Application of the Molecular Combing Technique to Starch Granules
Abstract
:1. Introduction
1.1. Starch granules
1.2. The classical model of starch structure
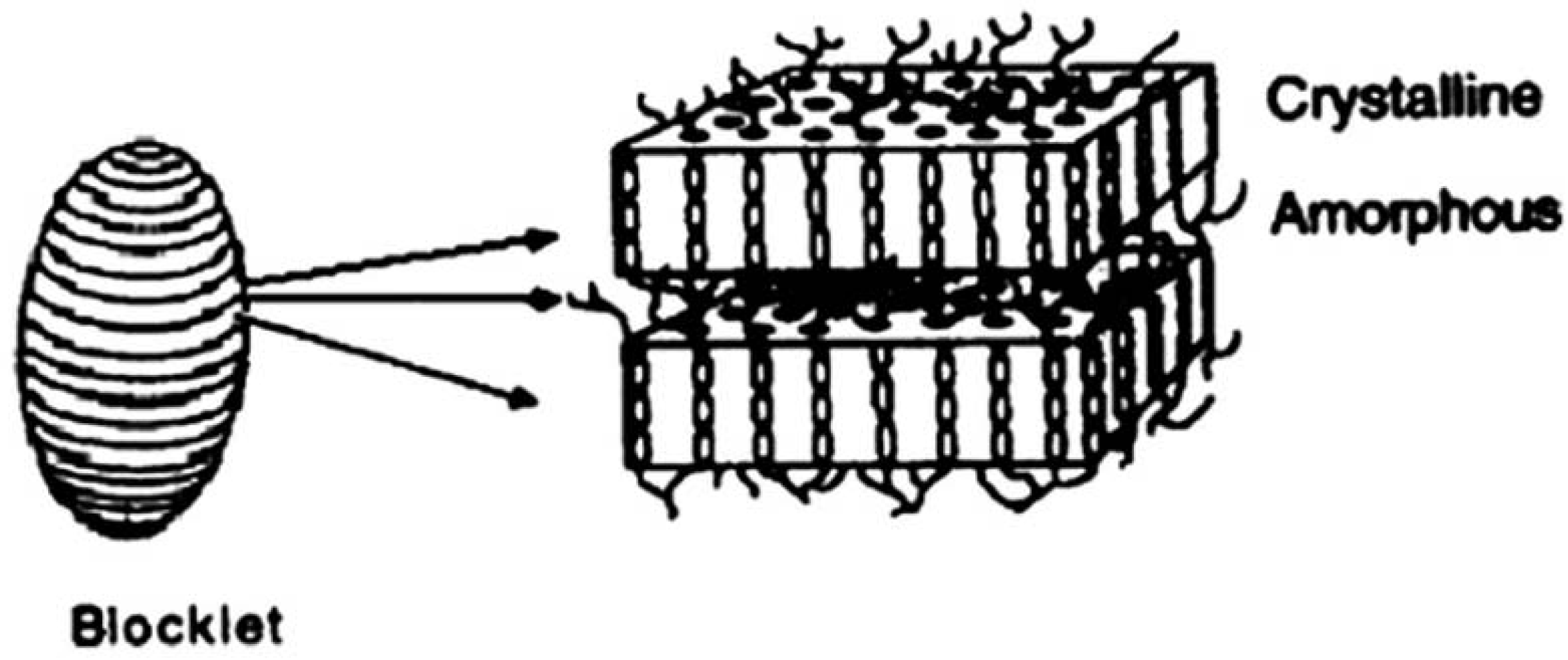
1.3. The technology of molecular combing
2. Results and Discussion


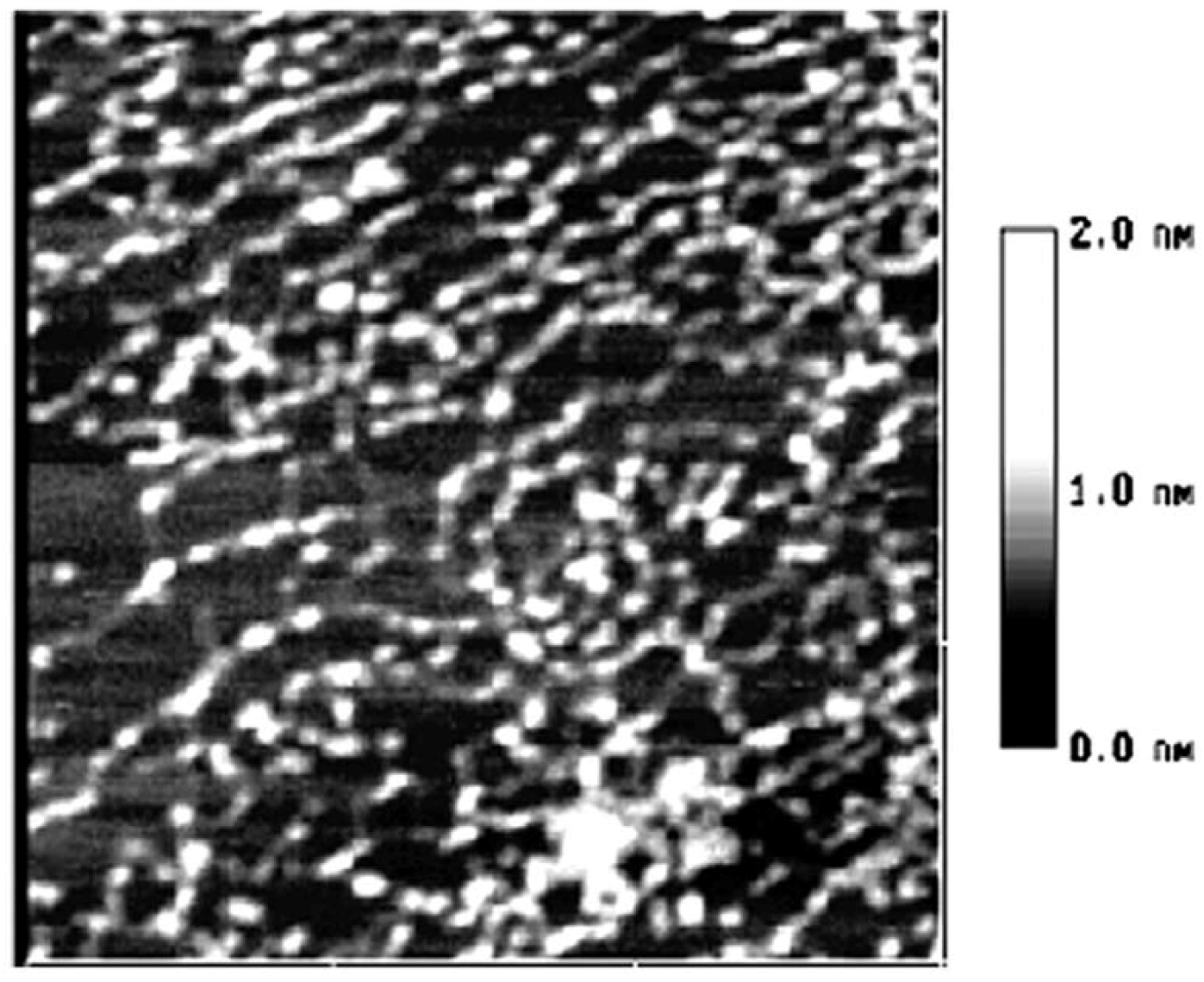
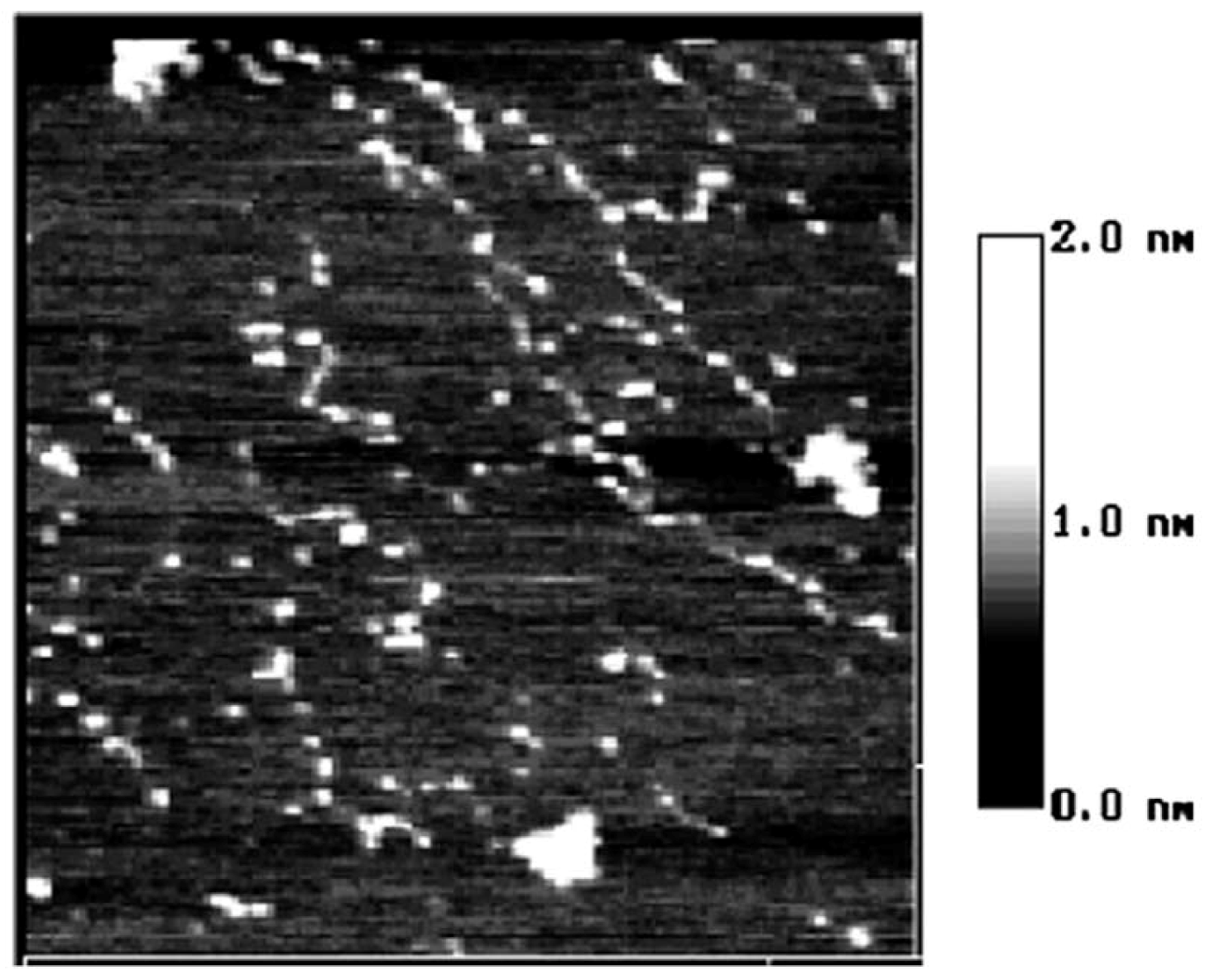
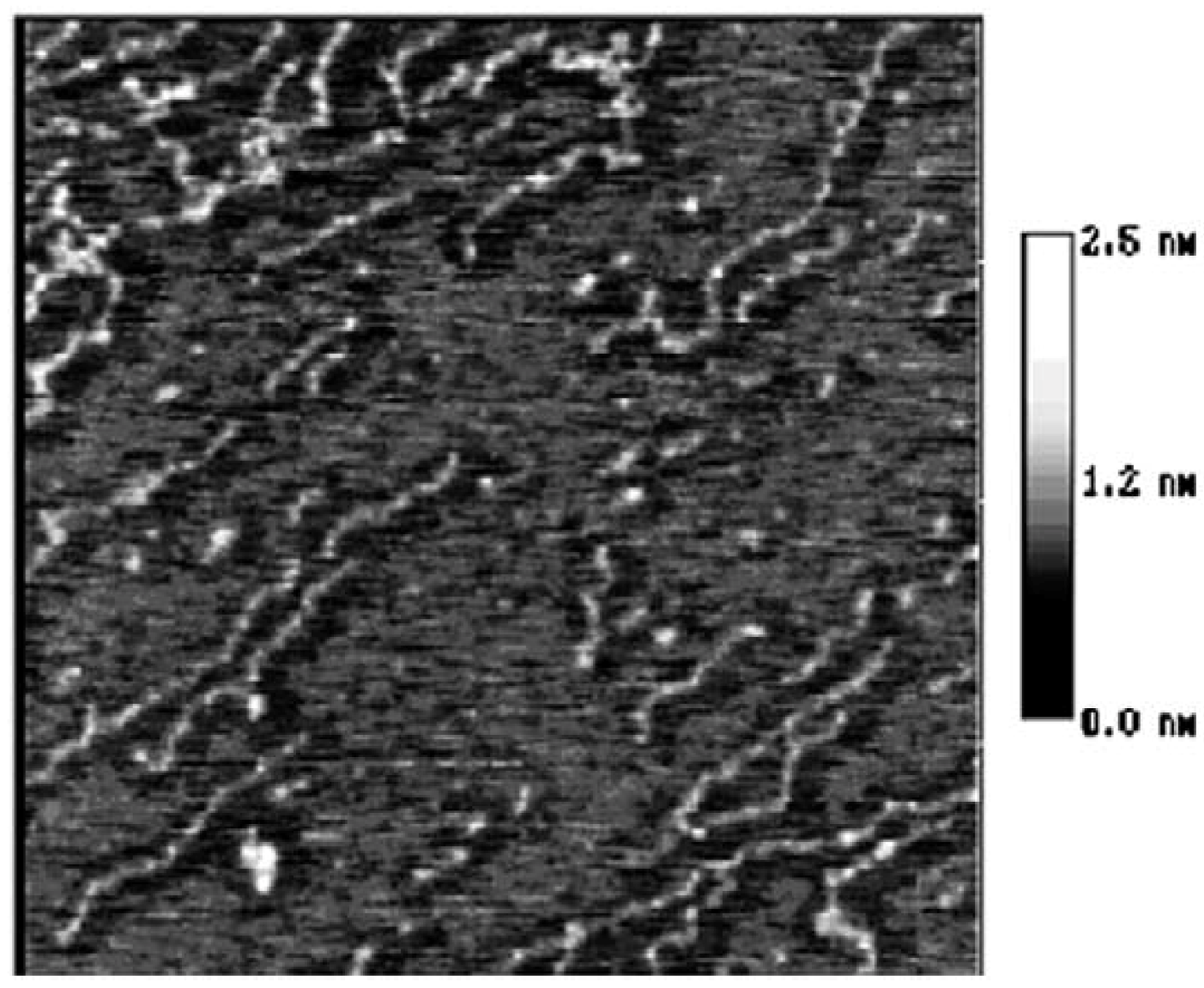
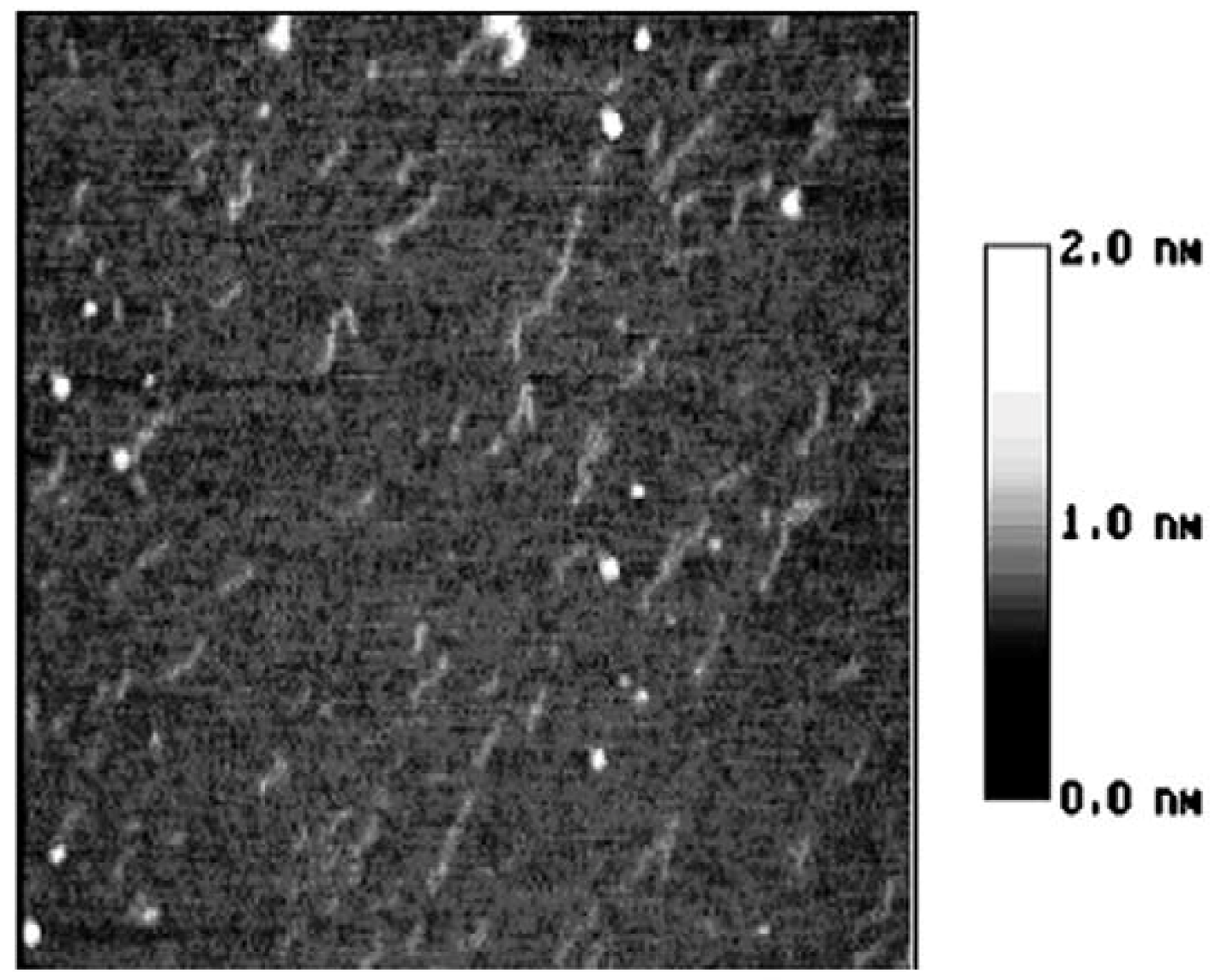
3. Experimental
3.1. Reagents and apparatus
3.2. Methods
4. Conclusions
Acknowledgments
- Sample Availability: Available from the authors.
References
- Zobel, H.F. The terminology and methodology associated with basic starch phenomena. Cereal Foods World 1988, 33, 306–311. [Google Scholar]
- Robin, J.P.; Mercier, C.; Charbonniere, R.; Guibot, A. Lintnerized starches. Gel filtration and enzymatic studies of insoluble residues from prolonged acid treatment of potato starch. Cereal Chem. 1974, 51, 389–406. [Google Scholar]
- Buleon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar]
- Bottomely, L.A. Scanning probe microscope. Anal. Chem. 1998, 70, 425–476. [Google Scholar]
- Li, M.Q.; Hansma, H.G.; Vesenka, J.; kelderman, G.; Hansma, P.K.J. Atomic force microscopy of uncoated plasmid DNA: Nanometer resolution with only nanogram amounts of sample. Biol. Struct. Dyn. 1992, 10, 607–617. [Google Scholar]
- Li, M. Q.; Xu, L.; Ikai, A. Atomic force microscope imaging of ribosome and chromosome. J. Vac. Sci. Technol. 1996, B14, 1410–1412. [Google Scholar]
- Hansma, H.G. Varieties of imaging with scanning probe microscopes. Proc. Natl. Acad. Sci. USA 1999, 96, 14678–14680. [Google Scholar]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of starch: Evidence of a new level of granule organization. Carbohydr. Polym. 1997, 32, 177–191. [Google Scholar]
- Baldwin, P.M.; Adler, J.; Davies, M.C.; Melia, C.D. High resolution imaging of starch granule surface by atomic force microscopy. J. Cereal Sci. 1996, 27, 255–265. [Google Scholar]
- Li, J.W.; Yingge, Z. Observation and manipulation of biological structure with AFM. ACTA Bioph. Sinica 2004, 20, 253–257. [Google Scholar]
- Ke, C.L. Advance in single biomolecule research. ACTA Bioph. Sinica 2001, 17, 411–418. [Google Scholar]
- Bensimon, A.; Simon, A. Alignment and sensitive detection of DNA by a moving interface. Science 1994, 265, 2096–2098. [Google Scholar]
- Zhong, Z.L.; Peng, L.; John, F.K. The technology of molecular manipulation and modiWcation assisted by microwave as applied to starch granules. Carbohydr. Polym. 2005, 61, 374–378. [Google Scholar] [CrossRef]
- Hong, X.L.; Zhao, T.C. Study on the carboxymethyl wheat starch. J. Zhengzhou Grain Coll. 1996, 17, 15–21. [Google Scholar]
- Zhong, Z.L.; Shen, F.C.; Zhen, Q.O. Study on chain structure of starch molecular by AFM. J. Vac. Sci. Technol. B 2001, 19, 111–114. [Google Scholar]
- Baker, A.A; Miles, M.J.; Helbert, W. Internal structure of the starch granule revealed by AFM. Carbohydr. Res. 2001, 330, 249–256. [Google Scholar]
- Ridout, M.J.; Paker, M.L.; Hedley, C.L.; Bogracheva, T.Y.; Morris, V.J. Atomic force microscopy of pea starch granules: Granule architecture of wild-type parent, r and rb single mutants, and the rrb double mutant. Carbohydr. Res. 2003, 338, 2135–2147. [Google Scholar]
- Szymonska, J.; Krok, F. Potato starch granule nanostructure studied by high resolution non-contact AFM. Int. J. Biol. Macromol. 2003, 33, 1–7. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.; Liu, Z.-D.; Boxiang, L.; Chen, J.-H.; Sun, Y.-N.; Lv, X.-L.; Zhang, Z.-S.; Sun, P.; Zhang, P.; Wang, Y.-L. Application of the Molecular Combing Technique to Starch Granules. Molecules 2009, 14, 4079-4086. https://doi.org/10.3390/molecules14104079
Li H, Liu Z-D, Boxiang L, Chen J-H, Sun Y-N, Lv X-L, Zhang Z-S, Sun P, Zhang P, Wang Y-L. Application of the Molecular Combing Technique to Starch Granules. Molecules. 2009; 14(10):4079-4086. https://doi.org/10.3390/molecules14104079
Chicago/Turabian StyleLi, Hua, Zhong-Dong Liu, Liu Boxiang, Jian-Hui Chen, You-Ning Sun, Xiao-Ling Lv, Ze-Sheng Zhang, Pin Sun, Pin Zhang, and Yang-Li Wang. 2009. "Application of the Molecular Combing Technique to Starch Granules" Molecules 14, no. 10: 4079-4086. https://doi.org/10.3390/molecules14104079
APA StyleLi, H., Liu, Z.-D., Boxiang, L., Chen, J.-H., Sun, Y.-N., Lv, X.-L., Zhang, Z.-S., Sun, P., Zhang, P., & Wang, Y.-L. (2009). Application of the Molecular Combing Technique to Starch Granules. Molecules, 14(10), 4079-4086. https://doi.org/10.3390/molecules14104079



