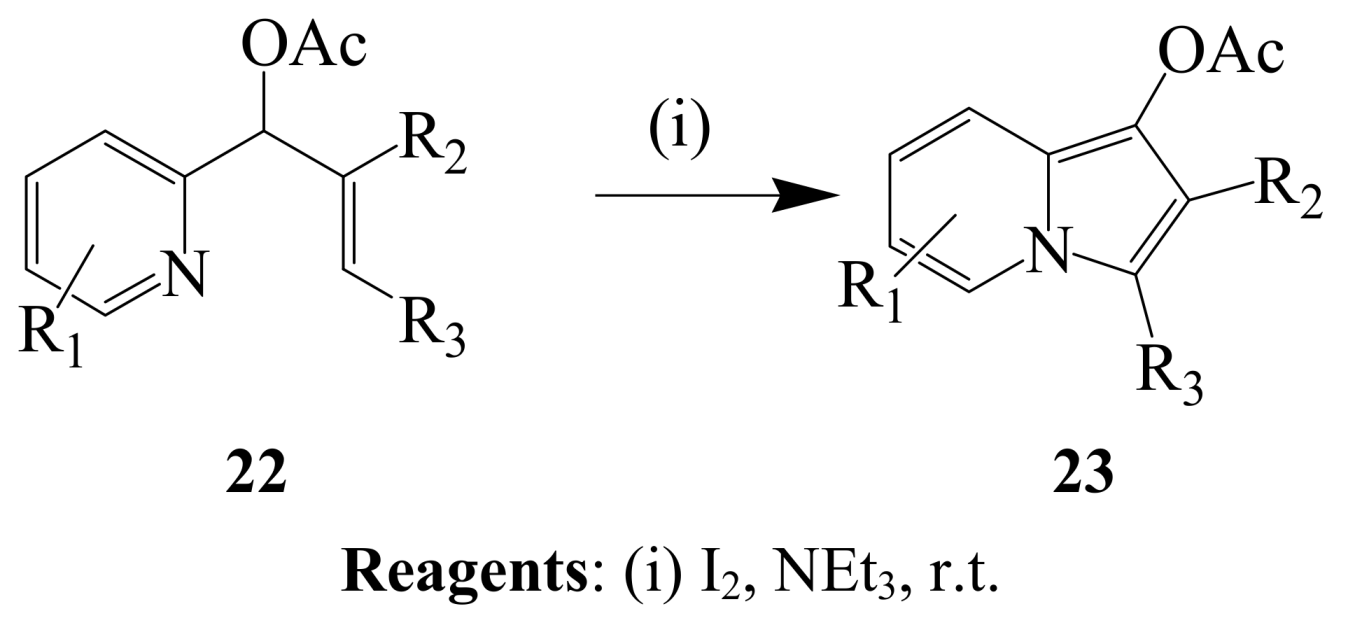
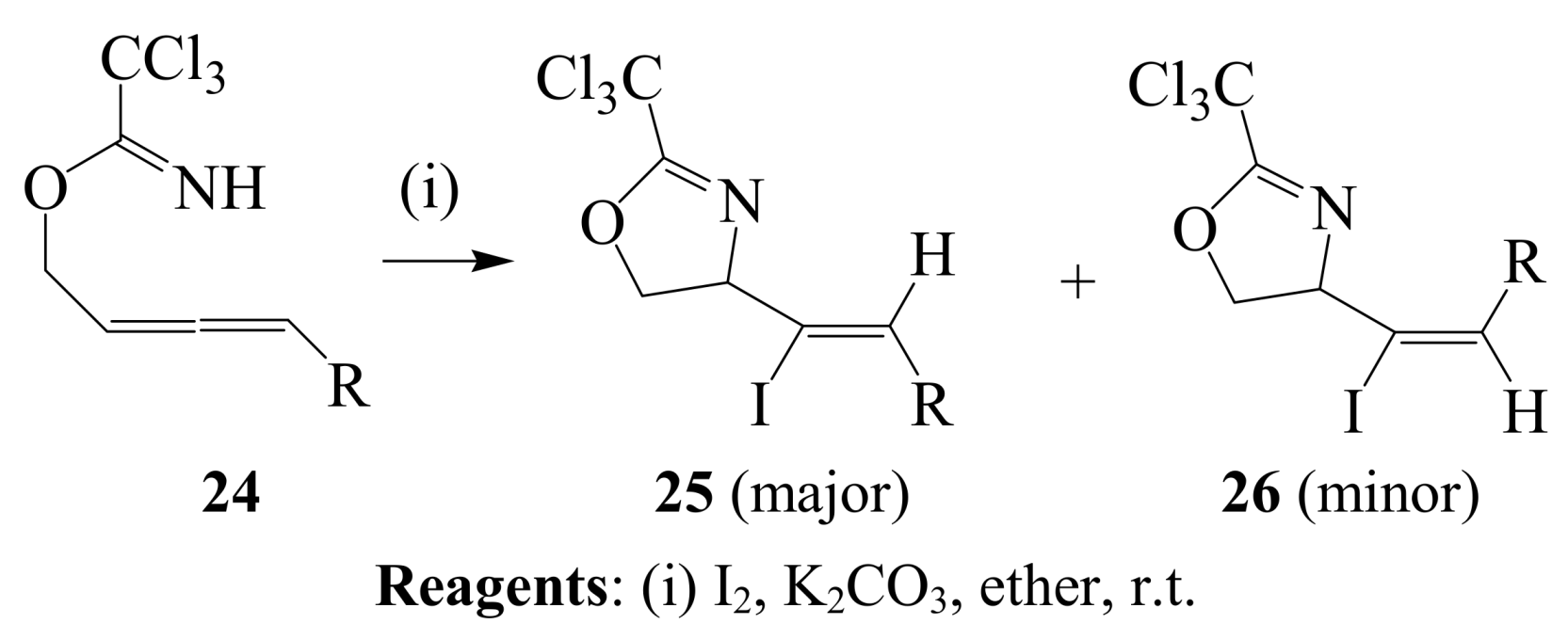
2.1.2. Iodocyclization of heteroatom-containing alkynyl and ynone derivatives
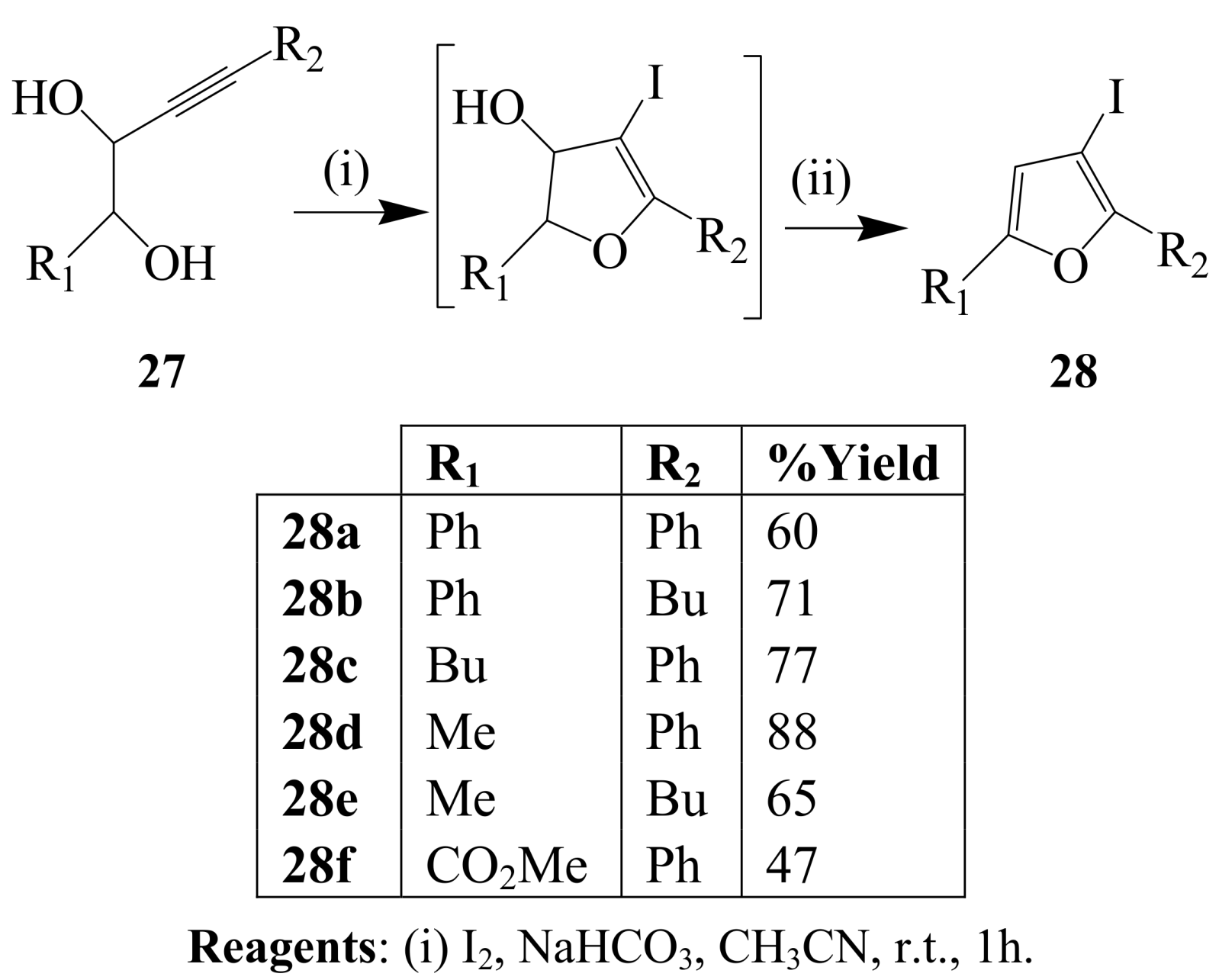
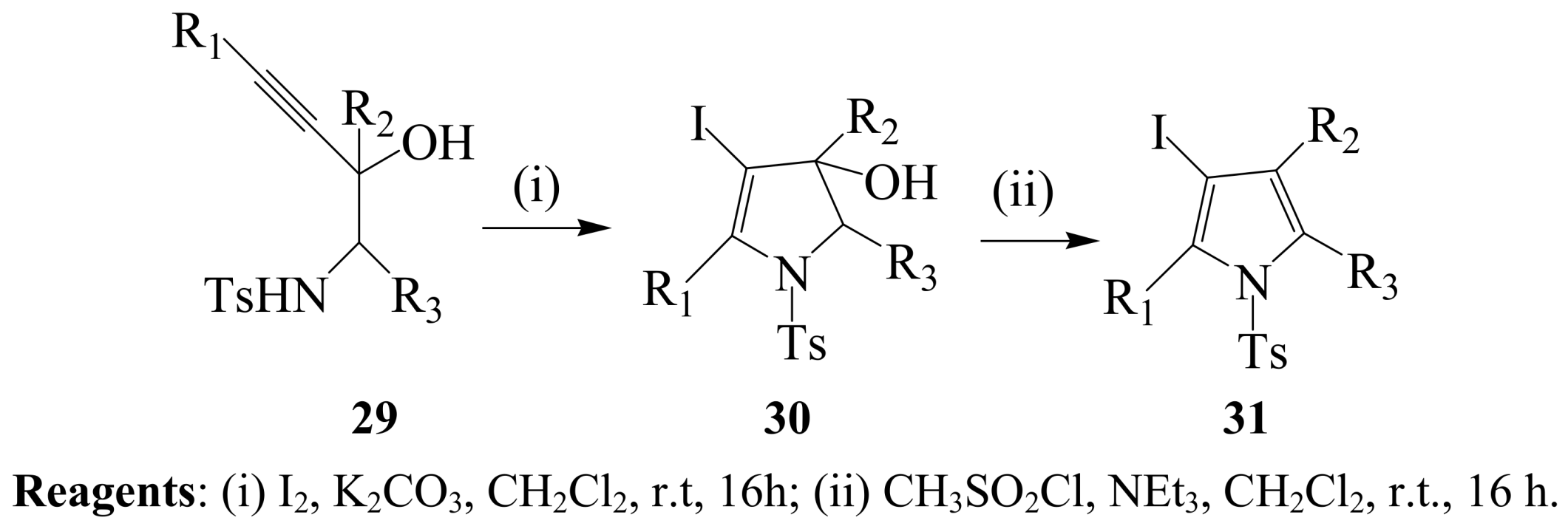
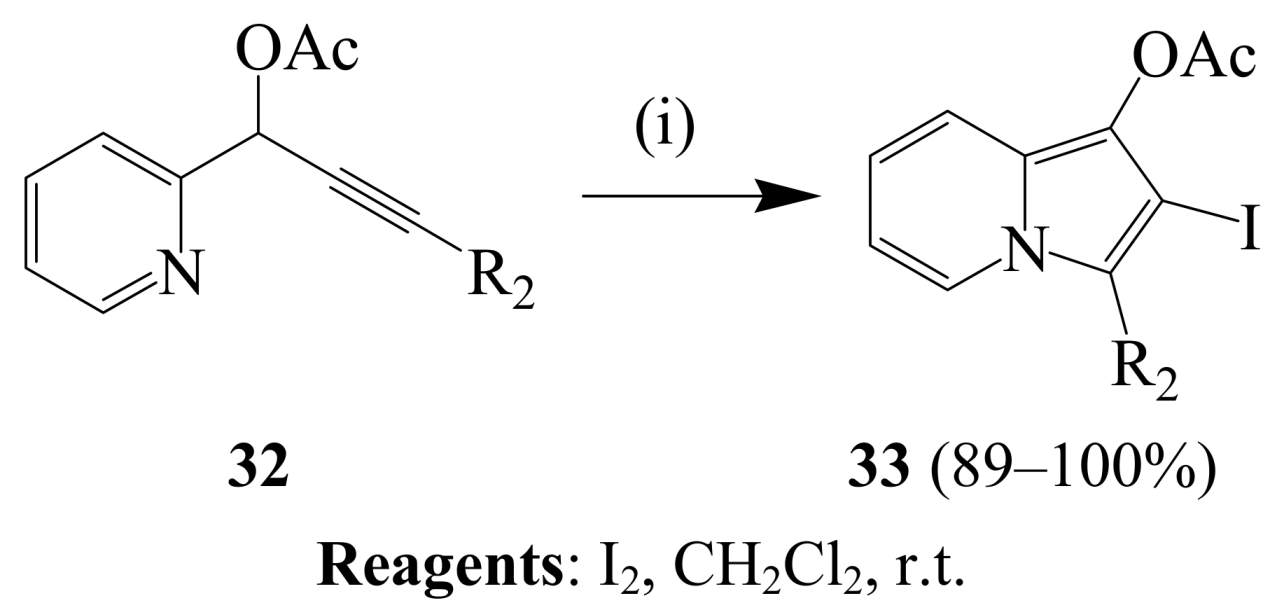
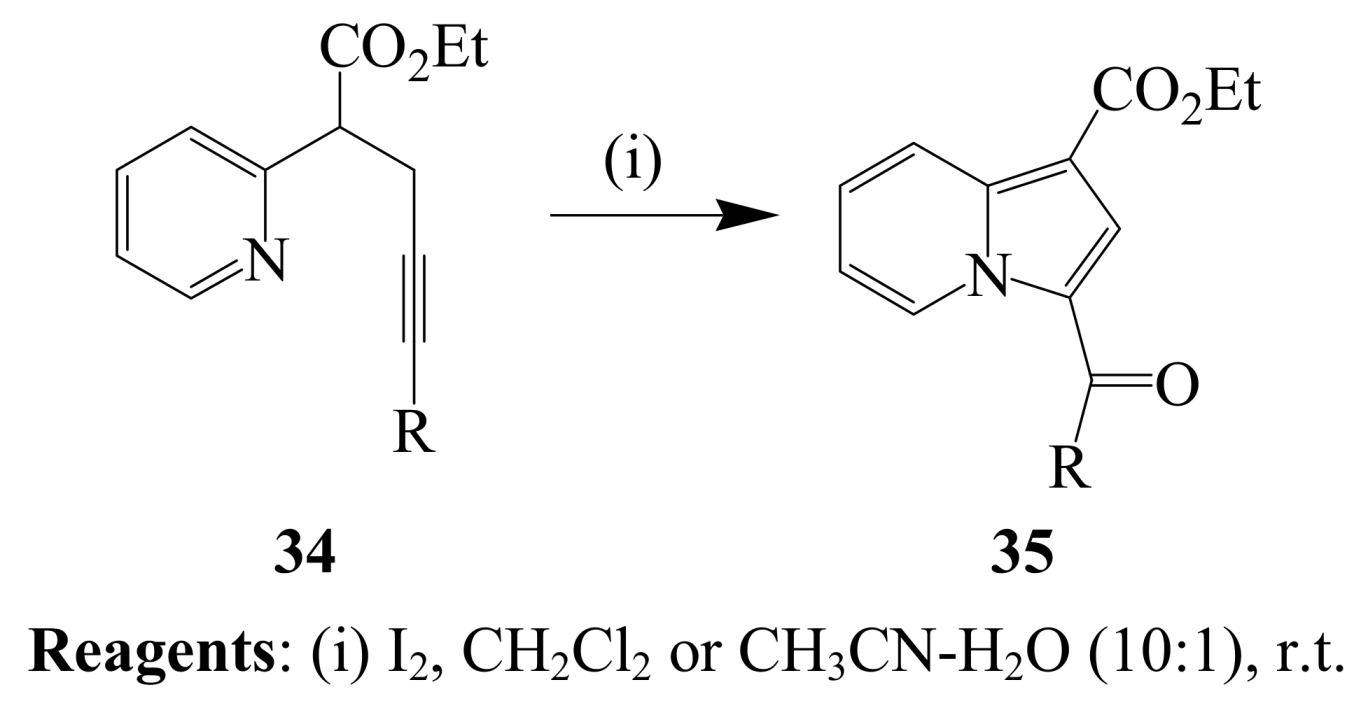
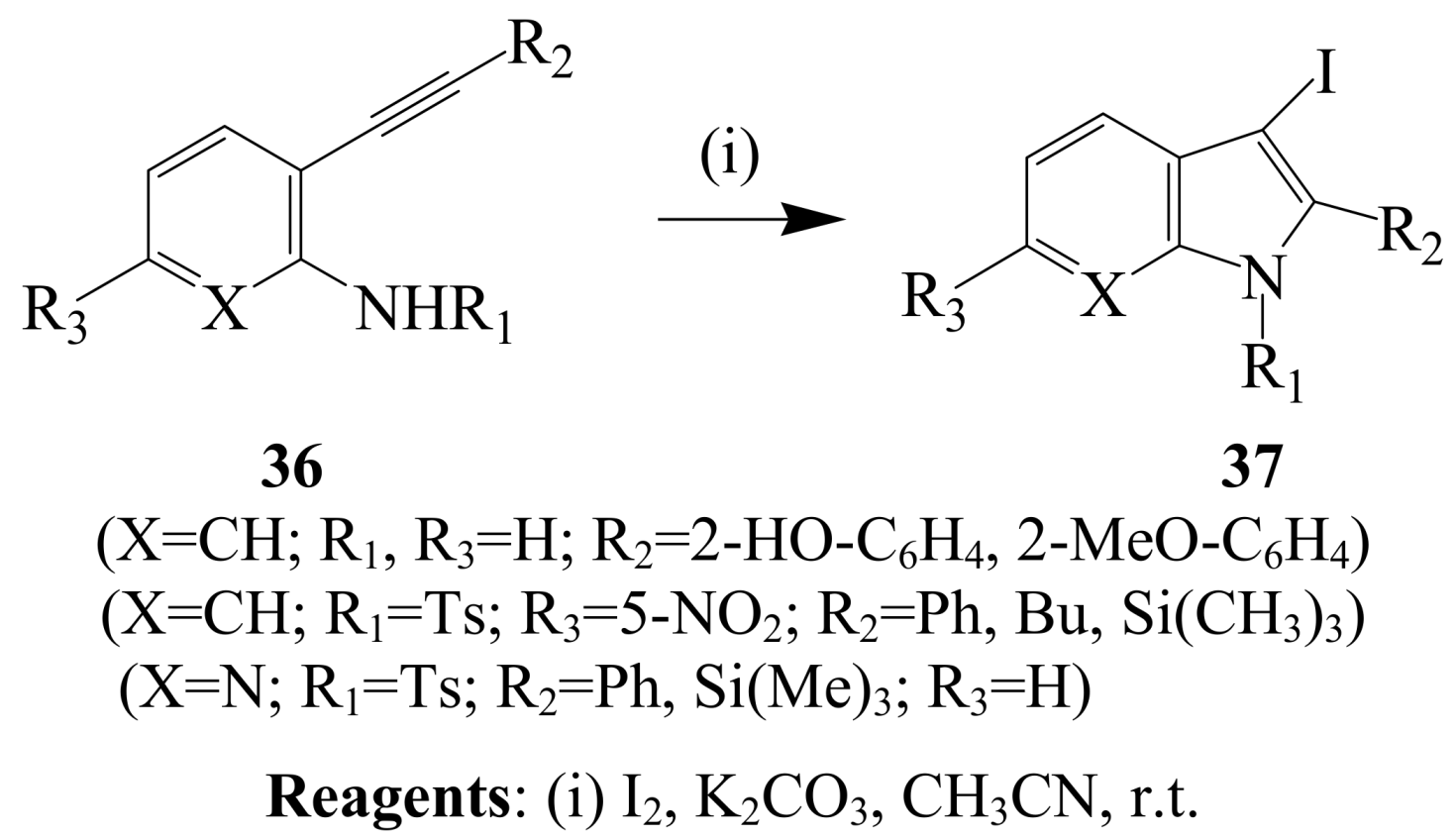
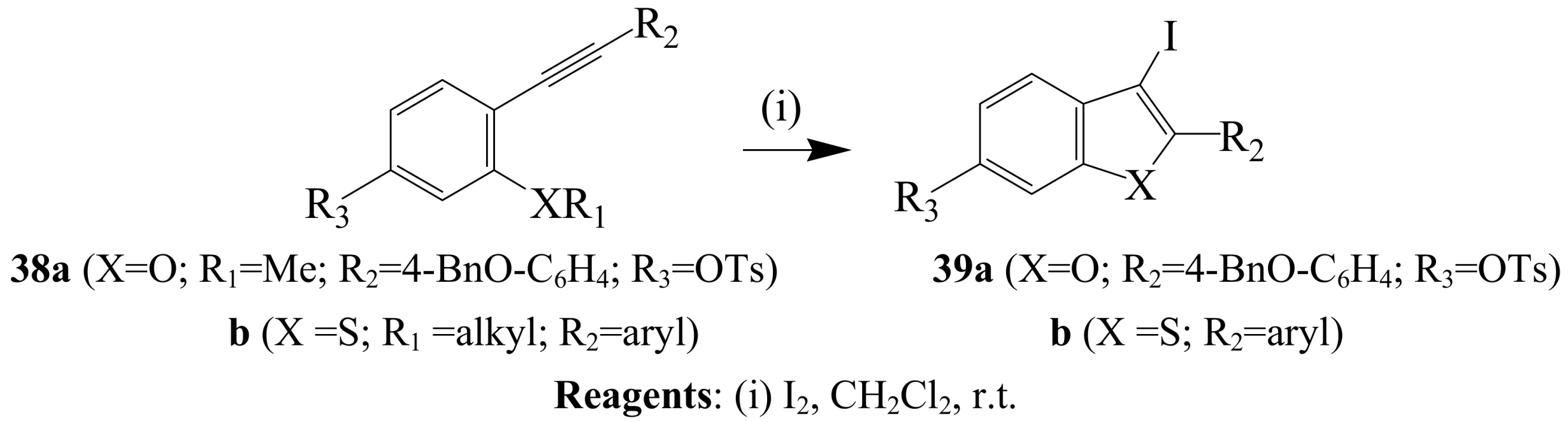
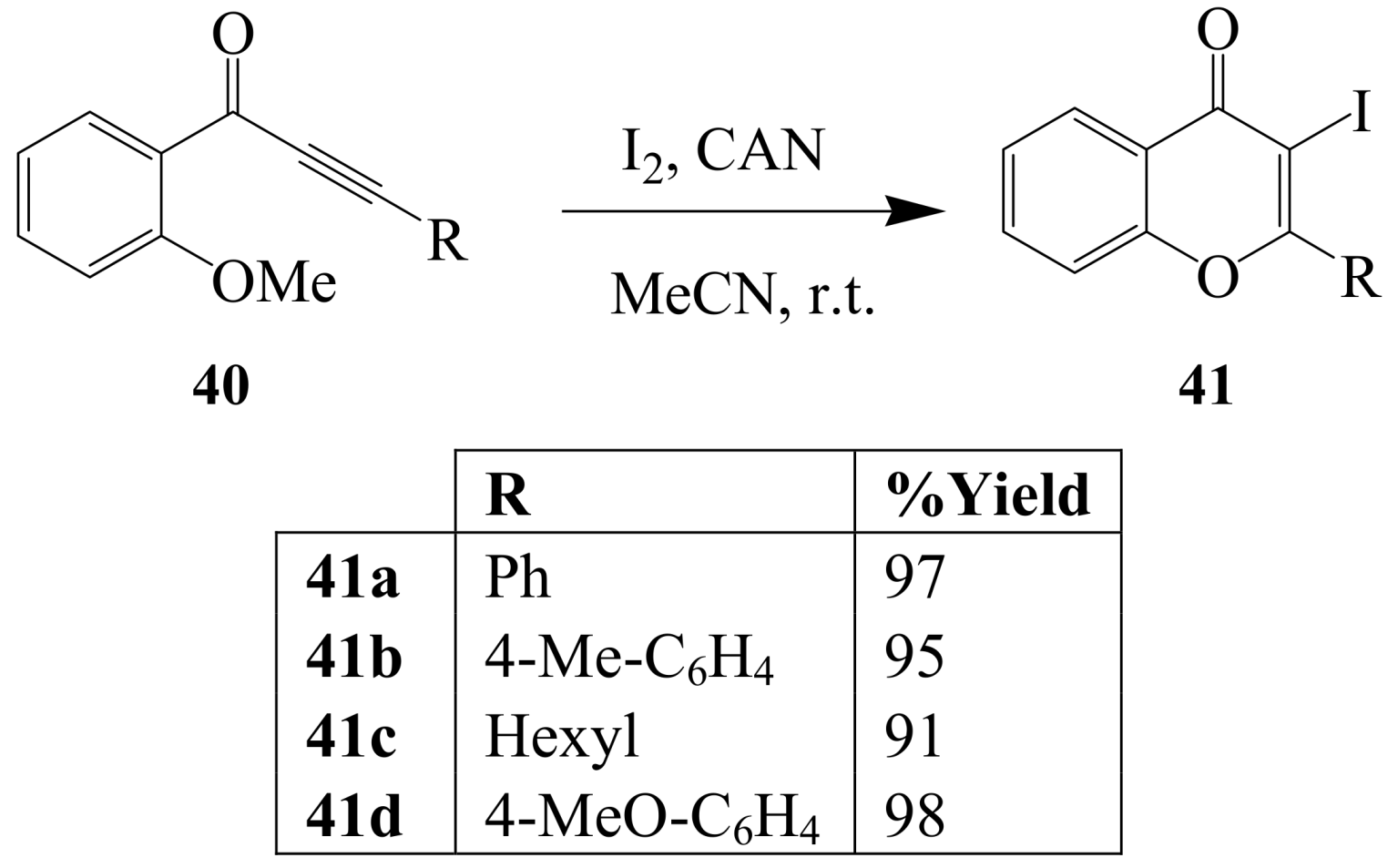
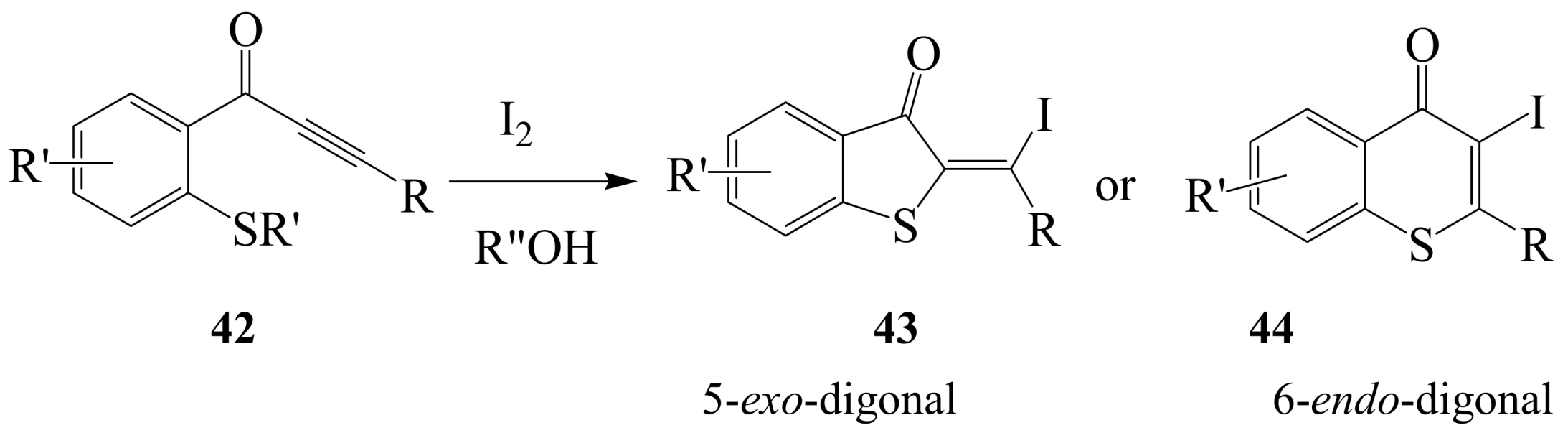
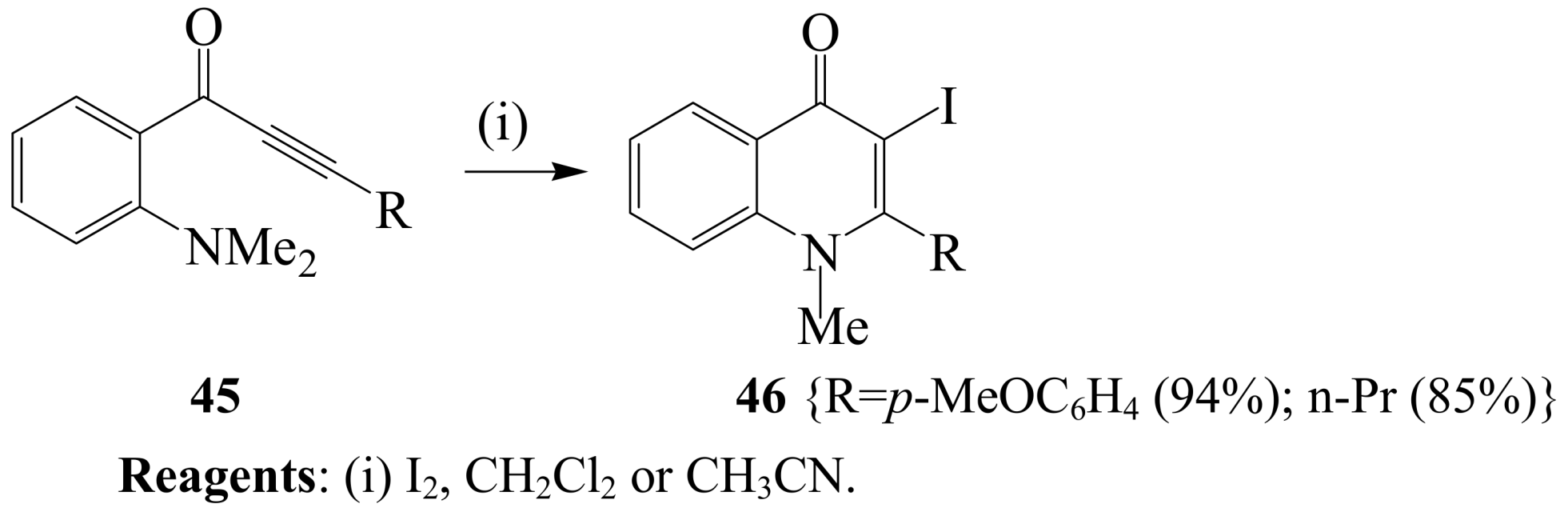
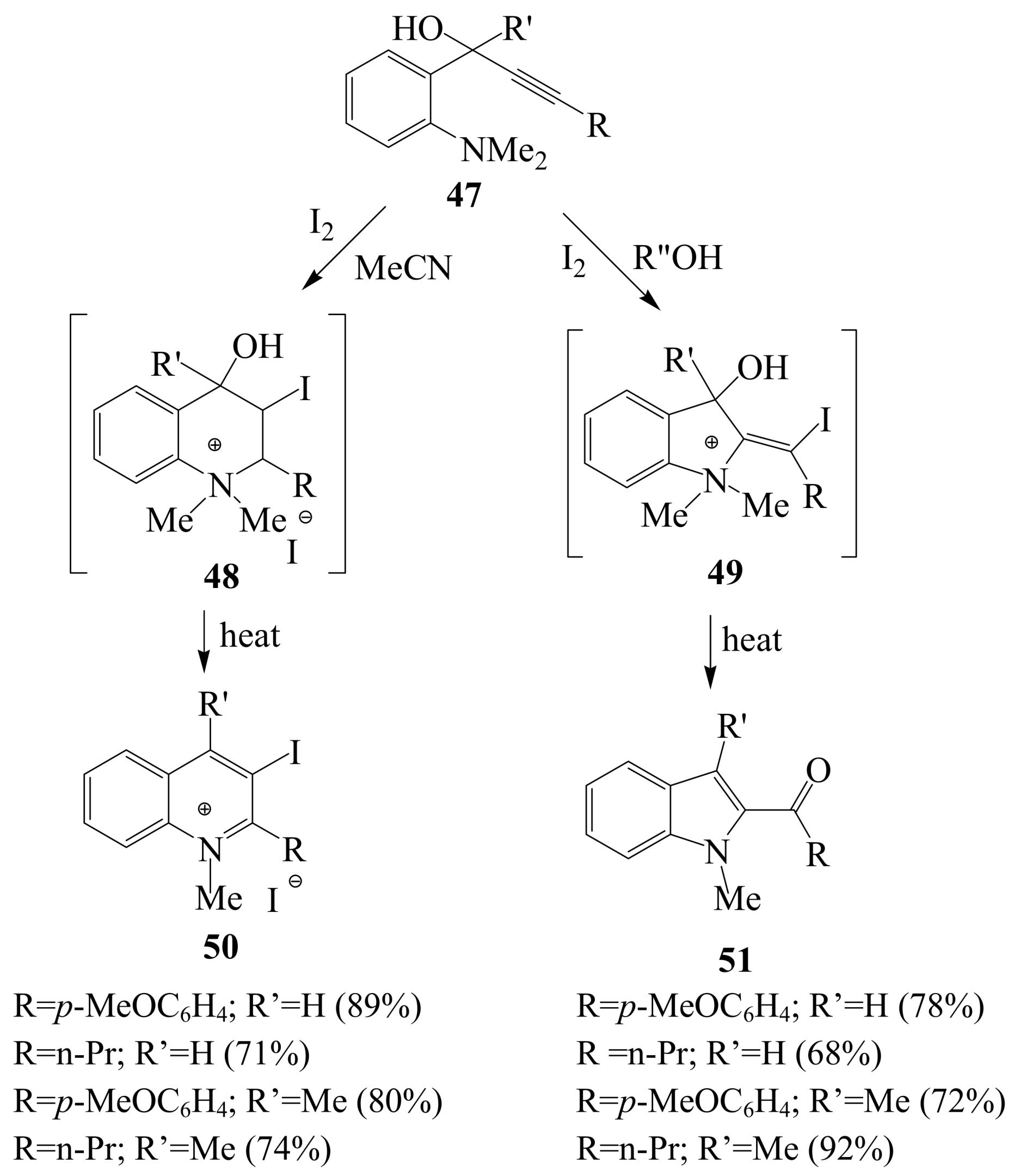
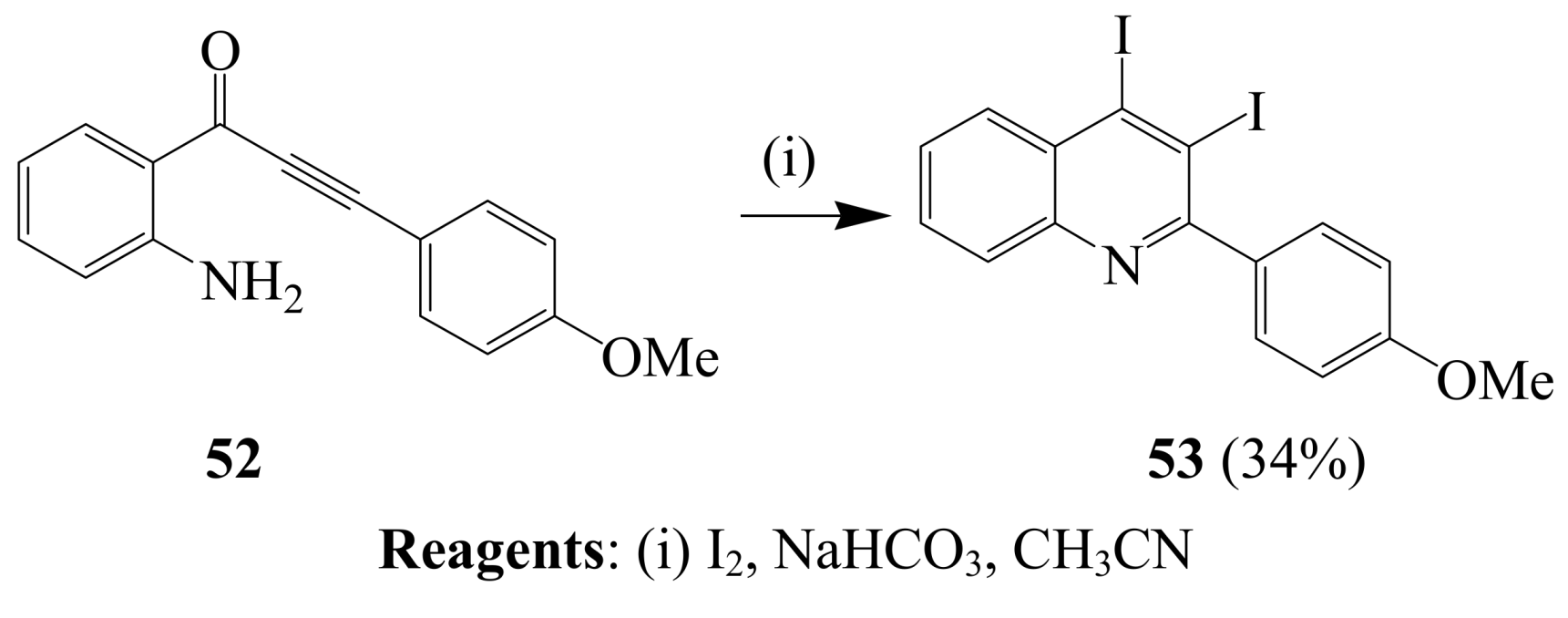
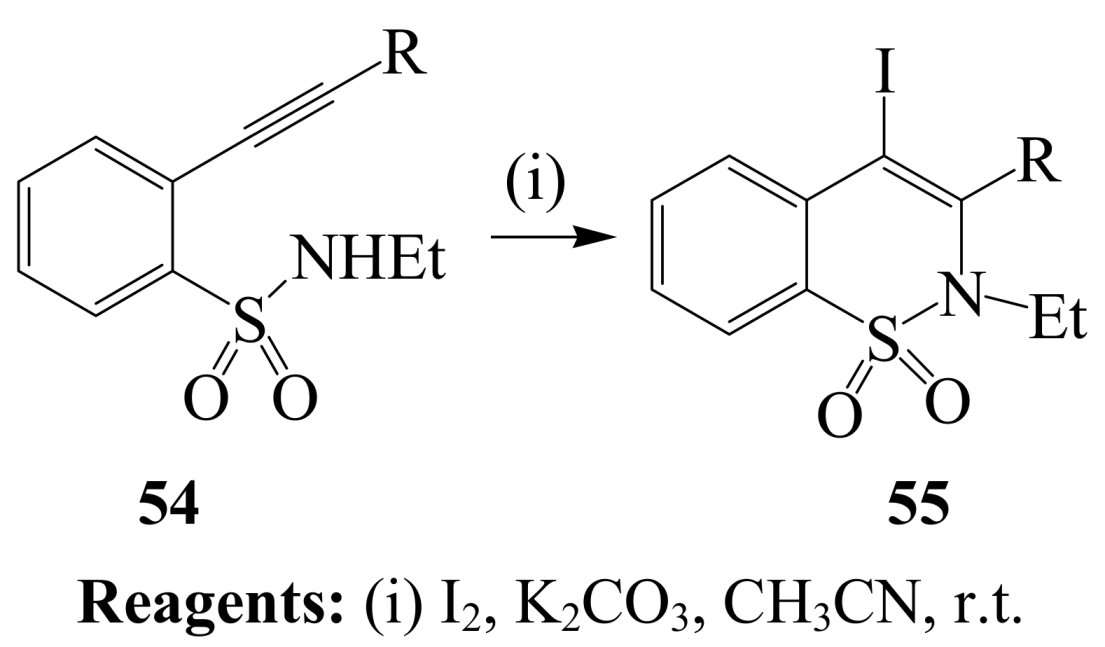
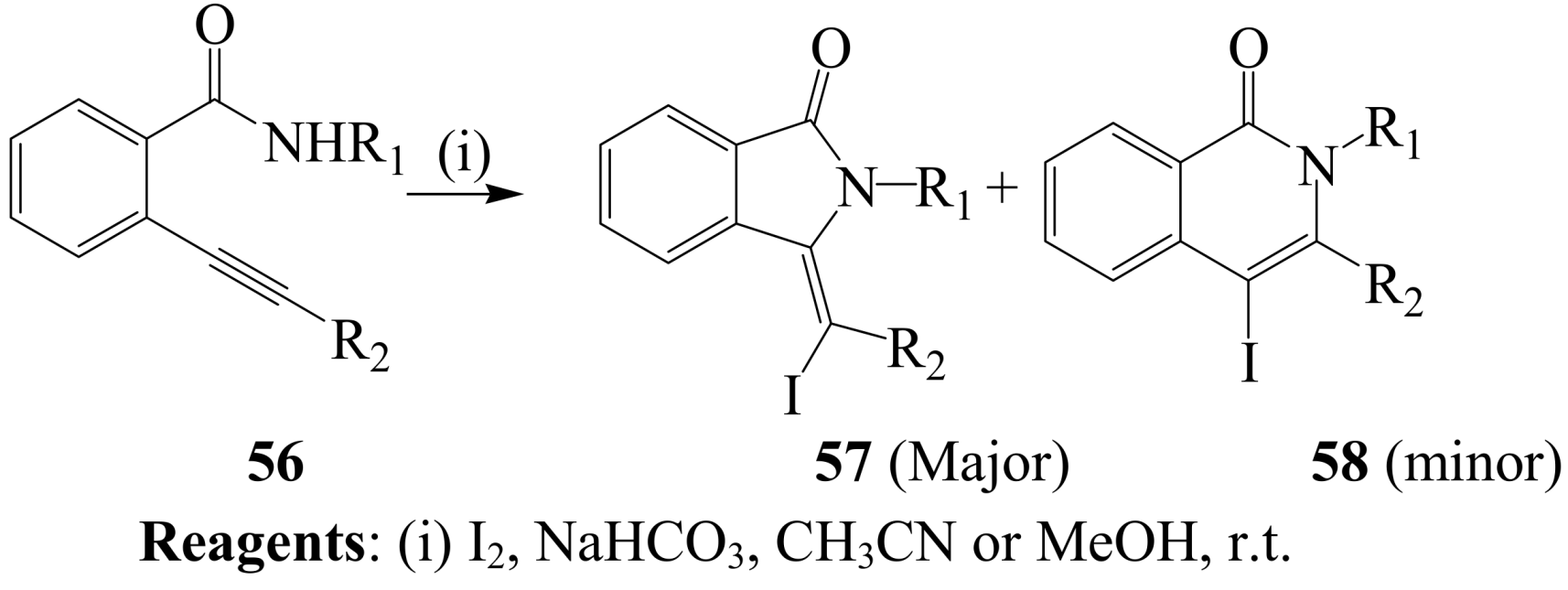
2.1.3. Iodocyclization of ortho-allylphenols
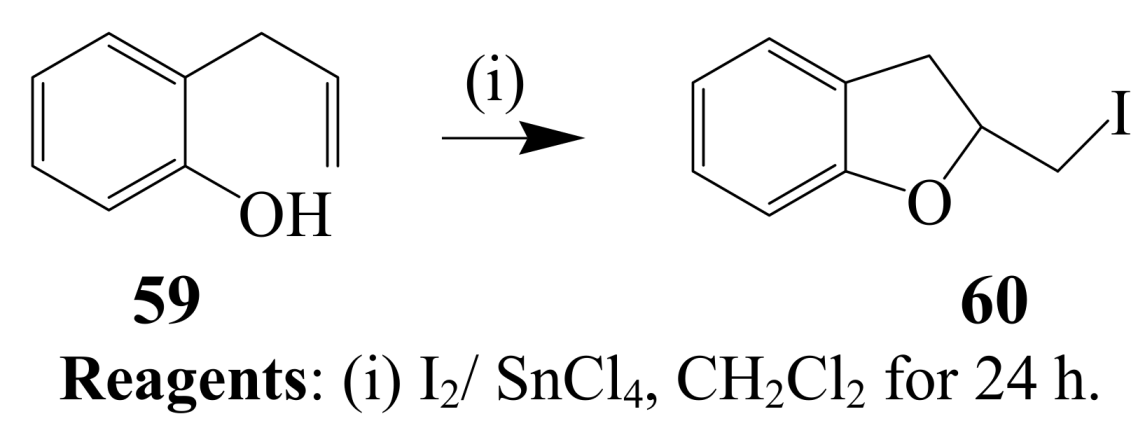
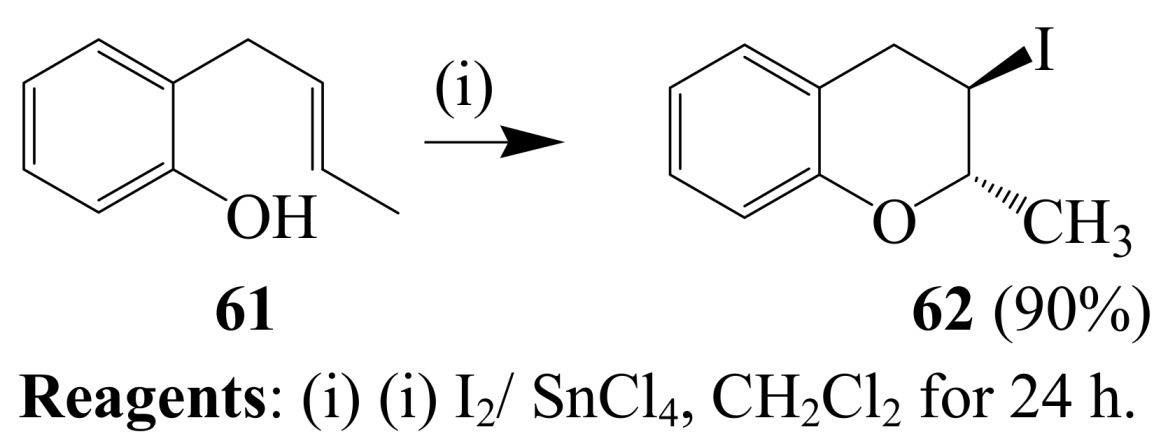
2.1.4. Iodocyclization of 2-allyl-1,3-dicarbonyl derivatives
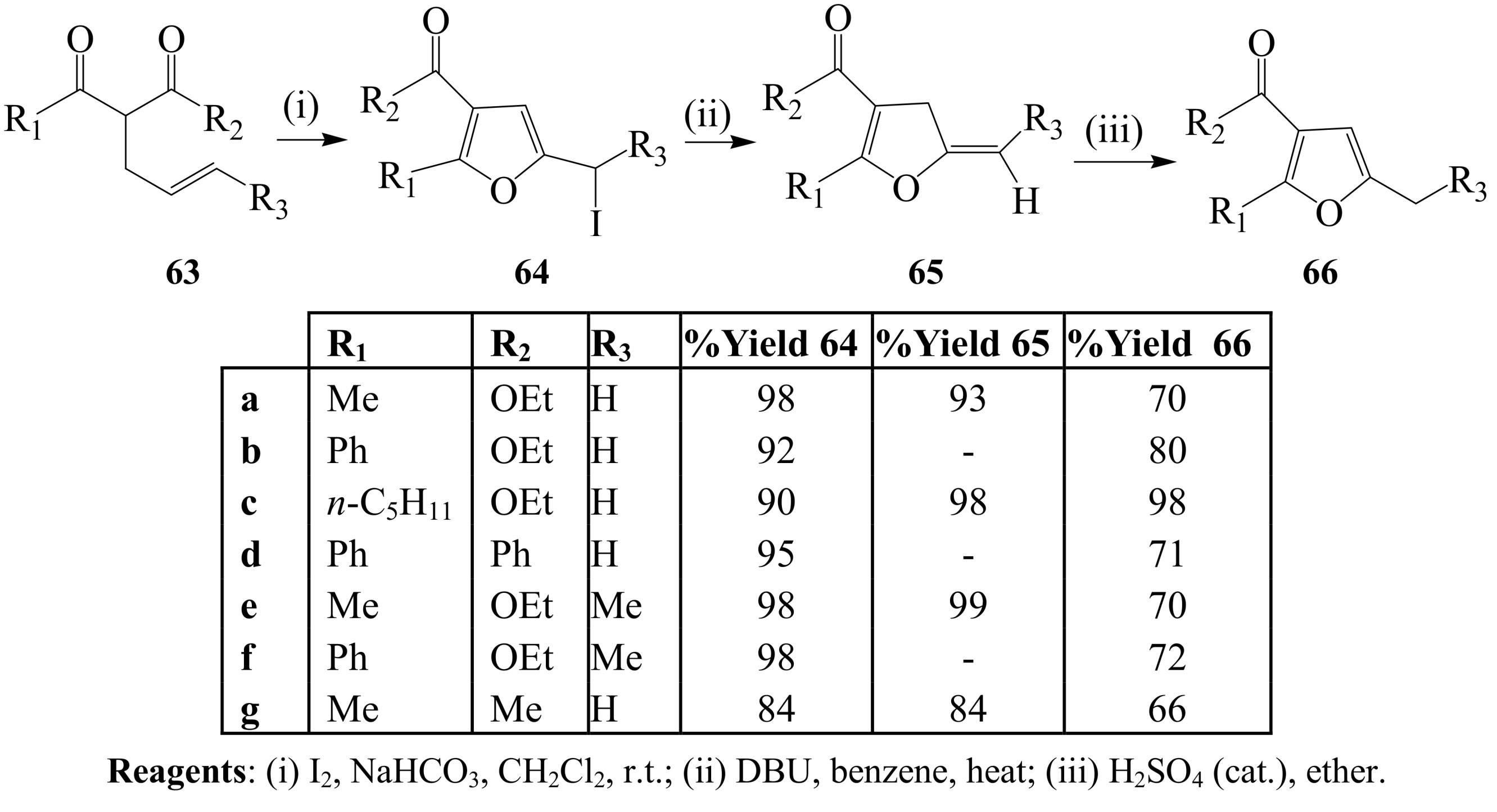
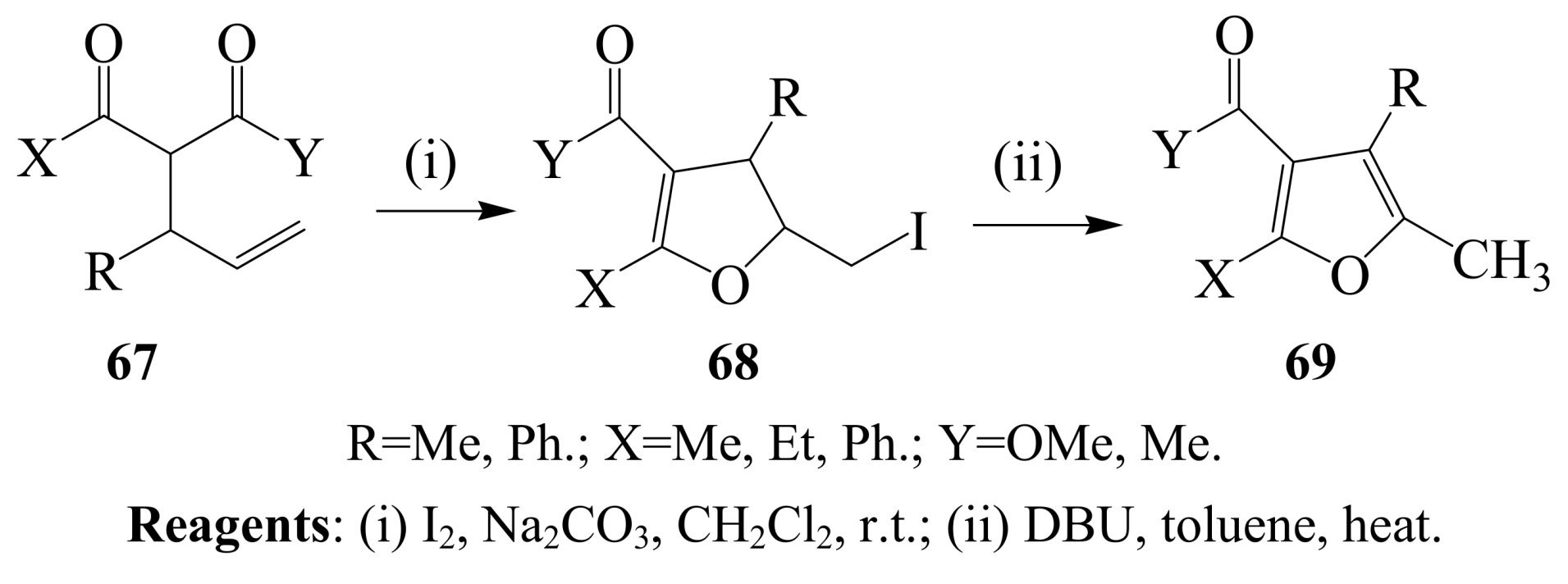
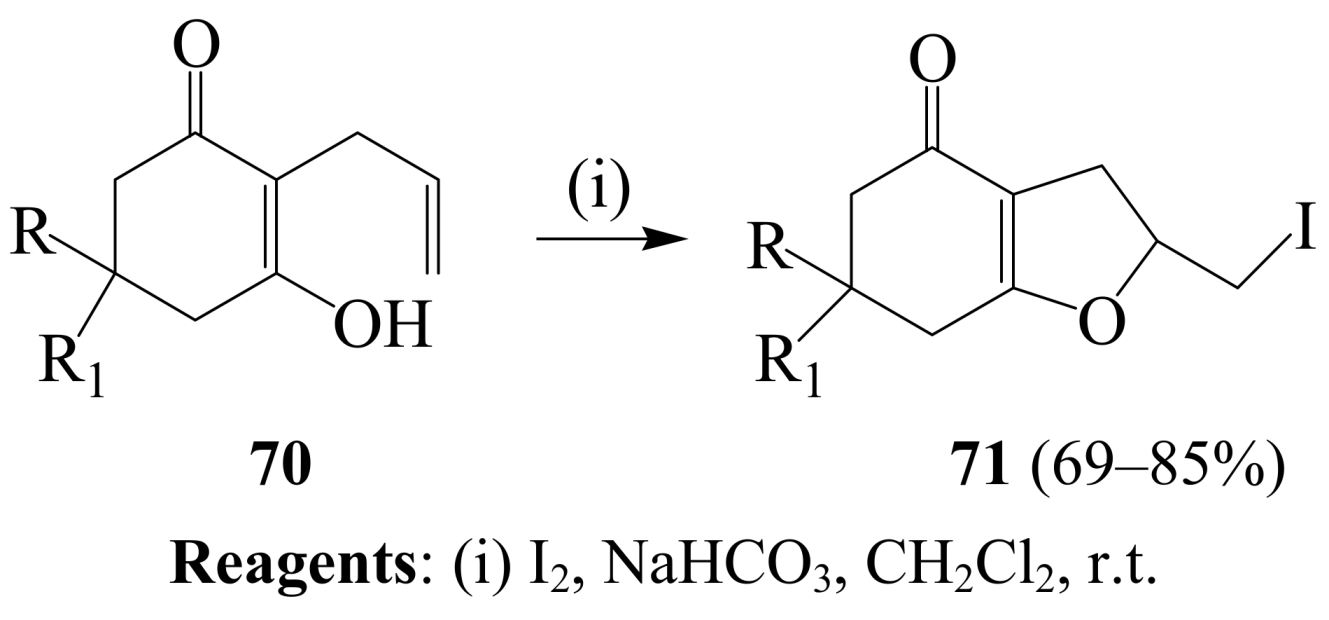
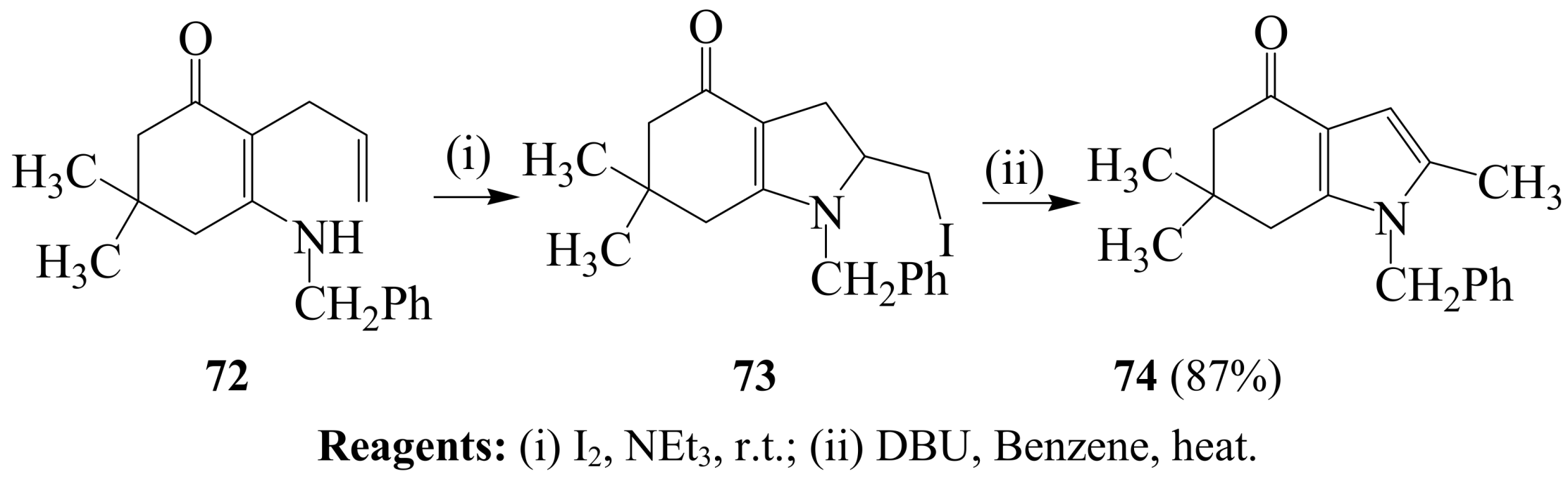
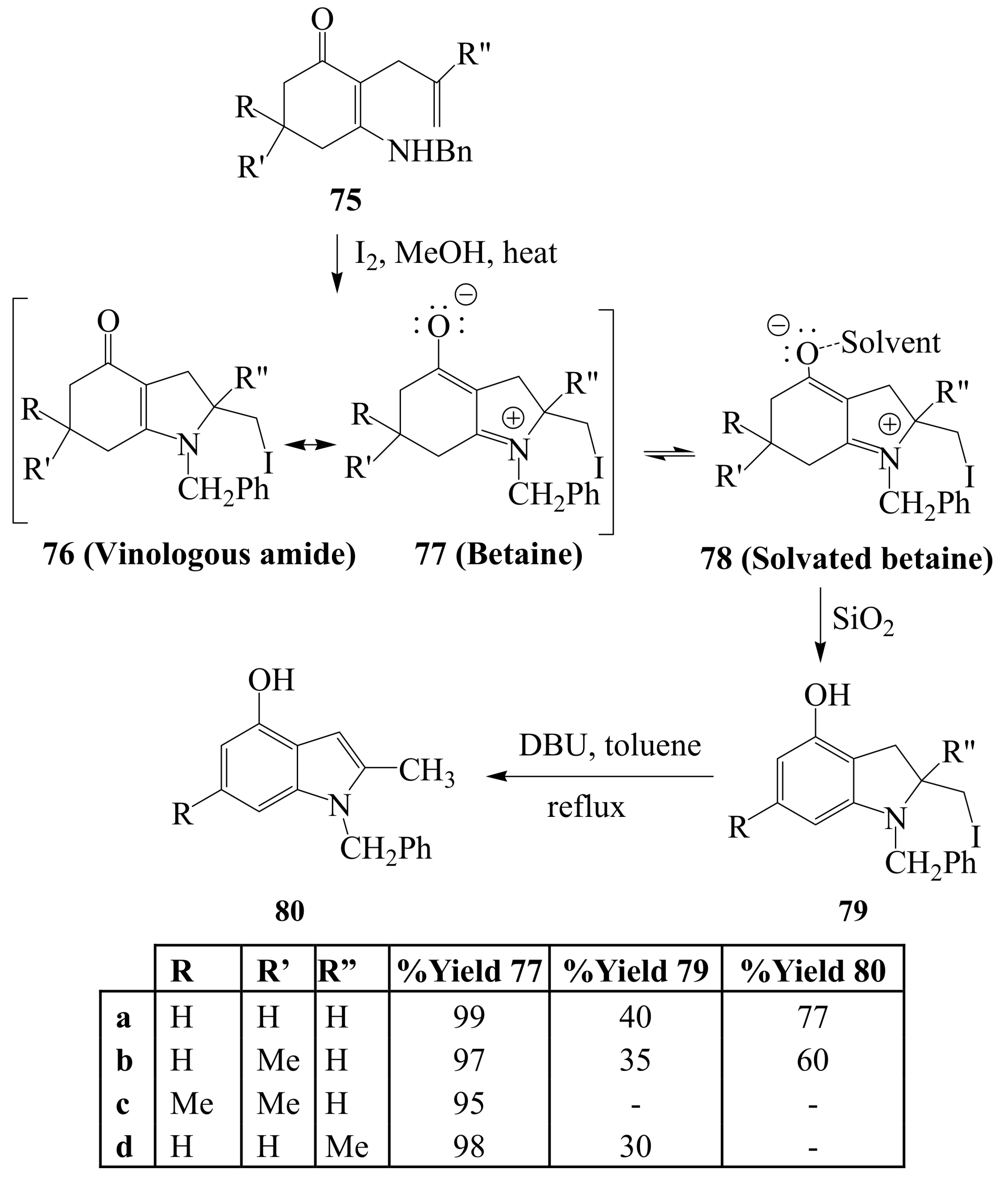
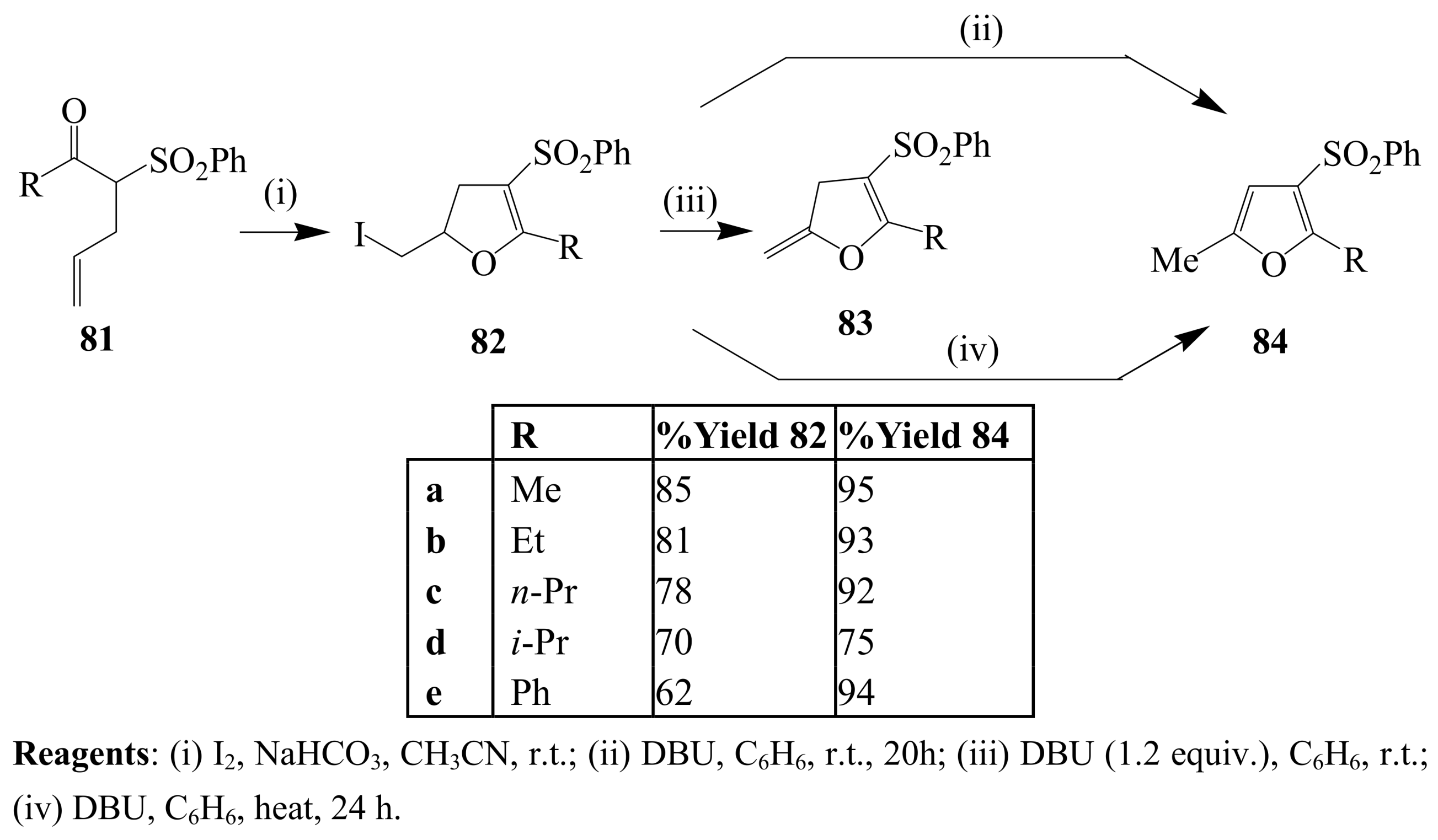
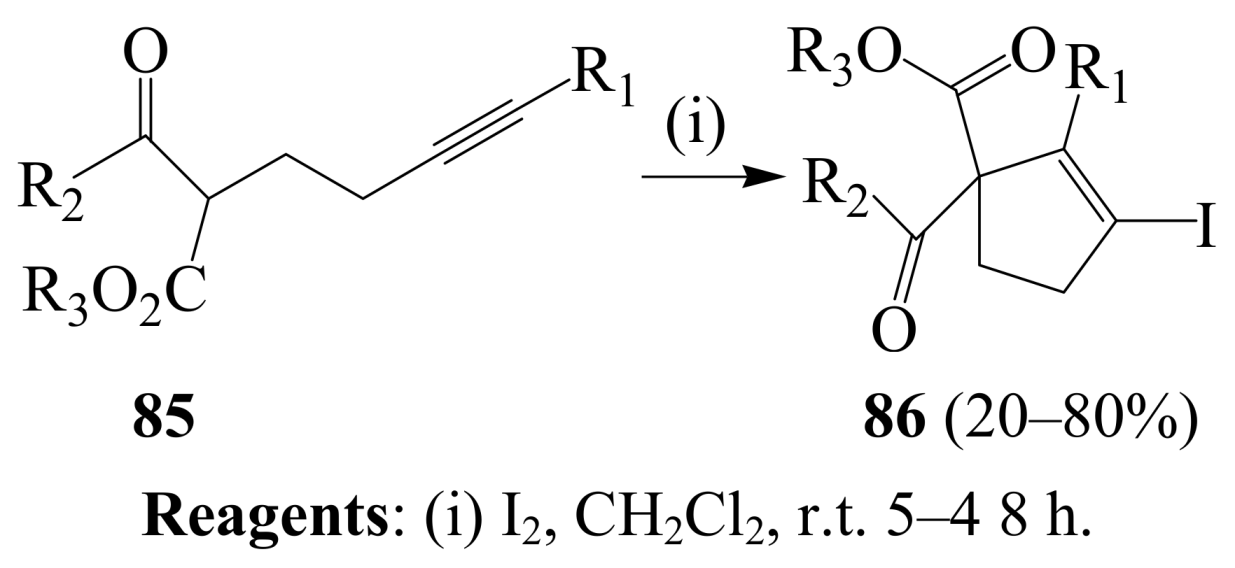
3. Combined Electrophilic and Oxidative Properties of Iodine in Cyclization Reactions
3.1. Iodine-mediated oxidative cyclization reactions
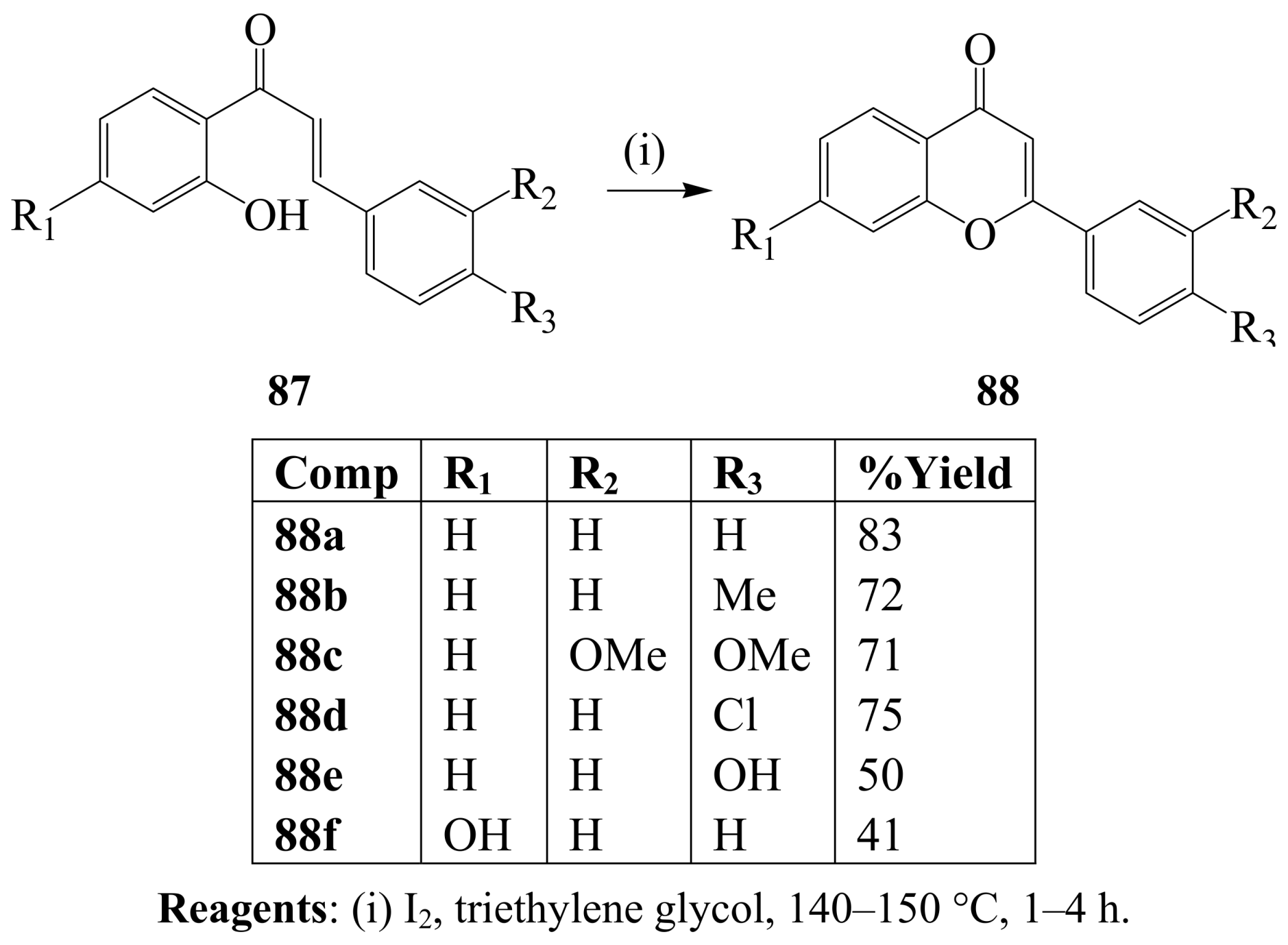
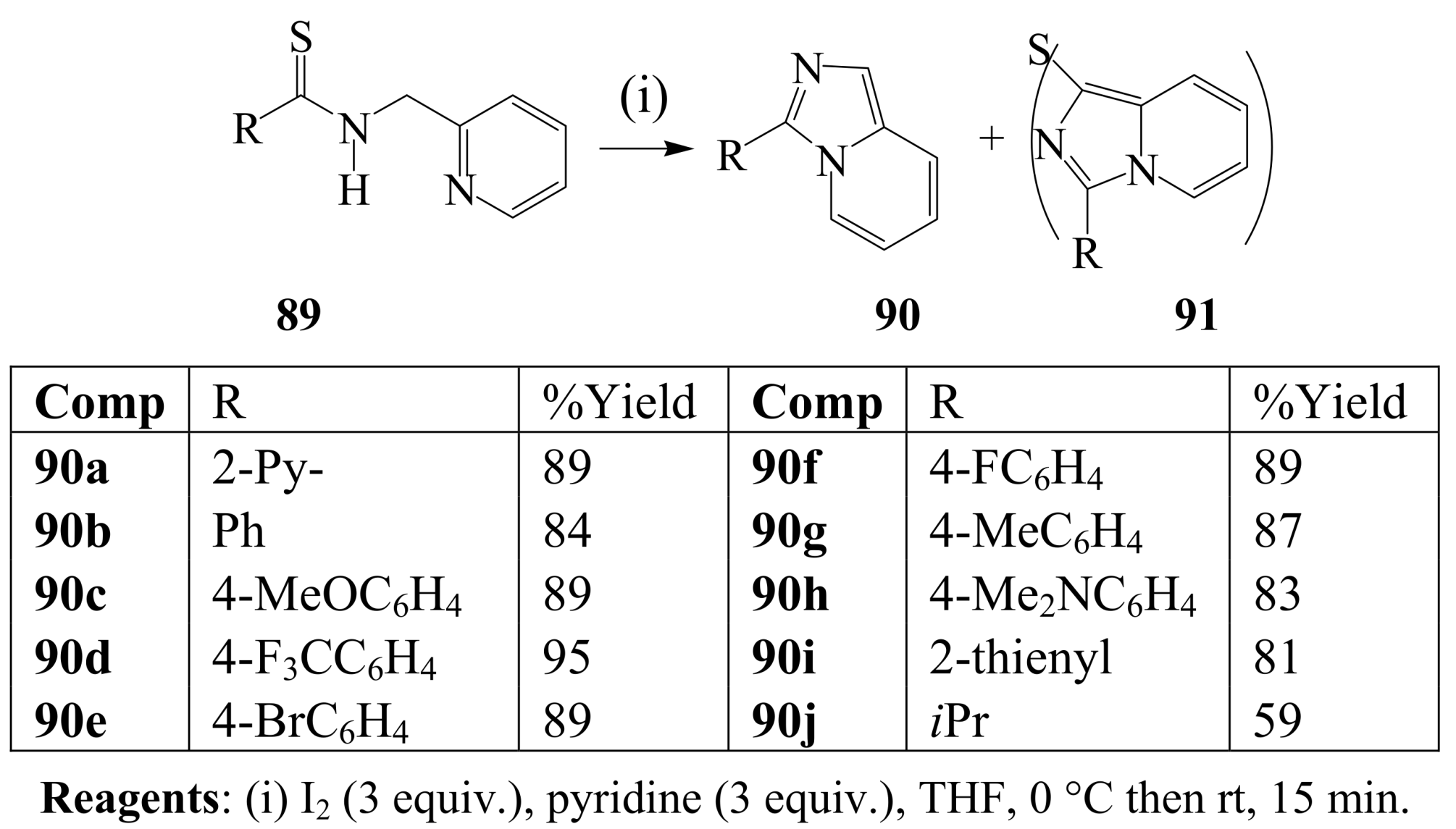
3.2. Iodine-mediated cyclization and oxidative aromatization reactions
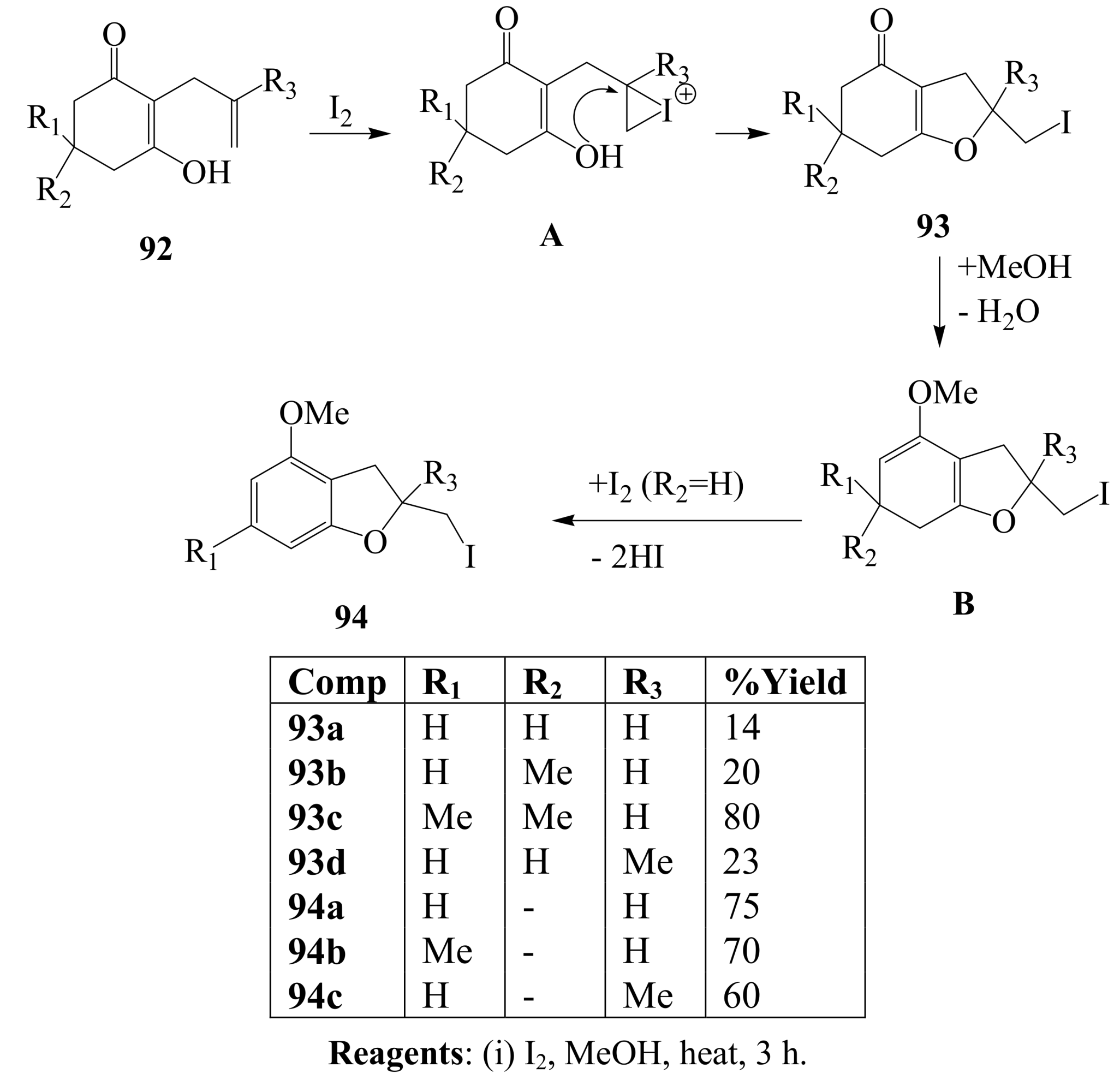
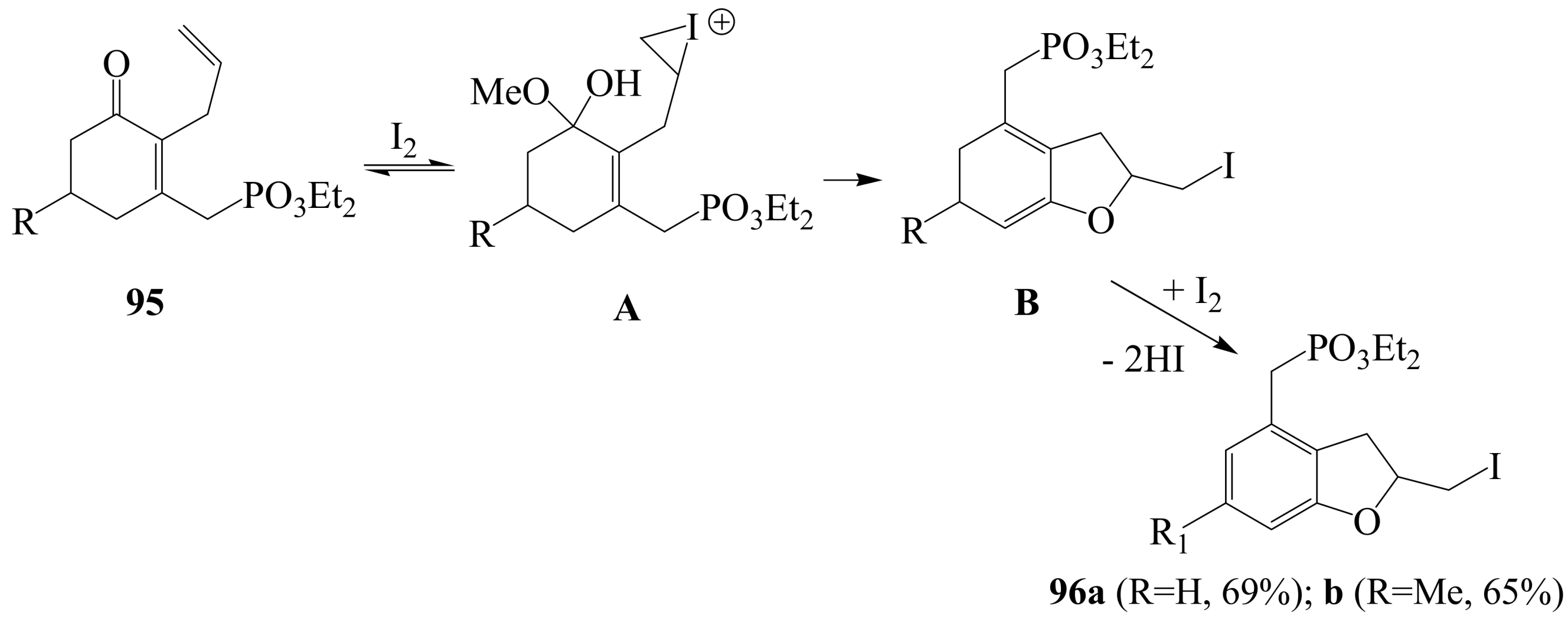
4. Conclusions
Acknowledgements
References
- El-Tach, G.M.M.; Evans, A.B.; Knight, D.W.; Jones, S. Practical alternatives for the synthesis of β-iodofurans by 5-endo-dig cyclizations of 3-alkyne-1,2-diols. Tetrahedron Lett. 2001, 42, 5945–5948. [Google Scholar]
- Sniady, A.A.; Wheeler, K.A.; Dembismski, R. 5-Endo-dig electrophilic cyclization of 1,4-disubstituted but-3-yn-1-ones: Regiocontrolled synthesis of 2,5-disubstituted 3-bromo- and 3-iodofurans. Org. Lett. 2005, 7, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Bew, S.P.; Knight, D.W. A brief synthesis of β-iodofurans. J. Chem. Soc. Chem. Commun. 1996, 1007–1008. [Google Scholar] [CrossRef]
- Knight, D.W.; Redfern, A.L.; Gilmore, J. The synthesis of 5-substituted proline derivatives and of 5-substituted pyrrole-2-carboxylates by 5-endo cyclisations featuring a method for effectively favouring these with respect to 5-exo cyclisations. J. Chem. Soc. Perkin Trans. I 2001, 2874–2883. [Google Scholar] [CrossRef]
- Knight, D.W.; Redfern, A.L.; Gilmore, J. An approach to 2,3-dihydropyrroles and β-iodopyrroles based on 5-endo-dig cyclizations. J. Chem. Soc. Perkin Trans. I 2002, 622–628. [Google Scholar] [CrossRef]
- Flynn, B.L.; Flynn, G.P.; Hamel, E.; Jung, M.K. The synthesis and tubulin binding activity of thiophene-based analogues of combretastatin A-4. Bioorg. Med. Chem. Lett. 2001, 11, 2341–2343. [Google Scholar] [CrossRef]
- Ren, X.F.; Turos, E.; Lake, C.H.; Churchill, M.R. Regiochemical and stereochemical studies on halocyclization reactions of unsaturated sulfides. J. Org. Chem. 1995, 60, 6468–6483. [Google Scholar] [CrossRef]
- Yue, D.; Larock, R.C. Synthesis of 3-iodoindoles by electrophilic cyclization of N,N-dialkyl-2-(1-alkynyl)anilines. Org. Lett. 2004, 6, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Knight, D.W. A simple, two-step synthesis of 3-iodoindoles. Tetrahedron Lett. 2004, 45, 539–541. [Google Scholar] [CrossRef]
- Banwell, M.G.; Flynn, B.L.; Wills, A.C.; Hamel, E. Synthesis, X-ray crystal structure and tubulin-binding properties of a benzofuran analog of the potent cytotoxic agent combretastatin A4. Aust. J. Chem. 1999, 52, 767–774. [Google Scholar] [CrossRef]
- Arcadi, A.; Cacchi, S.; Fabrizi, G.; Marinelli, F.; Moro, L. A new approach to 2,3-disubstituted benzo[b]furans from 2-alkynylphenols via 5-endo-dig-iodocyclisation/palladium-catalyzed reactions. Synlett 1999, 1432–1434. [Google Scholar] [CrossRef]
- Flynn, B.L.; Verdier-Pinard, P.; Hamel, E. A novel palladium-mediated coupling approach to 2,3-disubstituted benzo[b]thiophenes and its application to the synthesis of tubulin binding agents. Org. Lett. 2001, 3, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Larock, R.C. Synthesis of 2,3-disubstituted benzo[b]thiophenes via palladium-catalyzed coupling and electrophilic cyclization of terminal acetylenes. J. Org. Chem. 2002, 67, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Hessian, K.O.; Flynn, B.L. Iodine-induced reaction cascades for the rapid construction of variously substituted benzothiophenes. Org. Lett. 2003, 5, 4377–4380. [Google Scholar] [CrossRef] [PubMed]
- Hyashi, T.; Smith, F.T.; Lee, K.H. Antitumor agents. 89. Psychorubrin, a new cytotoxic naphthoquinone from Psychotria rubra and its structure-activity relationships. J. Med. Chem. 1987, 30, 2005–2008. [Google Scholar] [CrossRef]
- Yates, F.S. Comprehensive Heterocyclic Chemistry; Boulton, A.J., McKillop, A., Eds.; Pergamon: Oxford, UK, 1984; Vol. 2, Part 2A; p. 511. [Google Scholar]
- Tanaka, T.; Oba, T.; Okamure, N.; Watanabe, K.; Kurozumi, S.; Naruki, T. A convenient synthesis of 1,2,5-trisubstituted 3-benzoylpyrroles. Synth. Commun. 1980, 10, 773–789. [Google Scholar] [CrossRef]
- Daidone, G.; Maggio, B.; Schillari, D. Salicylanilide and its heterocyclic analogs. A comparative study of their antimicrobial activity. Pharmazie 1990, 45, 441–442. [Google Scholar] [PubMed]
- Lehuede, J.; Fauconneau, B.; Barrier, L.; Ourakow, M.; Piriou, A.; Vierfond, J.M. Synthesis and antioxidant activity of new tetraarylpyrroles. J. Med. Chem. 1999, 34, 991–996. [Google Scholar] [CrossRef]
- Rao, M.M.; Kingston, D.G.I. Plant anticancer agents. XII. Isolation and structure elucidation of new cytotoxic quinones from Tabebuia cassinoides. J. Nat. Prod. 1982, 45, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Hideo, T.; Shinpei, I. Synthetic use of molecular iodine for organic synthesis. Synlett 2006, 14, 2159–2175. [Google Scholar]
- Banerjee, A.K.; Vera, W.; Mora, H.; Laya, M.S.; Bedoya, L.; Cabrera, E.V. Iodine in organic synthesis. J. Sci. Ind. Res. 2006, 65, 299–308. [Google Scholar] [CrossRef]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976, 734–736. [Google Scholar] [CrossRef]
- Leonard, K.A.; Zhou, F.; Detty, M.R. Chalcogen(IV)-chalcogen(II) redox cycles. 1. Halogenation of organic substrates with dihaloselenium(IV) and -tellurium(IV) derivatives. dehalogenation of vicinal dibromides with diaryl tellurides. Organometallics 1996, 15, 4285–4292. [Google Scholar] [CrossRef]
- Singh, P.; Bhardwaj, A.; Kaur, S.; Kumar, S. Design, synthesis and evaluation of tetrahydropyran based COX-1/-2 inhibitors. Eur. J. Med. Chem. 2009, 44, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.O.; Brant, C.A.; Da Silverira, M.A.B.; Glennon, R.A. Synthesis of pyrrolidine-substituted benzamides via iodocyclization of β-enaminoesters. Tetrahedron Lett. 2007, 48, 6393–6396. [Google Scholar] [CrossRef]
- Amjad, M.; Knight, D.W. On the rapid synthesis of highly substituted proline analogues by 5-endo-trig iodocyclisation. Tetrahedron Lett. 2006, 47, 2825–2828. [Google Scholar] [CrossRef]
- Jones, D.A.; Knight, D.W.; Hibs, D.E. A stereochemically flexible approach to pyrrolidines based on 5-endo-trig iodocyclisations of homoallylic sulfonamides. J. Chem. Soc. Perkin Trans. 1 2001, 1182–1203. [Google Scholar] [CrossRef]
- Kitagawa, O.; Futjita, M.; Li, H.; Taguchi, T. Regiocontrolled iodoaminocyclization reaction of an ambident nucleophile mediated by LiAl(Ot-Bu)4. Tetrahedron Lett. 1997, 38, 615–618. [Google Scholar] [CrossRef]
- Ferraz, H.M.; Oliveria, E.; Payret-Arrua, M.E.; Brandt, C.A. A new and efficient approach to cyclic β-enamino esters and β-enamino ketones by iodine-promoted cyclization. J. Org. Chem. 1995, 60, 7357–7359. [Google Scholar] [CrossRef]
- Ferraz, H.M.; Pereira, F.L.C.; Leite, F.S.; Nunes, M.R.S.; Payret-Arrua, M.E. Synthesis of N-substituted pyrrole and tetrahydroindole derivatives from alkenyl β-dicarbonyl compounds. Tetrahedron 1999, 55, 10915–10924. [Google Scholar] [CrossRef]
- Gonzalez, F.B.; Bartlett, P.A. Stereocontrolled iodocyclization of acyclic olefinic acids. The trans and cis isomers of 4,5-dihydro-5-iodomethyl-4-phenyl-2(3-H)-furanone. Org. Synth. 1990, 7, 164. [Google Scholar]
- Laya, M.S.; Banerjee, A.K.; Cabrera, E.V. Iodolactonization. Past and present examples. Curr. Org. Chem. 2009, 13, 720–730. [Google Scholar] [CrossRef]
- Kim, I.; Won, H.K.; Choi, J.; Lee, G.H. A novel and efficient approach to highly substituted indolizines via 5-endo-trig iodocyclization. Tetrahedron 2007, 63, 12954–12960. [Google Scholar] [CrossRef]
- Friesen, R.W.; Giroux, A.; Cook, K.L. Iodocyclization reactions of α-allenic alcohol derivatives. Stereoselective formation of Z-4-(1-iodo-2-alkylethylene)-2-trichloromethyl-4,5-dihydro-1,3-oxazoles. Tetrahedron Lett. 1993, 34, 5983–5986. [Google Scholar] [CrossRef]
- Bew, S.P.; El-Taeb, G.M.M.; Jones, S.; Knight, D.W.; Tan, W.F. Expedient syntheses of β-iodofurans by 5-endo-dig cyclisations. Eur. J. Org. Chem. 2007, 5759–5770. [Google Scholar] [CrossRef]
- Knight, D.W.; Rost, H.C.; Sharland, C.M.; Singhornrat, J. A general approach to polysubstituted pyrroles. Tetrahedron Lett. 2007, 48, 7906–7910. [Google Scholar] [CrossRef]
- Kim, I.K.; Choi, J.; Won, H.K.; Lee, G.H. Expeditious synthesis of indolizine derivatives via iodine mediated 5-endo-dig cyclization. Tetrahedron Lett. 2007, 48, 6863–6867. [Google Scholar] [CrossRef]
- Kim, I.K.; Kim, S.G.; Kim, J.Y.; Lee, G.H. A novel approach to 3-acylated indolizine structures via iodine-mediated hydrative cyclization. Tetrahedron Lett. 2007, 48, 8976–8981. [Google Scholar] [CrossRef]
- Ahmed, N.; Dubuc, C.; Rousseau, J.; Bénard, F.; van Lier, J.E. Synthesis, characterization, and estrogen receptor binding affinity of flavone-, indole-, and furan-estradiol conjugates. Bioorg. Med. Chem. Lett. 2007, 17, 3212–3216. [Google Scholar] [CrossRef] [PubMed]
- Hessian, K.O.; Flynn, B.L. Selective endo and exo iodocyclizations in the synthesis of quinolines and indoles. Org. Lett. 2006, 8, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Cacchi, S.; Fabrizi, G.; Marinelli, F.; Moro, L. A new approach to 2,3-disubstituted benzo[b]furans from 2-alkynylphenols via 5-endo-dig-iodocyclisation/palladium-catalyzed reactions. Synlett 1999, 1432–1434. [Google Scholar] [CrossRef]
- Likhar, P.R.; Subhas, M.S.; Roy, M.; Roy, S.; Kantam, M.L. Copper-free Sonogashira coupling of acid chlorides with terminal alkynes in the presence of a reusable palladium catalyst: An improved synthesis of 3-iodochromenones (= 3-iodo-4H-1-benzopyran-4-ones). Helv. Chim. Acta 2008, 91, 259–264. [Google Scholar] [CrossRef]
- Arcadi, A.; Marinelli, F.; Rossi, E. Synthesis of functionalized quinolines through tandem addition/annulation reactions of β-(2-aminophenyl)-α,β-ynones. Tetrahedron 1999, 55, 13233–13250. [Google Scholar] [CrossRef]
- Barange, D.K.; Batchu, V.R.; Gorja, D.; Pattabiraman, V.R.; Tatini, L.K.; Babu, J.M.; Pal, M. Regioselective construction of six-membered fused heterocyclic rings via Pd/C-mediated C-C coupling followed by iodocyclization strategy: A new entry to 2H-1,2-benzothiazine-1,1-dioxides. Tetrahedron 2007, 63, 1775–1789. [Google Scholar] [CrossRef]
- Yao, T.; Larock, R.C. Regio- and stereoselective synthesis of isoindolin-1-ones via electrophilic cyclization. J. Org. Chem. 2004, 70, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Orito, K.; Hatakayama, T.; Takeo, M.; Suginome, H.; Tokuda, M. Synthesis of 5-iodobenzofurans and 6-iodobenzopyrans via direct iodination with mercury(II) oxide-iodine reagent. Synthesis 1997, 23–25. [Google Scholar] [CrossRef]
- Mahajan, V.A.; Shinde, P.D.; Gajare, A.S.; Karthikeyan, M.; Wakharkar, R.D. EPZ-10 catalyzed regioselective transformation of alkenes into β-iodo ethers, iodohydrins and 2-iodomethyl-2,3-dihydrobenzofurans. Green Chem. 2002, 4, 325–327. [Google Scholar] [CrossRef]
- Fousteris, M.; Chevrin, C.; Le Bras, J.; Muzart, J. Water-promoted iodocyclization of 2-allylphenols. Green Chem. 2006, 8, 522–523. [Google Scholar] [CrossRef]
- Antonioetti, R.; Bonadies, F.; Scettri, A. A convenient approach to furan derivatives by iodine-induced cyclization of 2-alkenyl substituted 1,3-dicarbonyl compounds. Tetrahedron Lett. 1988, 29, 4987–4990. [Google Scholar] [CrossRef]
- Antonioletti, R.; Cecchini, C.; Ciani, B.; Magnabti, S. Iodoenolycyclization. III. A general approach to tetrasubstituted furans from 2-alkenyl-1,3-dicarbonyl compounds. Tetrahedron Lett. 1995, 36, 9019–9022. [Google Scholar] [CrossRef]
- Antonioletti, R.; Malancona, S.; Bovicelli, P. Diastereoselective synthesis of 4,5-dihydrofurans by iodoenolcyclization of 2-allyl-1,3-dicarbonyl compounds. Tetrahedron 2002, 58, 8825–8831. [Google Scholar] [CrossRef]
- Antonioletti, R.; Malancona, S.; Cattaruzza, F.; Bovicelli, P. Iodoenolcyclization of 2-allyl-substituted β-keto esters under thermodynamic conditions. Tetrahedron 2003, 59, 1673–1678. [Google Scholar] [CrossRef]
- Ferraz, H.M.C.; Sano, M.K.; Nunes, M.R.S.; Bianco, G.G. Synthesis of cyclic enol ethers from alkenyl-beta-dicarbonyl compounds. J. Org. Chem. 2002, 67, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J.; El-Gogary, T.M. Synthesis and chemical transformation of 2-iodomethyl-1-(phenylmethyl)-1,5,6,7-tetrahydroindol-4-ones. J. Chem. Res. 2008, 227–231. [Google Scholar] [CrossRef]
- Lee, J.W.; Oh, D.Y. Iodine-induced enoletherification of α-allyl substituted β-keto sulfones; a route to 3-phenylsulfonyl-2,5-disubstituted furans. Heterocycles 1990, 31, 1417–1421. [Google Scholar]
- Barluenga, J.; Palomas, D.; Rubio, E.; Gonzalez, J.M. Iodocarbocyclization reaction of β-ketoesters and alkynes. Org. Lett. 2007, 9, 2823–2826. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Takizawa, E.; Sasaki, M. Syntheses of flavones via the iodine-mediated oxidative cyclization of 1,3-diphenylprop-2-en-1-ones. Bull. Chem. Soc. Jpn. 2003, 76, 835–836. [Google Scholar] [CrossRef]
- Joo, Y.H.; Kim, J.K.; Kang, S.H.; Noh, M.S.; Ha, J.Y.; Choi, J.K.; Lim, K.M.; Lee, C.H.; Chung, S. 2,3-Diarylbenzopyran derivatives as a novel class of selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 413–417. [Google Scholar] [CrossRef]
- Imafuku, K.; Honda, M.; McOmie, J.F.W. Cyclodehydrogenation of 2′-hydroxychalcones with 2,3-dichloro-5,6-dicyano-p-benzoquinone: A simple route for flavones and aurones. Synthesis 1987, 199–201. [Google Scholar] [CrossRef]
- Shibahara, F.; Kitagawa, A.; Yamaguchi, E.; Murai, T. Synthesis of 2-azaindolizines by using an iodine-mediated oxidative desulfurization promoted cyclization of N-2-pyridylmethyl thioamides and an investigation of their photophysical properties. Org. Lett. 2006, 8, 5621–5624. [Google Scholar]
- Mphahlele, M.J.; Moekwa, T.B. Iodo- and bromo-enolcyclization of 2-(2-propenyl)cyclohexanediones and 2-(2-propenyl)cyclohexenone derivatives using iodine in methanol and pyridinium hydrobromide perbromide in dichloromethane. Org. Biomol. Chem. 2005, 3, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Yoshimoto, Y. An improved method for the conversion of cyclohexenones into anisoles. Chem. Ind. 1980, 888–389. [Google Scholar]
- Kotnis, A.S. A rapid entry to highly functionalized p-methoxybenzoates: Synthesis of the aromatic portion of milbemycin β3. Tetrahedron Lett. 1990, 31, 481–484. [Google Scholar] [CrossRef]
- Kotnis, A.S. A convenient, practical synthesis of substituted resorcinols: Synthesis of DB-2073 and olivetol. Tetrahedron Lett. 1991, 32, 3441–344. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Pienaar, A.; Modro, T.A. Reactions of phosphorus-stabilized carbanions with cyclic enones. Aromatization of the substitution and addition products. J. Chem. Soc. Perkin Trans. II 1996, 1455–1460. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.Y.; Kim, J.N. Oxidative aromatization of 2-acylcyclohexane-1,3-dione derivatives using iodine in methanol. Bull. Korean. Chem. Soc. 2003, 24, 1057–1058. [Google Scholar] [CrossRef]
- Hedge, S.G.; Kassim, A.M.; Ingram, A.I. Aromatization of cyclohexenones with iodine/sodium alkoxide. A regioselective synthesis of 2-iodophenols. Tetrahedron Lett. 1995, 36, 8395–8398. [Google Scholar]
- Kim, J.M.; Lee, K.Y.; Kim, T.H.; Kim, J.N. Synthesis of anisole derivatives from 4-alkylidene-2-cyclohexen-1-ones with iodine in methanol. Bull. Korean. Chem. Soc. 2003, 24, 999–1001. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.Y.; Kim, J.N. Synthesis of 4-methylene-2-cyclohexenones and their aromatization reaction toward para-methoxylmethyl anisole derivatives. Bull. Korean. Chem. Soc. 2004, 25, 328–330. [Google Scholar] [CrossRef]
- Na, J.E.; GowriSankar, S.; Lee, S.; Kim, J.N. Selective methylation of the ninhydrin-phenol adducts with I2 in MeOH. Bull. Korean Chem. Soc. 2004, 54, 569–572. [Google Scholar] [CrossRef]
- Na, J.E.; Kim, J.M.; Lee, S.; Kim, J.N. Synthesis of benzo[b]indeno[2,1-d]furanone skeleton from ninhydrin and cyclohexane-1,3-dione derivatives. Bull. Korean. Chem. Soc. 2003, 24, 1725–1726. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Hlatshwayo, S.M.; Mogamisi, F.K.; Tsanwani, M.; Mampa, R. Iodine-Methanol–Promoted Aromatization of 2-Aryl-1,2,3,4-tetrahydro-4-quinolones to 2-Aryl-4-methoxyquinolines. M. J. Chem. Res. (S) 1999, 706–707. [Google Scholar] [CrossRef]
- Grieco, P.A.; Marinovic, N. Rhodium-catalyzed migration of double bonds: Preparation of substituted phenols and anilines. Tetrahedron Lett. 1978, 29, 2545–2548. [Google Scholar] [CrossRef]
- Blagbrough, I.S.; Pattenden, G.; Raphael, E.F. Isomerization of ω-alkenyl-substituted cyclohexane-1,3-dione enol derivatives using rhodium catalysis. A practical synthesis of substituted resorcinols. Tetrahedron Lett. 1982, 23, 4843–4846. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamamoto, K.; Ueda, T.; Morikawa, T.; Kawase, Y. A new preparative method of 4-hydroxybenzofuran-2-carboxylic acid derivatives. Bull. Chem. Soc. Jpn. 1989, 62, 4066–4068. [Google Scholar] [CrossRef]
- Hirao, T.; Mori, M.; Ohshiro, Y. VO(OR)Cl2-induced oxidative aromatization of α,β-unsaturated cyclohexenones. J. Org. Chem. 1990, 55, 358–360. [Google Scholar] [CrossRef]
- Lee, Y.R.; Morehead, A.T., Jr. A new route for the synthesis of furanoflavone and furanochalcone natural products. Tetrahedron 1995, 51, 4909–4922. [Google Scholar] [CrossRef]
- For review on the use of iodofunctionalized system in metal-catalyzed C–C formation see: Kirsch, G.; Hesse, S.; Comel, A. Synthesis of five- and six-membered heterocycles through palladium-catalyzed reactions. Curr. Org. Synth. 2004, 1, 47–63. [Google Scholar] [CrossRef]
- Worlikar, S.A.; Neuenswander, B.; Lushington, G.H.; Larock, R.C. Highly substituted indole library synthesis by palladium-catalyzed coupling reactions in solution and on a solid support. J. Comb. Chem. 2009, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mphahlele, M.J. Molecular Iodine-Mediated Cyclization of Tethered Heteroatom-Containing Alkenyl or Alkynyl Systems. Molecules 2009, 14, 4814-4837. https://doi.org/10.3390/molecules14124814
Mphahlele MJ. Molecular Iodine-Mediated Cyclization of Tethered Heteroatom-Containing Alkenyl or Alkynyl Systems. Molecules. 2009; 14(12):4814-4837. https://doi.org/10.3390/molecules14124814
Chicago/Turabian StyleMphahlele, Malose Jack. 2009. "Molecular Iodine-Mediated Cyclization of Tethered Heteroatom-Containing Alkenyl or Alkynyl Systems" Molecules 14, no. 12: 4814-4837. https://doi.org/10.3390/molecules14124814
APA StyleMphahlele, M. J. (2009). Molecular Iodine-Mediated Cyclization of Tethered Heteroatom-Containing Alkenyl or Alkynyl Systems. Molecules, 14(12), 4814-4837. https://doi.org/10.3390/molecules14124814