Flavonoids and a New Polyacetylene from Bidens parviflora Willd
Abstract
:Introduction

Results and Discussion
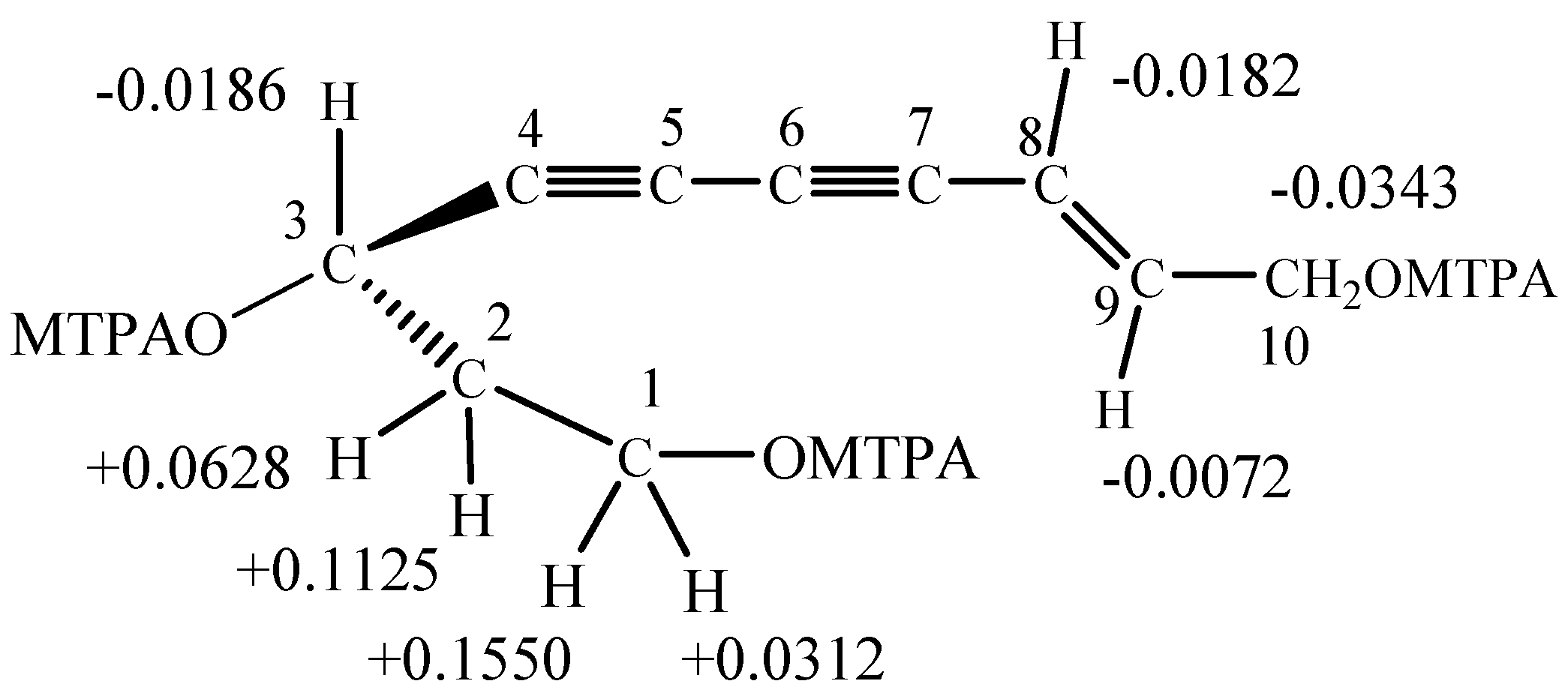
 : –16.9° (MeOH, c = 0.1), and its molecular formula was determined to be C10H12O3, as indicated by the [M+Na]+ ion at m/z 203.0679 (calcd. for 203.0687 [M+Na]+) in the HRESIMS spectrum. In the IR spectrum, absorption bands attributable to acetylene (2341 cm-1, 2360 cm-1), hydroxyl (3182 cm-1) and ethylene (1627cm-1) groups were observed. The UV spectrum of 8 was typical for an ene-diyne chromophore (λmax = 228, 240, 252, 266, 282 nm) [18]. The 13C-NMR (Table 1) and DEPT spectra of 8 present 10 carbon signals, including three methylene groups at δ 41.4, 59.1 and 62.6, one methine group at δ 60.4, two olefinic carbons at δ 108.6 and 148.2, and four quaternary carbons at δ 69.5, 74.1, 77.5 and 84.2 which were confirmed to be ethynyl carbons. Extensive analysis of the 1H-NMR spectrum, together with 1H-1H COSY and HMQC spectra, presented a methylene proton at δ 4.13 (2H, dd, J = 2.1, 4.7 Hz, H-10), coupled with two E-configured olefinic protons at δ 6.39 (1H, td, J = 4.7, 15.9 Hz, H-8) and δ 5.79 (1H, dtd, J = 0.7, 2.1, 15.9 Hz, H-9) indicating a methylene allyl moiety, two methylene protons at δ 3.69 (2H, m, H-1) and δ 1.88 (2H, m, J = 6.8 Hz, H-2), one methine proton at δ 4.57 (1H, t, J = 6.8 Hz, H-3). In the COSY spectrum, the correlations between δ 3.69 (H-1) and δ 1.88 (H-2), δ 1.88 (H-2) and δ 4.57 (H-3) suggested the presence of a CH2CH2CH moiety. In the HMBC spectrum, heteronuclear multiple-bond connectivity between the following: δH 5.79 (H-9)/δC 77.5, δH 6.39 (H-8)/δC 77.5 and δC 74.1, δH 4.13 (H-10)/δC 77.5 (C-7) could be observed; furthermore, the intensity of correlations between δH 6.39/δC 77.5 was weaker than that between δH 6.39/δC 74.1, suggesting that δC 74.1 and δC 77.5 form a alkynyl group and δC 77.5 directly connected with δC (148.2) of the CH=CHCH2 moiety, while δC 69.5 and δC 84.7 form another alkynyl. The peak at δH 4.57 (H-3) correlates simultaneously with δC 84.7, 69.5, 77.5 and 74.1, and together with δH 5.37 (H-9) presents a correlation with δC 69.5, suggesting two adjacent alkynyls, and δC 60.4 of the CH2CH2CH moiety is connected to δC 84.7. Thus, based on the chemical shifts of protons and carbons, the planar structure of compound 8 was determined to be 8-(E)-decene-4,6-diyne-1,3,10-triol. All 1H- and 13C-NMR signals as shown in Table 1 were assigned according to DEPT, HMQC, HMBC and 1H-1H COSY experiments. Figure 2 shows the key correlations presented in the 1H-1H COSY and HMBC spectra of 8.
: –16.9° (MeOH, c = 0.1), and its molecular formula was determined to be C10H12O3, as indicated by the [M+Na]+ ion at m/z 203.0679 (calcd. for 203.0687 [M+Na]+) in the HRESIMS spectrum. In the IR spectrum, absorption bands attributable to acetylene (2341 cm-1, 2360 cm-1), hydroxyl (3182 cm-1) and ethylene (1627cm-1) groups were observed. The UV spectrum of 8 was typical for an ene-diyne chromophore (λmax = 228, 240, 252, 266, 282 nm) [18]. The 13C-NMR (Table 1) and DEPT spectra of 8 present 10 carbon signals, including three methylene groups at δ 41.4, 59.1 and 62.6, one methine group at δ 60.4, two olefinic carbons at δ 108.6 and 148.2, and four quaternary carbons at δ 69.5, 74.1, 77.5 and 84.2 which were confirmed to be ethynyl carbons. Extensive analysis of the 1H-NMR spectrum, together with 1H-1H COSY and HMQC spectra, presented a methylene proton at δ 4.13 (2H, dd, J = 2.1, 4.7 Hz, H-10), coupled with two E-configured olefinic protons at δ 6.39 (1H, td, J = 4.7, 15.9 Hz, H-8) and δ 5.79 (1H, dtd, J = 0.7, 2.1, 15.9 Hz, H-9) indicating a methylene allyl moiety, two methylene protons at δ 3.69 (2H, m, H-1) and δ 1.88 (2H, m, J = 6.8 Hz, H-2), one methine proton at δ 4.57 (1H, t, J = 6.8 Hz, H-3). In the COSY spectrum, the correlations between δ 3.69 (H-1) and δ 1.88 (H-2), δ 1.88 (H-2) and δ 4.57 (H-3) suggested the presence of a CH2CH2CH moiety. In the HMBC spectrum, heteronuclear multiple-bond connectivity between the following: δH 5.79 (H-9)/δC 77.5, δH 6.39 (H-8)/δC 77.5 and δC 74.1, δH 4.13 (H-10)/δC 77.5 (C-7) could be observed; furthermore, the intensity of correlations between δH 6.39/δC 77.5 was weaker than that between δH 6.39/δC 74.1, suggesting that δC 74.1 and δC 77.5 form a alkynyl group and δC 77.5 directly connected with δC (148.2) of the CH=CHCH2 moiety, while δC 69.5 and δC 84.7 form another alkynyl. The peak at δH 4.57 (H-3) correlates simultaneously with δC 84.7, 69.5, 77.5 and 74.1, and together with δH 5.37 (H-9) presents a correlation with δC 69.5, suggesting two adjacent alkynyls, and δC 60.4 of the CH2CH2CH moiety is connected to δC 84.7. Thus, based on the chemical shifts of protons and carbons, the planar structure of compound 8 was determined to be 8-(E)-decene-4,6-diyne-1,3,10-triol. All 1H- and 13C-NMR signals as shown in Table 1 were assigned according to DEPT, HMQC, HMBC and 1H-1H COSY experiments. Figure 2 shows the key correlations presented in the 1H-1H COSY and HMBC spectra of 8. | Position | δC (ppm) | δH (ppm) | HMBC (H to C) |
|---|---|---|---|
| 1 | 59.1 | 3.69 (2H, m) | C-2, 3 |
| 2 | 41.4 | 1.88 (2H, m) | C-1, 3, 4 |
| 3 | 60.4 | 4.57 (1H, t, J = 6.8 Hz) | C-1, 2, 4, 5, 6, 7 |
| 4 | 84.2 | ||
| 5 | 69.5 | ||
| 6 | 74.1 | ||
| 7 | 77.5 | ||
| 8 | 148.2 | 6.39 (1H, td, J = 4.7, 15.9 Hz ) | C-6, 7 |
| 9 | 108.6 | 5.79 (1H, dtd, J = 0.7, 2.1, 15.9 Hz) | C-5, 6, 8, 9 |
| 10 | 62.6 | 4.13 (2H, dd, J = 2.1, 4.7 Hz ) | C-7, 8, 9 |

Experimental
General
Plant material
Extraction and Isolation
Acknowledgements
References
- Jiangsu New Medical College. Shanghai Technological Publisher: Shanghai, P.R. China, 1986; pp. 1413, 1694, 2239.
- Xia, G.; Zhang, J.; Chen, X.; Ma, L. The resource utilization of Bidens parviflora Willd. in Baoding district. Chin. Trad. Herb. Drugs 1985, 16, 37–40. [Google Scholar]
- Ma, T.B.; Li, J.L.; Yuan, J.R. Isolation and identification of flavonoid glycosides from the leaf of smallflower beggarticks (Bidens parviflora). Chin. Trad. Herb. Drugs 1991, 22, 531–533. [Google Scholar]
- Hoffman, B.; Hozel, J. Weitere acylierte Chalcone aus Bidens pilosa. Planta Med. 1988, 54, 450–451. [Google Scholar] [CrossRef]
- Hoffman, B.; Hozel, J. A methylated chalcone glucoside from Bidens pilosa. Phytochemistry 1988, 27, 3700–3703. [Google Scholar] [CrossRef]
- Tommasi, N.De; Piacente, S.; Pizza, C. Flavonol and Chalcone Ester Glycosides from Bidens andicola. J. Nat. Prod. 1998, 61, 973–977. [Google Scholar] [CrossRef]
- Sashida, Y.; Ogawa, K.; Kitada, M.; Karikome, H.; Mimaki, Y.; Shimomura, H. New aurone glucosides and phenylpropanoid glucosides from Bidens pilosa. Chem. Pharm. Bull. 1991, 39, 709–711. [Google Scholar] [CrossRef]
- Redl, K.; Davis, B.; Bauer, R. Chalcone glycosides from Bidens campylotheca. Phytochemistry 1993, 32, 218–220. [Google Scholar] [CrossRef]
- Bauer, R.; Redl, K.; Davis, B. Four polyacetylene glucosides from Bidens campyplotheca. Phytochemistry 1992, 31, 2035–2037. [Google Scholar] [CrossRef]
- Chen, A.H.; Lin, S.R.; Hong, C.H. Hua Hsueh 1975, 9, 42–48.
- Wang, N.L.; Yao, X.S.; Ishii, R.; Kitanaka, S. Bioactive sucrose esters from Bidens parviflora willd. Phytochemistry 2003, 62, 741–746. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.L.; Yao, X.S.; Kitanaka, S. Structures and anti-histamine activities of phenolic acid derivatives from Bidens parviflora Willd. Chin. J. Med. Chem. 2006, 16, 168–171. [Google Scholar]
- Wang, N.L.; Yao, X.S.; Ishii, R.; Kitanaka, S. Antiallergic agents from natural sources. 3.1) Structures and inhibitory effects on nitric oxide production and histamine release of five novel polyacetylene glucosides from Bidens parviflora Willd. Chem. Pharm. Bull. 2001, 49, 938–942. [Google Scholar] [CrossRef]
- Wang, N.L.; Wang, J.; Yao, X.S.; Kitanaka, S. Two new monoterpene glycosides and a new (+)-jasmololone glucoside from Bidens parviflora Willd. J. Asian Nat. Prod. Res. 2007, 9, 449–455. [Google Scholar] [CrossRef]
- Wang, N.L.; Wang, J.; Yao, X.S.; Kitanaka, S. Two neolignan glucosides and antihistamine release activities from Bidens parviflora Willd. Chem. Pharm. Bull. 2006, 54, 1190–1192. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.L.; Yao, X.S.; Kitanaka, S. Phenolic glucosides from Bidens parviflora and their anti-histamine activities. Chin. Trad. Herb. Drugs 2007, 38, 647–649. [Google Scholar]
- Wang, J.; Wang, N.L.; Yao, X.S.; Kitanaka, S. Caffeoylquinic acid derivatives from Bidens parviflora and their antihistamine release activites. Chin. Trad. Herb. Drugs 2006, 37, 966. [Google Scholar]
- Bohlman, E.; Burkhardt, T.; Zdero, C. Naturally Occuring Acetylenes; Academic Press: London, UK, 1973. [Google Scholar]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT-IR application of Mosher’s method-the absoluteconfiguration of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Wanjala, C.W.; Majinda, R.T. Flavonoid glycosides from Crotalaria podocarpa. Phytochemistry 1999, 51, 705–707. [Google Scholar] [CrossRef]
- Kawashty, S.A.; El-Garf, I.A. The flavonoid chemosystematics of Egyptian Verbena species. Biochem. System. Ecol. 2000, 28, 919–921. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, X.Y.; Ying, H.P.; Cheng, T.M.; Zhao, Y.Y. Studies on the Chemical Constituents of Carduus crispus L. China J. Chin. Mat. Med. 2001, 26, 837–839. [Google Scholar]
- Wang, J.H.; Wang, Y.L.; Luo, F.C. Study on the chemical constituents from seeds Sophora japonica. Chin. Trad. Herb. Drugs 2001, 32, 471–473. [Google Scholar]
- Harborne, J.B. The Flavonoids: Advances in Reseach since 1986; Chapman & Hall: London, UK, 1994; pp. 450–451. [Google Scholar]
- Jia, L.Y.; Sun, Q.S.; Huang, S.W. Isolation and identification of flavonoids from chrysanthemum moriflolium Ramat. Chin. J. Med. Chem. 2003, 13, 159–161. [Google Scholar]
- Markham, K.R.; Ternai, B.; Stanley, R.; Geiger, K.; Mabry, T.J. Carbon-13 NMR studies of flavonoids-Ⅲ naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 1978, 34, 1389–1397. [Google Scholar] [CrossRef]
- Nunziatina, D.T.; Cosimo, P. Flavonol and Chalcone Ester Glycosides from Bidens leucantha. J. Nat. Prod. 1997, 60, 270–273. [Google Scholar] [CrossRef]
- Nunziatina, D.T.; Sonia, P.; Cosimo, P. Flavonol and Chalcone Ester Glycosides from Bidens andicola. J. Nat. Prod. 1998, 61, 973–977. [Google Scholar] [CrossRef]
- Jia, Z.J.; Gong, N.C.; Du, M. Chemical constituents of Saussurea medusa Maxim. Chem. J. Chin. Univ. 1990, 11, 202–204. [Google Scholar]
- HU, H.Y.; Yang, Y.; Yu, N.J.; Zhao, Y.M. Study on chemical constituents of bark of Paeonia suffruticos. China J. Chin. Mat. Med. 2006, 31, 1795–1797. [Google Scholar]
- Phrutivorapongkul, A.; Lipipun, V.; Ruangrungsi, N.; Kirtikara, K.; Nishikkawa, K.; Maruyama, S.; Watanabe, T.; Ishikawa, T. Studies on the chemical constituents of stem bark of Millettia leucantha: Isolation of new chalcones with cytotoxic, anti-herpes simplex virus and anti-inflammatory activities. Chem. Pharm. Bull. 2003, 51, 187–190. [Google Scholar] [CrossRef]
- Wei, Y.H.; Wu, X.A.; Zhang, C.Z.; Li, C.; Song, L. Studies on Chemical Constituents of Rheum glabricaule Sam.(II). Chin. Pharm. J. 2006, 41, 253–254. [Google Scholar]
- Li, X.; Shi, R.B.; Liu, B.; Chen, Y.P. Study on chemical components from effective fraction of Qingnaoxuanqiao Formula (II). J. Beijing Univ. Trad. Chin. Med. 2006, 29, 545–550. [Google Scholar]
- Venkateswarlu, S.; Panchagnula, G.K.; Subbaraju, G.V. Synthensis and antioxidative activity of 3', 4', 6, 7-tetrahydroxyaurone, a metabolite of Bidens frondosa. Biosci. Biotechnol. Biochem. 2004, 68, 2183–2185. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.P.; Zhao, M.B.; Jiang, Y.; Tu, P.F. A novel chalcone from Coreopsis tinctoria Nutt. Biochem. System. Ecol. 2006, 34, 766–769. [Google Scholar]
- Tian, G.l.; Zhang, B.; Zhang, T.Y.; Yang, F.Q.; Yoichiro, I. Separation of flavonoids from the seeds of Vernonia anthelmintica Willd by high-speed counter-current chromatography. J. Chromatogr. A 1049, 219–222. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 3, 4, 6, 8, 9, 11-15 are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.-L.; Li, J.; Wang, N.-L.; Yao, X.-S. Flavonoids and a New Polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931-1941. https://doi.org/10.3390/molecules13081931
Li Y-L, Li J, Wang N-L, Yao X-S. Flavonoids and a New Polyacetylene from Bidens parviflora Willd. Molecules. 2008; 13(8):1931-1941. https://doi.org/10.3390/molecules13081931
Chicago/Turabian StyleLi, Yu-Lan, Jun Li, Nai-Li Wang, and Xin-Sheng Yao. 2008. "Flavonoids and a New Polyacetylene from Bidens parviflora Willd" Molecules 13, no. 8: 1931-1941. https://doi.org/10.3390/molecules13081931
APA StyleLi, Y.-L., Li, J., Wang, N.-L., & Yao, X.-S. (2008). Flavonoids and a New Polyacetylene from Bidens parviflora Willd. Molecules, 13(8), 1931-1941. https://doi.org/10.3390/molecules13081931




