Evaluation of Antioxidant and Antibacterial Activities of Aqueous, Methanolic and Alkaloid Extracts from Mitragyna Speciosa (Rubiaceae Family) Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total phenolic and flavanoid content
2.2. Antioxidant assays
| Extract | Total phenolic content mg/g | Total flavanoid content mg/g | IC 50µg/mL |
|---|---|---|---|
| MeOH extract | 105.58 ± 15.43 | 91.12 ± 17.27 | 37.08 ± 3.54 |
| Aqueous extract | 66.00 ± 1.23 | 28.19 ± 2.28 | 213.45 ± 31.31 |
| Alkaloid extract | 88.37 ± 0.70 | 20.03 ± 3.03 | 104.81 ± 5.77 |
| BHT | − | − | 4.50 ± 0.23 |
2.3. Antimicrobial activity
| Microorganism | Zone of inhibition (mm)a | MIC (mg/mL)b of the extract | ||||||
|---|---|---|---|---|---|---|---|---|
| MeOH extract | Aqueous extract | Alkaloid extract | Chloramphenicol | MeOH extract | Aqueous extract | Alkaloid extract | ||
| Salmonella typhi | 29 ± 2.3 | - | 30 ± 2.1 | 23 | 6.25 | - | 3.12 | |
| Bacillus subtilis | 30 ± 2.1 | - | 33 ± 2.5 | 24 | 6.25 | - | 3.12 | |
| Escherichia coli | - | - | - | 22 | - | - | - | |
| Pseudomonas aeruginosa | - | - | - | 23 | - | - | - | |
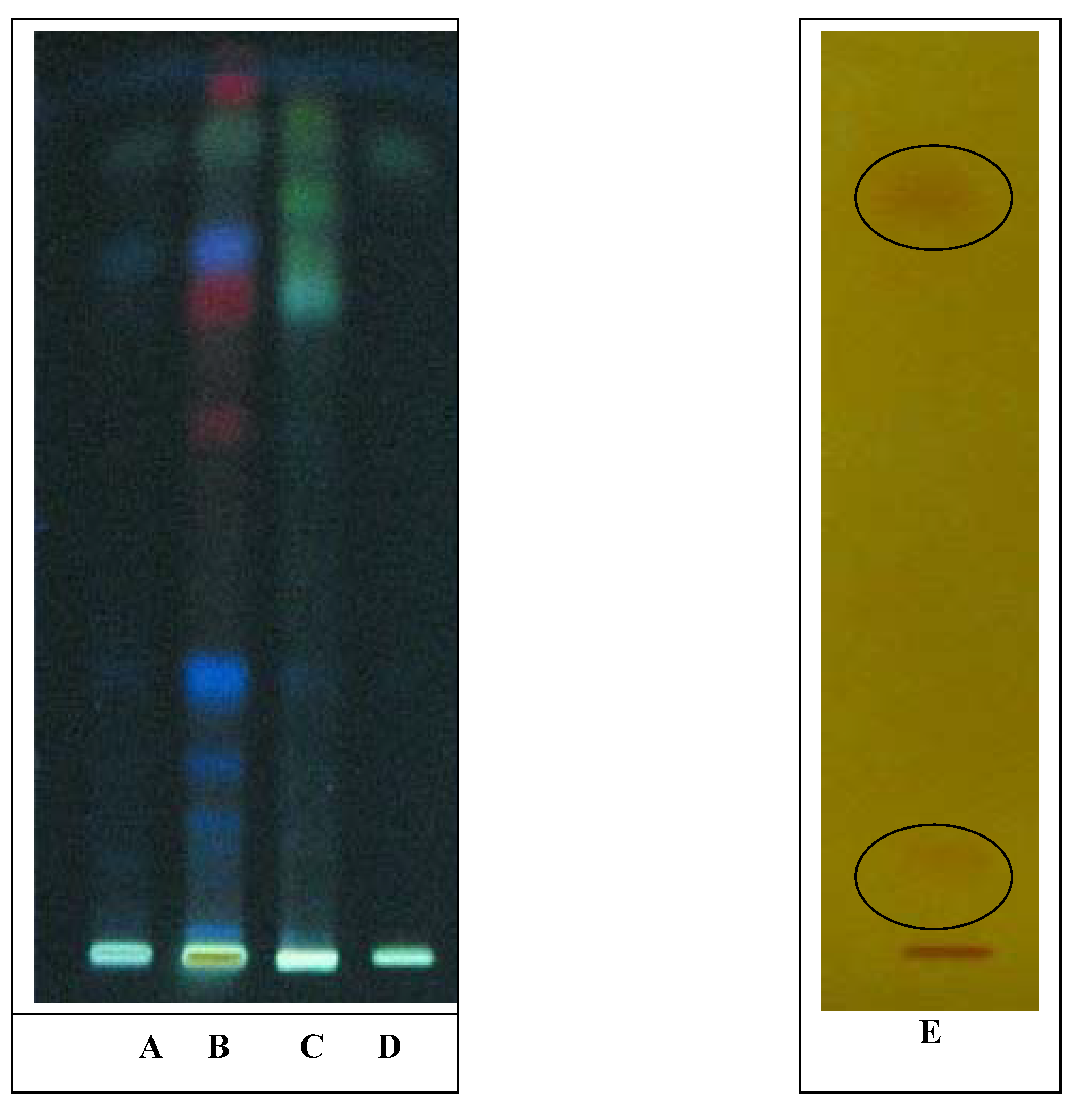
3. Experimental
3.1. Plant material
3.2. Chemicals
3.3. Plant extraction procedure
3.3.1. Hot water extraction
3.3.2. Methanolic and alkaloid extract
3.4. Standardize extracts
3.5. Test microorganisms and growth media
3.6. Antioxidant assay
3.6.1. Total phenolic assay
3.6.2. Total flavonoid content
3.6.3. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay
3.7. Antimicrobial assay
3.7.1. Disk diffusion assay
3.7.2. Determination of minimum inhibitory concentrations (MIC)
3.8. Statistical analysis
4. Conclusion
Acknowledgements
- Samples Availability: Available from the authors.
References
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Pavrez, M.; Mahboob, H.K.; Zahuul, I.; Shek, M.H. Antimicrobial activities of the petroleum ether, methanol and acetone extracts of kaempferia galangal. rhizome. J. Life Earth Sci. 2005, 1, 25–29. [Google Scholar]
- Khan, M.; Kibm, M.; Oinoloso, B. Antimicrobial activity of the alkaloidal comtituents of the root bark of Eupomatia lourina. Phannaceut. Biol. 2003, 41, 277–280. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Lipid peroxidation, oxygen radicals, cell damage and antioxydant therapy. Lancet 1984, 1, 1396–1397. [Google Scholar]
- Idid, S.Z.; Saad, L.B. Evaluation of Analgesia Induced by Mitragynine,Morphine and Paracetamolon Mice; ASEAN Review of Biodiversity and Environmental Conservation: Bangi, Malaysia, 1988; pp. 1–7. [Google Scholar]
- Macko, E.; Weisbach, J.A. Some observations on the pharmacology of mitragynine. Arch. Int. Pharmacodyn. Ther. 1972, 198, 145–161. [Google Scholar]
- Perry, L.M. Medicinal Plants of East and Southeast Asia; MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar]
- Karagozler, A.A.; Erdag, B.; Emek, Y.C.; Uygun, D.A. Antioxidant activity and proline content of leaf extracts from Dorystoechas hastate. Food Chem. 2008, 111, 400–407. [Google Scholar]
- González, E.M.; de Ancos, B.; Cano, M.P. Relation between bioactive compounds and free radical-scavenging capacity in berry fruits during frozen storage. J. Sci. Food Agric. 2003, 83, 722–726. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Comp. Anal. 2007, 20, 125–132. [Google Scholar]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibrerich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Diplock, A.T. Will the good fairies please prove to us that vitamin E lessens human degenerative disease. Free Radic. Res. 1997, 27, 511–532. [Google Scholar] [CrossRef]
- Leven, M.; VandenBerghe, D.A.; Mertens, F.; Vlictinck, A.; Lammens, E. Screening of higher plants for biological activities/-antimicrobial activity. Plant. Med. 1979, 36, 311–321. [Google Scholar]
- Guidelines for the Appropriate use of Herbal Medicines; WHO Regional Publications, Western Pacific Series No. 23. WHO Regional Office for the Western Pacific: Manila, 1998.
- Miliauskasa, G.; Venskutonisa, P.R.; Van Beekb, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar]
- Abdel-Hameed, E.S.S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2008, 1133–1138. [Google Scholar]
- Alzoreky, N.S.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food. Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by standardized single disc method. Am. J. Clin. Pathol. 1996, 36, 493–496. [Google Scholar]
- NCCLS, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 3rd ed; NC-275 CLS, Approved standard. NCCLS document M100-S12.: Wayne, PA, USA, 2002.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Parthasarathy, S.; Bin Azizi, J.; Ramanathan, S.; Ismail, S.; Sasidharan, S.; Said, M.I.M.; Mansor, S.M. Evaluation of Antioxidant and Antibacterial Activities of Aqueous, Methanolic and Alkaloid Extracts from Mitragyna Speciosa (Rubiaceae Family) Leaves. Molecules 2009, 14, 3964-3974. https://doi.org/10.3390/molecules14103964
Parthasarathy S, Bin Azizi J, Ramanathan S, Ismail S, Sasidharan S, Said MIM, Mansor SM. Evaluation of Antioxidant and Antibacterial Activities of Aqueous, Methanolic and Alkaloid Extracts from Mitragyna Speciosa (Rubiaceae Family) Leaves. Molecules. 2009; 14(10):3964-3974. https://doi.org/10.3390/molecules14103964
Chicago/Turabian StyleParthasarathy, Suhanya, Juzaili Bin Azizi, Surash Ramanathan, Sabariah Ismail, Sreenivasan Sasidharan, Mohd I. Mohd. Said, and Sharif Mahsufi Mansor. 2009. "Evaluation of Antioxidant and Antibacterial Activities of Aqueous, Methanolic and Alkaloid Extracts from Mitragyna Speciosa (Rubiaceae Family) Leaves" Molecules 14, no. 10: 3964-3974. https://doi.org/10.3390/molecules14103964
APA StyleParthasarathy, S., Bin Azizi, J., Ramanathan, S., Ismail, S., Sasidharan, S., Said, M. I. M., & Mansor, S. M. (2009). Evaluation of Antioxidant and Antibacterial Activities of Aqueous, Methanolic and Alkaloid Extracts from Mitragyna Speciosa (Rubiaceae Family) Leaves. Molecules, 14(10), 3964-3974. https://doi.org/10.3390/molecules14103964




