Abstract
Recently, World Health Organization (WHO) and Medicins San Frontieres (MSF) proposed a classification of diseases as global, neglected and extremely neglected. Global diseases, such as cancer, cardiovascular and mental (CNS) diseases represent the targets of the majority of the R&D efforts of pharmaceutical companies. Neglected diseases affect millions of people in the world yet existing drug therapy is limited and often inappropriate. Furthermore, extremely neglected diseases affect people living under miserable conditions who barely have access to the bare necessities for survival. Most of these diseases are excluded from the goals of the R&D programs in the pharmaceutical industry and therefore fall outside the pharmaceutical market. About 14 million people, mainly in developing countries, die each year from infectious diseases. From 1975 to 1999, 1393 new drugs were approved yet only 1% were for the treatment of neglected diseases [3]. These numbers have not changed until now, so in those countries there is an urgent need for the design and synthesis of new drugs and in this area the prodrug approach is a very interesting field. It provides, among other effects, activity improvements and toxicity decreases for current and new drugs, improving market availability. It is worth noting that it is essential in drug design to save time and money, and prodrug approaches can be considered of high interest in this respect. The present review covers 20 years of research on the design of prodrugs for the treatment of neglected and extremely neglected diseases such as Chagas’ disease (American trypanosomiasis), sleeping sickness (African trypanosomiasis), malaria, sickle cell disease, tuberculosis, leishmaniasis and schistosomiasis.
Introduction
Billions of dollars are invested each year in research and development (R&D) of drugs for conditions that affect people who can pay, which include obesity, baldness, and ageing. However, the World’s poor are left completely marginalized. Tropical infectious diseases, including protozoan infections, helminths, as well as other diseases such as sickle cell disease are known as neglected diseases [1,2]. Recently, these diseases were officially classified as neglected and/or extremely neglected diseases by the World Health Organization and Medicins San Frontieres. They affect areas of extreme poverty and are themselves poverty promoting. For this very reason, they fail to attract attention from the drug developers, who do not consider this group of diseases as a lucrative target [3,4]. The market logic is: no profit, no investment [5].
Only 10% of global health research is related to diseases that account for 90% of the global disease burden, which urgently need new drugs. From 1975 to 1999, 1,393 new drugs were launched in the market, but only 16 (1%) were for the treatment of tropical diseases [3]. Science has advanced in biology, molecular biology and also genetics of the etiologic agents that cause those diseases. However, this knlowledge has not been used for drug design [6] and this is not acceptable.
Different basic approaches to drug discovery for tropical diseases included combinations of existing drugs, new indications for existing drug, improvements to known drugs and compound classes and focused sample collections [7]. The prodrug approach was developed to overcome mainly pharmacokinetics problems of many therapeutic classes of drugs [8;9]. A prodrug is an inactive drug carrier form that requires a metabolic activation step before it exhibits its pharmacological effects in a target tissue. It can provide reduced toxicity, improved therapeutic index, slow release of the parent drug and also improved selectivity towards the target tissue or cell. In this work we have focused in prodrugs for the following neglected diseases: Chagas’disease, sleeping sickness, leishmaniasis, malaria, schistosomiasis, tuberculosis and sickle cell disease.
Chagas’disease
In 1909 Carlos Chagas discovered American trypanosomiasis, commonly known as Chagas´ disease, which is a parasitosis caused by Trypanosoma cruzi. The disease is mostly spread throughout Latin America, affecting 21 countries [10]. It is estimated that some 16 to 18 million people are infected by the parasite and 50,000 people die annually as a consequence of the illness [11]. Chagas’ disease remains essentially incurable. The pharmaceutical industry has had limited interest in developing new antichagasic drugs, due principally to a lack of commercial incentives [12]. Benznidazole and nifurtimox are the available drugs for the treatment of this parasitosis. However, both drugs are toxic nitroheterocyclic derivatives and almost only effective in the acute phase of the disease. They have been used as long-term treatment [13]. These drugs cure only a very low percentage of chronic patients [12] and natural resistance of T. cruzi to nitro derivatives has been suggested as an important factor to explain the low rates of cure detected in chagasic patients [12]. Thus, a search for new drugs against T. cruzi is of utmost importance and must be emphasized.
Sleeping sickness
Human African Trypanosomiasis (HAT), or sleeping sickness, is caused by infection with parasitic protozoa of the Trypanosoma brucei (T. brucei) subspecies, which are introduced to the human bloodstream by the bites of infected tsetse flies in the inter-tropical regions of Africa. Trypanosoma brucei gambiense, found in West and Central Africa, leads to a chronic form of the disease [14]. Over 60 million people living in 36 countries are at risk of acquiring sleeping sickness and the estimated number of people affected is between 300,000 and 500,000 [15].
Chemotherapy of African trypanosomiasis is still based on old drugs and some of these show toxic side effects. Suramin and pentamidine are the drugs of choice against the Rhodesian and Gambian forms of the disease, respectively, before the onset of central nervous system symptoms. Late-stage disease is treated with the melaminophenyl arsenical drug melarsoprol. In the case of the disease caused by T. b. gambiense, eflornithine (DFMO) is also used [16]. New drugs are urgently needed for human African trypanosomiasis and the emergence of drug resistance is likely to be a challenge that must be faced.
Leishmaniasis
Parasites from Leishmania genus are the causative agents of leishmaniasis, a group of protozoan diseases transmitted to mammals, including human beings, by phlebotomine sandflies. Globally, there are an estimate of 350 million people that are at risk of infection and disease [17]. Among all parasitic infections, it is considered the second most important from the socioeconomics point of view. It affects 12 million people in 88 countries, there are 1.5–2 million new cases and 70,000 deaths each year, and 350 milllion are under the risk of contamination [17]. Only malaria and schistosomiasis, respectively, present more expressive numbers than leishmaniasis [18, 19].
The clinical forms of the disease range from cutaneous leishmaniasis to the most severe visceral infection (known as kala-azar in India), fatal if untreated [20]. Chemotherapy for leishmaniasis is scarce and limited to pentavalent antimonials, pentamidine, amphotericin B, miltefosine, fluconazole and few other drugs at various stages of their development process [21,22,23], drugs with undesirable side effects. The latter and the emergence of drug resistance have been the driven force to researchers to search for new potential compounds [24,25,26].
Malaria
Malaria is a life-threatening parasitic infection caused by parasites of genus Plasmodium -- P. falciparum, P. vivax, P. ovale and P. malariae – from which P. falciparum is the only one capable of producing fatal complications. Over three billion people live under the threat of malaria and it has been estimated that there are 300 to 500 million new cases of malaria resulting in approximately two million deaths per year. The majority of these cases occur in the poorest countries [27]. With the absence of an effective vaccine and global emergence of multidrug resistance, malaria continues to be a worldwide epidemic. Resistant strains of malaria have been identified for most of the antimalarial drugs in clinical use today [28]. Many drugs, alone or in combination – chloroquine, primaquine, mefloquine, halofantrine, artemisinin, atovaquone, among others – have been used for malaria chemotherapy. However, drug or multi-drug resistance has been a challenge for chemotherapy effectiveness [29]. The need for drugs against tecidual forms as well as the emergence and spread of resistance against chloroquine and other major antimalarial drugs have brought the urgency to develop a new generation of safe and effective agents [1].
Schistosomiasis
Schistosomiasis is caused by a trematode of the genus Schistosoma and five major species, S. mansoni, S. japonicum, S. haematobium, S. intercalatun and S. mekong, infect humans. It is estimated that two billion people are chronically infected with soil transmitted helminths and schistosomes, many suffering from severe morbidity. World Health Organization has estimated that some 200 million people are infected, a further 600 million people are at risk of infection and more than 200,000 deaths per year ocurr just from schistosomiasis [30]. It ranks second only to malaria considering the extent of endemic areas and the number of infected people. It is distributted in around 52 countries in South America, Caribbean, Africa and East Mediterranean. The intermediate host of the parasite is a snail widespread in fresh water, and it is skin contact with this water that permits infection. Schistosomiasis represents both public health and socioeconomics challenges.
Chemotherapy has become the most effective means of endemic disease control and therapeutic drugs available are: metrifonate, oxamniquine and praziquantel. In some countries only oxamniquine and praziquantel are used. The latter has been the drug of choice as it is effective against all human schistosomose. However, there is considerable concern about the development of resistance. Oxamniquine is used as second drug of choice, due to its side effects, mainly those associated to CNS [30]. Since vaccine development is still far from practical application, the design of new schitosomicide drugs are urgently needed. However, as in other neglected diseases, this is too expensive and is not economically attractive to industry [31].
Tuberculosis
Parallel to the tropical endemic diseases, tuberculosis (TB) appears as great challenge for the world-wide organizations and related groups of research involved in health. TB is the most serious infectious lung disease in the world and the leading cause of death from a single infectious organism. It is spreaded worldwide but affects mainly African regions. It was estimated that it maintains hidden in two billion people [32] and between 2005 and 2020, one billion people will be newly infected, over 125 million people will get sick, and 30 million will die of TB if control is not further strengthened [33]. HIV infection, which compromises cell-mediated immunogenicity [34], increases to 50% the percentage of people infected with M. tuberculosis who would develop active TB during a shortened lifetime [35]. This and the growing TB emergence of drug resistant strains of Mycobacterium tuberculosis [36] has been the major challenges and claim, urgently, for new and better drugs.
Sickle cell disease
Sickle cell disease (SCD), an inherited disorder of haemoglobin synthesis, is a single point mutation that substitutes valine for glutamic acid in the β-globin subunit [37].It causes deformation of the red blood cells that can result in vaso-occlusive events [38], ischaemia and tissue and organ damage as well [39,40] and can be fatal. This condition is prevalent mainly in tropical areas and predominates in Africa. Millions of people throughout the world have been affected with this disease, mainly from Sub-Saharan Africa, Latin America, United States, Saudi Arabia, India, and Mediterranean countries as Turkey, Greece, and Italy. The sickle gene has however have a genetic advantage: it protects heterozygous carriers from succumbing to endemic Plasmodium falciparum malaria infection [41]. Hydroxyurea is the only US Food and Drug Administration (FDA)-approved drug for the treatment of SCD. Some compounds have been studied for sickle cell treatment like: butyric acids, aldehydes, decitabine, clotrimazole, L-arginine, zileuton [42,43,44].
For most of the neglected diseases the improvement of pharmaceutical, pharmacokinetic or even pharmacodinamic profile of the existant drugs using molecular modification processes is a recommended alternative. From those methods, prodrug design is one of the most promising [9,45]. We describe, in this paper, the main results of 20 years of research into prodrug design against some neglected diseases.
Antichagasic prodrugs
Prodrugs of primaquine and nitrofurazone
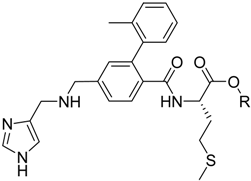
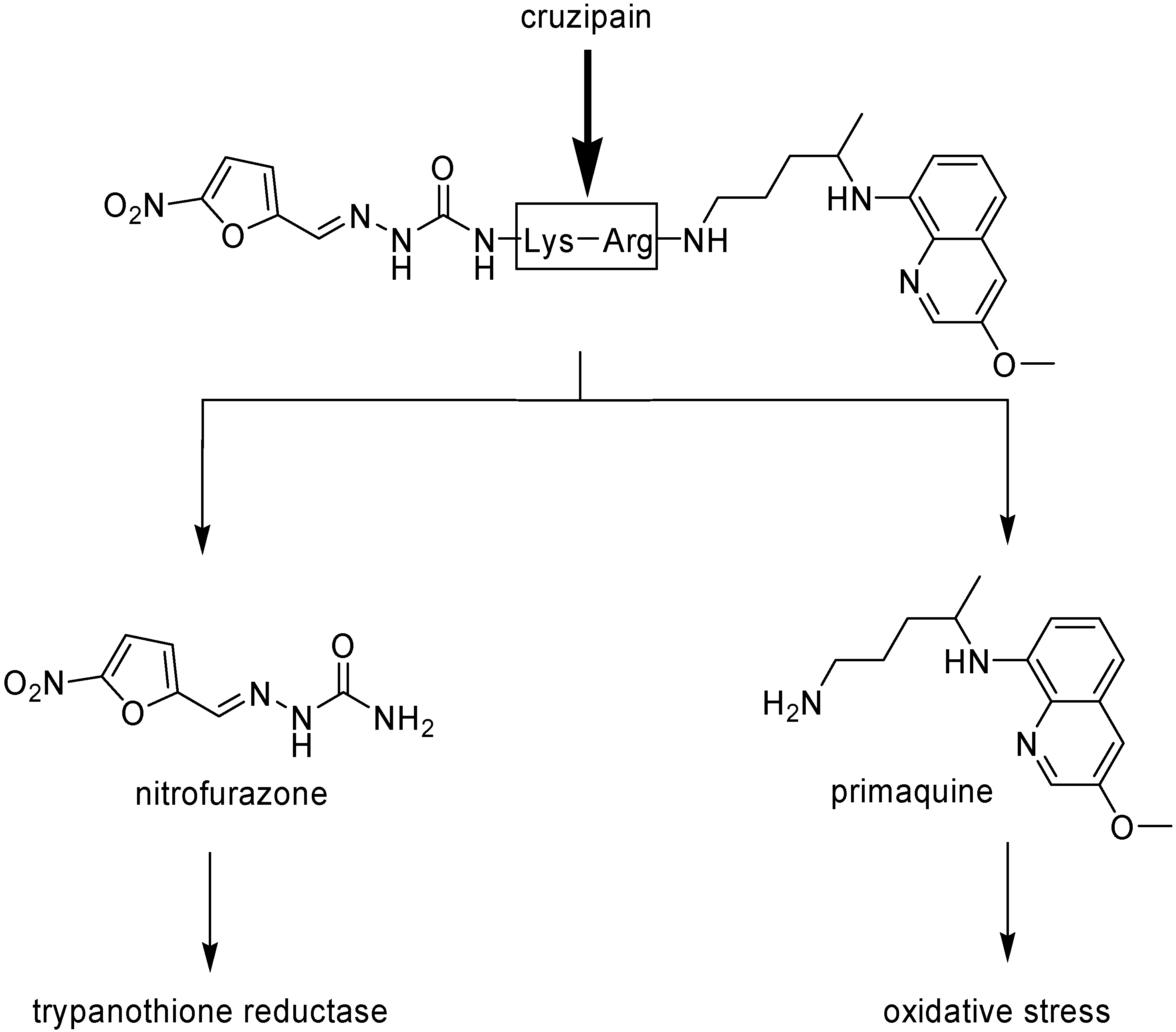
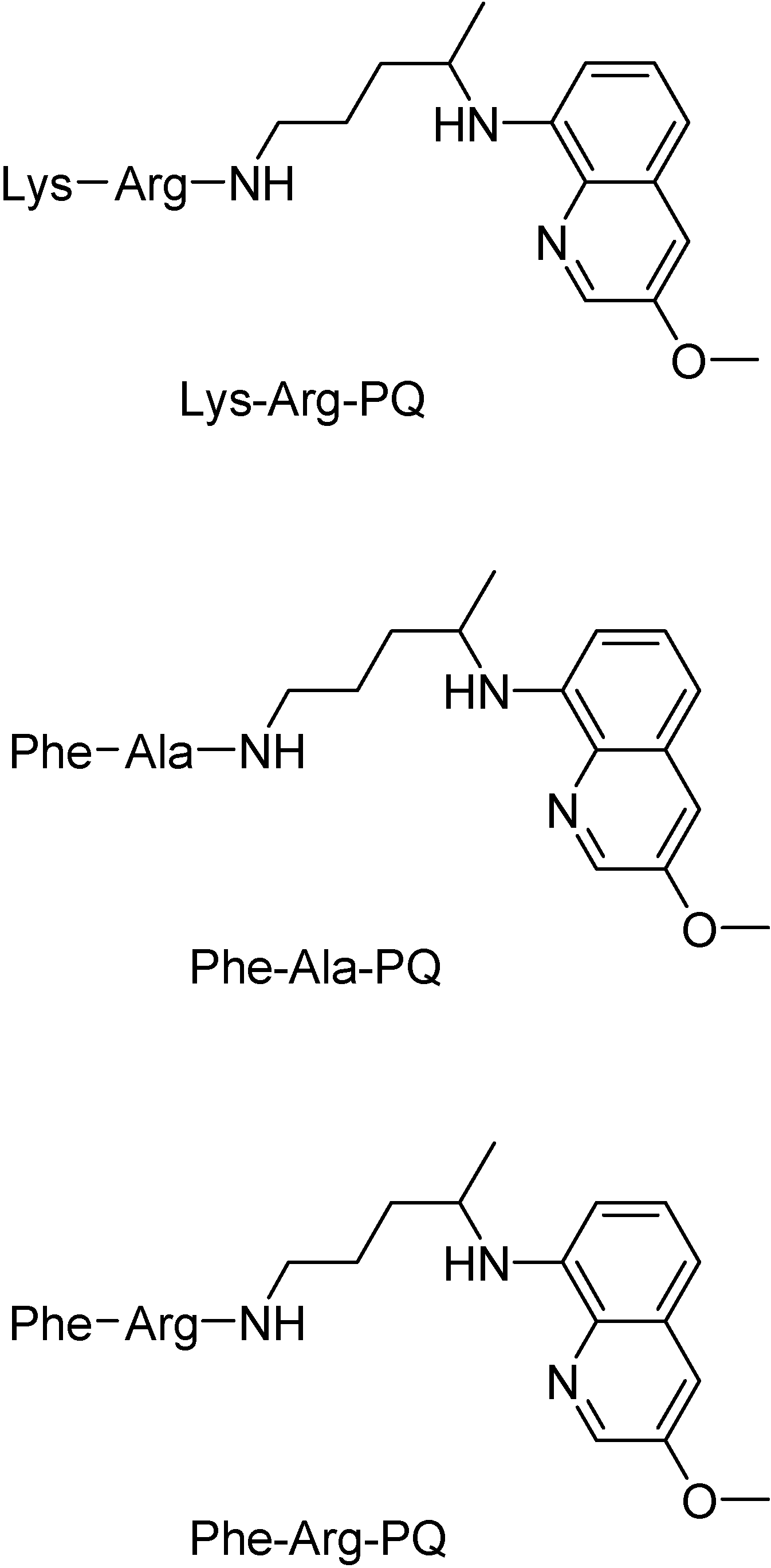
Chung, in 1996, synthesized mutual prodrugs of primaquine (PQ) and nitrofurazone (NF) using dipeptides as spacer groups. These peptides are selectively cleaved by cruzipain, a cysteinyl-protease found only in T. cruzi [46]. The rationale for this approach was the mechanism of the drugs: while primaquine increases the oxidative stress into the parasite, nitrofurazone (NF), as a trypanothione reductase inhibitor [47], does not allow the protective action of the enzyme, provoking the increase in the intracellular oxidative stress. The compound in which Lys-Arg was the spacer group has been the most active (Figure 1).
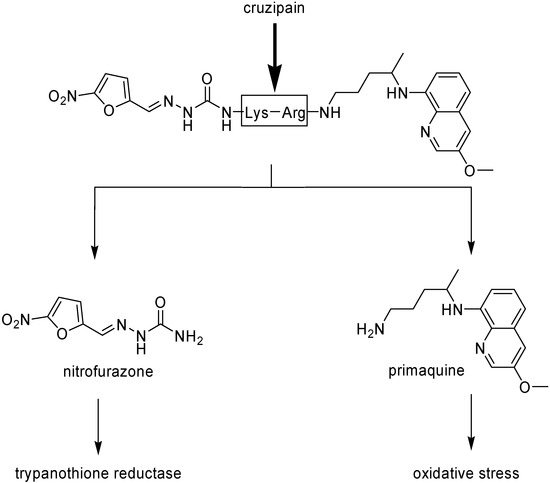
Figure 1.
Chemical structure of the mutual prodrug of nitrofurazone and primaquine and its conversion to the parent drugs by cruzipain.
Figure 1.
Chemical structure of the mutual prodrug of nitrofurazone and primaquine and its conversion to the parent drugs by cruzipain.
Dipeptide prodrugs of primaquine as synthesis intermediates were also shown to be active on LLC-MK2 cell culture infected with trypomastigotes forms of T. cruzi. It was demonstrated that Lys-Arg-PQ is active on T. cruzi development inside host cells, probably by interfering in the initial steps of trypomastigote-amastigote transformation. It was found that Lys-Arg-PQ is more active than Phe-Ala-PQ and Phe-Arg-PQ, suggesting that the specific cleavage has an important role in the release of PQ. The dipeptide Lys-Arg, putatively specific for cruzipain, was a good carrier for PQ and has potential to be used as a spacer group for the development of other PQ prodrugs in the future (Figure 2). Thus, Lys-Arg-PQ is a very promising compound for in vivo tests [48].
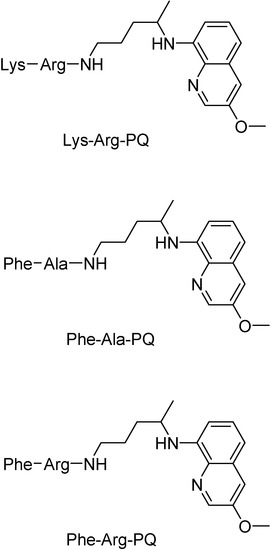
Figure 2.
Chemical structures of dipeptide prodrugs of primaquine.
Figure 2.
Chemical structures of dipeptide prodrugs of primaquine.
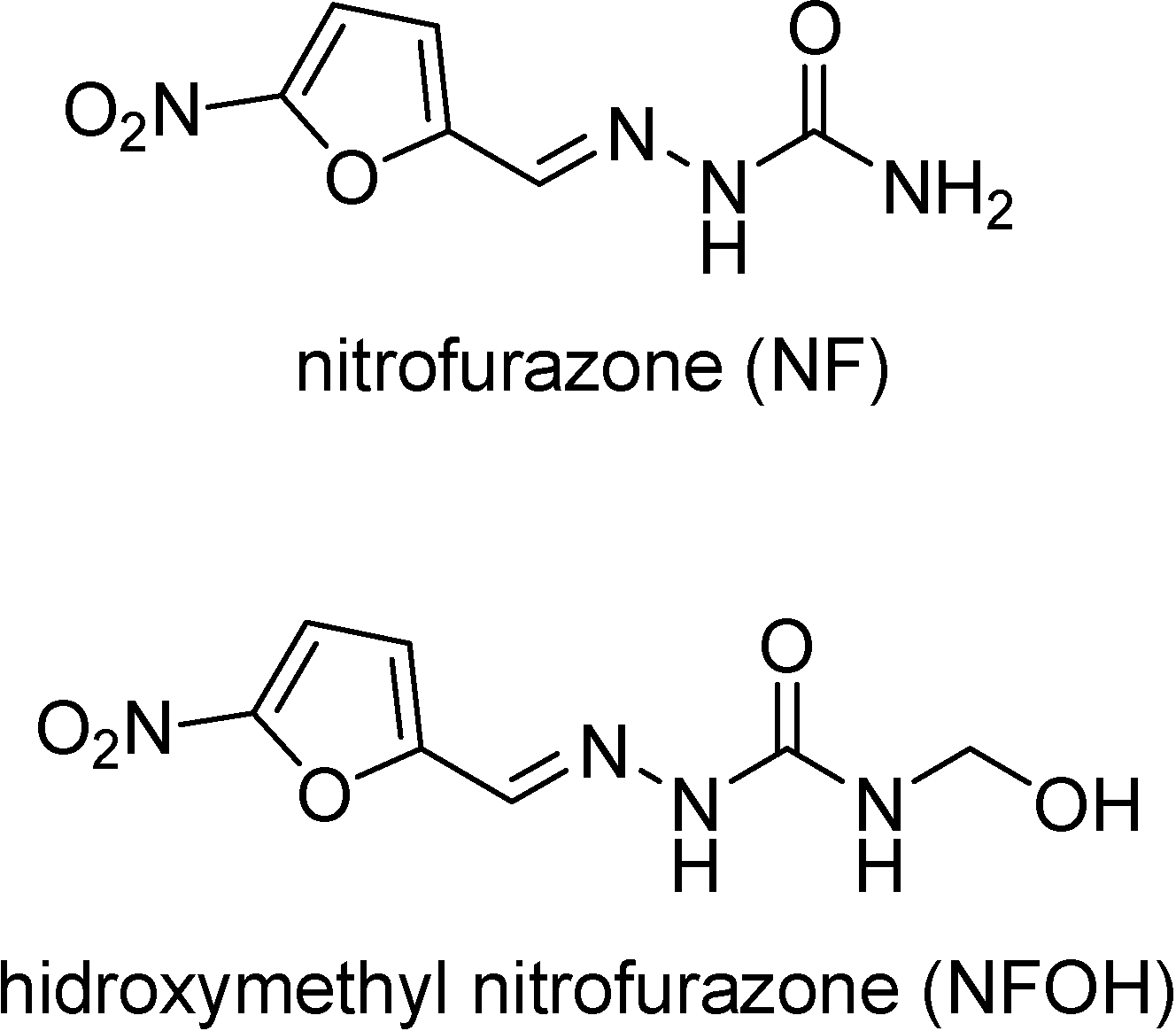
T. cruzi trypanothione reductase has been considered a key enzyme in the oxidative metabolism of the parasite [47] and nitrofuran derivatives have shown to produce irreversible inactivation of that enzyme under anaerobic as opposed to aerobic conditions. Hydroxymethyl derivatives are prodrugs whose main goal is to provide more hydrophilic derivatives than the parent compound. In general, NH acidic drugs such as amides, imides and ureides are potential targets to N-hydroxymethylation [49]. Chung synthesized the nitrofurazone hydroxymethyl derivative (NFOH) (Figure 3), as a synthesis intermediate of mutual prodrugs of primaquine and nitrofurazone, potentially active in Chagas’ disease. This compound was shown to be highly active in cell cultures infected with Trypanosoma cruzi and much less toxic than the drug, when subtmitted to Ames’ mutagenicity test [50].
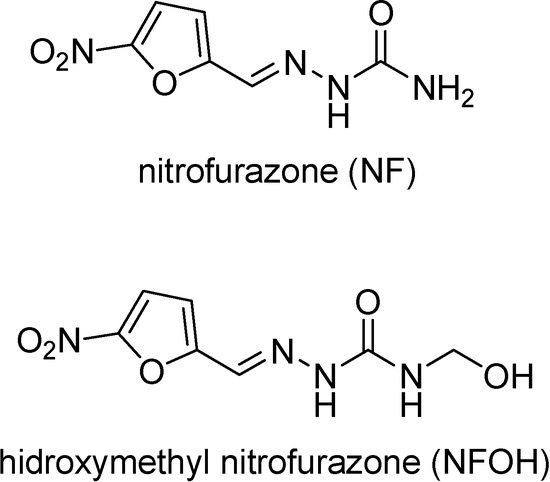
Figure 3.
Chemical structures of nitrofurazone and its prodrug hydroxymethyl nitrofurazone.
Figure 3.
Chemical structures of nitrofurazone and its prodrug hydroxymethyl nitrofurazone.
The chemical hydrolysis at pH 1.2 and 7.4 showed that NFOH was completed converted to NF within 16 hours; however the recovery of NF occurs just 1 hour after hydrolysis. This fact suggests that the conversion of NFOH into NF is carried out by a two-step pathway, in which there is the formation of an intermediate compound. The half-life at pH 1.2 was about 1.5 hours while at pH 7.4 it takes 134 hours to be 50% hydrolyzed with no formation of an intermediate compound. These facts suggest the great stability of this compound at physiological pH [50].
Later, the group extended their studies with NFOH and dipeptidic prodrugs to other fields. Molecular modeling studies have been performed to evaluate possible interactions between the prodrugs and cruzain, one of the possible molecular targets of these derivatives [51]. The AM1 study suggests that the aminoacid nature and dipetide sequence are determinant for the approximation and nucleophilic attack of cruzain, as observed in the irreversible inhibitors model. Steric and electrostatic interactions in the double prodrugs indicate the spacer group AlaPhe as the most favorable sequence relative to PheAla. In addition, the LysArg spacer group seems to be the best substrate for the enzyme.
Semi-empirical AM1 studies were applied to the mutual dipeptide prodrugs of primaquine and nitrofurazone to understand the release of the drugs by cruzain. The work suggested that the aminoacid sequence is determinant to this release. The volume and electronic nature of the aromatic nucleus of Phe, comparatively to those of Ala, and the possibility of steric and electrostatic interactions between the side chains of the aminoacids and the drugs lead to different rearrangements that allow or avoid cruzain approximation ant attack [52].
Also, in order to find the reason of higher activity of NFOH than NF, the voltametric behaviour of the drug and its prodrug was also determined by Scalea and co-workers [53]. No differences have been detected in the reduction capability of the nitrogroup.
Prodrugs of N-isopropyl oxamate
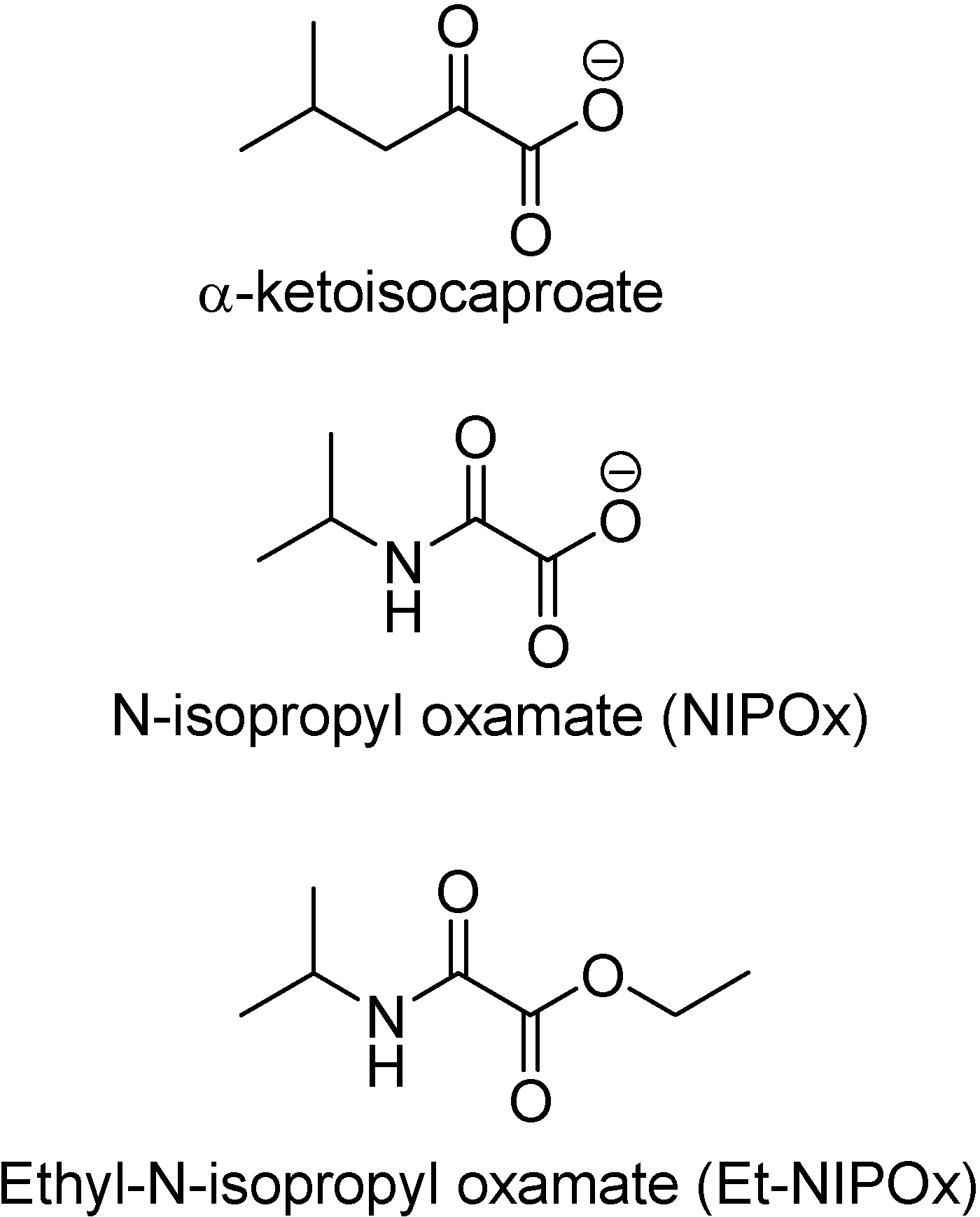
Trypanosoma cruzi possesses an enzyme similar to the lactate dehydrogenase isozyme-x (LDH-C4) from mammalian spermatozoa [54]. This enzyme has two molecular forms (HADH-isozyme I and HADH-isozyme II). Isozyme I is responsible for the weak lactate dehydrogenase activity found in T. cruzi extracts [55], while isoenzyme II is not active against pyruvate but on many linear and branch chain substrates, especially α-ketoisocaproate [56]. Its similarity to LDH-C4 leads to analogous functions in T. cruzi [57]. These enzymes display a role in supplying energy for the motility of the flagellum and the survival of the parasite [56;57] This hypotheses was advanced based on the structural similarity between N-isopropyl oxamate (NIPOx) and the substrate α-ketoisocaproate (Figure 4).
Elizondo and co-workers (2003) have investigated and demonstrated that NIPOx was a selective inhibitor of HADH-isozyme II from T. cruzi. However, this compound did not show any activity when tested on V2R T. cruzi epimastigote culture, unless it was transformed in its prodrug, the ethyl ester of N-isopropyl oxamate (Et-NIPOx). Actually, it was even better than nifurtimox and benznidazol [58]. A hypothesis advanced to explain this effect was the existance of carboxylesterases, aliphatic or non-specific esterases, in T. cruzi epimastigotes [59].
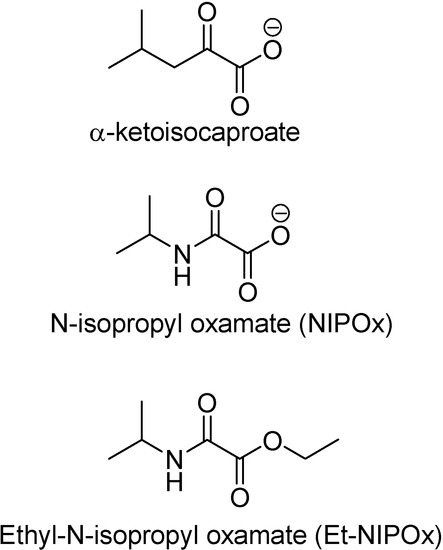
Figure 4.
Chemical structures of α-ketoisocaproate, NIPOx and Et-NIPOx.
Figure 4.
Chemical structures of α-ketoisocaproate, NIPOx and Et-NIPOx.
Increased effectiveness of Et-NIPOx most probably resulted from their better absorption by this parasite and its efficient hydrolysis by carboxylesterases in situ into the active HADH inhibitor [58]. This was later also confirmed in vivo [60], when Et-NIPOx was tested on mice parasitaemia, exhibiting trypanocidal activity in the five tested T. cruzi strains. It is worth to note that benznidazol and nifurtimox, submitted to the same test, were active only against three out of five tested T. cruzi strains, probably due to natural resistance of the parasite to nitroderivatives [61].
Prodrugs for Sleeping Sickness
ADEPT approach and mAb-drug conjugate for treatment of African trypanosomiasis
Trypanosomes protect themselves from host immune responses by continuously changing the VSGs that cover their entire membrane, lowering the possibility of developing a conventional vaccine [62,63,64]. Thus, it is important to search for chemotherapeutics to treat sleeping sickness (African trypanosomiasis).
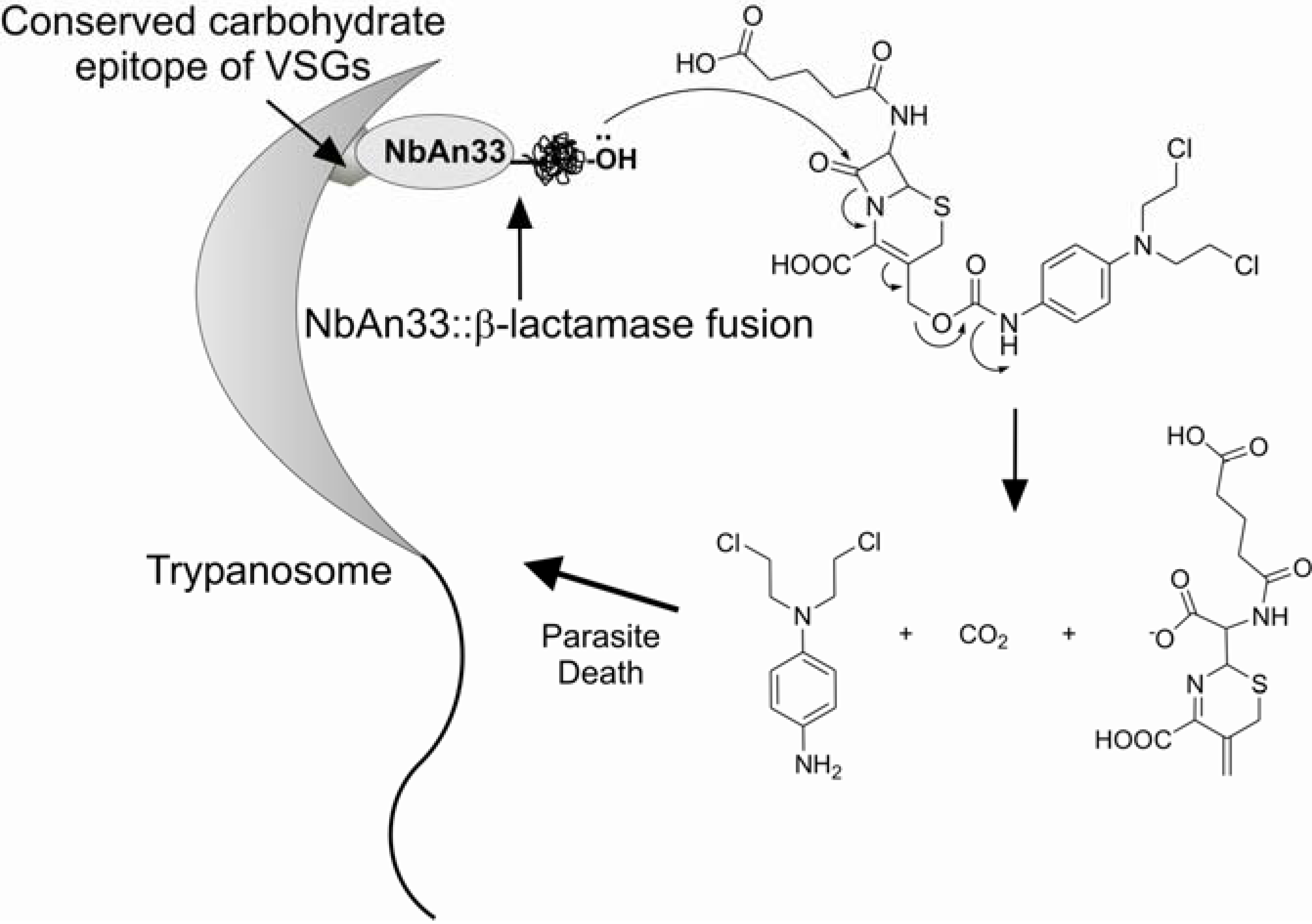
Antibody-Directed Enzyme Prodrug Approach, ADEPT, is one of the highest mean to accomplish selectivity in the prodrug design field [65]. It requires monoclonal antibodies and enzymes that are not common in the mammalian host. The system requires two phases: first, an interaction of the conjugate enzyme-antibody with the antigen and second, the relase of drug from classical prodrug by the enzyme of the previous conjugate. One of the most used enzymes in the conjugate has been β-lactamase along with cephalosporins as the carrier for the second phase. Pratt [66] has shown that when cephalosporins are hydrolysed by a β-lactamase the C-3’ substituent is expelled depending on its living-group reactivity (Figure 5).
In the case of African trypanosomiasis, isolation of a monoclonal antibody reactive toward multiple VSGs on viable parasites has been a challenge, as the conserved epitopes become cryptic upon the assembly of VSGs on the membrane of the parasite. To circumvent this problem, Stijlemans and co-workers developed an ADEPT approach potentially useful for the treatment of African trypanosomiasis using a nanobody named NbAn33, a mAb specific for the conserved carbohydrate epitope of VSGs fused to a β-lactamase enzyme for activation of a cephalosporin mustard prodrug [67,68]. Nanobodies were introduced by Stijlemans and co-workers [69] and are antigen-binding fragment with a molecular mass of only 15 kDa, coming from the serum of Camelidae which contains a high proportion of functional antibodies with no light chains [70]. The in vitro assay was carried out with sub-lethal doses of 0.5 to 10 μM of the prodrug following the incubation with the NbAn33::β-lactamase conjugate and succeeded.
Recently a human-specific serum protein, apoL-I, was found to be the trypanolytic factor of normal human serum (NHS) [71;72]. Also, it was discovered that trypanosomes become resistant to NHS after expression of the apoL-I-neutralizing serum resistance-associated (SRA) protein [73]. Based on these findings, a Tr-apoL-I, which cannot be neutralized by SRA and thus is capable of lysing both NHS-sensitive and NHS-resistant T. b. rhodesiense [74], was obtained. This compound is a natural trypanolytic agent that can be useful to cure T. b. rhodesiense infections providing its targeting Tr-apoL-I to the parasite surface could improve its effectiveness.
According to this and considering the nanobody approach and the use of NbAn33 as a trypanosoma monoclonal antibody [69], Baral and co-workers [75] tested the performance of NbAn33-Tr-apoL-I in mouse models of African trypanosomiasis and the promising results allowed them to conclude a new trypanocidal modality for the human parasitosis treatment had been developed. The successful use of single domain antibody derivatives to combat trypanosomiasis could also be repeated to various other infectious agents.
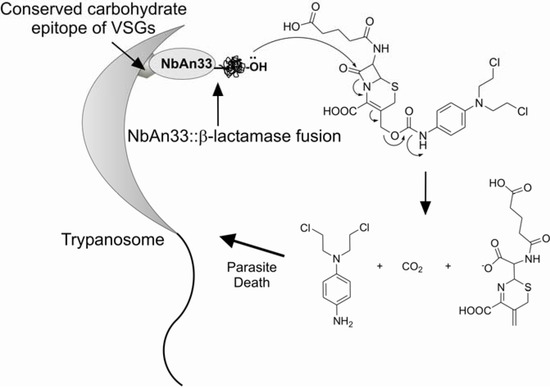
Figure 5.
ADEPT approach for African trypanosomiasis.
Figure 5.
ADEPT approach for African trypanosomiasis.
Prodrugs of aromatic diamines
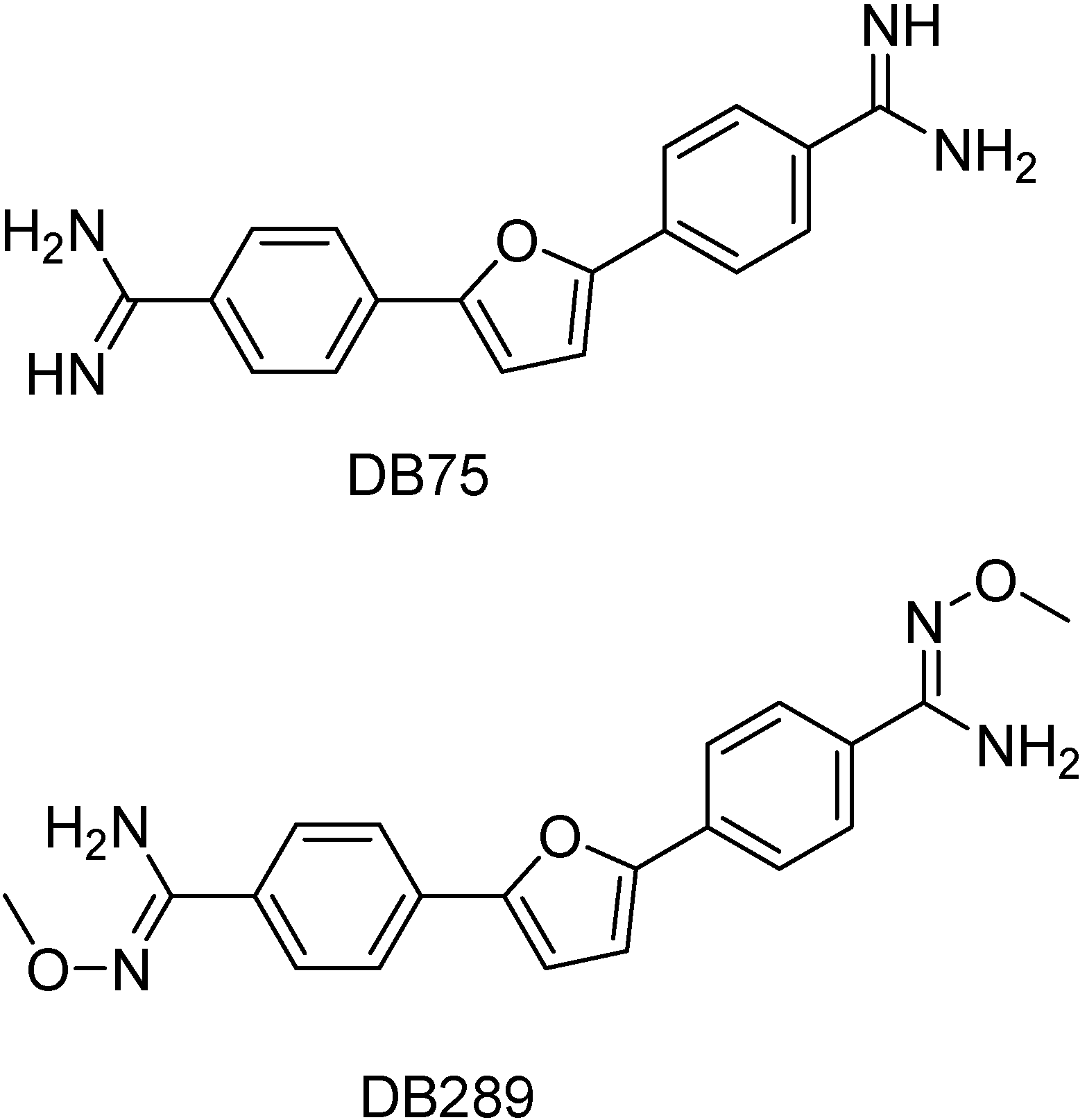
Aromatic diamines represent a class of compounds with broad-spectrum antimicrobial activity. DB75 [2,5-bis(4-amidenophenyl)furan] is an important aromatic diamine that is active against Trypanosoma sp in vitro [76;77] and DB289 [2,5-bis-(4-amidinophenyl)furan bis-O-methyl-amidoxime] is its orally active amidoxin prodrug (Figure 6). The latter is in phase IIb human clinical trials for treatment of early-stage human African trypanosomiasis, Pneumocystis jiroveci carinii pneumonia and malaria. Charlotte and co-workers found that mitochondrion is a cellular target of DB75 in yeast cell. This finding can aid in the target-based design of new antimicrobial aromatic diamines [62].
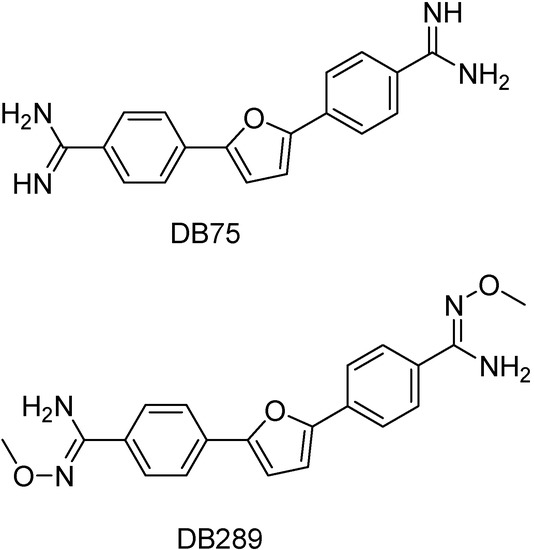
Figure 6.
Chemical structures of DB75 and its prodrug DB289.
Figure 6.
Chemical structures of DB75 and its prodrug DB289.
Prodrugs for Leishmaniasis
Glutathione prodrugs
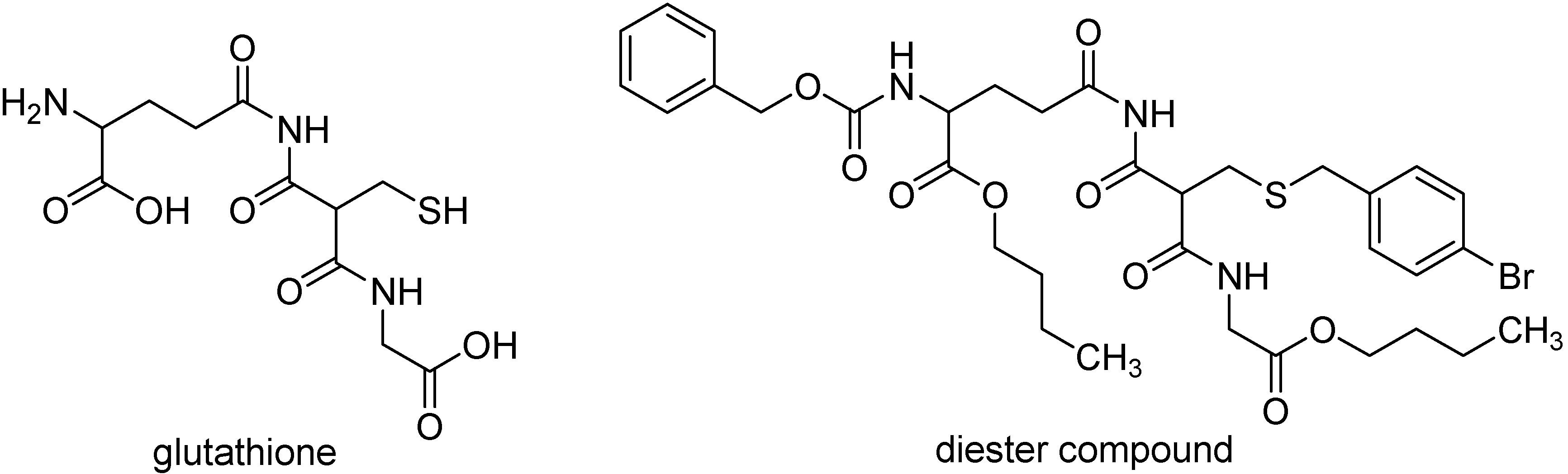
Trypanotione plays a central role in the metabolism of trypanosomatids, like T. brucei, T. cruzi and L. donovani, acting both as a source of reducing equivalents and as a defense against oxidative stress [78]. Diester compounds per se were considered to be ineffective inhibitors of trypanotione metabolism, suggesting that these compounds might act as prodrugs, increasing the membrane penetration of glutathione derivatives prior to cleavage into possible gluthatione derivatives, which includes combinations of monoesters, free acids, and amines, some of which are inhibitors of trypanothione metabolism [78]. The dibutyl ester compound presented in Figure 7 shows moderate activity against L. donovani in an in vitro assay, and a higher activity against T. brucei.
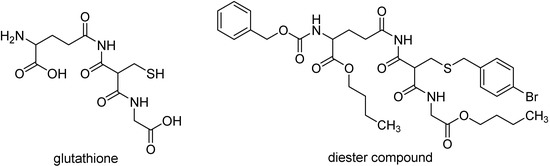
Figure 7.
Diester prodrug of glutathione derivative with antileishmaniasis activity.
Figure 7.
Diester prodrug of glutathione derivative with antileishmaniasis activity.
Thermolytically activated oligonucleotide prodrugs
Oligonucleotides such as those containing unmethylated CpG dinucleotides are known to trigger vertebrate imune system through the activation of Toll-like receptor 9 (TLR-9) [79,80]. The resulting innate immune response limits the early spread of infectious organisms, while promoting the development of adaptative immunity. These dinucleotides show promise as vaccine adjuvants and to improve clinical outcome in rhesus macaques challenged with L. major [79,80]. The administration of such compounds can improve the host response to parasitic, bacterial and viral infection in animal models [80,81,82]. Prodrugs of those oligonucleotides have been designed to facilitate cellular uptake, allowed them to have increased resistance to hydrolytic nucleases.
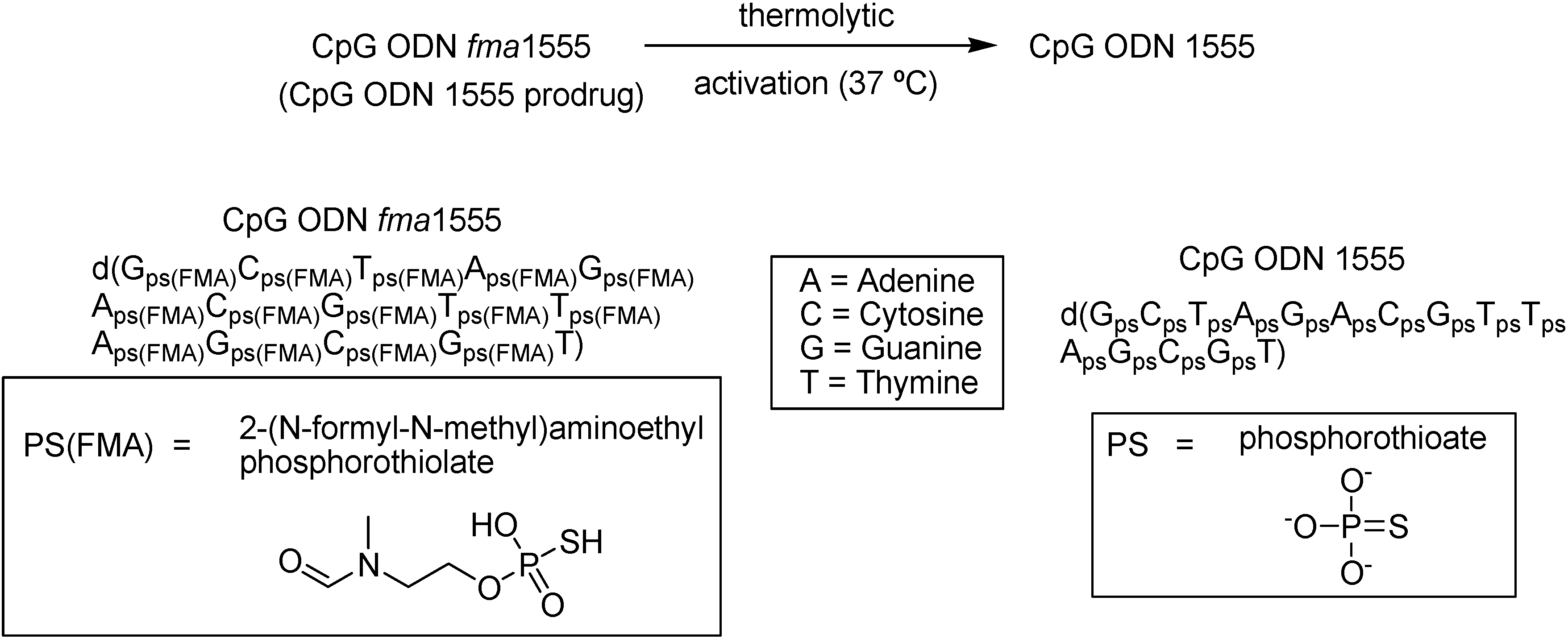
Grajowski and co-workers [83] reported the synthesis and characterization of a CpG ODN functionalized with thermolytic 2-(N-formyl-N-methyl)aminoethyl (fma) phosphorothioate triesters (Figure 8). Those compounds are activated only by temperature (37 oC). The half-life of thiophosphate deprotection was estimated to be 73 h and the complete deprotection was achieved within 600 h. Studies in mice infected with Leishmania major metacyclic promastigotes [83] prove that CpG ODN fma1555 was as effective as that of CpG ODN 1555 in reducing the size of Leishmania lesions in local intradermal administration.
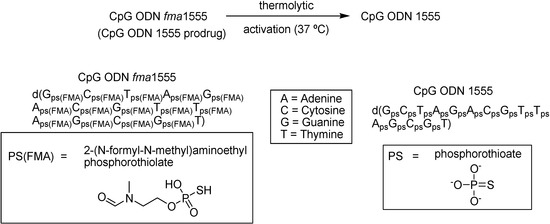
Figure 8.
Thermolytic activation of CpG ODN fma1555
Figure 8.
Thermolytic activation of CpG ODN fma1555
Previous studies demonstrated that administration of ODN type D, which has a 3´ and poli(G) motif [84] – a group of gaunine ribonucleotide with phosphate residues acting as bridges to form diester linkages between the ribose moieties – improves its performance in healthy and immunocompromised non-human primates challenge with Leishmania major [85;86]. However, this structure can lead to product polymorphisms, aggregation and precipitation, which negates their clinical applications [87,88,89]. A prodrug form of CpG ODN type D (Pro-D ODN) with fma groups was developed by Puig and co-workers [90]. The resultant prodrug (pro-D35, Figure 9) demonstrated the immunoprotective activity of pro-D ODN when applied to macaques infected with L. major.
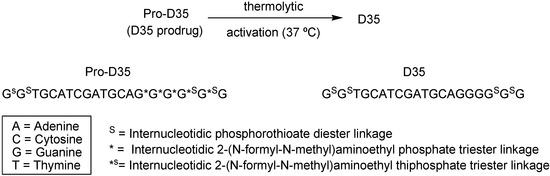
Figure 9.
Thermolytic activation of Pro-D35.
Figure 9.
Thermolytic activation of Pro-D35.
Pyrimethamine polymer prodrugs
Although classic antifolate drugs, such as pyrimethamine, have been used with success in most protozoan diseases, some of them have not been effective when applied to leishmaniasis [91]. Pterins are a chemical class that plays an important role in mammalian folate pathways. Bello and co-workers [92] identified a L. major pteridine reductase enzyme (PTR1) that reduces pterins to their tetrahydro derivatives, reducing folic acid to tetrahydrofolic acid. This enzyme is an alternative to the folate pathway, which explains the parasites resistance to the classic DHFR inhibitors [93]. This represents a new and potential target to be considered. Pyrimethamine, an antimalarial drug, was found to be able to inhibit both enzymes (DHFR-TS and PTR-1) of the leishmanial folate pathway, although this effect in vivo appears only in relatively high concentrations [94].
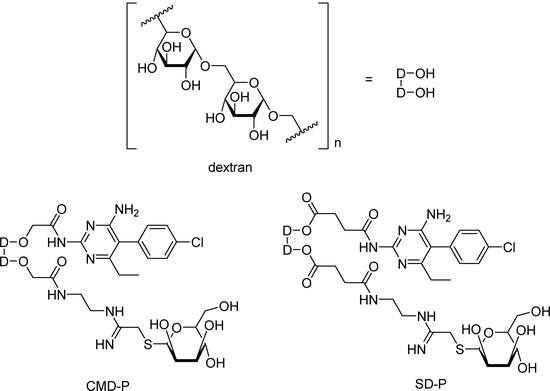
Figure 10.
Chemical structure of the prodrugs CMD-P and SD-P.
Figure 10.
Chemical structure of the prodrugs CMD-P and SD-P.
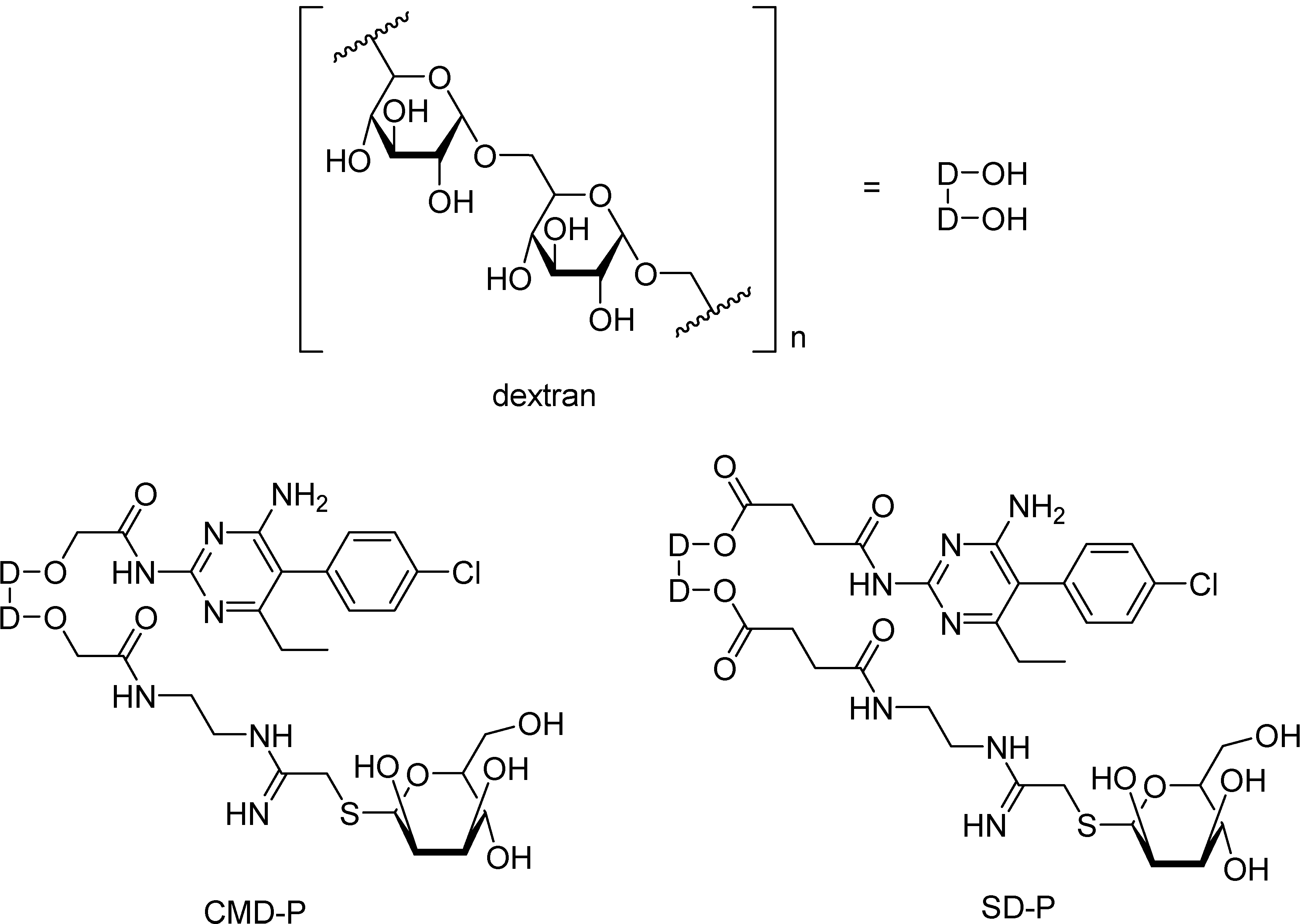
Based on that finding and on Leishmania amastigotes living inside mammal’s macrophages [95], where mannose receptors are found on the surface membrane [96], Carvalho and co-workers [94] have synthesized targeted drugs of pyrimethamine, carboxymethyldextran-thiomannopyranoside-pyrimethamine (CMD-P), and succinyldextran-thiomannopyranoside-pyrimethamine (SD-P) (Figure 10), in order to release the drug into those cells. The targeted drugs were tested against macrophages infected with L. (L.) amazonensis amastigotes in the presence or absence of CMD-P and SD-P at concentrations ranging from 100 to 200 μg/mL for 24 h. After treatment with a 200 μg/mL CMD-P dosis a 46,4% reduction of the infection rate was observed and similar results were verified with a lower dosis of CMD-P (100 μg/mL). However, SD-P in similar doses did not show good results, probably due to the release of succinylpyrimethamine, still a prodrug, instead of the free drug. Further studies will test the in vivo effects of CMD-P prodrug.
Prodrugs of buparvaquone
Various hydroxynaphtoquinones have high antileishmanial activity in vitro against species that cause both cutaneous (CL) and visceral leishmaniasis (VL) [97;98]. Buparvaquone is a hydroxynaphtoquinone derivative (Figure 11) more active against in vitro than in vivo L. donovani amastigotes, after subcutaneous administration [97]. Two factors can account for this lack of in vivo activity: poor distribution from the site of injection to the intracellular target, when administered subcutaneously, and a combination of low aqueous solubility and high lipophilicity, that lowers the drug bioavailability [99].
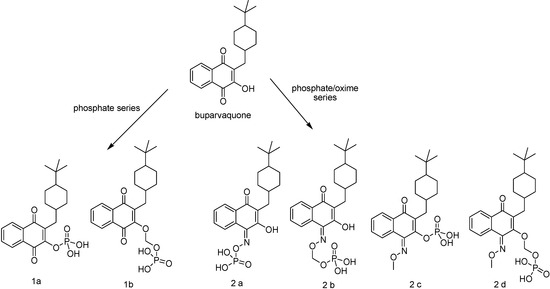
Figure 11.
Chemical structures of buparvaquone and its prodrugs.
Figure 11.
Chemical structures of buparvaquone and its prodrugs.
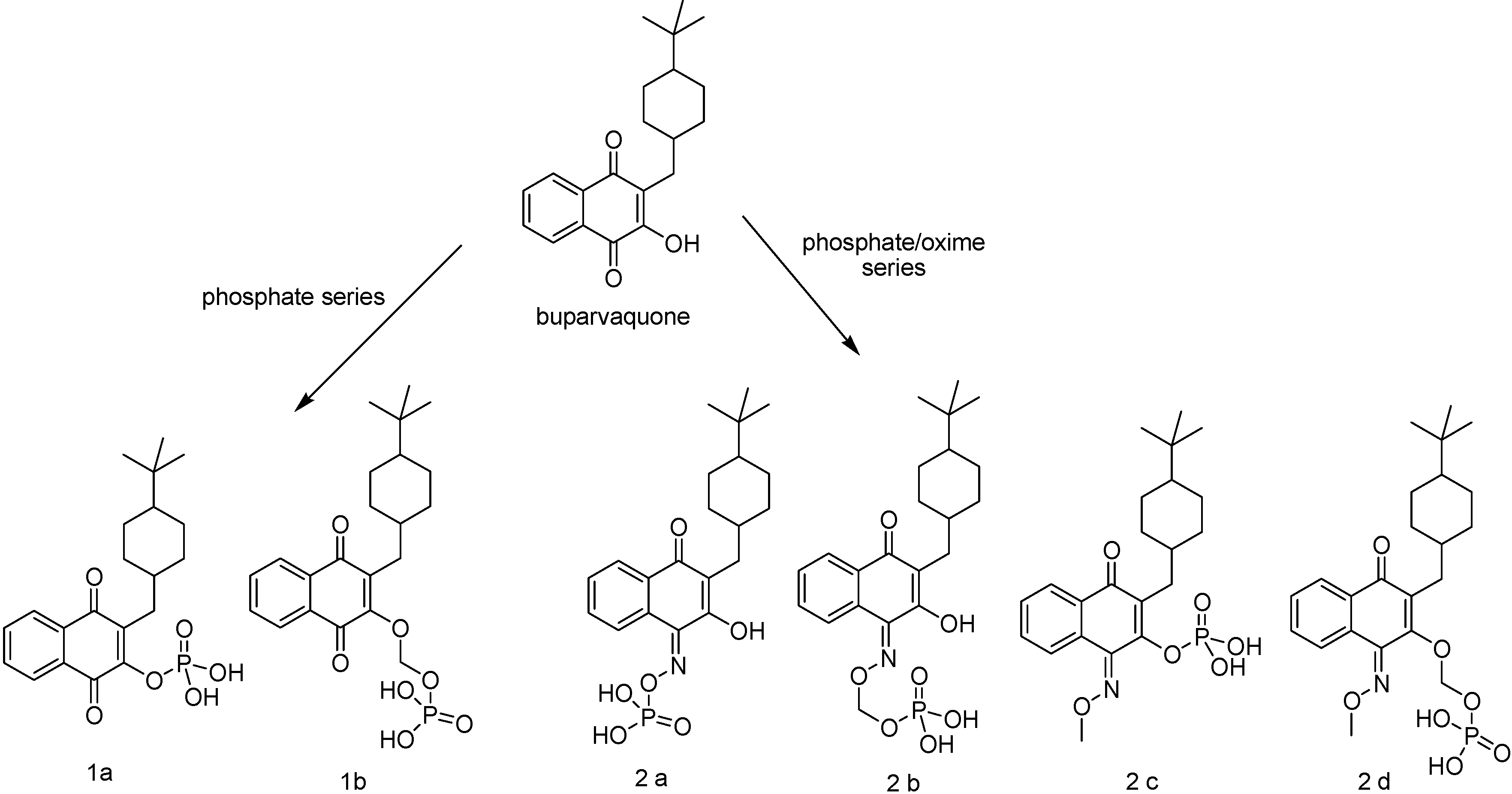
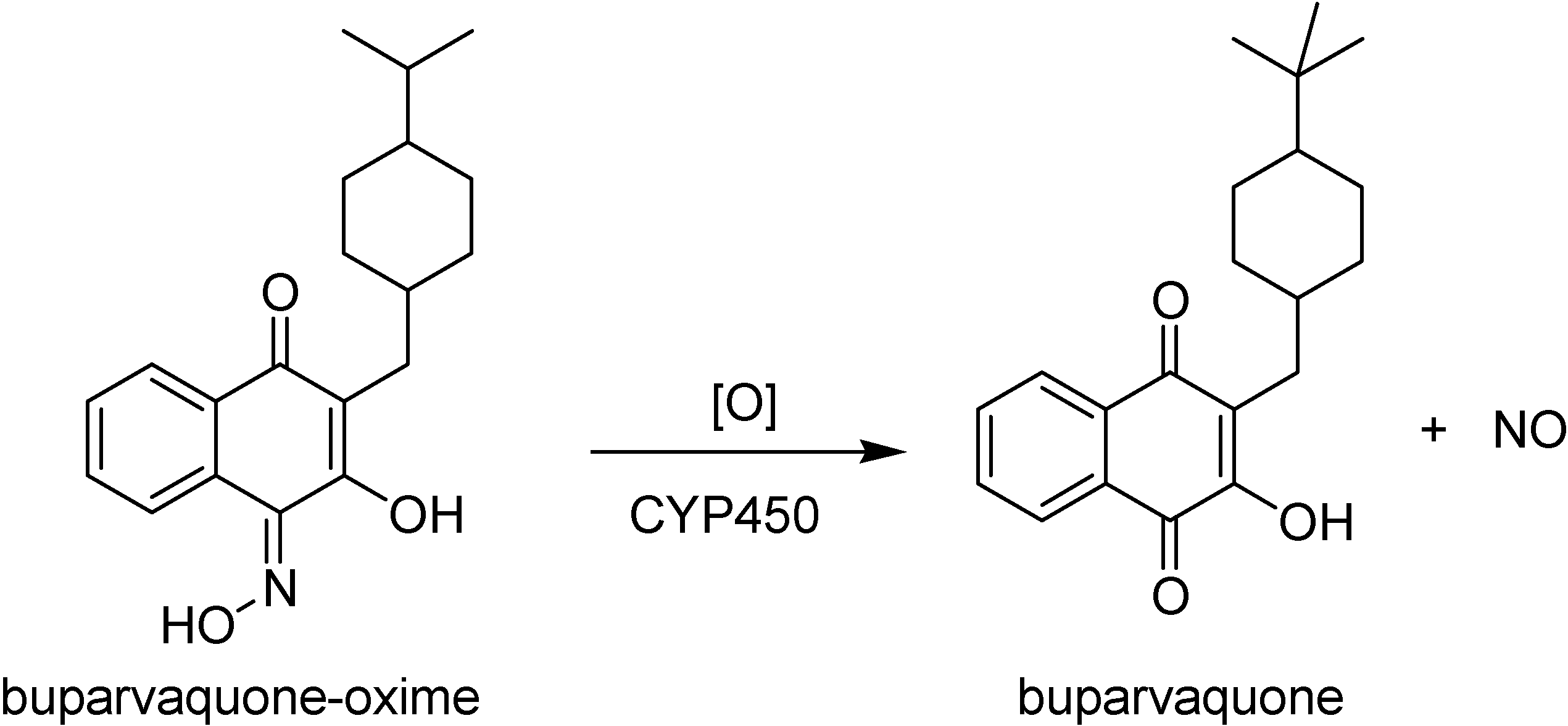
Mäntylä and co-workers [99,100] have synthesized two series of phosphate prodrugs (Figure 11) with the objective to enhance the drug bioavailability, increasing its aqueous solubility. This goal can be reached by the introduction of a phosphate promoiety in a hydroxyl group [101]. Neverthless, a phosphate carrier protecting a hydroxyl group is not always cleaved enzymatically due to steric hindrance. Phosphonooxymethyl prodrugs [102,103] can have more lability, due to a two-step hydrolysis reaction, one with enzyme participation and the other with a spontaneous release of the parent drug [99]. Compounds 1a and 1b presented a rapid buparvaquone release in alkaline phosphate solution, while only 1b was able to release the drug in human skin homogenate. These prodrugs had the same potency as buparvaquone against the promastigote and amastigote form of most species investigated. They also presented improved efficacy in in vivo Leishmania major and Leishmania donovani infections [104].
Oxime derivatives 2a-2d (Figure 11) have also been evaluated in vitro [100]. All prodrugs showed increased water solubilities and released corresponding oxime derivative in alkaline phosphate solution. The prodrug phosphonooxymethyl-buparvaquone-oxime (2b in Figure 11) was the most interesting, not only because of its high aqueous solubility and chemical stability over a pH range of 3.0 to 7.4, but also due to a quick alkaline phosphatase hydrolysis to the lipophilic parent buparvaquone-oxime. The mechanism of its conversion to buparvaquone (Figure 12) takes place in the body after absorption, probably by a oxidation cytochrome P450 mediated thorugh the formation nitrogen oxides formation, including NO, and the corresponding parent compounds containing a C=O group. The generation of NO in addition to the drug may account for the elimination of Leishmania [105,106]. This is an interesting example of mixed prodrug-bioprecursor that can be useful to improve physicochemical properties and bioavailability of the drug, besides releasing other therapeutic agent, NO in this case.
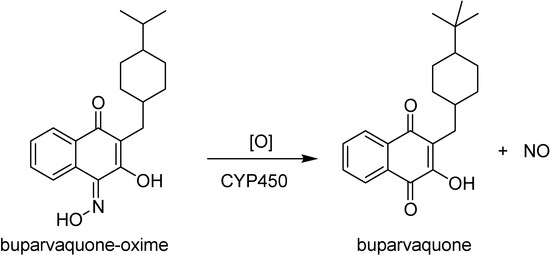
Figure 12.
The conversion of buparvaquone-oxime into buparvaquone by enzymatic oxidation and release of NO.
Figure 12.
The conversion of buparvaquone-oxime into buparvaquone by enzymatic oxidation and release of NO.
Antimalarial prodrugs
Polymeric antimalarial prodrugs
Current antimalarial chemotherapy consists of several compounds mainly active against the bloody form of Plasmodium parasites. Nonetheless, the need for drugs against tecidual forms as well as the emergence and spread of resistance against chloroquine and other major antimalarial drugs have brought the urgency to develop a new generation of safe and effective agents [107].
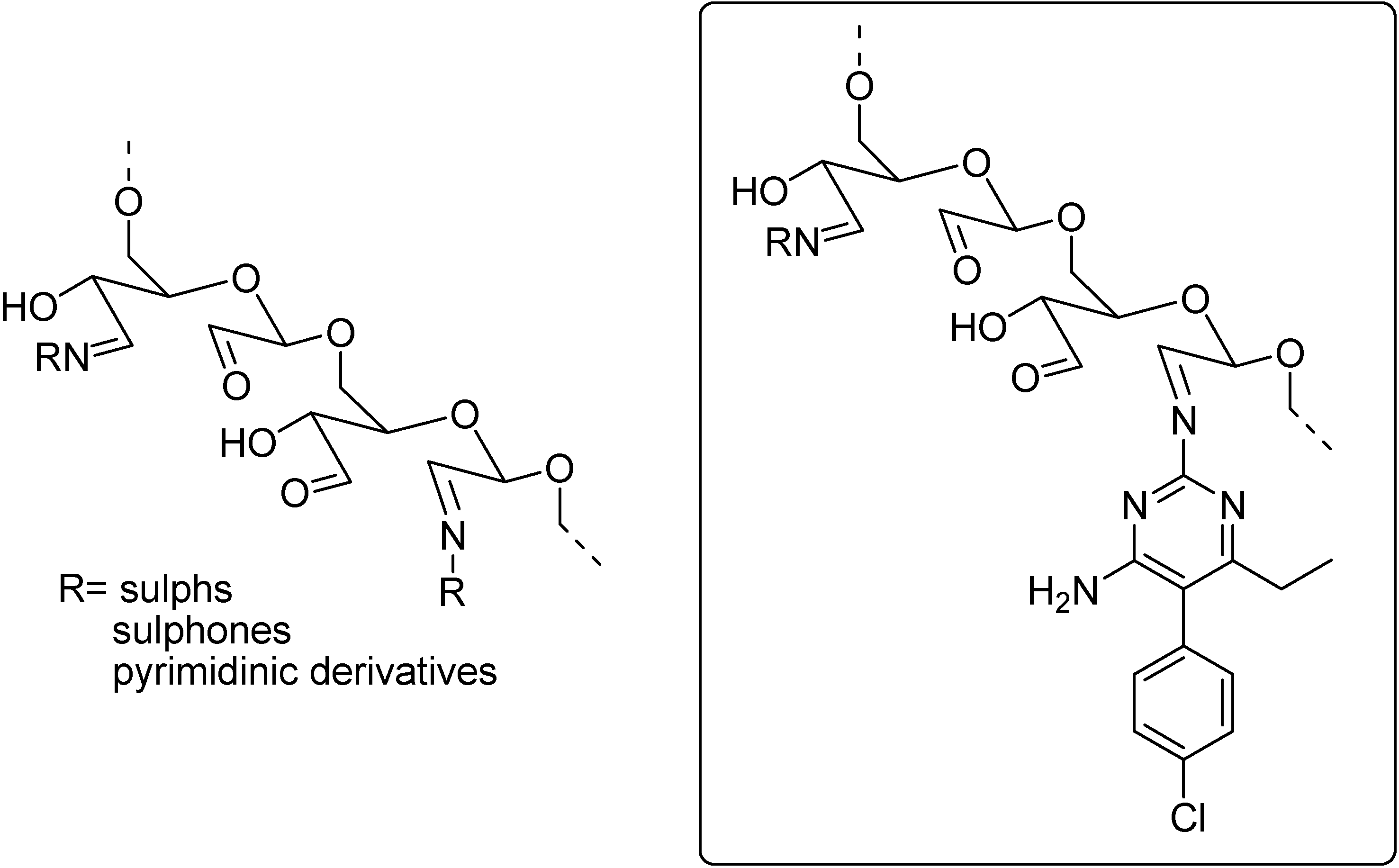
Ferreira and co-workers used polysaccharides as polymeric drug carriers to develop slow releasing prodrugs. The group developed several prodrugs using oxidized polysaccharides of antimalarial drugs as sulphadoxine, sulphadiazine, dapsone as well as some pyrimidinic derivatives, for instance, pyrimethamine and trimethoprim (Figure 13) [108,109,110].
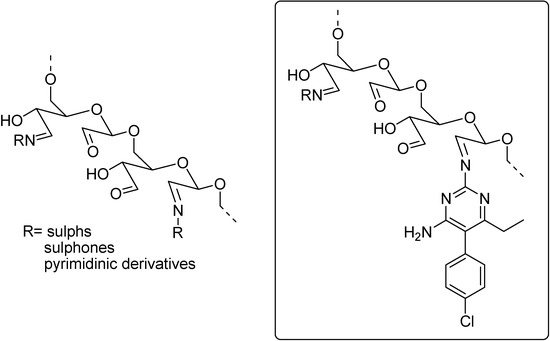
Figure 13.
Oxidized polysaccharides applied at the development of prodrugs.
Figure 13.
Oxidized polysaccharides applied at the development of prodrugs.
These polymeric prodrugs can also present better bioavailability profile and low toxicity when compared to the drug by itself whose problems with bioavailability is questioned as being related to the parasite resistant emergence.
Prodrugs of bisthiazolium salts
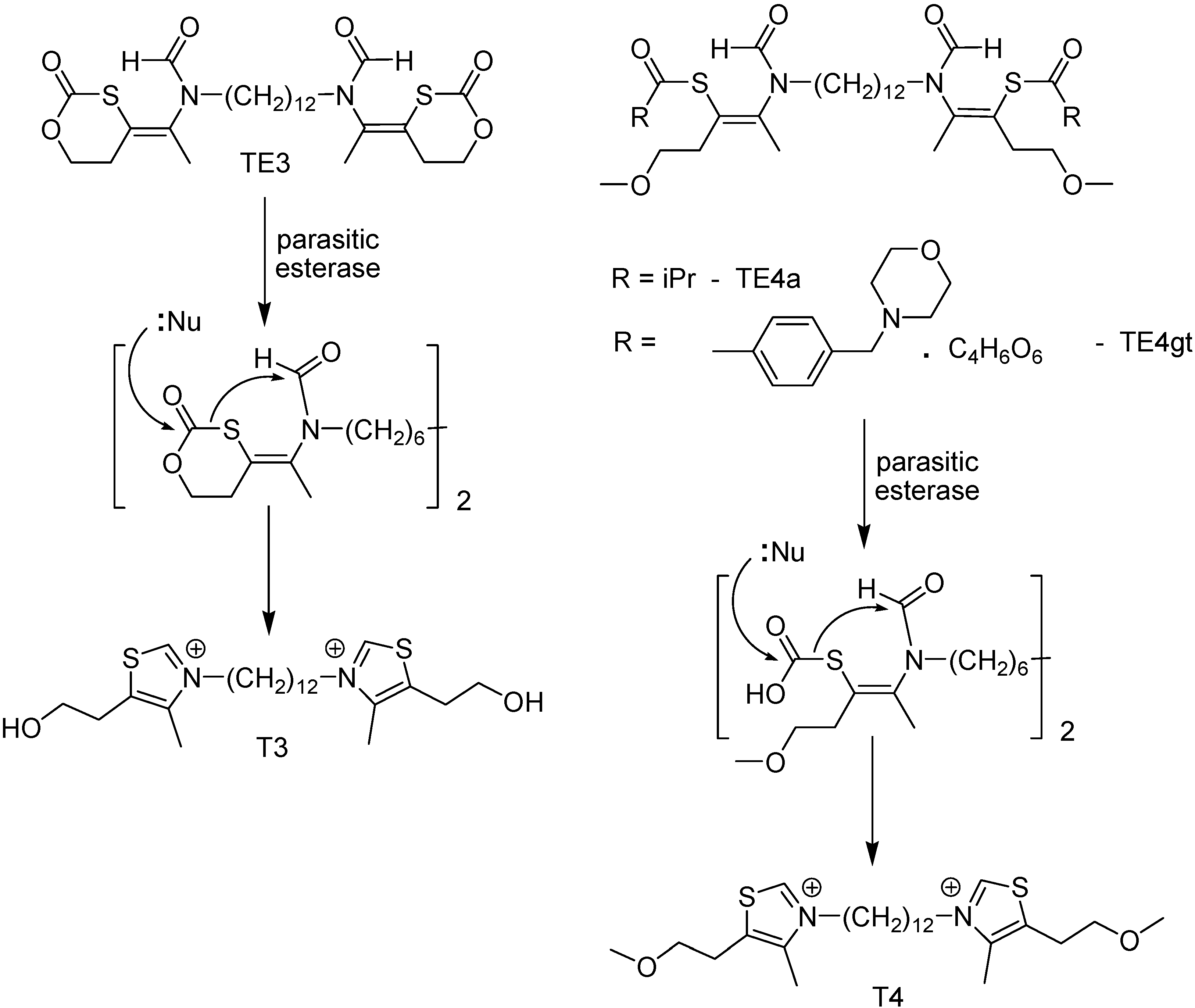
Among the targets available for chemotherapeutic design, phospholipid metabolism has been considered as an attractive biochemical step for developing new malaria chemotherapy. It is important for the parasite, is absent from normal mature human erythrocytes [111], and these cells have their content increased by around 500% because of the parasite metabolic activity. Because phosphatidylcholine is the major phospholipids, some monoquartenary and bisquartenary ammonium compounds have been used for malaria chemotherapy, showing exceptional in vitro and in vivo antimalarial proprieties, with no mutagenic activity [112,113]. Nevertheless, these compounds, structuraly similar to choline, present a cationic group which impairs their oral absorption [114]. Thus, prodrugs that mask the ionizable groups through thioester function were designed and synthesized (Figure 14) [112,113].
Prodrugs TE3, TE4a and TE4gt showed promising results: IC50 < 10 nM against 3D7 and Nigerian chloroquine (CQ)-sensitive strains and FCB1 and FCM29 CQ-resistant strains; rapid release to the active compounds in plasma and high effectiveness in vivo, with ED50 < 4 mg/kg after intraperitonial administration. Prodrugs TE4a and TE3 had higher activity (ED50 of 11 and 5 mg/kg, respectively) [113].
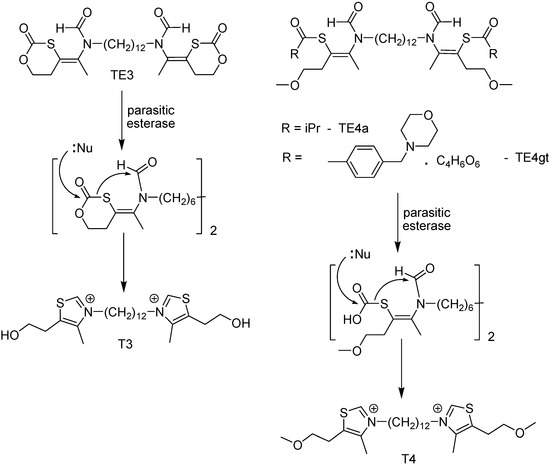
Figure 14.
Chemical structures of bisthiazolium salt prodrugs.
Figure 14.
Chemical structures of bisthiazolium salt prodrugs.
Prodrugs of fosmidomycin and FR900098
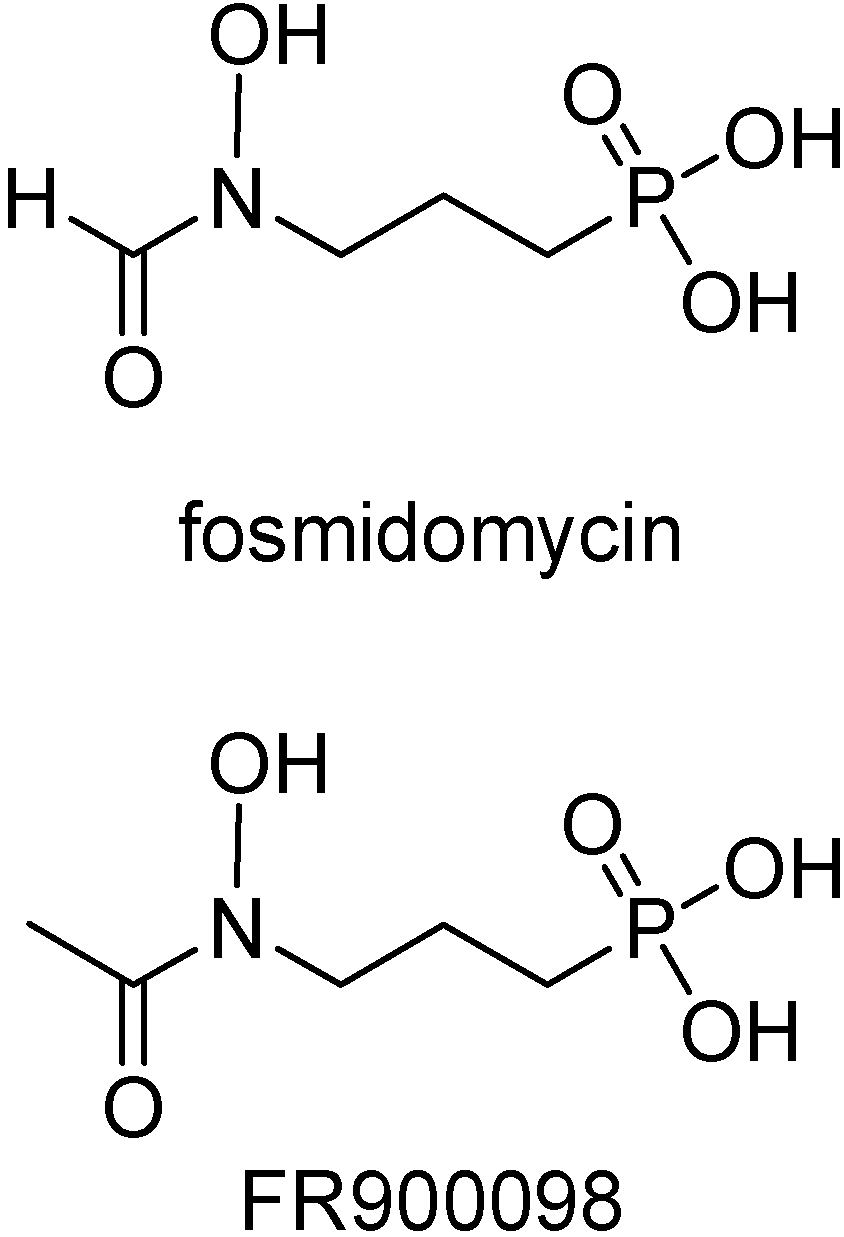
Fosmidomycin (Figure 15), involved in the inhibition of 1-deoxy-D-xylulose 5-phosphate (DOXP) reductoisomerase, an important and specific pathway in the isoprenoid synthesis [115], was shown to be active in recent clinical trials conducted in Gabon and Thailand with uncomplicated P. falciparum malaria [116]. Its acetyl derivative is around twice as active in vitro against P. falciparum [117] and in a P. vinckei mouse model [118]. Besides, it inhibited the growth of multidrug resistant P. falciparum strains in vitro [117].
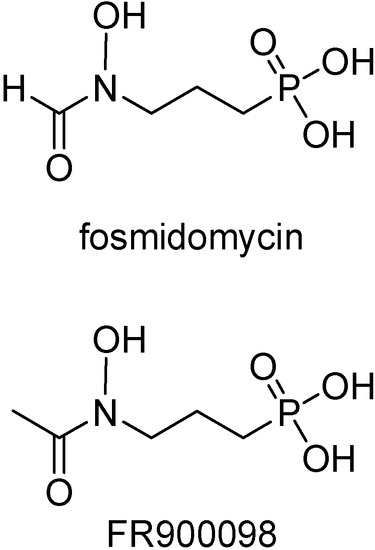
Figure 15.
Chemical structures of fosmidomycin and FR900098.
Figure 15.
Chemical structures of fosmidomycin and FR900098.
The problem for both compounds is their poor oral biovailability, probably because of their low lipophilicity provoked to the high ionization of the phosphono moiety at physiological pH values [118]. In order to overcome this unwanted effect, Reichenberg and co-workers synthesized diaryl ester prodrugs of the acetyl derivative (Figure 16) [119]. The diaryl prodrugs, although more bioavailable in P. vinckei infected mice, could release toxic phenol derivatives. Therefore, a series of acyloxyalkyl esters expected to be hydrolysed by non-specific esterases was prepared [120] (Figure 16) and the bis[(acetyloxy)ethyl]ester was twice more active than the prototype.
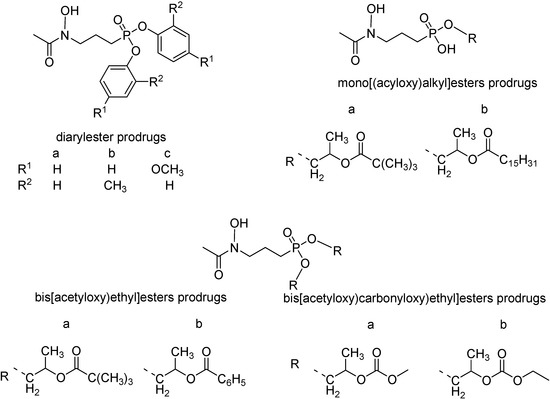
Figure 16.
Chemical structures of FR900098 ester prodrugs.
Figure 16.
Chemical structures of FR900098 ester prodrugs.
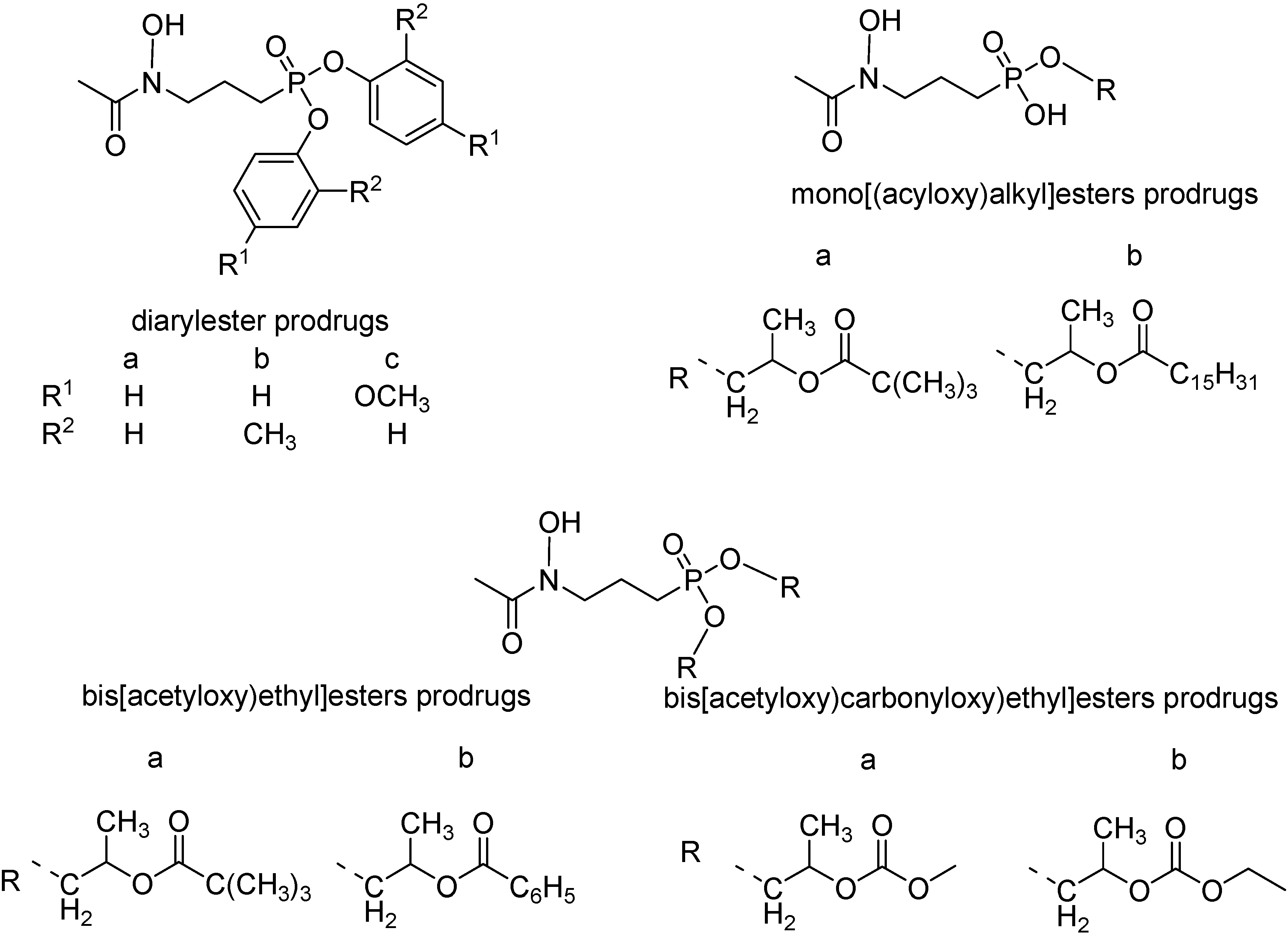
Also mono[(acyloxy)alkyl] and bis[(alkoxycarbonyloxy)ethyl] esters were prepared and were significantly more active than the parent compound [112,119,120,121].
Prodrugs of trioxane and dioxane derivatives (endoperoxidic agents)
Artemisinin (qinghaosu) is a sesquiterpene lactone, a 1,2,4-trioxane, which has been used clinically in China for the treatment of multidrug-resistant P. falciparum malaria [122]. It is believed that it acts through an interaction with heme, or ferrous ions, in the acidic parasite food vacuole, generating radical species responsible for membrane lipoperoxidation.
Despite the high activity of artemisinin, its clinical use has been limited by the inadequate pharmacokinetic profile. Prodrugs of its active metabolite, dihydroartemisinin, have been designed to improve its properties (Figure 17) [122,123].
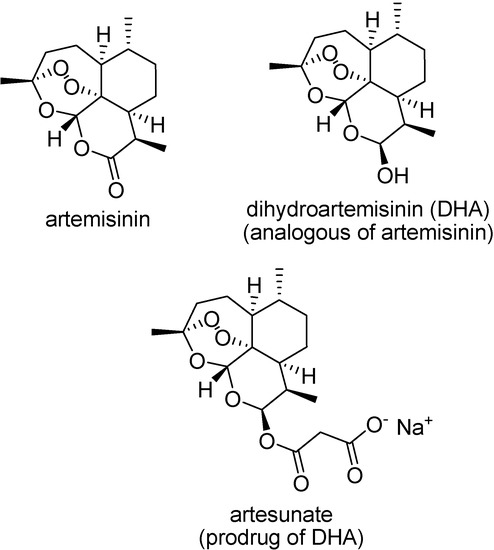
Figure 17.
Chemical structures of artemisinin, dihydroartemisinin and artesunate sodium salt.
Figure 17.
Chemical structures of artemisinin, dihydroartemisinin and artesunate sodium salt.
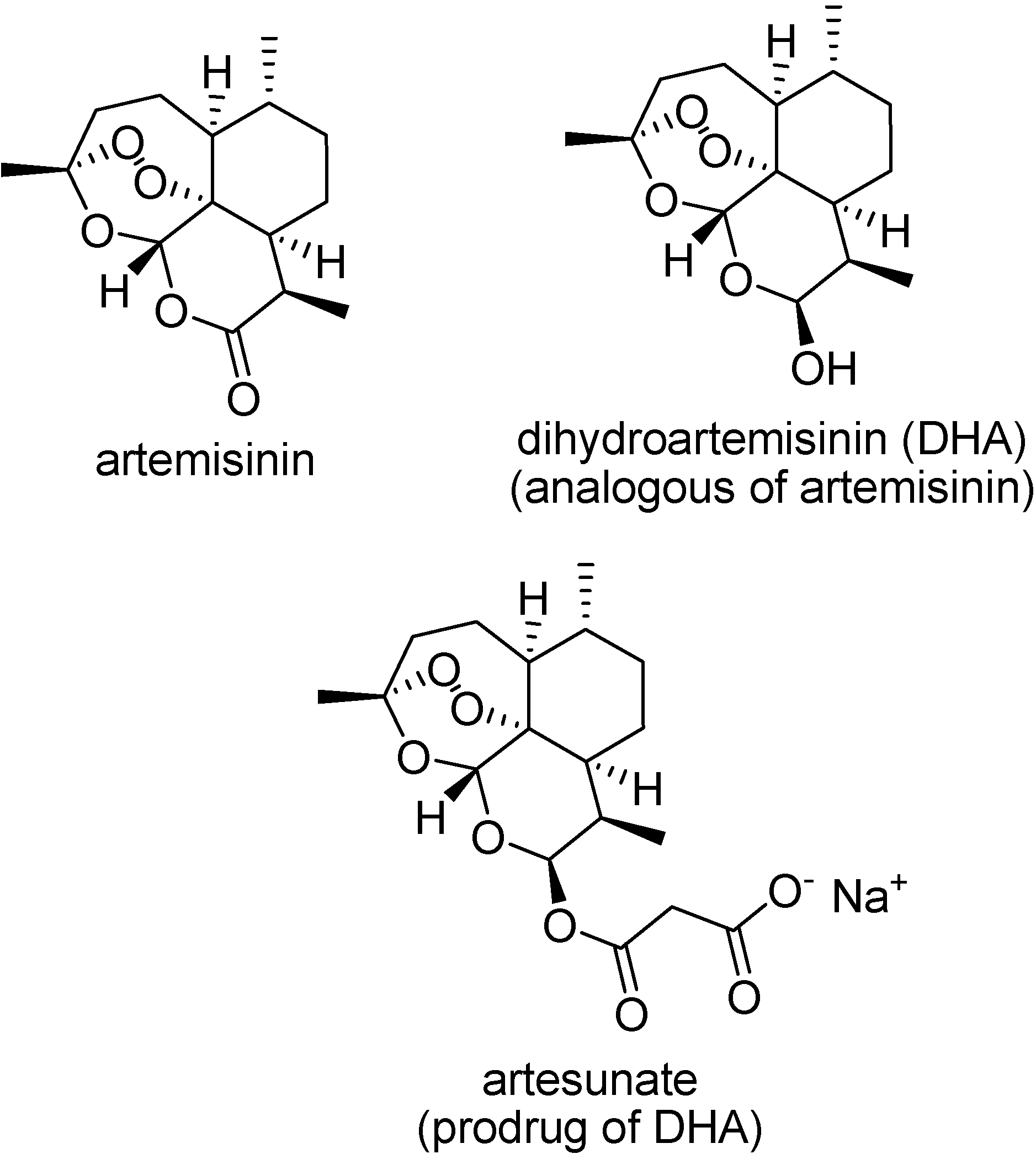
The sodium salt of artesunic acid is a water soluble prodrug, able to reduce parasitemia and to recover comatose cerebral malaria patients [124]. Other artemisinin prodrugs are artemether and arteether, more lipophilic than the parent drug, and also have been used in malaria chemotherapy.
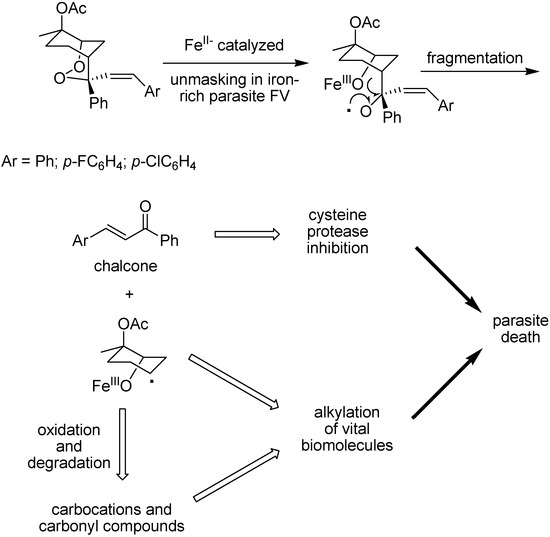
Figure 18.
FeII-catalysed degradation of endoperoxide prodrugs.
Figure 18.
FeII-catalysed degradation of endoperoxide prodrugs.
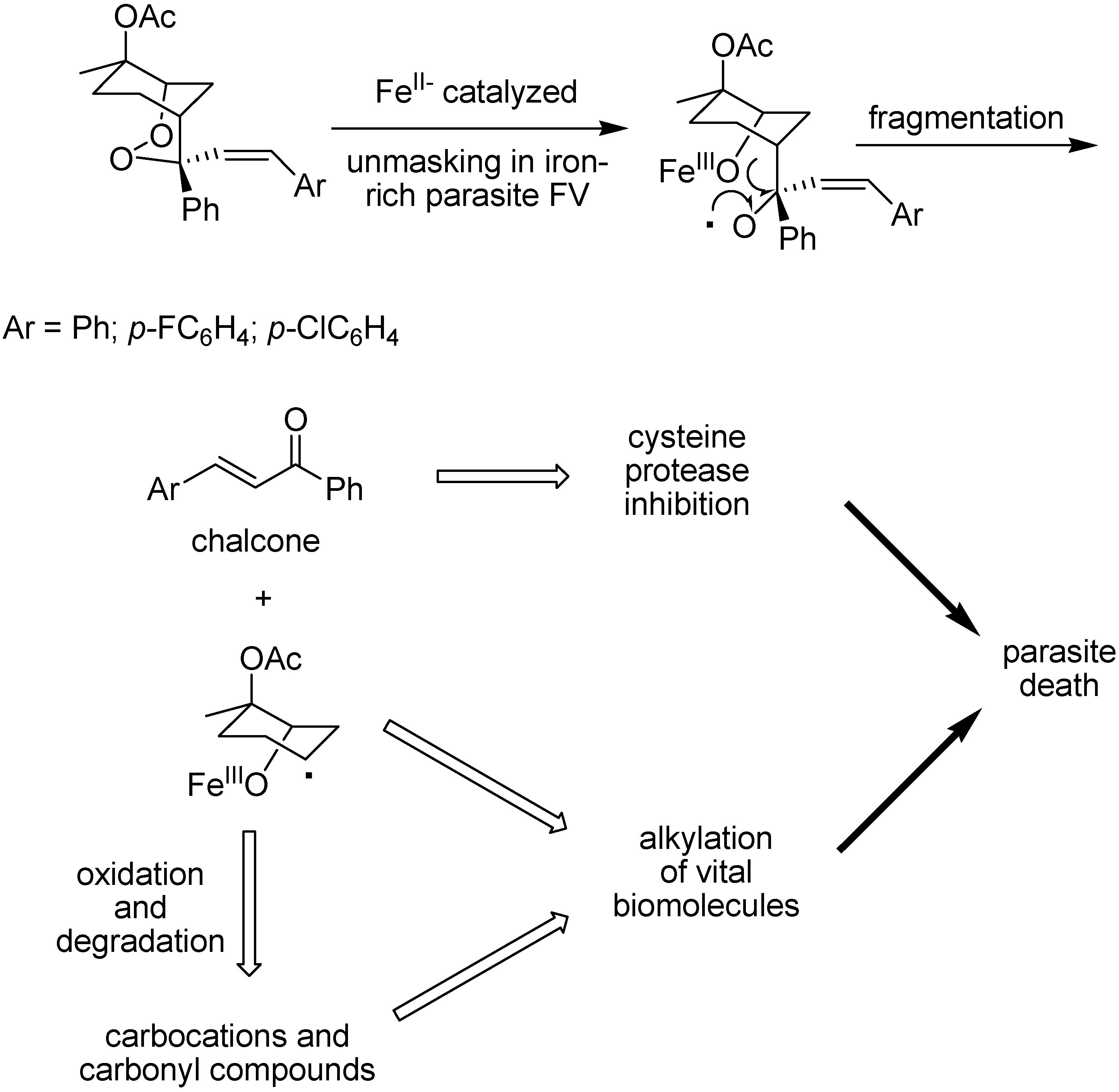
Compounds containing two antimalarial pharmacophores, such as a 2,3-dioxane and a cysteine protease inhibitor within the same molecule are part of new approaches using endoperoxide derivatives [125]. O’Neill and co-workers designed dual mechanism [125,126,127,128] prodrugs composed of a bicyclo endoperoxide prototype [126] in which the substituents at position 4 in prodrug prototype generates chalcone, through iron (II) mediation, alongside additional noxious species as shown in Figure 18. There are species that look like those generated by artemisinin and related trioxanes and presumably kill the parasite through a similar mode of action, and chalcones, that are considered to be effective antimalarial cisteine protease inhibitors [129].
Prodrugs of 4-aminoquinoline
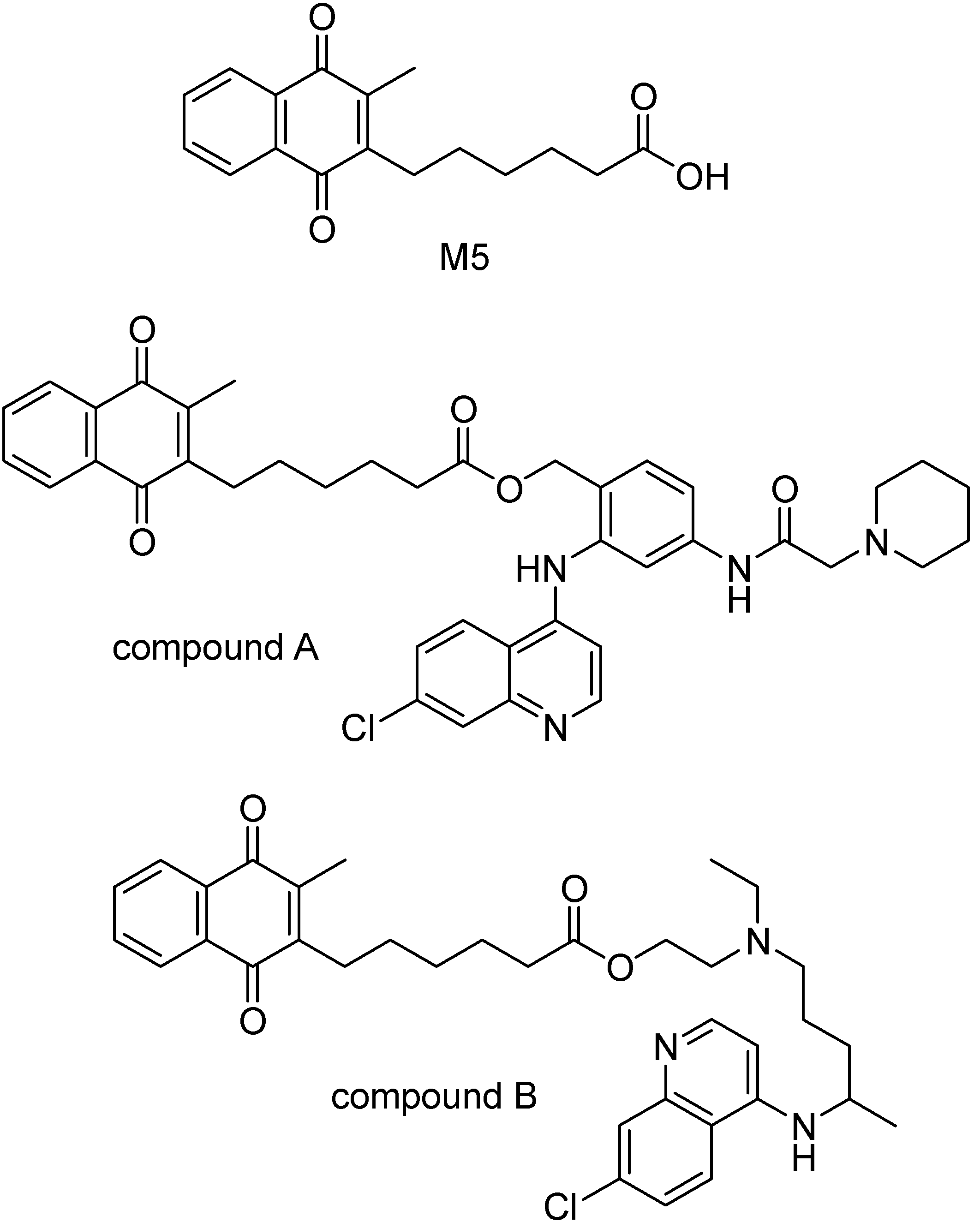
The glutathione-dependent heme modification is inhibited by chloroquine (CQ) and other related 4-aminoquinolines. This suggest glutatione (GSH) is implied in the development of CQ resistance [130]. Glutathione reductase (GR) displays an important role in glutatione disulfide production and parasite detoxification, and GR inhibitors were proposed to protect from malaria and to reverse the CQ-resistance [131,132,133].M5 is one of GR inhibitors that has a carboxilate function. Prodrugs of 4-aminoquinoline derivatives and M5 were prepared via an ester bond (Figure 19), that showed to be very stable in biological compartments but releases the drug in the food vacuole of the parasite [132]. The IC50 value of compound A in Figure 19 was 23.1 nM and that of the compound B was 107 nM against FeB1R strain of P. falciparum [134]. Compound A presented ED50 values around 30 nM on average. It was equally active against CQ-sensitive and CQ-resistant P. falciparum strains.
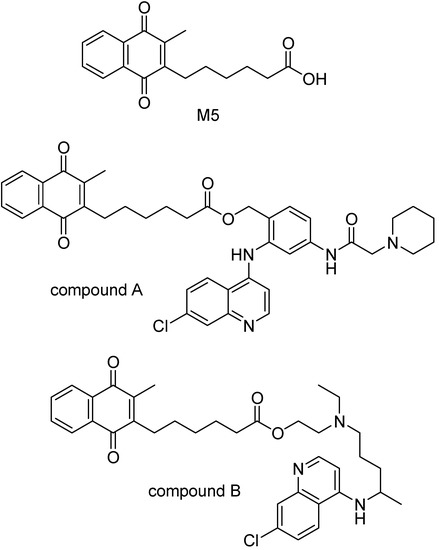
Figure 19.
Chemical structures of M5 and 4-aminoquinoline derivative prodrugs.
Figure 19.
Chemical structures of M5 and 4-aminoquinoline derivative prodrugs.
Prodrugs of 8-aminoquinoline
Primaquine is the only currently available drug as tissue schizonticidal agent. It is active against both the latent liver forms of the relapsing malaria caused by P. vivax and P. ovale and the gametocytes from all species of parasite causing human malaria including CQ-resistant P. falciparum [135]. Although it has been useful for those malaria form, its rapid metabolic inactivation to form carboxyprimaquine (Figure 20) together with methemoglonbinemia, a serious blood toxicity it provokes, prevent its wide use as antimalarial [136]. Several prodrugs with the goal of reducing toxicity and metabolic oxidative deamination have been synthesized.
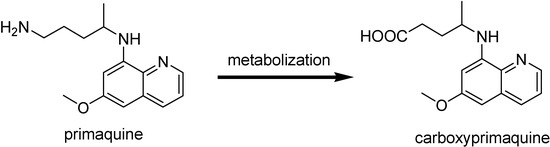
Figure 20.
Metabolism of primaquine.
Figure 20.
Metabolism of primaquine.
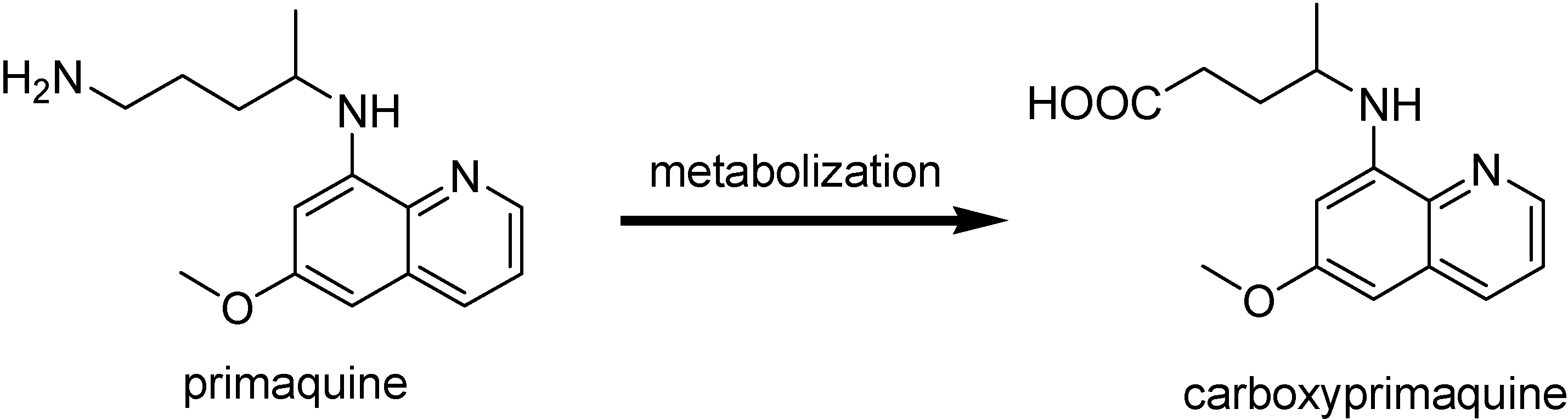
Trouet and co-workres [137] reported the preparation, antimalarial activity and toxicity of several amino acid and peptide derivatives of primaquine. The activity was assessed against P. berghei in mice and the toxicity was established by determining the LD50. Compounds A and B in Figure 21 were found to be less toxic and more active than primaquine.
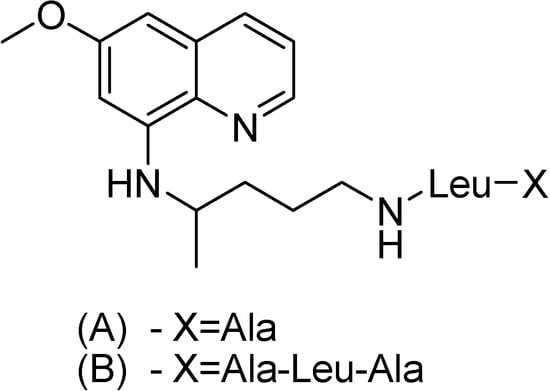
Figure 21.
Chemical structures of (A) alanylleucylprimaquine and (B) alanylleucylalanylleucylprimaquine.
Figure 21.
Chemical structures of (A) alanylleucylprimaquine and (B) alanylleucylalanylleucylprimaquine.
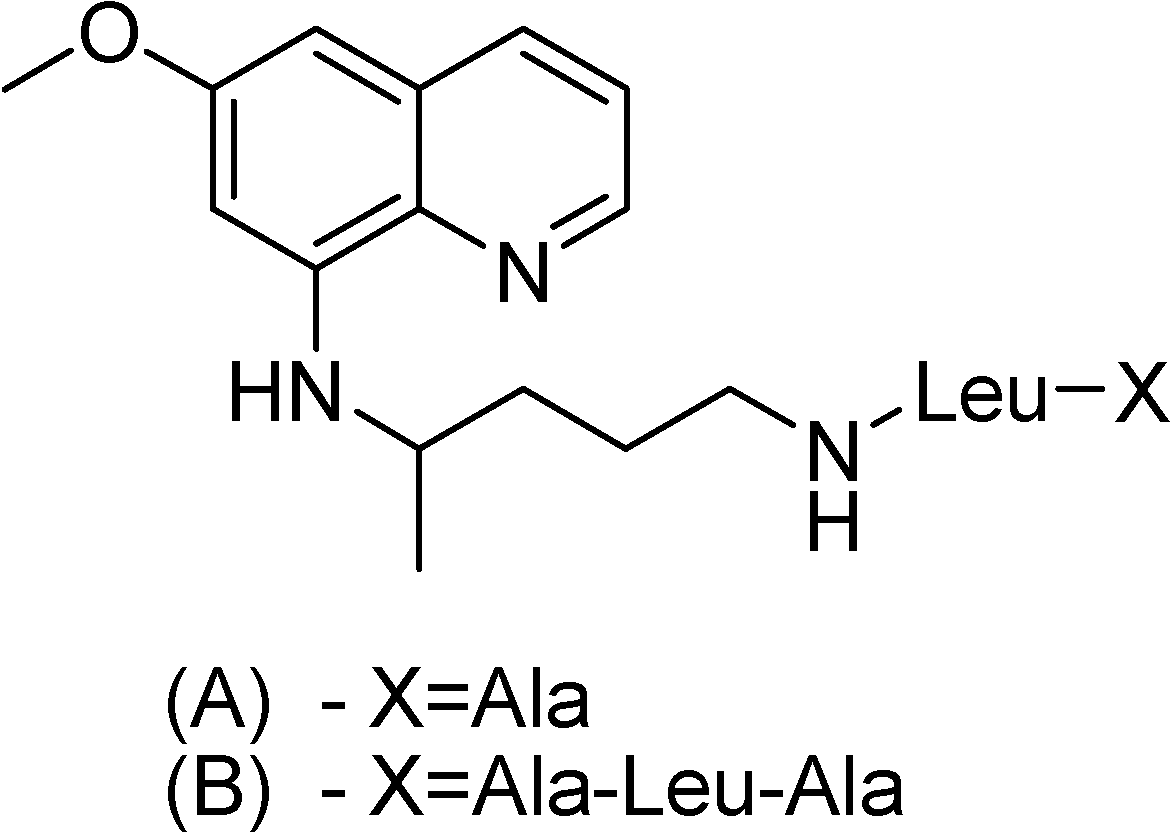
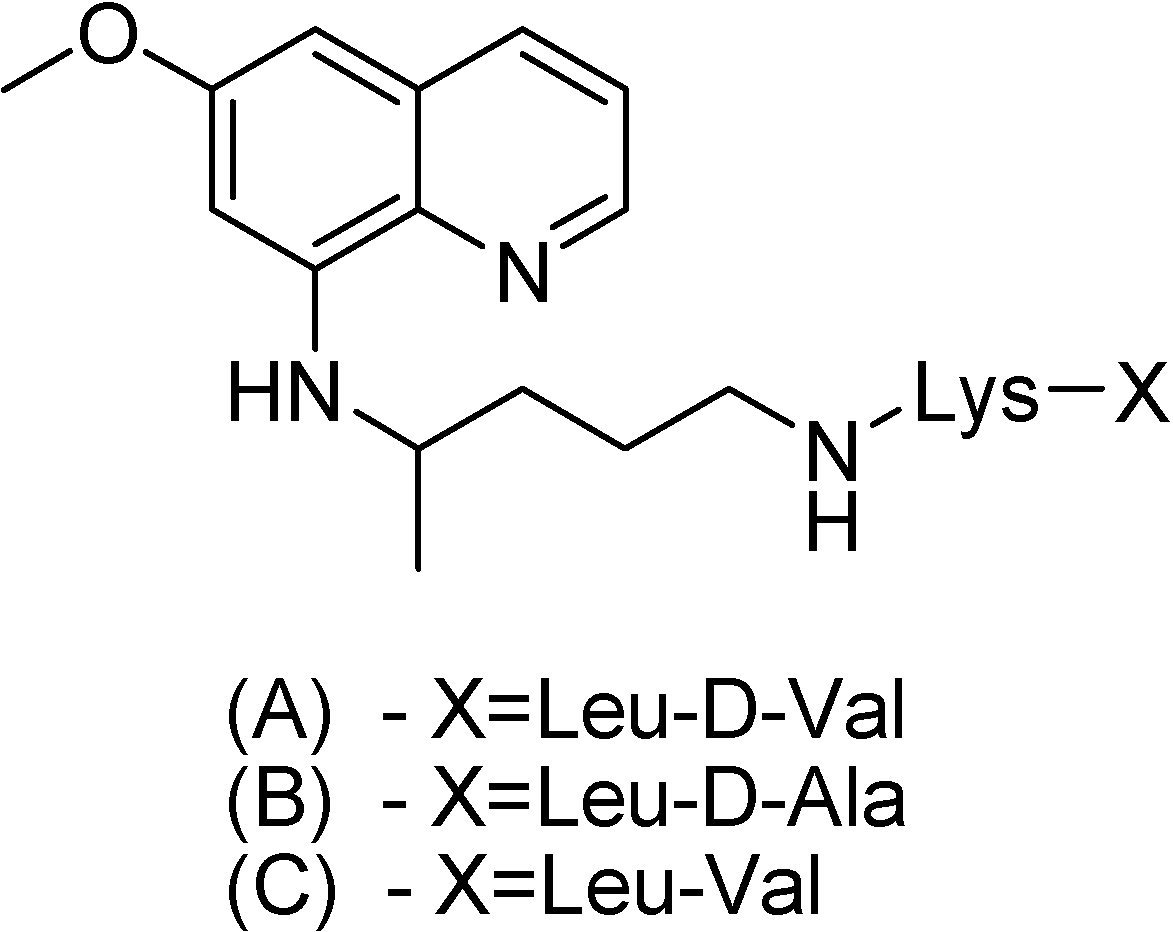
Philip and co-workers [138] have synthesized other three peptide derivatives of primaquine (Figure 22). All prodrugs showed activity against P. cynomolgi greater than the parent compound. The toxicity of compound A in Figure 22 was lower than that of primaquine in mice.
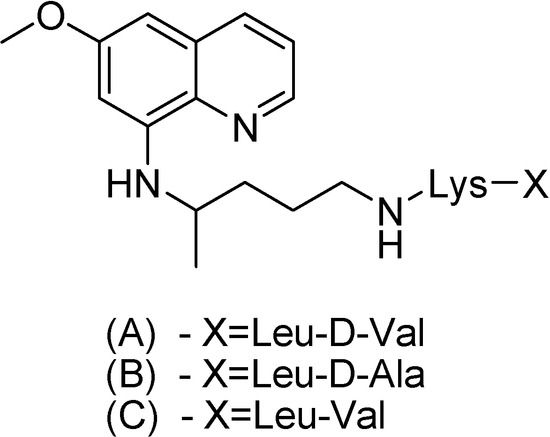
Figure 22.
Chemical structures of (A) d-valylleucyllysylprimaquine, (B) d-alanylleucyl-lysylprimaquine and (C) valylleucyllysylprimaquine.
Figure 22.
Chemical structures of (A) d-valylleucyllysylprimaquine, (B) d-alanylleucyl-lysylprimaquine and (C) valylleucyllysylprimaquine.
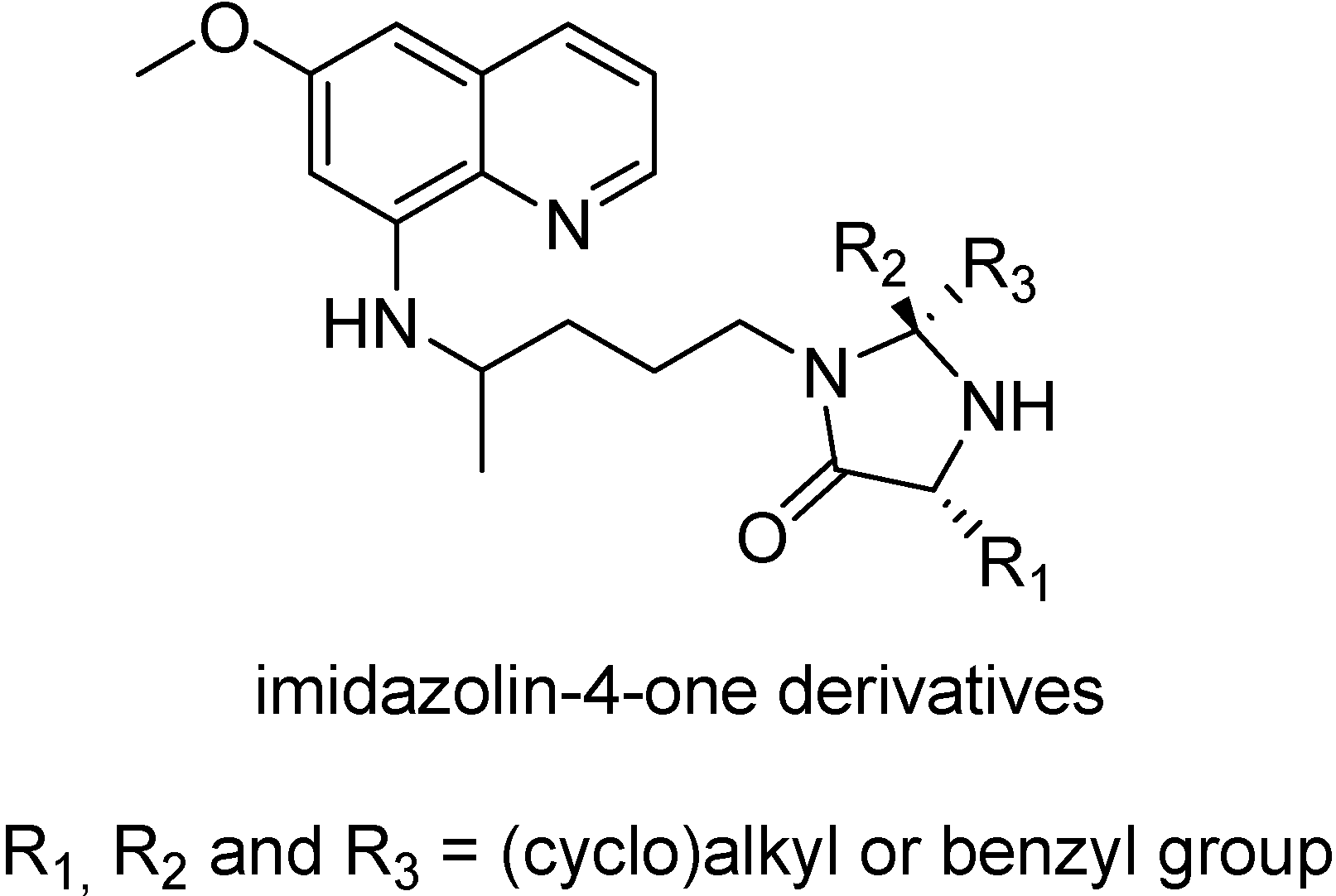
The problem of the peptide prodrugs, in general, is their instability [139,140], and this also happens with peptide primaquine prodrugs. They are rapidly hydrolyzed to primaquine by aminopeptidases and endopeptidases [141,142]. This suggests an extensive hydrolysis in the intestinal lumen when the prodrugs are orally administered, therefore, more stable peptide derivatives were needed [146]. One example is the use of imidazolidin-4-one formation (Figure 23) to protect the N-terminal amino acid residue of di-tri- and penta peptides against aminopeptidases-catalyzed hydrolysis [143].
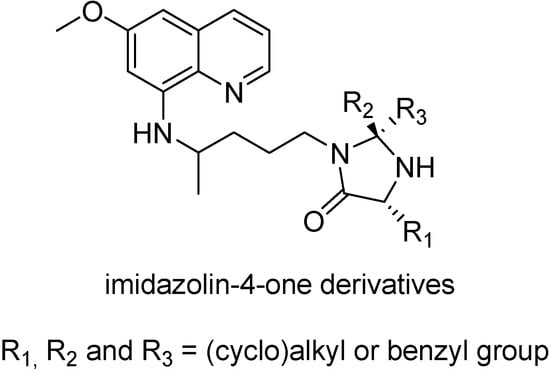
Figure 23.
Chemical structures of imidazolin-4-one derivatives of primaquine.
Figure 23.
Chemical structures of imidazolin-4-one derivatives of primaquine.
These primaquine prodrugs were synthesized as potential double prodrugs of primaquine and may be useful as slow-release forms of the parent amino acid derivatives. These compounds showed to be highly potent as gametocytocidal against P. berghei [144].
Vagapandu and co-workers [145] have used modified 8-aminoquinoline compounds with increased blood-schizontocidal activity and activity against drug-resistant strains. Attempts to improve the properties of 8-quinolinamine antimalarial agents through prodrug approach indicated, however, that a single chemical modification is not sufficient to achieve the desired alteration in the biological properties to enhance blood-schizontocidal activity [138]. Two series of “double prodrug” or “pro-prodrug” were therefore proposed (Figure 24). They need two independent reactions in order to regenerate the parent drug.
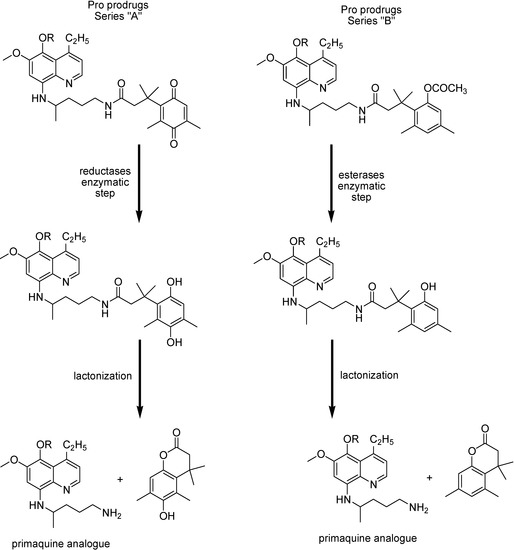
Figure 24.
Pro-prodrug series of primaquine analogue (R=C5H11 or C7H15), and the regeneration of parent drug.
Figure 24.
Pro-prodrug series of primaquine analogue (R=C5H11 or C7H15), and the regeneration of parent drug.
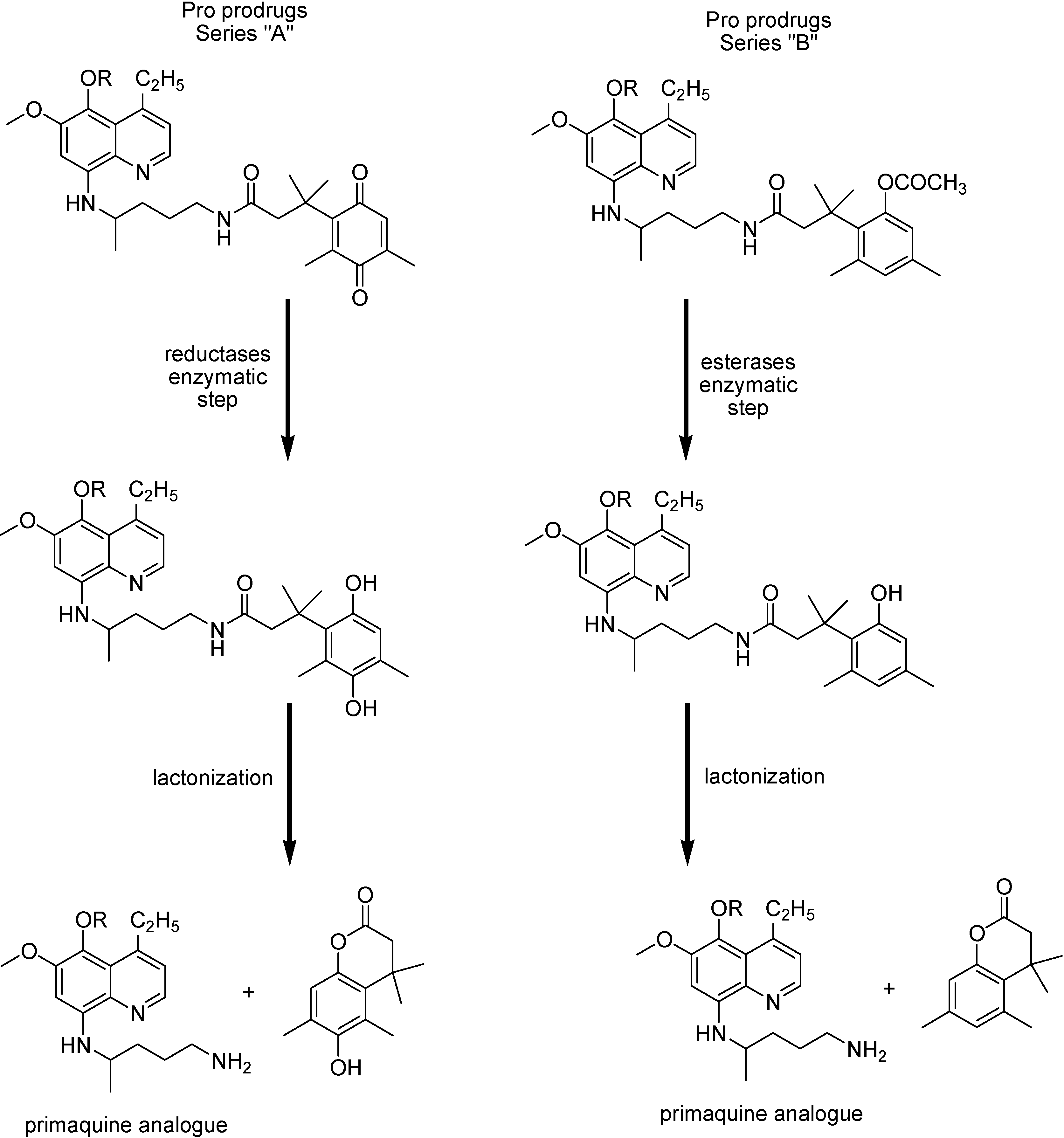
Pro-prodrug from series “A”, where R=C5H11 or C7H15, and from series “B”, where R=C5H11, have shown curative activity at initial test dosis of 100 mg/kg. The most potent compound of series “A”, where R= C5H11 produced suppressive effects at the dose of 50 mg/kg, and was the most potent compound from the pro-prodrug series, leading to antimalarial activity comparable to its parent drug [145].
Pro-prodrugs, mutual prodrugs in the following examples, with statine-based inhibitor of protease plasmepsinII (PLMII) and primaquine peptide derivative have been linked by an alkyl diacid or an aromatic diacid (Figure 25) [146]. They have been designed based on the fact that inhibition of leads to starvation of the parasite. This enzyme is a potential target for antimalarial agents. The most active compound was 4,4’-oxybis(benzoic acid) and the primaquine derivative was leucyllysylprimaquine. Its IC50 was 0.59 nM in in vitro test against P. falciparum [147].
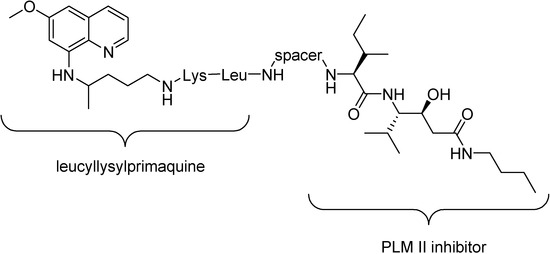
Figure 25.
Primaquine-statine pro-prodrugs.
Figure 25.
Primaquine-statine pro-prodrugs.
Prodrugs of dihydrotriazine
Malaria parasites have shown drug resistance for most of the antimalarials currently used. They include antifolate drugs such as cycloguanil, the active metabolite of proguanil (Figure 26).
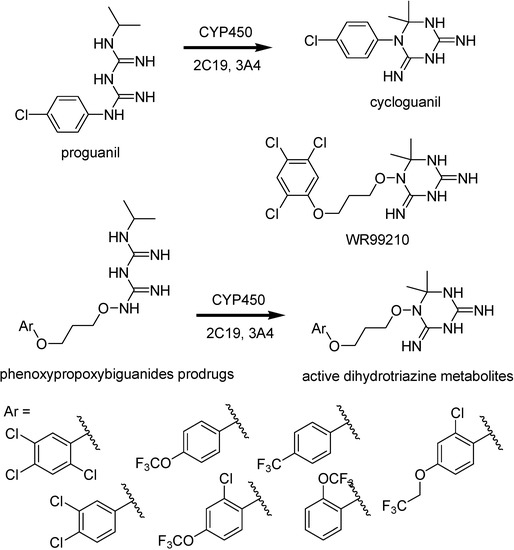
Figure 26.
Chemical structures of proguanil, cycloguanil, WR99210, phenoxypropoxy-biguanides prodrugs and their active metabolites.
Figure 26.
Chemical structures of proguanil, cycloguanil, WR99210, phenoxypropoxy-biguanides prodrugs and their active metabolites.
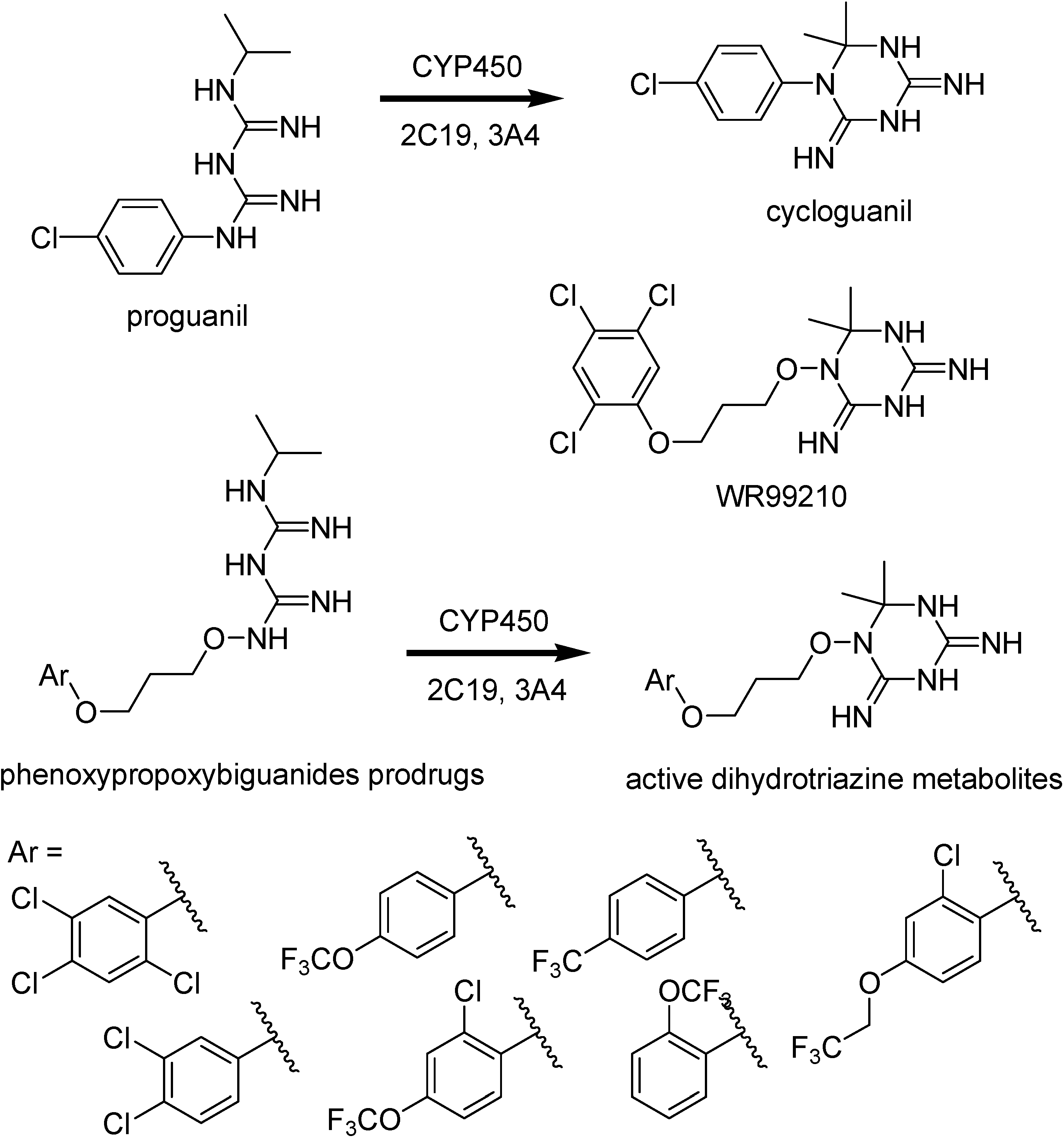
Mutations on the P. falciparum dihydrofolate reductase thymidilate synthase (pfDHFR-TS) enzyme lead to a decreased binding affinity for the drugs [148]. Rieckmann, in 1973 [149], demonstrated compound WR99210 to be potent in vitro against a CQ- and pyrimethamine-restistant strain of malaria and high level of efficacy against mutant pfDHFR-TS enzymes and antifolate resistant isolates of the malaria parasite [150]. Nevertheless, this interesting compound provokes significant gastrointestinal intolerance in animal studies and poor biovailability.
Phenoxypropoxybiguanide prodrugs, including the prodrug of WR99210 (Figure 26), were then synthesized. They have similar metabolization to that of proguanil [151,152]. Shearer and co-workers [153] have studied these prodrugs and prove that the active dihydrotriazines metabolites exhibited IC50 values less than 0.04 ng/mL against the CQ/pyrimethamine- sensitive (D6) and CQ/pyrimethamine-resistant DHFR mutant strain (W2).
Prodrugs of protein farnesyltransferase inhibitors
Another potential target for the design of new antimalarial drugs is protein farnesyltransferase (PFT) [154]. PFT catalyzes the covalent transfer of a farnesyl group to cysteine residues near to the C-terminus of protein substrates. It displays a critical role in cellular signaling and other regulatory paths [155].
Ohkanda and co-workers [156] have tested a series of peptidomimetics PFT inhibitors against P. falciparum in vitro. In this study, compound FTI-2148 has no activity against P. falciparum (ED50>30 μg/mL), despite being a potent mammalian PFT inhibitor (IC50=1 nM). On the other hand, the methyl ester prodrug of FTI-2148 (Figure 27) inhibited P. falciparum growth with an ED50=2 μg/mL. Other prodrugs are depicted in Table I and resulted from further work of Carrico and co-workers [157]. Although not active in the P. falciparum PFT, they were able to cross biological barriers due to their higher hydrophobicity and membrane permeability when compared to the free acid. Not only the hydrophobicity may affect the delivery of the compound into the parasite cell. Other factors, such as size and conformation of the ester side chain also display a role in the activity.
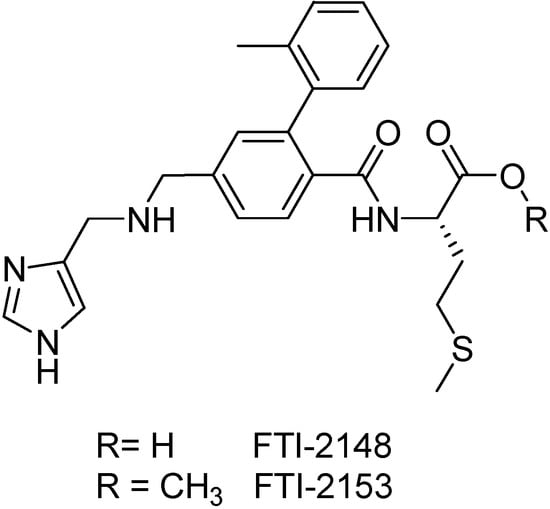
Figure 27.
Chemical structures of compound FTI-2148 and its methyl ester prodrug FTI-2153.
Figure 27.
Chemical structures of compound FTI-2148 and its methyl ester prodrug FTI-2153.

Table 1.
Inhibition activity of FTI-2148 prodrugs against P. falciparum PFT and P. falciparum infected red blood cell
| CH3 (FTI-2153) | 4.44 | >1000 | 3300 |
| C2H5 | 4.88 | 750 | 1900 |
 | 5.19 | >1000 | 1800 |
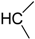 | 5.19 | >1000 | 800 |
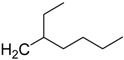 | 7.09 | >1000 | 700 |
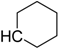 | 4.94 | >1000 | 350 |
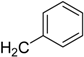 | 6.09 | 1000 | 150 |
| H (FTI-2148) | 1.77 | 15 | >66,000 |
Prodrugs of pyrroloquinazolinediamine
Pyrroloquinazolinediamine (PQD) and its derivatives possess antimalarial among other activities [158]. They are very active antimalarial agents in vivo and in vitro [159,160,161], but are also highly toxic. Several prodrugs were prepared and two of these derivatives, tetraacetamido-pyrroloquinazolinediamine (PQD-A4) and bis-ethylcarbamyl pyrroloquinazolinediamine (PQD-BE), were more potent and less toxic than the parent compound (Figure 28) [162]. They show high in vitro activity against P. falciparum, with a IC50=0.01 ng/mL, and against P. berghei in a rodent model, with a 100% curative oral dose between 0.1 and 4 μg/kg [162]. These two derivatives were also highly active in Aotus monkeys (oral curative doses, 1 mg/kg) and no toxicity was reported to the mammalian host. The high activity of PQD-A4 together with its high safety account for its interest as a promising candidate for antimalarial treatment [163].
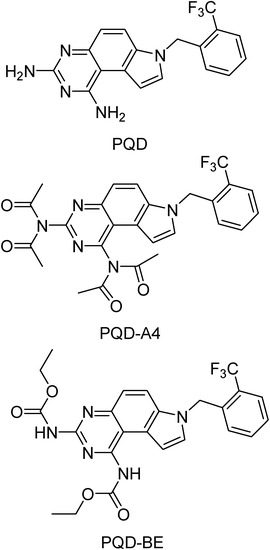
Figure 28.
Chemical structures of PQD, PQD-A4 and PQD-BE.
Figure 28.
Chemical structures of PQD, PQD-A4 and PQD-BE.
Prodrugs of aromatic amidine
Due to the potential interest of benzamidine groups found in many drugs, bezamidoxime and derivatives or carbamates are more lipophilic and less basic prodrugs with decreased pKa, overcoming the low oral biovailability of this group of compounds [164,165,166,167,168]. These modifications improved biovailability up to 30-fold and oral activity by 20-fold [164,168,169] and reduced adverse effects [164,168], although little effect was seen on the half-life eliminations of these compounds [164]. The conversion of benzamidoxime groups to benzamidine, both in vitro and in vivo, is performed by a microsomal reductase system present in all mammalian species [170].
Ouattara and co-workers [171] have synthesized a series of prodrugs of the lead compound 1,12-bis(N,N’-acetamidinyl)dodecane (M64, Figure 29). These prodrugs were tested in mice infected with P. vinckei and were administered intraperitoneally and orally once daily for four consecutive days. Prodrug 2 was not very effective probably due to its low conversion to drug. Prodrugs 1 and 3 showed to be effective and prodrug 4 was even more potent by oral route with an activity at least 3 times higher than the parent drug M64 and the best after oral administration.
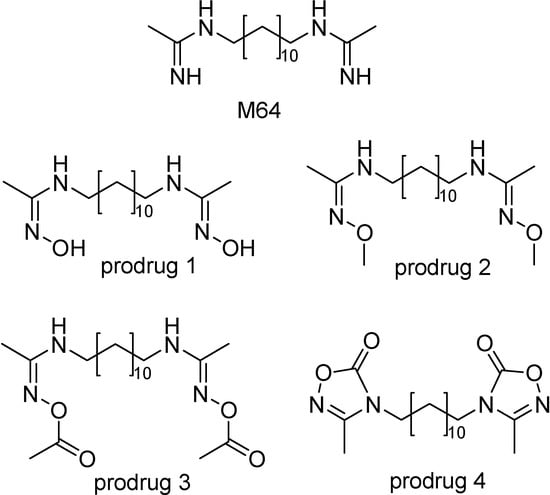
Figure 29.
Chemical structures of compound M64 and its prodrugs.
Figure 29.
Chemical structures of compound M64 and its prodrugs.
CD-CS approach
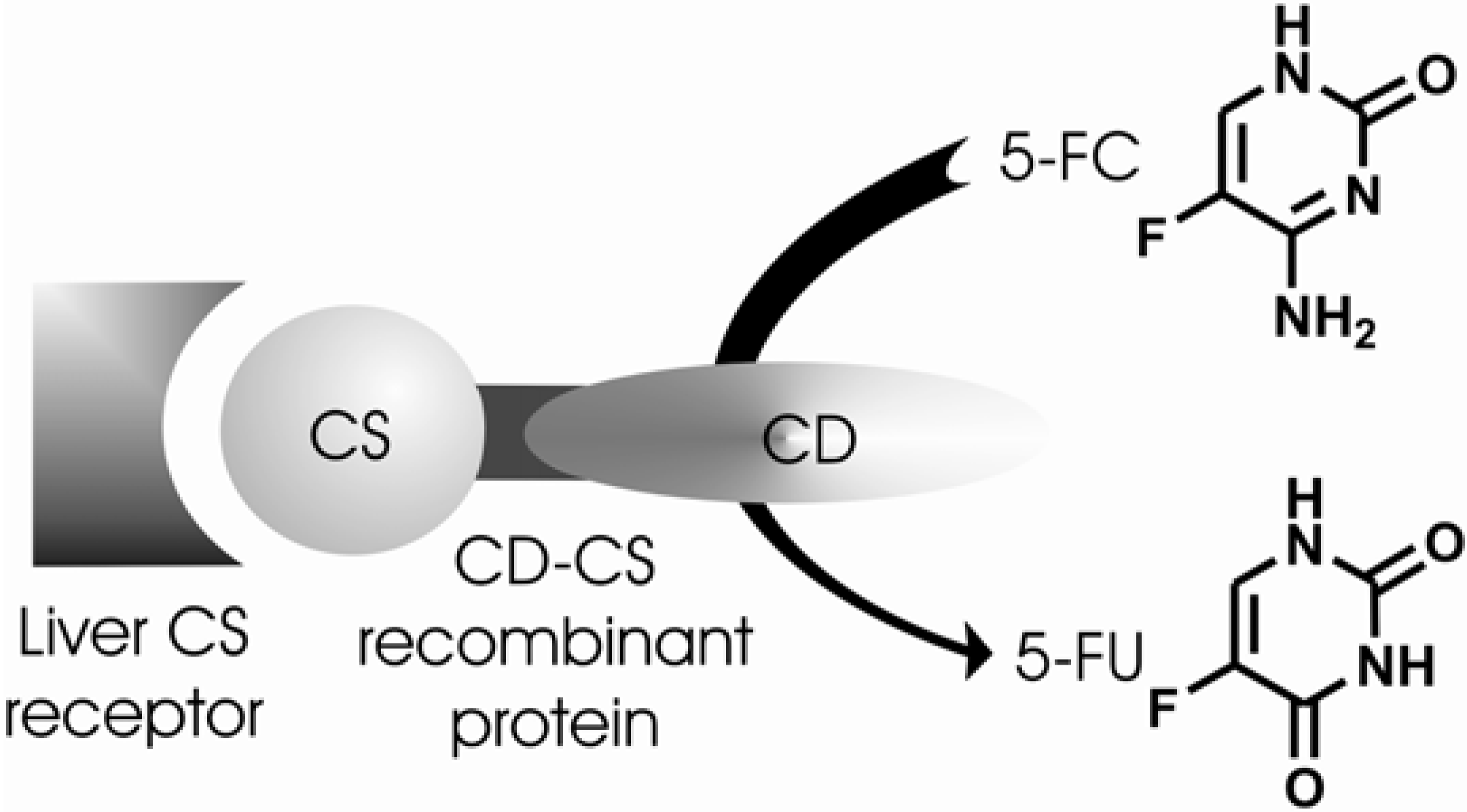
Cerami and co-workers [172] demonstrated that CS protein (malaria circunsporozoite) [173] found in the outer surfaces of sporozoites and responsible for the liver-specific invasion of malaria parasite could be used as a delivery vehicle to introduce recombinant DNA into hepatocytes. They used adenovirus as an endosomal lysing agent. On the other hand, Lin-Lee and co-workers [174] used nonviral vectors for the same purpose. They studied a receptor-mediated delivery strategy using recombinant fusion protein consisting of CS protein as a ligand and bacterial cytosine deaminase (CD). This enzyme catalyzes the production of 5-fluorouracil (5-FU) from its prodrug 5-fluorocytosine (5-FC) (Figure 30). Receptor for CS protein is distributed predominantly at the basolateral domain of hapatocytes [175]. This CD-CS system could be cell type-specific and in the presence of exogenous 5-FC would be prone to kill the parasites [174].
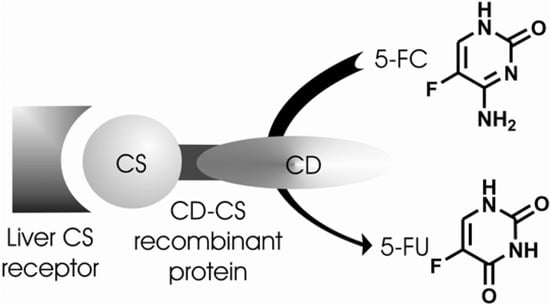
Figure 30.
CD-CS approach. Conversion of 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU) by cytosine deaminase (CD).
Figure 30.
CD-CS approach. Conversion of 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU) by cytosine deaminase (CD).
Prodrugs for Schistosomiasis
Prodrugs of oxamniquine
With the purpose of obtaining new and better oxamniquine derivatives, polymeric prodrugs have been designed. Polymethacrylic and polyacrylic are known to anchor drugs, promoting improved bioavailability, prolonged action and reduced adverse effects. Thus, Parise Filho and co-workers [176] selected methacrylic and acrylic monomers as carriers and synthesized oxamniquine methacrylate, acrylamide and methacrylate copolymer (Figure 31).
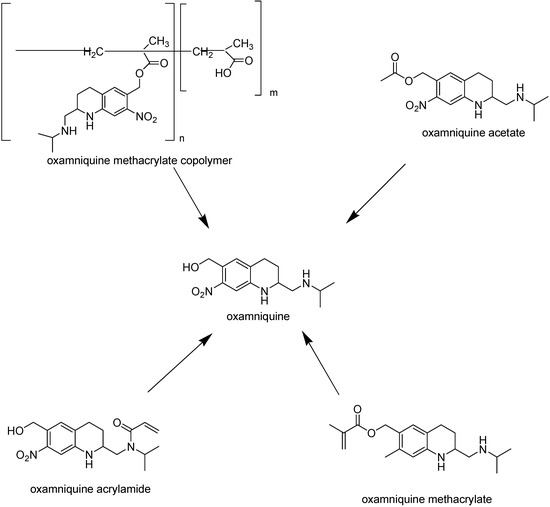
Figure 31.
Structures and hydrolysis of prodrugs of oxamniquine.
Figure 31.
Structures and hydrolysis of prodrugs of oxamniquine.
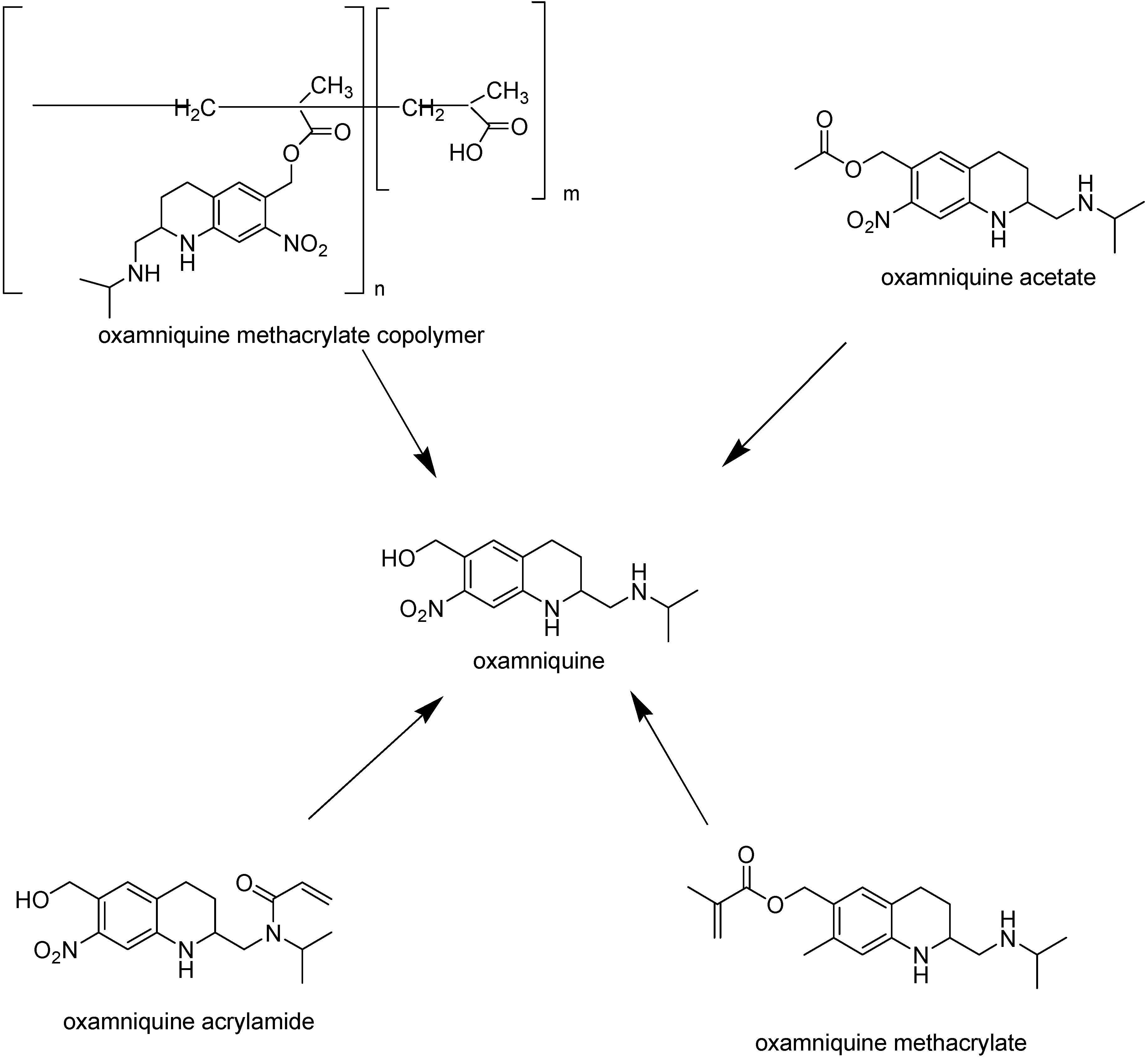
Preliminary in vivo evaluation showed that oxamniquine methacrylate and oxamniquine acrylamide have prolonged action profiles and reduced worm charge. Concerning parasite charge, similar profiles to oxamniquine were observed. Otherwise, for the compound oxamniquine methacrylate copolymer, a significant increase in the number of worms was observed, suggesting that the methacrylate copolymer did not exhibit antiparasitic activity. This behavior may be related to several factors such as: the compound might not have achieved the effective dosis in the studied period; problems of drug release from the polymeric matrix, due to its complexity, and also to the high molecular weight of the prodrugs obtained, which may have inhibited the endocytosis process.
Almeida and co-workers synthesized an oxamniquine polymeric prodrug [177], using dextran T-70 (Figure 32) as a carrier, in order to improve the oxamniquine action and minimize its side effects. The preliminary tests of biological activity of this compound showed similar activity of the polymeric prodrug when compared to the commercial oxamniquine (Mansil®).
Based on the oxamniquine mechanism of action – enzyme esterification to a reactive ester that acts as an alkylating agent and binds covalently to schistosomal DNA -- El-Hamouly synthesized oxamniquine acetate (Figure 31) and studied the effect on the survival of hycanthone-sensitive and hycanthone-resistant strains of S. mansoni [178]. In addition, the effect on schistosome [3H]uridine incorporation was determined, since it has been shown that there is a good correlation between this effect and schistosomicidal activity. The prodrug inhibited [3H]uridine incorporation and caused death in the hycanthone-sensitive strain, but no effective in hycanthone-resistant strain.
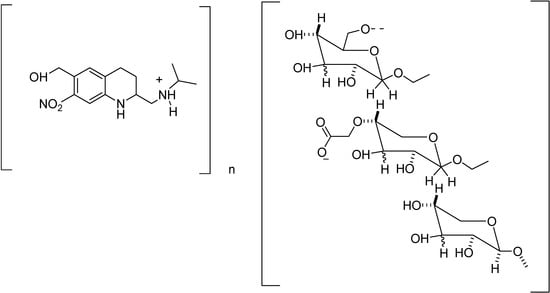
Figure 32.
Chemical structure of the dextran-methylcarboxylate prodrug of oxamniquine.
Figure 32.
Chemical structure of the dextran-methylcarboxylate prodrug of oxamniquine.
Prodrugs of dihydroartemisinin
Derivatives of artemisinin, which are already widely and effectively used in the treatment of malaria, also display antischsitosomal properties [179]. Artesunate acts as a prodrug of dihydroartemisinin (DHA) is rapidly hydrolysed to DHA in presence of water (Figure 17). Utzinger and co-workers showed this prodrug is highly active against the juvenile stages of S. mansoni, whereas adult worms are considerably less susceptible [180]. Another study [181] lead to the conclusion that artesunate is more active than praziquantel in causing damage to the tegument of adult S. mekongi. Further studies are needed to answer whether S. mekongi has the same typical stage specific susceptibilities to artesunate or artemether as previously observed in S. mansoni and S. japonicum.
Tuberculostatic prodrugs
Bioprecursors for tuberculosis chemotherapy
Several antituberculosis compounds are bioprecursor prodrugs that require activation by Mycobacterium enzymes to acquire bacterial toxicity (Figure 33). These include pyrazinamide (PZA), isoniazid (INH) and ethionamide (ETA). PZA is activated by the mycobacterial pyrazinamidase (PncA) to pyrazinoic acid, that lowers the pH enhancing the intracellular accumulation of the latter. This is unable to diffuse across the mycobacterial cell wall, leading to the disruption of membrane transport and energy depletion [182,183]. Because no pyrazinoic acid efflux mechanism exists, this accumulation process causes a remarkable susceptibility of M. tuberculosis to pyrazinamide. Mutations in the gene encoding pyrazinamidase/nicotinamidase (pncA), cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus [184]. Both isoniazid and the structurally analogous thioamide, ethionamide, act as inhibitors of InhA (enoyl-acyl carrier protein reductase). However, the large majority of isoniazid-resistant strains remain full susceptible to ethionamide [185]. This is due the fact that INH and ETA are activated by different mechanisms; thereby there is no cross-resistance [186,187].
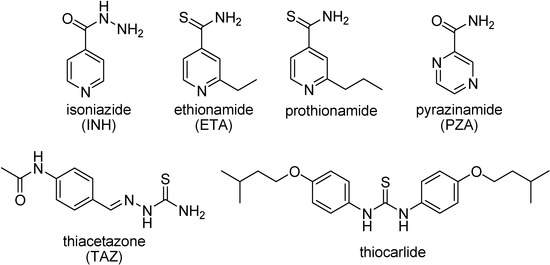
Figure 33.
Chemical structures of some antimycobacterial agents.
Figure 33.
Chemical structures of some antimycobacterial agents.
The INH is activated by the catalase/peroxidase (KatG) [187;188], generating an isonicotinoyl radical, which then couples to NADH [189], giving an INH-NADH adduct, which is a potent inhibitor of InhA. Monooxygenase (EthA)-mediated conversion of ETA results in the formation of the corresponding sulfoxide product (Figure 34). This has to be activated in a subsequent reaction and the second activation step was also found to be catalyzed by EthA yielding 2-ethyl-4-amidopyridine as final product with no antitubercular activity [190]. This indicates that the key toxic species is formed as an unstable reactive product intermediate [186]. It has been suggested that the initial sulfinate product formed after two consecutive EthA-mediated sulfoxidations of the parent drug decomposes to form a labile toxic intermediate [191]. Besides ethionamide, several others thioamide prodrugs and probably thiacetazone (TAZ) are activated by EthA. However, the final target for these antitubercular prodrugs differ for each one, although ethionamide and several others thioamide prodrugs but not TAZ affect the synthesis of short-chain fatty acids [186,190,192,193].
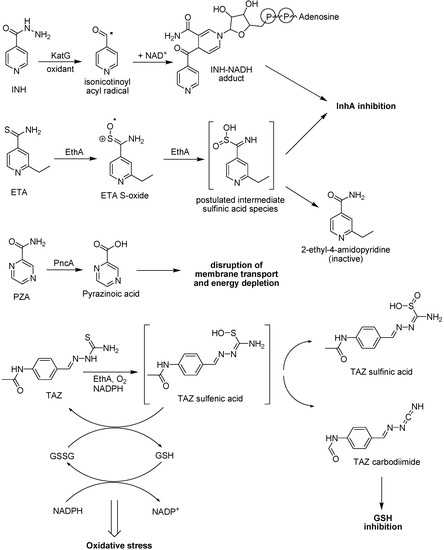
Figure 34.
Activation pathway of the bioprecursors for tuberculosis and targets of the active compounds.
Figure 34.
Activation pathway of the bioprecursors for tuberculosis and targets of the active compounds.
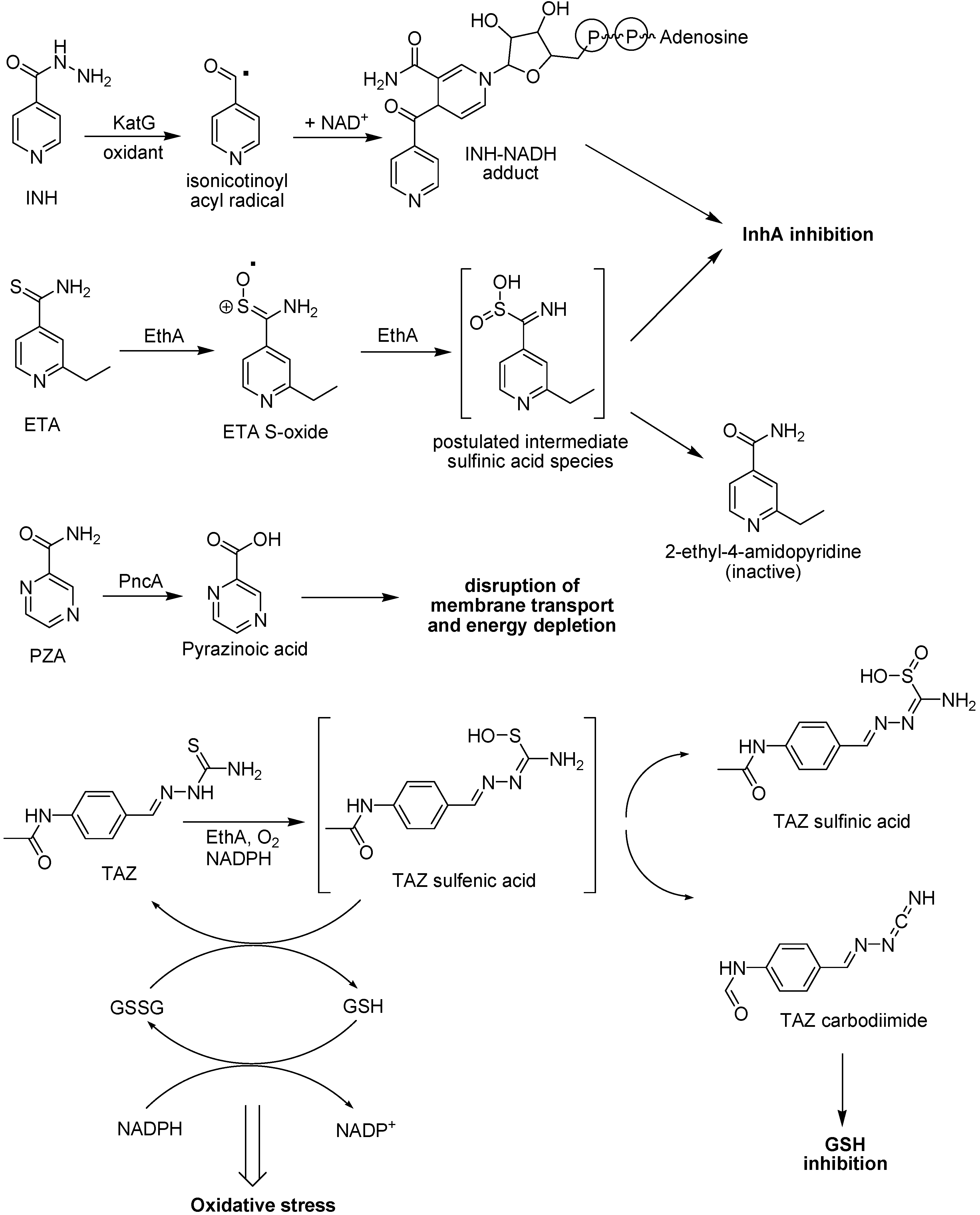
Oxidation of TAZ by EthA at basic pH favors formation of the carbodiimide, whereas neutral or acidic conditions lead to the sulfinic acid. These metabolites derive from an initial sulfenic acid intermediate. The sulfenic acid and carbodiimide metabolites, but not the sulfinic acid product, readily react with glutathione. These reactions may be involved in the antitubercular activity of TAZ (Figure 34) [194].
Mutual prodrug of isoniazid, para-aminosalicylic acid, and ethambutol
Antitubercular therapy is given for a long time and involves combination of drugs as 4-amino-salicylic acid (PAS), isoniazid (INH), ethambutol (EB) along with rifampicin, which is marketed in combination dosage form.
The free acid carboxylic group in PAS accounts for its gastrointestinal irritation effects. This drug is extensively metabolized by acetylation of the amino group and conjugation with glucuronic acid and glycine at the carbonyl group. Since its half-life for the metabolism is one hour, large doses are needed to maintain a minimum effective level of the PAS. INH is readily absorbed on oral administration, extensively metabolized to inactive metabolics (diacetylhydrazide, acetylhydrazide, N-acetylisoniazid and hydrazine) [195] and when co-administered with PAS, this drug is found to reduce its acetylation increasing its plasma level
EB is a water-soluble, bacteriostatic agent which is readily absorbed (75–80%) following oral administration. Most of the administered EB is excreted unchanged, with not more than 50% appearing in urine in oxidized form of aldehyde or carboxylic acid [196,197]. Since this drug can be used in combination with PAS and INH, Rawat and co-workers synthesized mutual prodrugs. The objectives were the reduction of gastrointestinal toxicity of PAS; the reduction of intestinal acetylation of isoniazid and the increase in duration of action, besides decreasing the dose of drugs (Figure 35) [198].
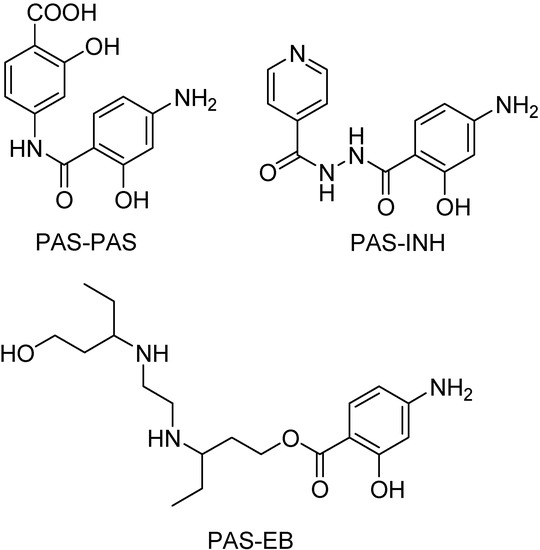
Figure 35.
Chemical structures of the mutual prodrugs (PAS = para-aminosalicylic acid; INH = isoniazid; EB = ethambutol).
Figure 35.
Chemical structures of the mutual prodrugs (PAS = para-aminosalicylic acid; INH = isoniazid; EB = ethambutol).
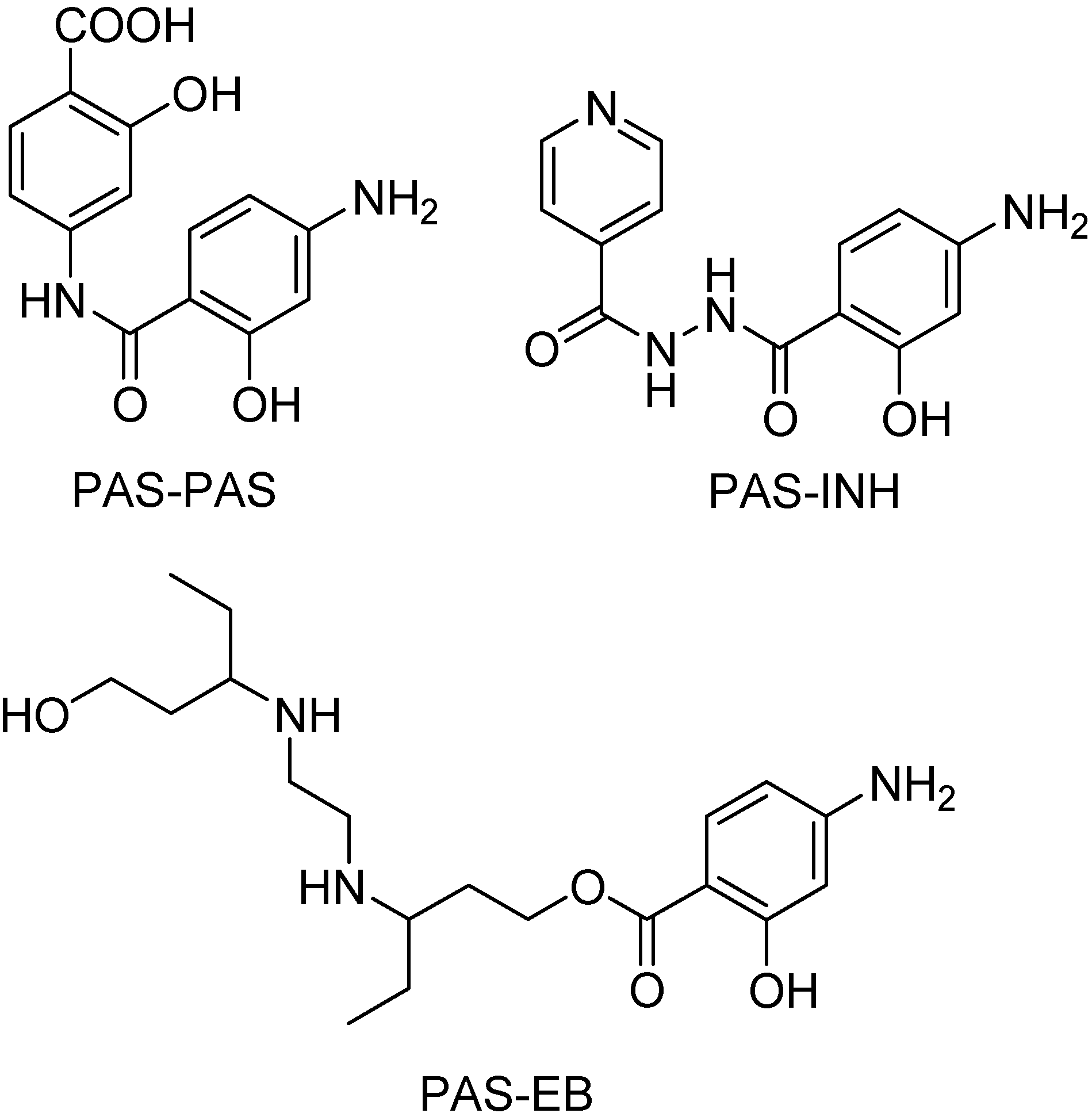
Those mutual prodrugs were absorbed unhydrolyzed. In vivo studies were also performed to confirm the release profile of these prodrugs and greater serum concentrations of EB, PAS and INH than their concentrations when given alone were found. Therefore, those mutual prodrugs eliminate significantly the problem of catabolism by acetylation in gastrointestinal tract and also PAS and INH toxicity [198].
Prodrugs of nitroimidazoles
One-third of the world population is currently infected with latent TB associated with the presence of the latent, non-replicating and resistant to conventional antimycobacterium drugs forms of the bacterium [199,200]. The latent bacilli have adapted to anaerobiosis and can maintain viability for extended periods of time. The first compound to show selective activity against those forms of M. tuberculosis was metronidazole. It undergoes reduction of its nitro group to reactive species that causes DNA damage and subsequent cell death [201]. However, metronidazole failed to show activity in an in vivo mouse model [202].
Based on the activity of nitroimidazoles, a series of 6-substituted-2-nitroimidazo[2,1-b][1,3]oxazimes prodrugs (Figure 36) were synthesized, which require bioreductive activation of an aromatic nitro group to exert antitubercular effects. PA-284 showed significative antitubercular activity against sensitive, mono-resistant and multidrug-resistant strains of M. tuberculosis, as well as against latent bacilli. It was also active against M. tuberculosis in in vivo mouse models [203]. This prodrug is in phase I clinical trials sponsored by the Global Alliance. However, M. tuberculosis mutant relatively to a specific protein, a F420-dependent glucose-6-phosphate dehydrogenase, that metabolizes PA-824 and also CGI17341, showed to be resistant those compounds [204;205].
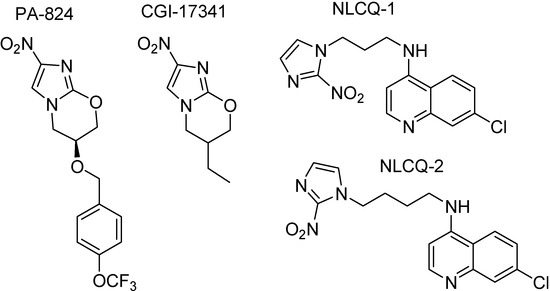
Figure 36.
Chemical structures of nitroimidazole prodrugs.
Figure 36.
Chemical structures of nitroimidazole prodrugs.
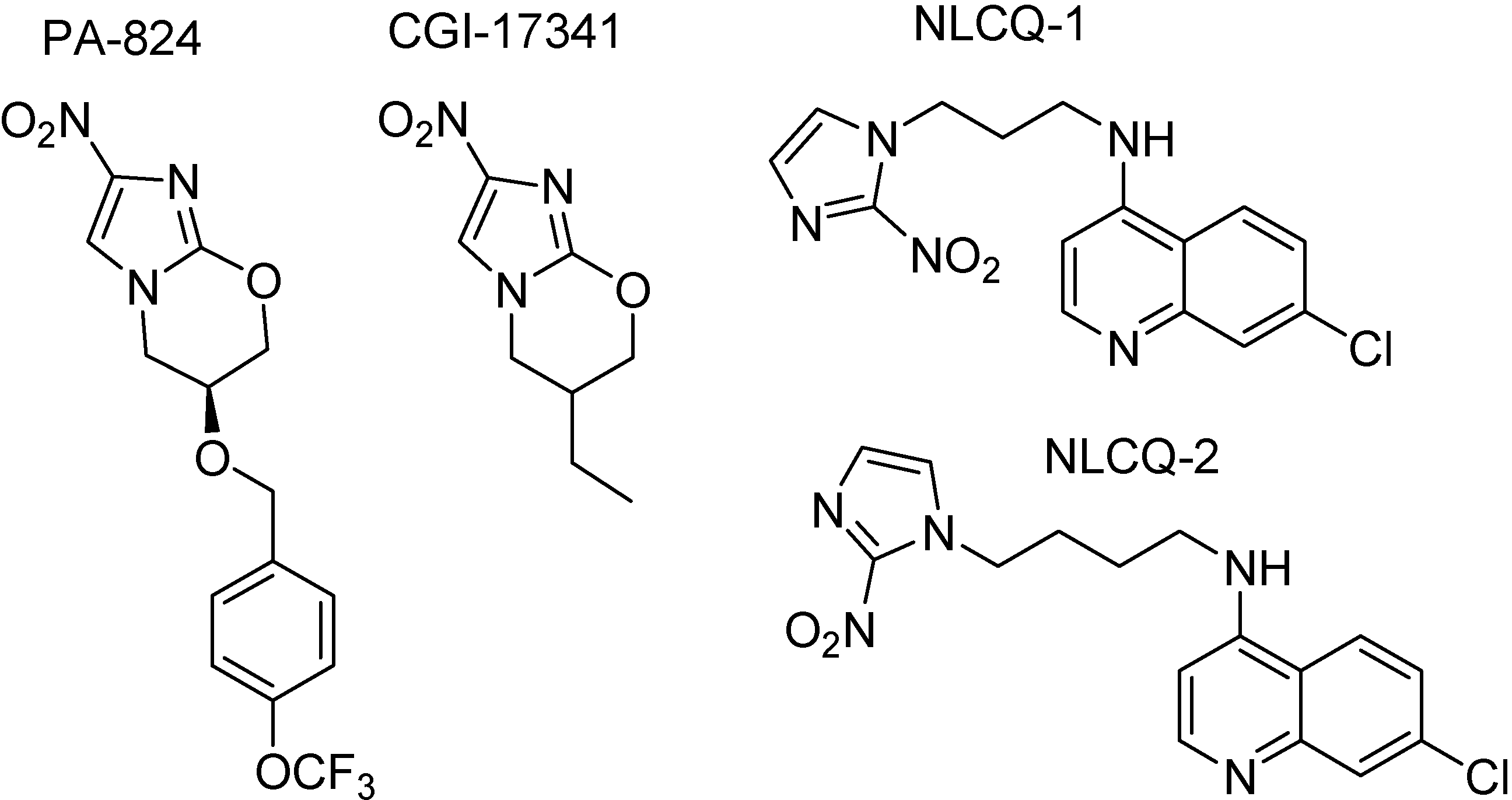
Compounds NLCQ-1 and NLCQ-2 hydrochloride (Figure 36) were tested against dormant M. tuberculosis H37Rv [204]. Both compounds demonstrated significant activity, comparable with PA-824. They showed Minimal bactericidal concentrations (MBCs) of 3.1-18.4 and 4.9-9.8 μg/mL, respectively while PA-824 MBC was 6.4-12.8 μg/mL. These compounds, mainly NLCQ-1 are activated by different reductive enzymes (cytochrome P450 reductase and cytochrome B5 reductase) and therefore resistance to those prodrugs does not appear in M. tuberculosis H37Rv mutant [205,206].
Nucleoside analogues prodrugs
Co-infection of AIDS with TB is now the leading cause of death for one out of three people who die with the former. For this very reason, an ideal drug for HIV/AIDS patients should have anti HIV activity while treating TB [207,208,209].
Shriram and co-workers developed and evaluated mutual prodrugs composed of an antimycobacterial agent, as ciprofloxacin, norfloxacin and isoniazid, and a anti HIV nucleoside analogues, as zidovudine, stavudine and lamivudine [209,210,211,212].
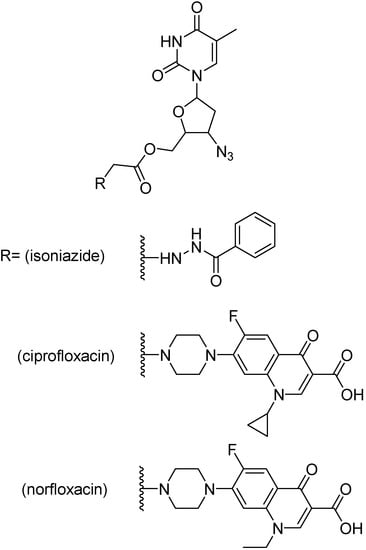
Figure 37.
Ester prodrugs of zidovudine.
Figure 37.
Ester prodrugs of zidovudine.
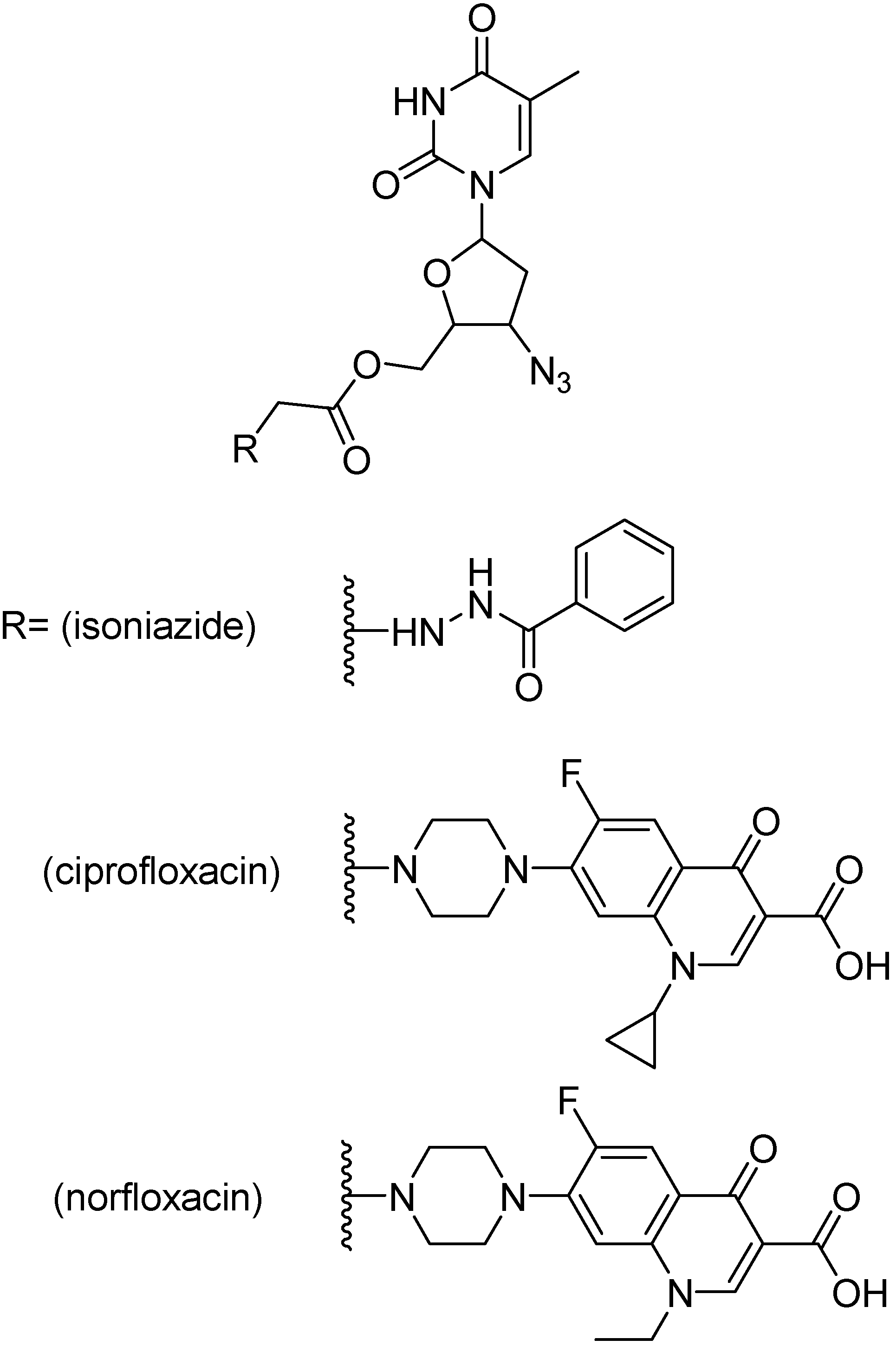
Mutual prodrugs of zidovudine (Figure 37) with INH, ciprofloxacin and norfloxacin were tested against M. tuberculosis strain H37Rv at a single concentration, 6.25 μg/mL. The prodrugs bearing ciprofloxacin or norfloxacin were the most active, showing 99% inhibition while isoniazid containing prodrug showed 90% inhibition [213]. Prodrugs of stavudine containing a fluoroquinolone moiety (Figure 38) showed 100% inhibition, while INH containing prodrug presented 90% inhibition [210]. Lamivudine prodrugs with fluoroquinolones (Figure 39) showed 92–100% inhibition against H37Rv M. tuberculosis strains [213].
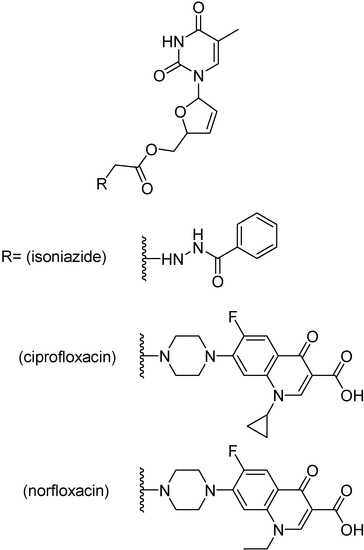
Figure 38.
Ester prodrugs of stavudine.
Figure 38.
Ester prodrugs of stavudine.
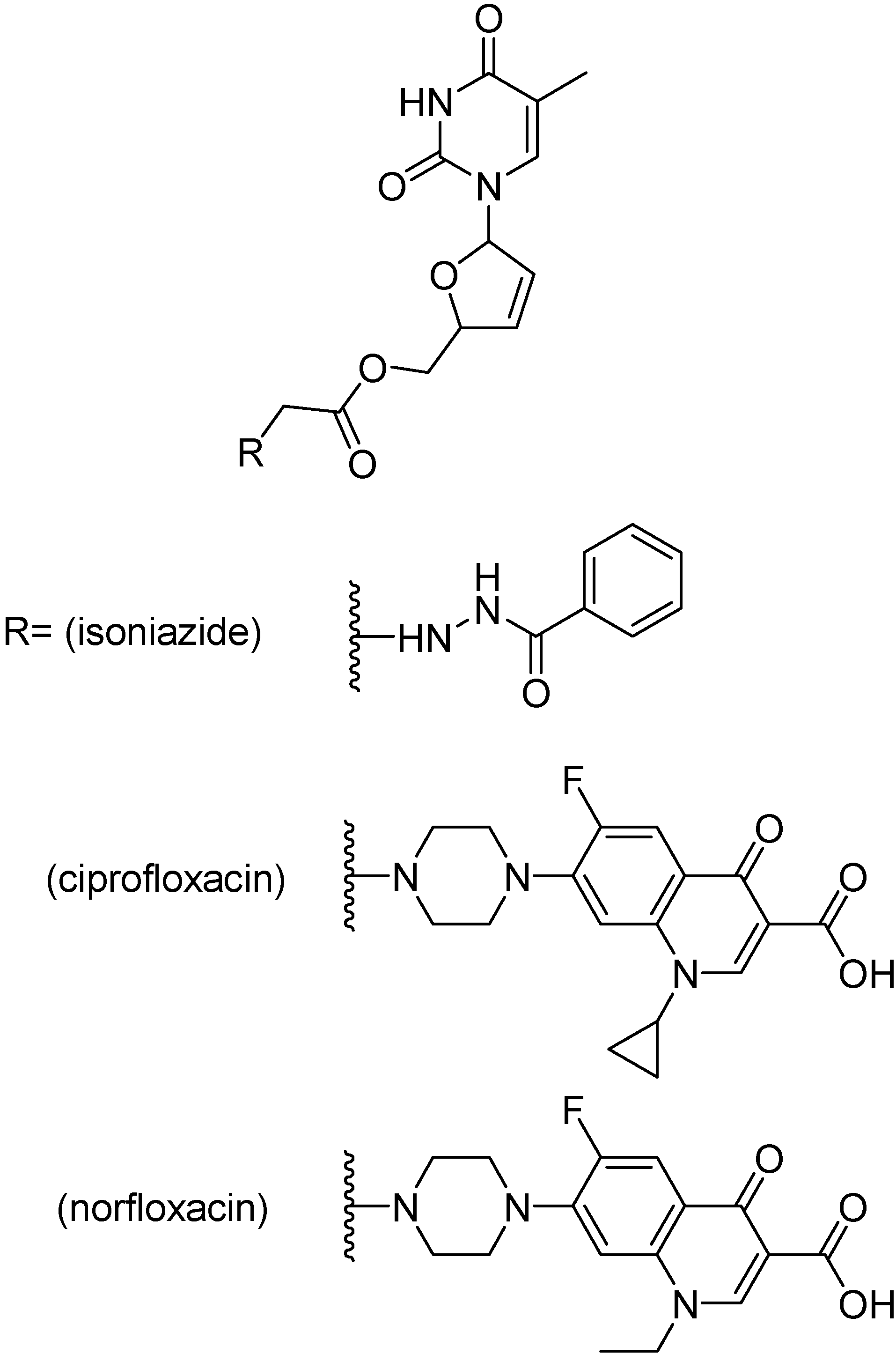
Figure 39.
Prodrugs of lamivudine.
Figure 39.
Prodrugs of lamivudine.
The stability for these mutual prodrugs concerning the transport across the cell membrane and their intracellular reversion to the parent compound, especially in the virally infected cells, display a determinant role in their interest as antituberculous agents in co-infection TB and HIV. Those prodrugs were hydrolized in the range of 20 to 240 min, and 120 to 240 for lamivudine prodrugs. The lamivudine prodrug C (Figure 39) inhibited HIV-1 replication and was responsible for 100% inhibition of M. tuberculosis and those properties account for its effective treatment of HIV/AIDS co-infection [213].
Polymeric prodrugs of norfloxacin
Mycobacteria are facultative intracellular pathogens and scape killing during phagocytosis by blocking phagosome-lysosome fusion. Intracellular mycobacteria are also largely protected against drugs and in this condition, they are difficult to be destroyed [214].
To be effective against intracellular bacteria, the drugs must penetrate the cells. Drug delivery systems capable of specifically targeting the drug into the macrophages can help to face this challenge [215]. Drugs linked to a homing device, that interact with a specific cell receptor, through a macromolecular carrier could induce the endocytosis of the drug, improving its effectiveness and imparting a selective action. Mannosyl ligands are director groups that can be used to target macrophages [216].
Covalent linking of norfloxacin to a polymeric carrier is one approach to improve its pharmacological properties (Figure 40). The prodrug is uptaken by cells, enters through endocytosis, and since the conjugate is large enough to impair renal filtration, the drug delivery and consequently its effectiveness are improved [217,218,219].
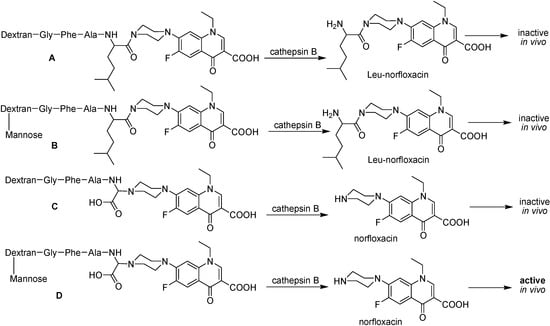
Figure 40.
Schematic representation of the polymeric prodrugs and the product of enzymatic hydrolysis activity. A and B, norfloxacin linked through amide bond and C and D, norfloxacin linked through an α bond.
Figure 40.
Schematic representation of the polymeric prodrugs and the product of enzymatic hydrolysis activity. A and B, norfloxacin linked through amide bond and C and D, norfloxacin linked through an α bond.
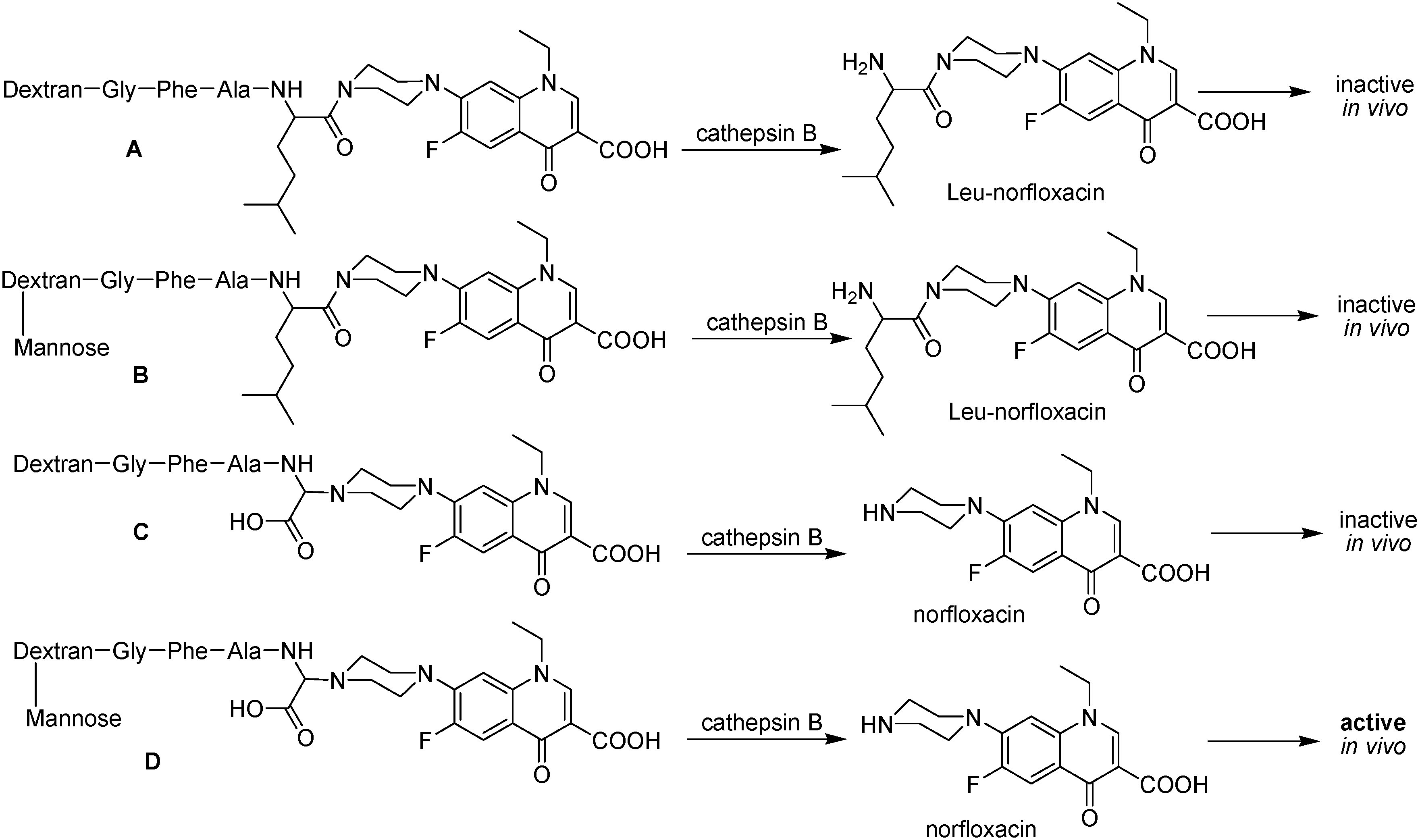
In this example, two different peptide spacer arms, Gly-Phe-Ala-Leu and Gly-Phe-Gly-Gly were used to link norfloxacin to dextran. In the former, Leu-norfloxacin was released in the presence of the lisosomal enzyme cathepsin B, instead of free norfloxacin. On the other hand, more than 60% of norfloxacin was released at pH 5.5 in presence of cathepsin B if the peptide was Gly-Phe-Gly-Gly (α-norfloxacin)-O-methyl linked to dextran with or without mannose [217]. The first conjugate is 20 times less active than the other conjugate, when tested in vitro against M. bovis BCG. The test in mice infected with M. bovis BCG prove that only the conjugate containing both an α bond and mannose was as active as isoniazid. This conjugate was able to deliver norfloxacin directly into macrophages and controls micobacterial growth in the liver, where isoniazid is inactive due to the low pO2, which turns M. tuberculosis insensitive to isoniazid and rifampicin [220].
Polymeric prodrug of isoniazid and pyrazinamide
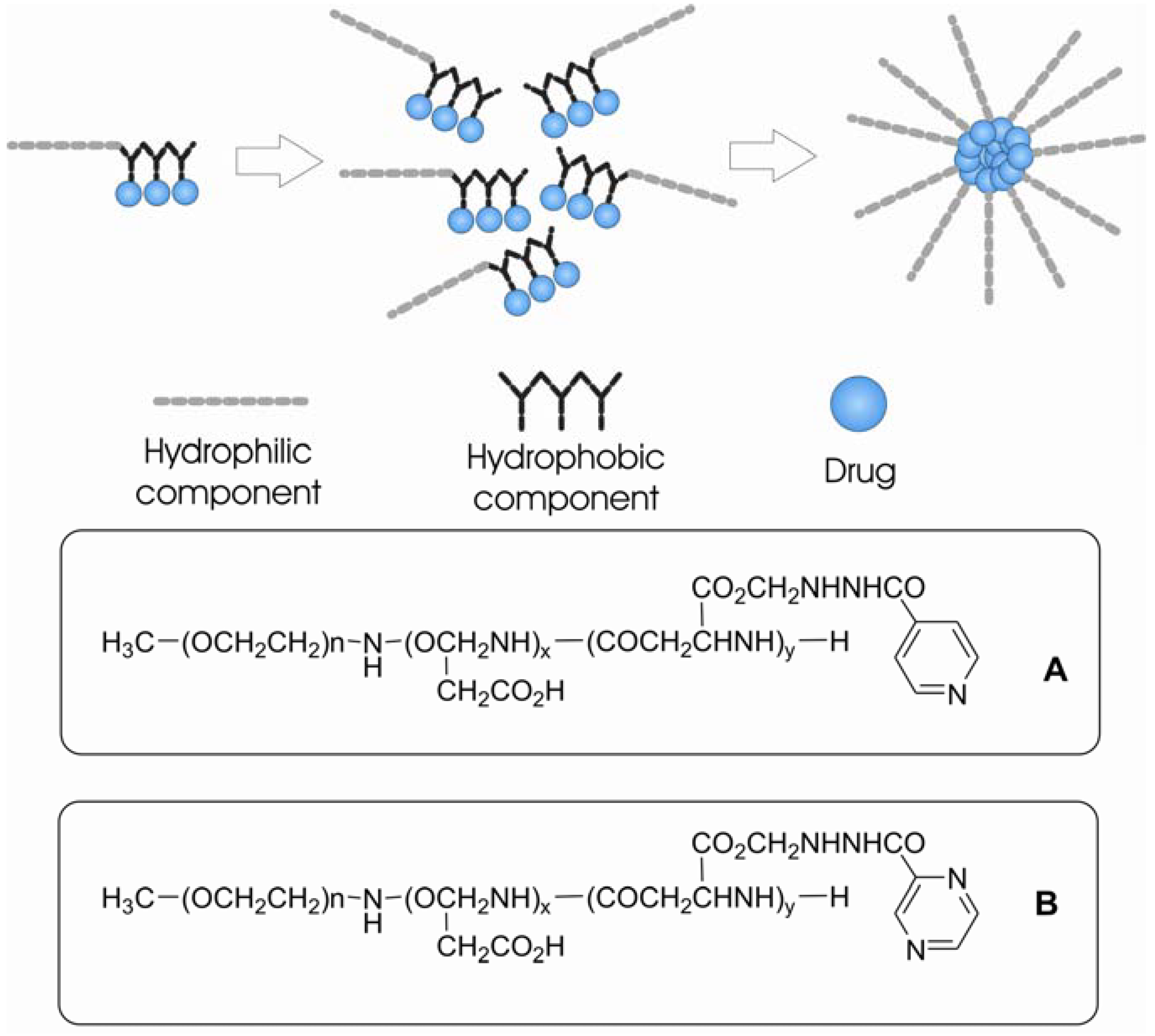
Micellar systems of polyethyleneglycol-poly(aspartic acid) copolymer were used by Silva and co-workers [221] as carrier to the tuberculostatic drug isoniazid, which was covalently bounded to the system (Figure 41A).
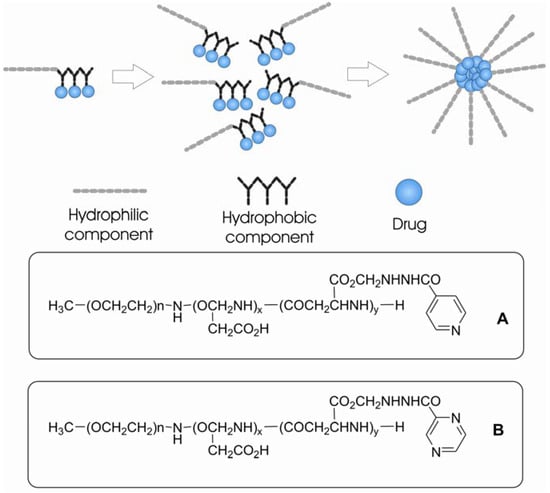
Figure 41.
Micelles of PEG-PAS covalently linked to drugs. A. isoniazide; B: pyrazinamide.
Figure 41.
Micelles of PEG-PAS covalently linked to drugs. A. isoniazide; B: pyrazinamide.
This polymeric prodrug envisioned not only the prolonged delivery of the drug, but also the lowering of toxicity and extension of the period between two consecutive doses, so patients would be more willing to follow the treatment schedule.
Later on, the same group extended their work to another antitubercular polymeric prodrug, derived from a poly(ethyleneglycol)-poly(aspartic acid) block copolymer by substituting pyrazinamide on the aspartate free carboxylic groups (PEG-PASP-PZA) (Figure 41B) [222]. The substituted polymer forms a micelle, with a hydrophobic core consisting of the drug-ligand group and a hydrophilic outer coat [221,222].
The advantage of this micellar carrier over others lies in the ability to control the particle size, its structural stability and good solubility in water. In addition, this structure allows a controlled rate of delivery of the drug, reduced toxicity and selective action on the chosen target [223,224]. The potential for prolonged drug action at low toxicity level means that this system could lead to greater patient approval. This micelle-forming polymeric prodrug offers the advantages of reducing toxicity and immunogenicity.
The activity of pyrazinamide is pH-dependent and is limited to slow-growing bacilli. Owing to its toxic effect on the liver, it has to be used under closed medical supervision, accompanied by regular tests of liver function. Thereby, this polymeric prodrug can be very useful to reduce pyrazinamide toxicity to the liver. Furthermore, the PEG-PASP-PZA derivative, when assayed for its anti-Mycobacterium activity, exhibited stronger activity than the simple drug [222].
In 2004, Rando and co-workers. [225] used N-methylene phosphonic chitosan (NMPC) to obtain a polymeric prodrug of isoniazid, defined as chitosan-isoniazid hemisuccinate. This polymeric system aimed, not only to slow the drug delivery as proposed by Silva and co-workers [221;222], but also to obtain a specific action (Figure 42).
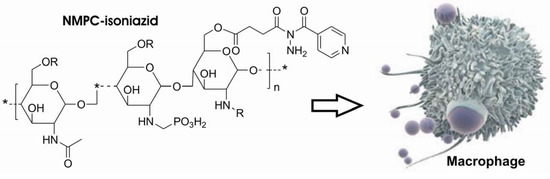
Figure 42.
Chitosan-Isoniazid hemisuccinate.
Figure 42.
Chitosan-Isoniazid hemisuccinate.
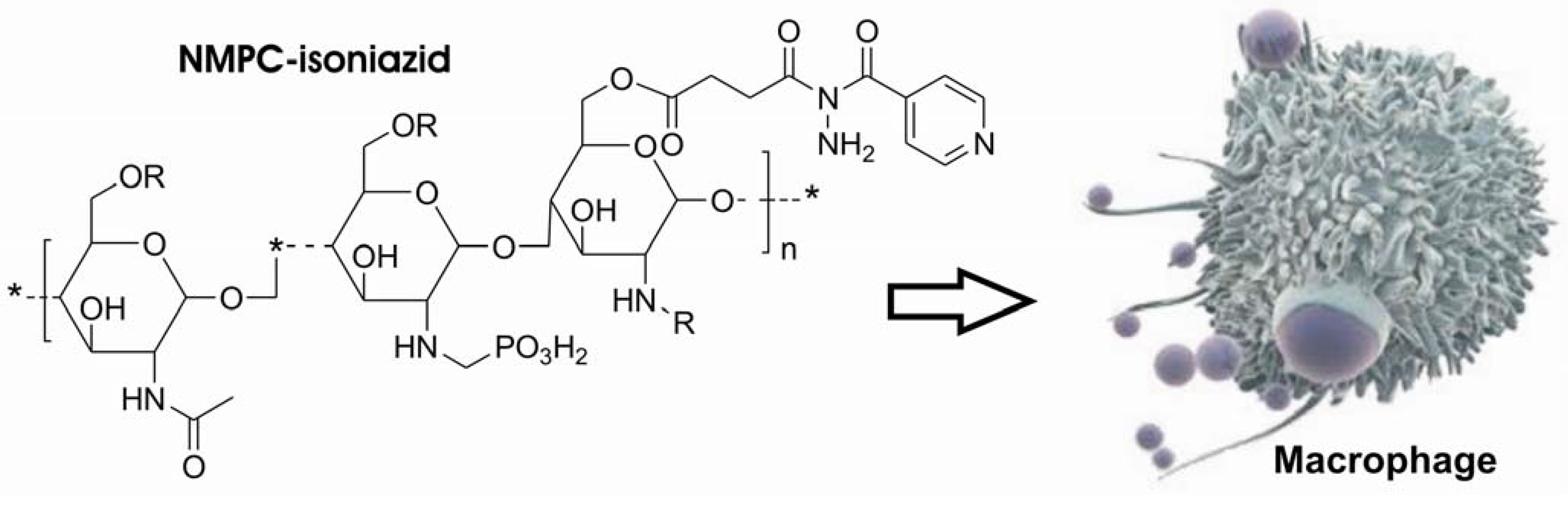
Generally, chitosan is a largely used carrier because of the presence of free NH2 group that provides a reactional center either to modification or drug linkage [225]. This derivative is almost atoxic and has immunopotentiating effect by stimulating macrophages and increasing the humoral response [226]. Since the macrophages are the host cell of M. tuberculosis, prodrugs with chitosan as carrier could act as a targeted prodrug.
The N-methylene phosphonic group account for the higher hydrosolubility of the resulting prodrug which can be easily handled and intravenously administrated [227].
DA-7218 prodrugs
Oxazolidinones are a new, unique class of synthetic antibacterial agents effective against many gram-positive bacteria, including aerobic pathogenic actinomycetes of the genera Mycobacterium, Nocardia, and Actinomadura [228,229,230,231,232]. Linesolid (Figure 43) was the first oxazolidinone introduced in the market. It was active in animal models and also in clinical trials with patients infected with M. tuberculosis [233]. Oxazolidinones selectively inhibit bacterial protein synthesis by avoiding the formation of a functional 70S initiation complex [234]. DA-7157 was shown to be more active than linesolid in in vitro test against several gram-positive species [235].
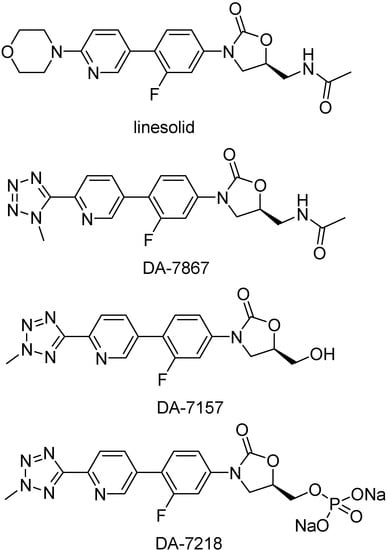
Figure 43.
Chemical structures of oxazolidinone derivatives.
Figure 43.
Chemical structures of oxazolidinone derivatives.
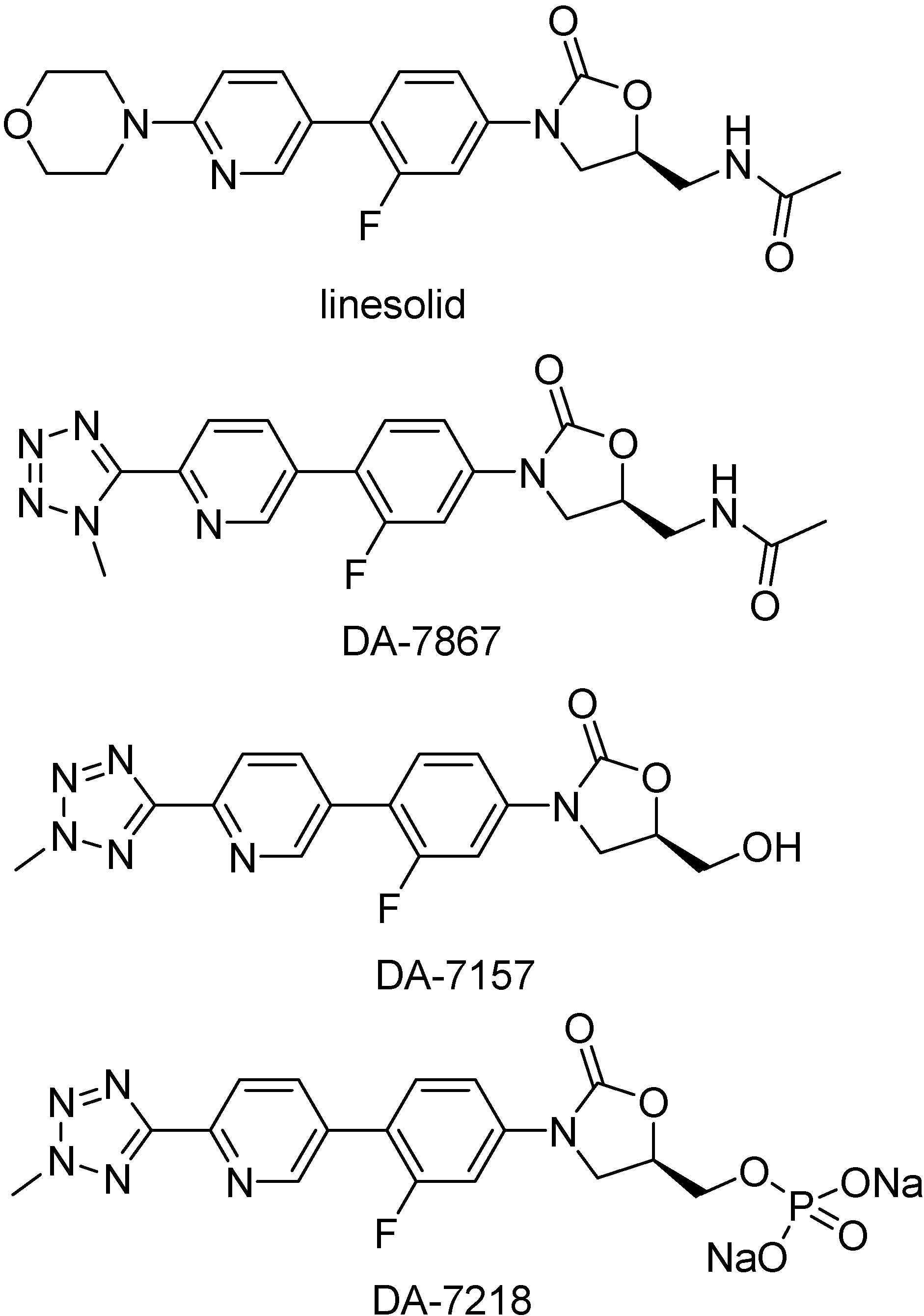
This compound is generated by the metabolism of DA-7218, a highly hydrophilic prodrug (Figure 43). Both compounds were tested against clinical isolates of Mycobacterium tuberculosis, their MIC90s against M. tuberculosis was about 0.5 μg/mL [235]. DA-7218 is an excellent candidate to be used in humans, although the in vivo test has not been performed yet. It has good water solubility, which allows high availability and perfusion in the tissues.
Prodrugs for Sickle Cell Disease
Butyric acid prodrugs
Recently, butyric acid (BA) and derivatives [236,237,238] were proposed to be useful in the treatment of β-globin disorders such as sickle cell disease and β-thalassemia. Their effect is associated with an increase in HbF production that leads to the minimization of the clinical symptoms of this disease [239]. However, BA is poorly absorbed in human gastrointestinal tract. In order to solve this problem, a series of butyric acid prodrugs were designed [238]. BA prodrugs, which also display low toxicity, may have significant advantages for the treatment of β-thalassemia and sickle cell anemia, when compared to the cytotoxic agent hydroxyurea approved for the chronic treatment of the latter [240].
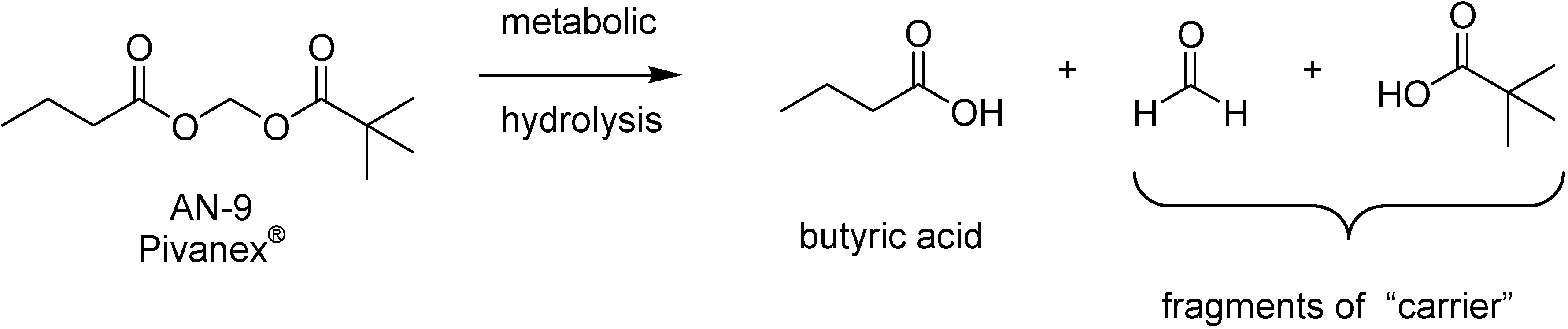
BA possesses a carboxylic acid function and there are suitable prodrugs for this functional group [9]. Esterification of BA improves its permeability across cell membranes and imparts this acidic compound with an efficient delivery to a subcellular target. Therefore, a series of acyloxyalkyl esters prodrugs designed with the aim of increasing stability and lipofilicility [238] was designed. Those compounds release equimolar amounts of BA, an aldehyde and a second acid. This is mainly biologically inactive and acts as “carrier” moieties. An example of this strategy was the compound AN-9 (Pivanex®) that after metabolic hydrolysis releases one molecule of butyric acid and fragments of carrier represented by pivalic acid and formaldehyde (Figure 44).
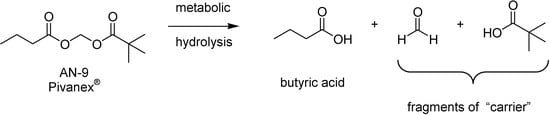
Figure 44.
Metabolic hydrolysis of Pivanex®.
Figure 44.
Metabolic hydrolysis of Pivanex®.
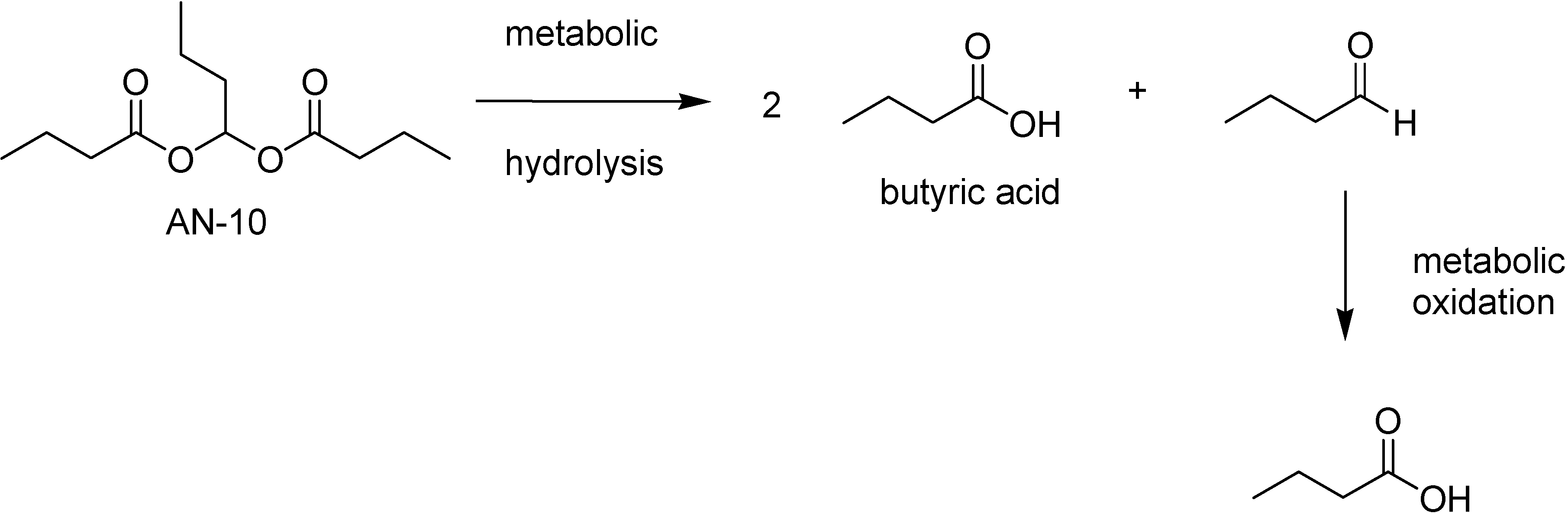
The most active prodrug was the butylidene dibutyrate (AN-10), that releases 2 equivalents of BA and 1 of butyraldehyde. This undergoes further in vivo oxidation to BA (Fig 45).
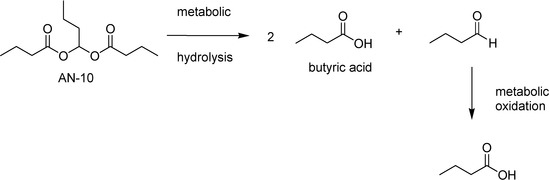
Figure 45.
Metabolic hydrolysis of AN-10.
Figure 45.
Metabolic hydrolysis of AN-10.
Although AN-9 (Figure 44) and AN-10 (Figure 45) are highly lipophilic derivatives that better cross cell membranes than BA, they have poor solubility in water, with leads to a non-aqueous media for clinical formulation. To solve this problem, a series of acid, basic and neutral prodrugs was obtained. The neutral and the acid prodrugs 1 and 2, respectively, in Figure 46, could induce fetal hemoglobin. They increase hemoglobin levels and showed lower toxicity than BA [239;241].
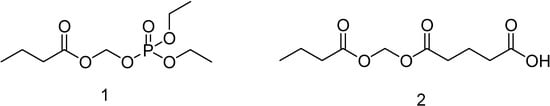
Figure 46.
Neutral (1) and acid (2) derivatives of butyric acid.
Figure 46.
Neutral (1) and acid (2) derivatives of butyric acid.
Vanillin prodrugs
Vanillin (Figure 47), obtained from vanilla beans, increases oxygen affinity by hemoglobin S (Hb S), when sickle erythrocytes (SS cells) is incubated with this flavouring agent [243]. This compound reacts covalently with Hb S and shifted the oxygen equilibrium curve (OEC) towards the left which inhibits cell sickling.
Other aldehydes like five-membered heterocyclic aldehydes (Figure 47) increase the oxygen affinity of hemoglobin (Hb), inhibiting the sickling of homozygous sickle red blood (SS). This aldehyde group form Schiff base-adducts with Hb, through a specific binding to the N-terminal αVal1 of Hb T state, leading to opposite allosteric shifts [242,243].
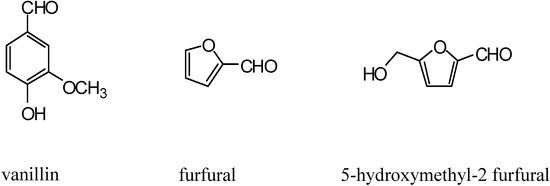
Figure 47.
Some aldehyde derivatives with potent antisickling effect.
Figure 47.
Some aldehyde derivatives with potent antisickling effect.
Vanillin did not have a significant anti-sickling effect when it was administered orally because of its gastrointestinal (GI) unstability, due to aldehyde chemical and metabolic labiality, what hinders its use in the therapeutics [244;245]. A vanilin prodrug, MX-1520, is more stable and improve the bioavailability of the parent compound [246].
Hydroxyurea and nitrate derivatives
Hydroxyurea (HU) is the only US Food and Drug Administration (FDA)-approved drug for the treatment of SCD [40,41]. It inhibits ribonucleoside diphosphate reductase, the enzyme that converts ribonucleotides into deoxyribonucleotides (dNTPs), involved in DNA synthesis and repair [247,248]. It increases the level of fetal hemoglobin (HbF), a genetically distinct hemoglobin (Hb) that inhibits the polymerization of deoxygenated sickle cell hemoglobin [249,250]. HU display other mechanism of action [249,251,252], being also considered as a source of nitric oxide (NO). This plays an important role in the maintenance of normal blood pressure and flow, and has drawn considerable interest as a sickle cell disease treatment.
Hydroxyurea could be consider a bioprecursor prodrug because after its oxidation, it may decompose into NO, ammonia and formamide or carbon dioxide, depending on the conditions and type of oxidant (Figure 48).
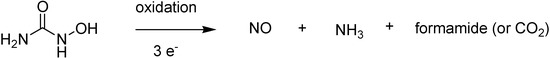
Figure 48.
Hydroxyurea oxidation to nitric oxide (NO).
Figure 48.
Hydroxyurea oxidation to nitric oxide (NO).
Nitrate derivatives
Nitrate organic compounds have vasodilatador acivity and are effective against stable angina. They could be metabolized into nitric oxide (NO) through bioactivation catalyzed by various enzymes such as glutathione S-transferase, cytochrome P-450, and possibly esterases [253]. Since NO is unstable, the prodrug approach can solve this problem, through more stable NO-donors, as nitrate prodrugs. The compounds NO-donors have vasodilatation and plalet aggregation inhibition activities among others [254].
Recently nitrate prodrugs with NO-donor properties were developed in our laboratory. These compounds increased gama-globin expression in K562 cell culture and presented important analgesic and anti-inflammatory activity that could be useful to the treatment of the SCD [255].
Conclusions
Prodrug approaches are interesting and promising alternatives to solve problems presented by current drugs or also by those that are under development. We have shown valuable examples of their use as a molecular modification tool to improve mainly pharmacokinetics properties of existing drugs, or also to experimental compounds, for the treatment of some neglected diseases.
Although many examples have been presented herein, prodrug design could be more explored by researchers in order to minimize the lack of suitable chemotherapeutic agents for the diseases considered in this review. It is worth noting that improving drug properties can, generally, lead to better alternatives associated with low cost and less time-consuming processess. These characteristics are of utmost importance for drug development mainly considering people affected by the neglected diseases, their needs and also the limited interest of pharmaceutical companies due to the low profit involved.
Acknowledgements
We wish thank to thank our funding agencies, FAPESP; CNPq, CAPES, PADC/FCF; FINEP; FUNDUNESP, for financial support.
List of abbreviations used
| 5-FC | 5-fluorocytosine |
| 5-FU | 5-fluorouracil |
| ADEPT | antibody-directed enzyme prodrug therapy |
| AN-10 | butylidene dibutyrate |
| apoL-I | apolopoprotein L1 |
| BA | butyric acid |
| CD-CS | recombinant fusion protein consisting of CS protein as a ligand and bacterial CD: cytosine deaminase |
| CL | cutaneous leishmaniasis |
| CMD-P | carboxymethyldextran-thiomannopyranoside-pyrimethamine |
| CpG ODN | CpG oligodeoxynucleotides |
| CpG | a dinucleotide constituted by a cytosine followed by guanine separated by a phosphate |
| CQ | chloroquine |
| CS | malaria circunsporozoite |
| DFMO | eflornithine |
| DHA | dihydroartemisinin |
| DHFR | dihydrofolate reductase |
| DHFR-TS | dihydrofolate reductase thymidilate synthase |
| dNTPs | deoxyribonucleotides |
| DOXP | 1-deoxy-D-xylulose 5-phosphate |
| EB | ethambutol |
| ETA | ethionamide |
| EthA | a flavin monooxygenase found to catalyze the Baeyer-Villiger reaction |
| Et-NIPOx | ethyl ester of N-isopropyl oxamate |
| fma | 2-(N-formyl-N-methyl)aminoethyl |
| GI | gastrointestinal |
| GR | glutathione reductase |
| GSH | glutatione |
| HADH-isozyme | α-hydroxyacid dehydrogenase-isozyme |
| HAT | Human African Trypanosomiasis |
| Hb | hemoglobin |
| Hb F | fetal hemoglobin |
| Hb S | hemoglobin S |
| HU | hydroxyurea |
| INH | isoniazid |
| InhA | enoyl-acyl carrier protein reductase |
| KatG | catalase/peroxidase |
| LDH-C4 | lactate dehydrogenase isozyme-x |
| mAb | monoclonal-antiboby |
| mAb-drug | monoclonal-antiboby drug conjugate |
| MBCs | minimal bactericidal concentrations |
| NbAn33 | a mAB specific for the conserved carbohydrate epitope of VSGs |
| NF | nitrofurazone |
| NFOH | nitrofurazone hydroxymethyl derivative |
| NHS | normal human serum |
| NIPOx | N-isopropyl oxamate |
| NMPC | N-methylene phosphonic chitosan |
| OEC | oxygen equilibrium curve |
| PAS | 4-aminosalicylic acid |
| PEG-PAS | polyethyleneglycol-poly(aspartic acid) |
| PEG-PASP-PZA | poly(ethyleneglycol)-poly(aspartic acid) block copolymer by substituting pyrazinamide on the aspartate free carboxylic groups |
| pfDHFR-TS | P. falciparum dihydrofolate reductase thymidilate synthase |
| PFT | protein farnesyltransferase |
| PLMII | plasmepsinII |
| PncA | mycobacterial pyrazinamidase |
| PQ | primaquine |
| PQD | pyrroloquinazolinediamine |
| PQD-A4 | tetra-acetamido pyrroloquinazolinediamine |
| PQD-BE | bis-ethylcarbamyl pyrroloquinazolinediamine |
| Pro-D ODN | a prodrug form of CpG ODN type D |
| Pro-D35 | D-35 prodrug |
| PTR1 | pteridine reductase enzyme |
| PZA | pyrazinamide |
| SCD | sickle cell disease |
| SD-P | succinyldextran-thiomannopyranoside-pyrimethamine |
| SRA | serum resistance-associated |
| SS cells | sickle erythrocytes |
| TAZ | thiacetazone |
| TB | tuberculosis |
| TLR-9 | Toll-like receptor 9 |
| Tr-apoL-I | truncated apoL-I |
| VL | visceral leishmaniasis |
| VSG | variant surface glycoprotein |
References and Notes
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Ottesen, E.; Ehrlich, S.S.; Sachs, J.D. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS M. 2006, 3, e102. [Google Scholar] [CrossRef]
- Beyrer, C.; Villar, J.D.; Suwanvanichkij, V.; Singh, S.; Baral, S.D.; Mills, E.J. Neglected diseases, civil conflicts, and the right to health. Lancet 2007, 370, 619–627. [Google Scholar] [CrossRef]
- Trouiller, P.; Olliaro, P.; Torreele, E.; Orbinski, J.; Laing, R.; Ford, N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet 2002, 359, 2188–2194. [Google Scholar] [CrossRef]
- Ehrenberg, J.P.; Ault, S.K. Neglected diseases of neglected populations: thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health 2005, 5, 119–132. [Google Scholar] [CrossRef]
- Medecins Sans Frontieres. Drugs for Neglected Diseases Initiative: Teaming up to address neglect. 12 March 2003. Accessed from: www.accessmed.msf.org in Nov. 2007.
- Pécoul, B. New drugs for neglected diseases: from pipeline to patients. PloS Med. 2004, 1, 19–22. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriès, M.A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nature Rev. Drug Disc. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Lee, H. J.; Cooperwood, J. S.; You, Z.; Ko, D. H. Prodrug and antedrug: two diametrical approaches in designing safer drugs. Arch. Pharm. Res. 2002, 25, 111–136. [Google Scholar] [CrossRef]
- Silva, A.T.A.; Castro, L.F.; Guido, R.V.C.; Chung, M. C.; Ferreira, E.I. Advances in prodrug design. Mini-Rev. Med. Chem. 2005, 5, 893–914. [Google Scholar] [CrossRef]
- Brener, Z. A descoberta. Mem. Inst. Oswaldo Cruz 1989, 84 (supl II), 1–6. [Google Scholar]
- World Health Organization. Chagas Disease: TDR. Acessed from: http://www.who.int/tdr/diseases/chagas/ in Dec. 2007.
- Urbina, JA. Parasitological cure of Chagas disease: Is it possible? Is it relevant? Mem. Inst. Oswaldo Cruz. 1999, 94, 349–355. [Google Scholar] [CrossRef]
- Rodrigues-Coura, J; de Castro, SL. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz. 2002, 97, 3–24. [Google Scholar]
- Urbaniak, M.D.; Tabudravu, J.N.; Msaki, A.; Matera, K.M.; Brenk, R.; Jaspars, M.; Ferguson, M.A.J. Identification of novel inhibitors of UDP-Glc 40-epimerase, a validated drug target for african sleeping sickness. Bioorg. Med. Chem. Lett. 2006, 16, 5744–5747. [Google Scholar] [CrossRef]
- Nkemngu, N.J.; Grande, R.; Hansell, E.; McKerrow, J.H.; Caffrey, C.R.; Steverding, D. Improved trypanocidal activities of cathepsin L inhibitors. Int. J. Antimicrob. Agents 2003, 22, 155–159. [Google Scholar] [CrossRef]
- Kuzoe, FAS. Perspectives in research on and control of African trypanosomiasis. Ann. Trop. Med. Parasitol. 1991, 85, 33–41. [Google Scholar]
- Barrett, M.P. The fall and rise of sleeping sickness. Lancet 1999, 353, 1113–1114. [Google Scholar] [CrossRef]
- World Health Organization. Strategic Direction for Research: Leishmaniosis 2002.
- Desjeux, P. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Inf. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef]
- Szyniarowski, P.; Bettendorff, L.; Schweingruber, M.E. The antitrypanosomal drug melarsoprol competitively inhibits thiamin uptake in mouse neuroblastoma cells. Cell Biol. Toxicol. 2006, 22, 183–187. [Google Scholar] [CrossRef]
- WHO. The world health report 2004. Changing history. WHO: Geneva, 2004. http://www.who.int/whr/2004/en/index.html (accessed June 12, 2007).
- Herwaldt, B. L. Miltefosine: The long-awaited therapy for visceral leishmaniasis? N. Engl. J. Med. 1999, 341, 1840–1842. [Google Scholar] [CrossRef]
- Berman, J. Current treatment approaches to leishmaniasis. Curr. Opin. Infect. Dis. 2003, 16, 397–401. [Google Scholar] [CrossRef]
- Croft, S.L.; Coombs, G.H. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003, 19, 502–508. [Google Scholar] [CrossRef]
- Davis, A.J.; Murray, H.W.; Handman, E. Drugs against leishmaniasis: a synergy of technology and partnerships. Trends Parasitol. 2004, 20, 73–76. [Google Scholar] [CrossRef]
- Ouellette, M.; Drummelsmith, J.; Papadopoulou, B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updat. 2004, 7, 257–266. [Google Scholar] [CrossRef]
- World Health Organization. Global Malaria Programme: Epidemics and Emmergences. http://www.who.int/malaria/epidemicsandemergencies.html Acessed in December 2007.
- Biot, C.; Chibale, K. Novel Approaches to Antimalarial Drug Discovery. Infect. Disord. Drug Targets 2006, 6, 173–204. [Google Scholar] [CrossRef]
- Menezes, C.M.S.; Ferreira, E.I. Modulating agents in resistant malaria. Drug Design Rev. Online 2005, 2, 409–418. [Google Scholar] [CrossRef]
- World Health Organization. Report of the third global meeting of the partners for parasite control Deworming for Health and Development. 2005. http://www.who.int/wormcontrol Acessed in Dec. 2007.
- Ribeiro-dos-Santos, G.; Verjovski-Almeida, S.; Leite, L.C.C. Schistosomiasis--a century searching for chemotherapeutic drugs. Parasitol. Res. 2006, 99, 505–521. [Google Scholar] [CrossRef]
- Murray, J. F. A century of tuberculosis. Am. J. Respir. Crit. Care Med. 2004, 169, 1181–1186. [Google Scholar] [CrossRef]
- World Health Organization. Global tuberculosis control: surveillance, planning, financing; WHO Report; 2007. [Google Scholar]
- Bower, D. L.; Lane, H. C.; Fauci, A. S. Immunopathogenesis of the acquired immunodeficiency syndrome. Ann. Intern. Med. 1985, 103, 704–709. [Google Scholar] [CrossRef]
- Reider, H. L.; Cauthen, G. M.; Comstock, G. W; Snider, D. E., Jr. Epidemiology of tuberculosis in the United States. Epidemiol. Rev. 1989, 11, 79–98. [Google Scholar]
- Cardoso, E. M. Multiple drug resistance: a threat for tuberculosis control. Rev. Panam. Salud Publica 2004, 16, 68–73. [Google Scholar] [CrossRef]
- Elliott, L.; Ashley-Koch, A. E.; De Castro, L.; Jonassaint, J.; Price, J.; Ataga, K. I.; Levesque, M.C.; Weinberg, J.B.; Eckman, J.R.; Orringer, E.P.; Vance, J.M.; Telen, M.J. Genetic polymorphisms associated with priapism in sickle cell disease. Br. J. Haematol. 2007, 137, 262–267. [Google Scholar] [CrossRef]
- Hebbel, R.P. Special issue of microcirculation: Examination of the vascular pathobiology of sickle cell anemia. Microcirculation 2004, 11, 99–100. [Google Scholar] [CrossRef]
- Hiran, S. Multiorgan dysfunction syndrome in sickle cell disease. J. Assoc. Physicians India 2005, 53, 19–22. [Google Scholar]
- Buchanan, G.R.; Debaun, M.R.; Quinn, C.T.; Steinberg, M.H. Sickle cell disease. Hematology 2004, 1, 35–47. [Google Scholar]
- Stuart, M.J.; Nagel, R.L. Sickel-cell disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef]
- Perrine, S.P.; Ginder, G.D.; Faller, D.V.; Dover, G.H.; Ikuta, T.; Witkowska, H.E.; Cai, S.P.; Vichinsky, E.; Olivieri, N.F. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N. Engl. J. Med. 1993, 328, 81–86. [Google Scholar] [CrossRef]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef]
- Powars, D.R.; Weiss, J.N.; Chan, L.S.; Schroeder, W.A. Is there a threshold level of fetal hemoglobin that ameliorates morbidity on sickle cell anaemia? Blood 1983, 63, 921–926. [Google Scholar]
- Chung, M-C.; Silva, A. T de A.; Castro, L. F.; Güido, R. V C.; Nassute, J. C.; Ferreira, E. I. Latenciação e formas avançadas de transporte de fármacos. Rev. Bras. Cienc. Farm/Braz. J. Pharm Sci. 2005, 41, 155–179. [Google Scholar] [CrossRef]
- McKerrow, J.H.; McGrath, M.E.; Engel, J.C. The cysteine protease of Trypanosoma cruzi as a model for antiparasite drug design. Parasitol. Today 1995, 11, 272–82. [Google Scholar] [CrossRef]
- Schirmer, R. H.; Müller, J. G.; Krauth-Siegel, L. Disulfide-reductase inhibitors as chemotherapeutic agents: The design of drugs for trypanosomiasis and malaria. Angew. Chem., Int. Ed. Engl. 1995, 34, 141–154. [Google Scholar] [CrossRef]
- Chung, M-C.; Gonçalves, M. F.; Colli, W.; Ferreira, E. I.; Miranda, M. T. Synthesis and in vitro evaluation of potential antichagasic dipeptide prodrugs of primaquine. J. Pharm. Sci. 1997, 86, 1127–1131. [Google Scholar] [CrossRef]
- Bundgaard, H. (Ed.) Prodrug Design; Elsevier: Amsterdam, 1985.
- Chung, M. C.; Guido, R..V.; Martinelli, T. F.; Goncalves, M. F.; Polli, M. C.; Botelho, K. C.; Varanda, E. A.; Colli, W.; Miranda, M. T.; Ferreira, E. I. Synthesis and in vitro evaluation of potential antichagasic hydroxymethylnitrofurazone (NFOH-121): a new nitrofurazone prodrug. Bioorg. Med. Chem. 2003, 11, 4779–4783. [Google Scholar] [CrossRef]
- Trossini, G. H. G.; Ferreira, E. I. ; Menezes, C. M. S. A substrate-specificity evaluation of cruzain by the AM1 semi-empirical method. In The International Chemical Congress of Pacific Basin Societies - PACIFCHEM 2005, Honolulu, 2005: The International Chemical Congress of Pacific Basin Societies - PACIFCHEM 2005; Abstract Book. 2005. [Google Scholar]
- Menezes, C. M. S.; Trossini, G. H. G.; Chung, Mc ; Ferreira, E. I. A semi-empirical evaluation of mutual dipeptide prodrugs of primaquine and nitrofurazone toward antichagasic leads. In Molecular helminthology & drugs against protozoan parasites. Keystone Symposia - Drugs Against Protozoan Parasites: Target Selection, Structural Biology and Medicinal Chemistry, Silverthone, 2005.
- Scalea, M. A. L.; Juliao, M. S. S.; Chung, M. C.; Serrano, S. H. P.; Ferreira, E. I. Voltammetric behavior of nitrofurazone and its hydroxymethyl prodrug with potential anti-Chagas´ activity. J. Braz. Chem Soc. 2005, 16, 774–782. [Google Scholar] [CrossRef]
- Gerez de Burgos, N.M.; Burgos, C.; Blanco, A.; Paulone, I.; Segura, E.L. Actividad hidroxiácido-dehidrogenasa en Trypanosoma cruzi. Acta. Physiol. Latinoam. 1976, 26, 10–19. [Google Scholar]
- Coronel, C.; Gerez de Burgos, N.M.; Burgos, C.; Blanco, A. Separación y propiedades catalíticas de las izo enzimas de la α-hidroxiácido deshidrogenasa de Trypanosoma cruzi. Medicina (Buenos Aires) 1980, 40, 159–164. [Google Scholar]
- Coronel, C.E.; Rovai, L.E.; Gerez de Burgos, N.M.; Burgos, C.; Blanco, A. Properties of α-hydroxyacid dehydrogenase isozymes from Trypanosoma cruzi. Mol. Biochem. Parasitol. 1981, 4, 29–38. [Google Scholar] [CrossRef]
- Montamat, E.E.; Arauzo, S.S.; Blanco, A. Subcellular localization of leucine aminotransferase and a-hydroxiacid dehydrogenase in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1987, 22, 185–193. [Google Scholar] [CrossRef]
- Elizondo, S.; Chena, M.A,; Rodríguez-Páez, L.; Nogueda, B.; Baeza, I.; Wong, C. Inhibition of Trypanosoma cruzi α-Hydroxyacid Dehydrogenase-isozyme II by N-Isopropyl Oxamate and its Effect on Intact Epimastigotes. J. Enz. Inhib. Med. Chem. 2003, 18, 265–271. [Google Scholar] [CrossRef]
- Aldunate, J.; Repetto, Y.; Letelier, M.E.; Morello, A. The carboxyl esterases of Trypanosoma cruzi epimastigotes. Comp. Biochem. Physiol. 1987, 86, 67–71. [Google Scholar] [CrossRef]
- Chena, M.A,; Elizondo, S.; Rodríguez-Páez, L.; Nogueda, B.; Baeza, I.; Wong, C. Trypanocidal activity of N-isopropyl oxamate on cultured epimastigotes and murine trypanosomiasis using different Trypanosoma cruzi strains. J. Enz. Inhib. Med. Chem. 2005, 2, 189–197. [Google Scholar]
- Brener, Z. Recent advances in the chemotherapy of Chagas’ disease. Mem. Inst. Oswaldo. Cruz. 1984, 79, 149–155. [Google Scholar] [CrossRef]
- Lanteri, C. A.; Trumpower, B. L.; Tidwell, R. R.; Meshnick, S, R. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob. Agents Chemoter. 2004, 48, 3968–3974. [Google Scholar] [CrossRef]
- Dann, O.; Fick, H.; Pietzner, B.; Walkenhorst, E.; Fernbach, R.; Zeh, D. Trypanocidal diamidine with three isolated ring systems. Justus Liebigs. Ann. Chem. 1975, 160–194. [Google Scholar]
- Das, B. P.; Boykin, D. W. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J. Med. Chem. 1977, 20, 531–536. [Google Scholar] [CrossRef]
- Syrigos, K. N; Epenetos, A. A. Antibody directed enzyme prodrug therapy (ADEPT): a review of the experimental and clinical considerations. Anticancer Res. 1999, 19, 605–614. [Google Scholar]
- Pratt, R. F.; Faraci, W. S. Direct observation by proton NMR of cephalosporoate intermediates in aqueous solution during the hydrazinolysis and .beta.-lactamase-catalyzed hydrolysis of cephalosporins with 3' leaving groups: kinetics and equilibria of the 3' elimination reaction. J. Am. Chem. Soc. 1986, 108, 5328–5333. [Google Scholar] [CrossRef]
- Nguyen, V. K.; Desmyter, A.; Muyldermans, S. Functional heavy-chain antibodies in Camelidae. Adv. Immunol. 2001, 79, 261–296. [Google Scholar] [CrossRef]
- Lauwereys, M.; Arbabi, Ghahroudi, M.; Desmyter, A.; Kinne, J.; Holzer, W.; De Genst, E.; Wyns, L.; Muyldermans, S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998, 17, 3512–3520. [Google Scholar] [CrossRef]
- Stijlemans, B.; Conrath, K.; Cortez-Retamozo, V.; Xong, H. V.; Wyns, L.; Senter, P.; Revets, H.; De Baetselier, P.; Muyldermans, S.; Magez, S. Efficient targeting of conserved cryptic epitopes of infectious agents by a single domain antibodies. J. Biol. Chem. 2004, 279, 1256–1261. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robison, G.; Hamers, C.; Songa, E. B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature. 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Van Meirvenne, N.; Maginus, E.; Janssens, P. G. The effect of normal human serum on trypanosomes of distinct antigenic type (ETat 1 to 12) isolated from a strain of Trypanosoma brucei rhodesiense. Ann. Soc. Belg. Med. Trop. 1976, 56, 55–63. [Google Scholar]
- Vanhamme, L; Pays, E. The trypanosome lytic factor of human serum and the molecular basis of sleeping sickness. Int. J. Parasitol. 2004, 34, 887–898. [Google Scholar] [CrossRef]
- Vanhamme, L.; Paturiaux-Hanocq, F.; Poelvoorde, P.; Nolan, D. P.; Lins, L.; Abbeele, J. V. D.; Pays, A.; Tebabi, P.; Xong, H. V.; Jacquet, A.; Moguilevsky, N.; Dieu, M.; Kane, J. P.; De Baetselier, P.; Brasseur, R.; Pays, E. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 2003, 422, 83–87. [Google Scholar] [CrossRef]
- Xong, H. V.; Vanhamme, L.; Chamekh, M.; Chimfwembe, C. E. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 1998, 95, 839–846. [Google Scholar] [CrossRef]
- Baral, T. N.; Magez, S.; Stijlemans, B.; Conrath, K.; Vanhollebeke, B.; Pays, E.; Muyldermans, S.; De Baetselier, P. Experimental therapy of African trypanosomiasis with a nanobody-conjugated Human Trypanolitic Factor. Nat. Med. 2006, 12, 580–584. [Google Scholar] [CrossRef]
- Pays, E.; Vanhamme, L.; Perez-Morga, D. Antigenic variation in Trypanosoma brucei: facts, challenges and mysteries. Curr. Opin. Microbiol. 2004, 7, 369–374. [Google Scholar] [CrossRef]
- Horn, D. The molecular control of antigenic variation in Trypanosoma brucei. Curr. Mol. Med. 2004, 4, 563–576. [Google Scholar] [CrossRef]
- D’Silva, C.; Daunes, S. Structure-Activity Study on the in Vitro Antiprotozoal Activity of Glutathione Derivatives. J. Med. Chem. 2000, 43, 2072–2078. [Google Scholar] [CrossRef]
- Krieg, A. M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef]
- Klinman, D. M.; Verthelyi, D.; Takeshita, F.; Ishii, K. J. Immune recognition of foreign DNA: a cure for bioterrorism? Immunity 1999, 11, 123–129. [Google Scholar] [CrossRef]
- Klinman, D. M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef]
- Agrawal, S.; Kandimalla, E. R. Antisense and siRNA as agonists to Toll-like receptors. Nat. Biotechnol. 2004, 22, 1533–1537. [Google Scholar] [CrossRef]
- Grajkowski, A.; Pedras-Vasconcelos, J.; Wang, V.; Ausín, C.; Hess, S.; Verthelvi, D.; Beaucage, S. L. Thermolytic CpG-containing DNA oligonucleotides as potential immunotherapeutic prodrugs. Nucleic Acids Res. 2005, 33, 3550–3560. [Google Scholar] [CrossRef]
- Verthelyi, D.; Ishii, K. J.; Gursel, M.; Takeshita, F.; Klinman, D. M. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J. Immunol. 2001, 166, 2372–2377. [Google Scholar] [CrossRef]
- Verthelyi, D.; Kenney, R. T.; Seder, R. A.; Gam, A. A.; Friedag, B.; Klinman, D. M. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 2002, 168, 1659–1663. [Google Scholar] [CrossRef]
- Verthelyi, D.; Gursel, M.; Kenney, R. T.; Lifson, J. D.; Shuying, L.; Mican, J.; Klinman, D. M. CpG Oligodeoxynucleotides protect normal and SIV infected macaques from Leishmania infection. J. Immunol. 2003, 170, 4717–4723. [Google Scholar] [CrossRef]
- Guiducci, C.; Ott, G.; Chan, J. H.; Damon, E.; Calacsan, C.; Matray, T.; Lee, K. D.; Coffman, R. L.; Barrat, F. J. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 2006, 203, 1999–2008. [Google Scholar] [CrossRef]
- Wu, C. C. N.; Lee, J.; Raz, E.; Corr, M.; Carson, D. Necessity of oligonucleotide aggregation for Toll-like receptor 9 activation. J. Biol. Chem. 2004, 279, 33071–33078. [Google Scholar] [CrossRef]
- Kerkmann, M.; Costa, L. T.; Richter, C.; Rothenfusser, S.; Battiany, J.; Hornung, V.; Johnson, J.; Englert, S.; Ketterer, T.; Heckl, W.; Thalhammer, S.; Endres, S.; Hartmann, G. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-{alpha} induction by CpG-A in plasmacytoid dendritic cells. J. Biol. Chem. 2005, 280, 8086–8093. [Google Scholar] [CrossRef]
- Puig, M.; Grajkowski, A.; Bockowska, M.; Ausín, C.; Beaucage, S. L.; Verthelvi, D. Use of thermolytic protective groups to prevent G-tetrad formation in CpG ODN type D: structural studies and immunomodulatory activity in primates. Nucleics Acid Res. 2006, 34, 6488–6495. [Google Scholar] [CrossRef]
- Mansour, N. S.; Mcconnel, E. Leishmaniasis in the Sudan Republic 27. Lack of effect of chloroquine and pyrimethamine on visceral Leishmaniasis in the hamster. Am. J. Trop. Med. Hyg. 1966, 15, 146–148. [Google Scholar]
- Bello, A. R.; Nare, B.; Freedman, D.; Hardy, L.; Beverley, S. M. PTR1: A reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA. 1994, 9, 11442–11446. [Google Scholar]
- Nare, B.; Hardy, L. W.; Beverley, S. M. The roles of pteridine reductase 1 and dihydrofolate reductasethymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J. Biol. Chem. 1997, 272, 13883–13891. [Google Scholar]
- De Carvalho, P. B.; Ramos, D. C. C.; Cotrim, P. C.; Ferreira, E. I. Synthesis and in vitro evaluation of potential anti-leishmanial targeted drugs of pyrimethamine. J. Pharm. Sci. 2003, 92, 2109–2116. [Google Scholar] [CrossRef]
- Molyneux, D. H.; Ashford, R. W. (Eds.) The biology of Trypanosoma and Leishmania, parasites of man and domestic animals; International Publications: New York, 1983; pp. 211–249.
- Chakraborty, R.; Chakraborty, P.; Basu, M. K. Macrophage mannosyl fucosyl receptor: Its role in invasion of virulent and avirulent L. donovani promastigotes. Biosci. Rep. 1998, 18, 129–142. [Google Scholar] [CrossRef]
- Croft, S. L.; Hogg, J.; Gutteridge, W. E.; Hudson, A. T.; Randall, A. W. The activity of hydroxynaphthoquinones against Leishmania donovani. J. Antimicrob. Chem. 1992, 30, 827–832. [Google Scholar] [CrossRef]
- Mäntylä, A.; Garnier, T.; Rautio, J.; Nevalainen, T.; Vepsälainen, J.; Koskinen, A.; Croft, S. L.; Järvinen, T. Synthesis, in vitro evaluation and antileishmanial activity of water-soluble prodrugs of buparvaquone. J. Med. Chem. 2003, 47, 188–195. [Google Scholar]
- Mäntylä, A.; Rautio, J.; Nevalainen, T.; Vepsälainen, J.; Juvonen, R.; Kendrick, H.; Garnier, T.; Croft, S.L.; Järvinen, T. Synthesis and antileishmanial activity of novel buparvaquone oxime derivatives. Bioorg. Med. Chem. 2004, 12, 3497–3502. [Google Scholar] [CrossRef]
- Mäntylä, A.; Garnier, T.; Rautio, J.; Nevalainen, T.; Vepsälainen, J.; Koskinen, A.; Croft, S. L.; Järvinent, T. Synthesis, in Vitro Evaluation, and Antileishmanial Activity of Water-Soluble Prodrugs of Buparvaquone. J. Med. Chem. 2004, 47, 188–195. [Google Scholar] [CrossRef]
- Fleisher, D.; Bong, R.; Stewart, B. H. Improved oral drug delivery: solubility limitations overcome by the use of prodrugs. Adv. Drug Delivery Rev. 1996, 19, 115–130. [Google Scholar] [CrossRef]
- Varia, S. A.; Schuller, S.; Sloan, K. B.; Stella, V. J. Phenytoin prodrugs III: water-soluble prodrugs for oral and/or parenteral use. J. Pharm. Sci. 1984, 73, 1068–1073. [Google Scholar] [CrossRef]
- Krise, J. P.; Zygmunt, J.; Georg, G. I.; Stella, V. J. Novel prodrug approach for tertiary amines: synthesis and preliminary evaluation of n-phosphonooxymethyl prodrugs. J. Med. Chem. 1999, 42, 3094–3100. [Google Scholar] [CrossRef]
- Garnier, T.; Mäntylä, A.; Järvinen, T.; Lawrence, J.; Brown, M.; Croft, S. In vivo studies on the antileishmanial activity of buparvaquone and its prodrugs. J. Antimicrob. Chemother. 2007, 60, 802–810. [Google Scholar] [CrossRef]
- Mäntylä, A.; Rautio, J.; Nevalainen, T.; Keski-Rahkonen, P.; Vepsälainen, J.; Järvinen, T. Design, synthesis and in vitro evaluation of novel water-soluble prodrugs of buparvaquone. Eur. J. Pharm. Sci. 2004, 23, 151–158. [Google Scholar] [CrossRef]
- Jousserandot, A.; Boucher, J. L.; Henry, Y.; Niklaus, B.; Clement, B.; Mansuy, D. Microsomal cytochrome P450 dependent oxidation of N-hydroxyguanidines, amidoximes, and ketoximes: mechanism of the oxidative cleavage of their C=N(OH) bond with formation of nitrogen oxides. Biochemistry 1998, 37, 17179–17191. [Google Scholar] [CrossRef]
- Chattopadhyay, R.; Mahajan, B.; Kumar, S. Assessment of safety of the major antimalarial drugs. Expert Opin Drug Saf. 2007, 6, 505–21. [Google Scholar] [CrossRef]
- Ferreira, E. I.; Cruz, M. L.; Korolkovas, A. Latentiation of chemotherapeutic agents. Part 1: synthesis of oxidized starch imine derivatives and antimalarials. Starch/starke 1992, 44, 21–24. [Google Scholar] [CrossRef]
- Ohara, M. T.; Sakuda, T. M.; Cruz, M. L.; Ferreira, E. I.; Korolkovas, A. Antimalarial Activity Of Imine Oxidized Starch And Cellulose Derivatives. Boll. Chim. Farm. 1995, 9, 522–527. [Google Scholar]
- Cruz, M. L.; Ferreira, E. I.; Korolkovas, A. Latentiation of chemotherapeutic agents. Part 2: synthesis of oxidized cellulose imine derivatives and antimalarials. Starch/Stärke 1997, 49, 66–70. [Google Scholar] [CrossRef]
- Van Deenen, L. L. M.; De Gier, J. Lipids of the red cell membrane. In The red blood cell; Surgenor, G., Ed.; Academic Press, Inc.: New York, N.Y, 1975; pp. 147–211. [Google Scholar]
- Nicolas, O.; Margout, N. T.; Wein, S.; Calas, M.; Vial, H. J.; Bressollei, F. M. Pharmacological Properties of a New Antimalarial Bisthiazolium Salt, T3, and a Corresponding Prodrug, TE3. Antimicrob. Agents Chemother. 2005, 49, 3631–3639. [Google Scholar] [CrossRef]
- Vial, H.J.; Wein, S.; Farenc, C.; Kocken, C.; Nicolas, O.; Ancelin, M. L.; Bressolles, F.; Thomas, A.; Calas, M. Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 15458–15463. [Google Scholar] [CrossRef]
- Calas, M.; Ancelin, M. L.; Cordina, G.; Portefaix, P.; Piquet, G.; Vidal-Sailhan, V.; Vial, H. Antimalarial Activity of Compounds Interfering with Plasmodium falciparum Phospholipid Metabolism: Comparison between Mono- and Bisquaternary Ammonium Salts. J. Med. Chem. 2000, 43, 505–516. [Google Scholar] [CrossRef]
- Wiesner, J.; Hintz, M.; Altincicek, B.; Sanderbrand, S.; Weidemeyer, C.; Beck, E.; Jomaa, H. Plasmodium falciparum: Detection of the Deoxyxylulose 5-Phosphate Reductoisomerase Activity. Exp. Parasitol. 2000, 96, 182–186. [Google Scholar] [CrossRef]
- Lell, B.; Ruangweerayut, R.; Wiesner, J.; Missinou, M. A.; Schindler, A.; Baranek, T.; Hintz, M.; Hutchinson, D.; Jomaa, H.; Kremsner, P. G. Fosmidomycin, a Novel Chemotherapeutic Agent for Malaria. Antimicrob. Agents Chemother. 2003, 47, 735–738. [Google Scholar] [CrossRef]
- Jomaa, H.; Wiesner, J.; Sanderbrand, S.; Altincicek, B.; Weidemeyer, C.; Hintz, M.; Turbachova, I.; Eberl, M.; Zeidler, J.; Lichtenthaler, H. K.; Soldati, D.; Beck, E. Inhibitors of the Nonmevalonate Pathway of Isoprenoid Biosynthesis as Antimalarial Drugs. Science. 1999, 285, 1573–1576. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ishibashi, K.; Terakawa, M.; Nishiyama, M.; Itoh, N.; Noguchi, H. Pharmacokinetics and metabolism of fosmidomycin, a new phosphonic acid, in rats and dogs. Eur. J. Drug Metab. Pharmacokinet. 1982, 7, 59–64. [Google Scholar] [CrossRef]
- Reichenberg, A.; Wiesner, J.; Weidemeyer, C.; Dreiseidler, E.; Sanderbrand, S.; Altincicek, B.; Beck, E.; Schlitzer, M.; Jomaa, H. Diaryl Ester Prodrugs of FR900098 with Improved In Vivo Antimalarial Activity. Bioorg. Med. Chem. Lett. 2001, 11, 833–835. [Google Scholar] [CrossRef]
- Ortmann, R.; Wiesner, J.; Reichenberg, A.; Henscker, D.; Beck, E.; Jomaa, H.; Schlitzer, M. Acyloxyalkyl ester prodrugs of fr900098 with improved in vivo anti-malarial activity. Bioorg. Med. Chem. Lett. 2003, 13, 2163–2166. [Google Scholar] [CrossRef]
- Ortmann, R.; Wiesner, J.; Reichenberg, A.; Henschker, E. B.; Jomaa, H.; Schlitzer, M. Alkoxycarbonyloxyethyl Ester Prodrugs of FR900098 with Improved In Vivo Antimalarial Activity. Arch. Pharm. Chem. Life. Sci. 2005, 338, 305–314. [Google Scholar] [CrossRef]
- Hindley, S.; Ward, S. A.; Storr, R. C.; Searle, N. L.; Bray, P. G.; Park, K.; Davies, J.; O’Neil, P. M. Mechanism-Based Design of Parasite-Targeted Artemisinin Derivatives: Synthesis and Antimalarial Activity of New Diamine Containing Analogues. J. Med. Chem. 2002, 45, 1052–1063. [Google Scholar] [CrossRef]
- O’Neil, P.; Posner, G. H. A Medicinal Chemistry Perspective on Artemisinin and Related Endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [Google Scholar] [CrossRef]
- Borstnik, K.; Paik, I-H.; Posner, G. H. Malaria: New Chemotherapeutic Peroxide Drugs. Mini Rev. Med. Chem. 2002, 2, 573–583. [Google Scholar] [CrossRef]
- Cumming, J. N.; Ploypradith, P.; Posner, G. H. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv. Pharmacol. 1997, 37, 253–297. [Google Scholar]
- O’Neil, P.; Stocks, P. A.; Pugh, M. D.; Araujo, N. C.; Korshin, E.; Bickley, J. F.; Ward, S. A.; Bray, P. G.; Pasini, E.; Davies, J.; Verissimo, E.; Bach, M. D. Design and Synthesis of Endoperoxide Antimalarial Prodrug Models. Angew. Chem. 2004, 43, 4193–4197. [Google Scholar] [CrossRef]
- Jefford, A. W. Why Artemisinin and Certain Synthetic Peroxides are Potent Antimalarials. Implications for the Mode of Action. Curr. Med. Chem. 2001, 8, 1803–1826. [Google Scholar] [CrossRef]
- Eckstein-Ludwig, U.; Webb, R. J.; van Goethem, I. D. A.; East, J. M.; Lee, A. G.; Kimura, M.; O’Neil, P. M.; Bray, P. G.; Ward, s. A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Li, R.; Kenyon, G. L.; Cohen, F. E.; Chen, X.; Gong, B.; Dominguez, J. N.; Davidson, E.; Kurzban, G.; Miller, R. E.; Nuzum, E. O.; Rosenthal, P. J.; McKerrol, J. H. In Vitro Antimalarial Activity of Chalcones and Their Derivatives. J. Med. Chem. 1995, 38, 5031–5037. [Google Scholar] [CrossRef]
- Atamna, H.; Ginsburg, H. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J. Biol. Chem. 1995, 270, 24876–24883. [Google Scholar] [CrossRef]
- Schirmer, R. H.; Müller, J. G.; Krauth-Siegel, R. L. Disulfide reductase inhibitors as chemotherapeutic agents: the design of drugs for trypanosomiasis and malaria. Angew. Chem. Int. Edn. 1995, 34, 141–154. [Google Scholar]
- Davioud-Charvet, E.; Delarue, S.; Biot, C.; Schwobel, B.; Boehme, C. C.; Mussigbrodt, A.; Maes, L.; Sergheraert, C.; Grellier, P.; Schirmer, R. H.; Becker, K. A prodrug for of a Plasmodium falciparum glutathione reductase inhibitor conjugated with a 4-anilinoquinoline. J. Med. Chem. 2001, 44, 4268–4276. [Google Scholar] [CrossRef]
- Meierjohann, S.; Walter, R. D.; Muller, S. Regulation of intracellular glutathione levels in erythrocytes infected with chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Biochem. J. 2002, 368, 761–768. [Google Scholar] [CrossRef]
- Biot, C.; Dessolin, J.; Grellier, P.; Davioud-Charvet, E. Double-drug development against antioxidant enzymes from Plasmodium falciprurn. Redox Rep. 2003, 8, 280–283. [Google Scholar]
- Gomes, P.; Araujo, M. J.; Rodriguez, M.; Vale, N.; Azevedo, Z.; Iley, J.; Chambel, P.; Morais, J.; Moreira, R. Synthesis of imidazolidin-4-one and 1H-imidazo[2,1-a]isoindole-2,5(3H,9bH)-dione derivatives of primaquine: scope and limitations. Tetrahedron. 2004, 60, 5551–5562. [Google Scholar] [CrossRef]
- Brueckner, R. P.; Ohrt, C.; Baird, J. K.; Milhous, W. K. Antimalarial Chemotherapy; Rosenthal, P. J., Ed.; Humana: Totowa, 2001; pp. 123–151. [Google Scholar]
- Trouet, A.; Pirson, P.; Steiger, R.; Masquelier, M.; Baurain, R.; Gillet, J. Development of new derivatives of primaquine by association with lysosomotropyc carriers. Bull. World Health Org. 1981, 59, 449–458. [Google Scholar]
- Philip, A.; Kepler, J A.; Johnson, B. H.; Carroll, F. I. Peptide Derivatives of Primaquine as Potential Antimalarial Agents. J. Med. Chem. 1988, 31, 870–874. [Google Scholar] [CrossRef]
- Pauletti, G. M.; Gangwar, S.; Siahaan, T. J.; Aube, J.; Borchardt, R. T. Improvement of oral peptide bioavailability: Peptidomimetics and prodrug strategies. Adv. Drug. Del. Rev. 1997, 27, 235–256. [Google Scholar] [CrossRef]
- Bundgaard, H. (C) Means to enhance penetration: (1) Prodrugs as a means to improve the delivery of peptide drugs. Adv. Drug. Del. Rev. 1992, 8, 1–38. [Google Scholar]
- Borissova, R.; Stjarnkvist, P.; Karlsson, M. O.; Sjoholm, I. Biodegradable microspheres. 17. Lysosomal degradation of primaquine-peptide spacer arms. J. Pharm. Sci. 1995, 84, 256–262. [Google Scholar] [CrossRef]
- Portela, M. J.; Moreira, R.; Valente, E.; Constantino, L.; Iley, J.; Pinto, J.; Rosa, R.; Cravo, P.; do Rosario, V. E. Dipeptide Derivatives of Primaquine as Transmission-Blocking Antimalarials: Effect of Aliphatic Side-Chain Acylation on the Gametocytocidal Activity and on the Formation of Carboxyprimaquine in Rat Liver Homogenates. Pharm. Res. 1999, 16, 949–955. [Google Scholar]
- Bak, A.; Fich, M.; Larsen, B. D.; Frokjaer, S.; Friis, G. J. N-terminal 4-imidazolidinone prodrugs of Leu-enkephalin: synthesis, chemical and enzymatic stability studies. Eur. J. Pharm. Sci. 1999, 7, 317–323. [Google Scholar] [CrossRef]
- Araujo, M.. J.; Bom, J.; Capela, R.; Casimiro, C.; Chambel, P.; Gomes, P.; Iley, J.; Lopes, F.; Morais, J.; Moreira, R.; de Oliveira, E.; do Rosario, V.; Vale, N. Imidazolidin-4-one Derivatives of Primaquine as Novel Transmission-Blocking Antimalarials. J. Med. Chem. 2005, 48, 888–892. [Google Scholar] [CrossRef]
- Vagapandu, S.; Sachdeva, S.; Jain, M.; Singh, S.; Singh, P. P.; Kaul, C. L.; Jain, R. 8-Quinolinamines and Their Pro Prodrug Conjugates as Potent Blood-Schizontocidal Antimalarial Agents. Bioorg. Med. Chem. 2003, 11, 4557–4568. [Google Scholar] [CrossRef]
- Goldberg, D. E.; Slater, A. F.; Beavis, R.; Chait, B.; Cerami, A.; Henderson, G. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J. Exp. Med. 1991, 173, 961–969. [Google Scholar] [CrossRef]
- Romeo, S.; Dell'Agli, M.; Parapini, S.; Rizzi, L.; Galli, G.; Mondani, M.; Sparatore, A.; Taramelli, D.; Bosisio, E. Plasmepsin II inhibition and antiplasmodial activity of Primaquine–Statine ‘double-drugs’. Bioorg. Med. Chem. Lett. 2004, 14, 2931–2934. [Google Scholar] [CrossRef]
- Yuthavong, Y. Basis for antifolate action and resistance in malaria. Microbes Infect. 2002, 4, 175–182. [Google Scholar] [CrossRef]
- Rieckmann, K. H. The in vitro activity of experimental antimalarial compounds against strains of Plasmodium falciparum with varying degrees of sensitivity to pyrimethamine and chloroquine. In Chemotherapy of malaria and resistance to antimalarials; (World Health Organization Technical Report Series); World Health Organization: Geneva, Switzerland, 1973; p. 58. [Google Scholar]
- Yuvaniyama, J.; Chitnumsub, P.; Kamchonwongpaisan, S.; Vanichtanankul, J.; Sirawaraporn, W.; Taylor, P.; Walkinshaw, M. D.; Yuthavong, Y. Insights into antifolate resistance from malarial DHFR-TS structures. Nat. Struct. Biol. 2003, 10, 357–365. [Google Scholar] [CrossRef]
- Rieckmann, K. H.; Yeo, A. E.; Edstein, M. D. Activity of PS-15 and its metabolite, WR99210, against Plasmodium falciparum in an in vivo-in vitro model. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 568–571. [Google Scholar] [CrossRef]
- Canfield, C. J.; Milhous, W. K.; Ager, A. L.; Rossan, R. N.; Sweeney, T. R.; Lewis, N. J.; Jacobus, D. P. PS-15: a potent, orally active antimalarial from a new class of folic acid antagonists. Am. J. Trop. Med. Hyg. 1993, 49, 121–126. [Google Scholar]
- Shearer, T. W.; Kozar, M. P.; O’Neil, M. T.; Smith, P. L.; Schiehser, G. A.; Jacobus, D. P.; Diaz, D. S.; Yang, Y-S.; Milhous, W. k.; Skillman, D. R. In Vitro Metabolism of Phenoxypropoxybiguanide Analogues in Human Liver Microsomes to Potent Antimalarial Dihydrotriazines. J. Med. Chem. 2005, 48, 2805–2813. [Google Scholar] [CrossRef]
- Wiesner, J.; Kettler, K.; Sakowski, J.; Ortmann, R.; Katzin, A. M.; Kimura, E. A.; Silber, K.; Klebe, G.; Jomaa, H.; Schlitzer, M. Farnesyltransferase inhibitors inhibit the growth of malaria parasites in vitro and in vivo. Angew. Chem., Int. Ed. 2004, 43, 251–254. [Google Scholar] [CrossRef]
- Casey, P. J.; Seabra, M. C. Protein Prenyltransferases. J. Biol. Chem. 1996, 271, 5289–5292. [Google Scholar] [CrossRef]
- Ohkanda, J.; Lockman, J. W.; Yokoyama, K.; Gelb, M. H.; Croft, S. L.; Kendrick, H.; Harrell, M. I.; Feagin, J. E.; Blaskovich, M. A.; Sebti, S. M.; Hamilton, A. D. Peptidomimetic inhibitors of protein farnesyltransferase show potent antimalarial activity. Bioorg. Med. Chem. Lett. 2001, 11, 761–764. [Google Scholar] [CrossRef]
- Carrico, D.; Ohkanda, J.; Kendrick, H.; Yokoyama, K.; Blasklovich, M. A.; Bucher, C. J.; Buckner, F. S.; van Voorhis, W. C.; Chakrabarti, D.; Croft, S.; Gelb, M. H.; Sebti, S. M.; Hamilton, A. D. In vitro and in vivo antimalarial activity of peptidomimetic protein farnesyltransferase inhibitors with improved membrane permeability. Bioorg. Med. Chem. 2004, 12, 6517–6526. [Google Scholar] [CrossRef]
- Ledig, K. W. 7-(Substituted)-7H-pyrrolo[3,2,-f]quinazoline-1,3-diamines. U.S. Patent 4,118,561, 1978. [Google Scholar]
- Davoll, J.; Johnson, A. M.; Davies, H. J.; Bird, O. D.; Clarke, J.; Elslager, E. F. Folate antagonists. 2. 2,4-Diamino-6-((aralkyl and (heterocyclic) methyl)amino)quinazolines, a novel class of antimetabolites of interest in drug-resistant malaria and Chagas’ disease. J. Med. Chem. 1972, 15, 812–826. [Google Scholar] [CrossRef]
- Elslager, E. F.; Bird, O. D.; Clarke, J.; Perricone, S. C.; Worth, D. F. Folate antagonists. 9. 2,4-Diamino-6-((aralkyl)alkylamino)quinazolines, a potent class of antimetabolites with prodigious antimalarial effects. J. Med. Chem. 1972, 15, 1138–1146. [Google Scholar] [CrossRef]
- Hua, H.; Cheng, M.; Li, X.; Pei, Y. A new pyrroloquinazoline alkaloid from Linaria vulgaris. Chem. Pharm. Bull. 2002, 50, 1393–1394. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, Q.; O’Neil, M.; Obaldia III, N.; Ager, A.; Gerena, L.; Lin, A. J. Antimalarial activity of new pyrrolo[3,2-f]Quinazoline-1,3-Diamine derivatives. Antimicrob. Agents Chemother. 2005, 49, 4928–4933. [Google Scholar] [CrossRef]
- Xie, L. H.; Li, Q.; Lin, A. J.; Smith, K.; Zhang, J.; Skillman, D. New Potential Antimalarial Agents: Therapeutic-Index Evaluation of Pyrroloquinazolinediamine and Its Prodrugs in a Rat Model of Severe Malaria. Antimicrob. Agents Chemother. 2006, 50, 1649–1655. [Google Scholar] [CrossRef]
- Weller, T.; Alig, L.; Beresini, M.; Blackburn, B.; Bunting, S.; Hadvary, P.; Muller, M. H.; Knopp, D.; Levet-Trafit, B.; Lipari, M. T.; Modi, N. B.; Muller, M.; Refino, C. J.; Schmitt, M.; Schonholzer, P.; Weiss, S.; Steiner, B. Orally Active Fibrinogen Receptor Antagonists. 2. Amidoximes as Prodrugs of Amidines. J. Med. Chem. 1996, 39, 3139–3147. [Google Scholar] [CrossRef]
- Zhou, L.; Lee, K.; Thakker, D. R.; Boykin, D. W.; Tiwell, R. R.; Hall, J. E. Enhanced Permeability of the Antimicrobial Agent 2,5-Bis(4-Amidinophenyl)Furan Across Caco-2 Cell Monolayers Via Its Methylamidoxime Prodrug. Pharm. Res. 2002, 19, 1689–1685. [Google Scholar]
- Kitamura, S. F. H.; Miyawaki, T.; Kawamura, M.; Terashita, Z-I.; Naka, T. Orally Active GPIIb/IIIa Antagonists: Synthesis and Biological Activities of Masked Amidines as Prodrugs of 2-[(3S)-4-[(2S)-2-(4-Amidinobenzoylamino)-3-(4-methoxyphenyl)propanoyl]-3-(2-methoxy-2-oxoethyl)-2-oxopiperazinyl]acetic Acid. Chem. Pharm. Bull. 2001, 49, 268–277. [Google Scholar] [CrossRef]
- Rehse, K.; Bade, S.; Harsdorf, A.; Clement, B. New NO-Donors with Antithrombotic and Vasodilating Activities, Part 17. Arylazoamidoximes and 3-Arylazo-1,2,4-oxadiazol-5-ones. Arch. Pharm. 1997, 330, 392–398. [Google Scholar] [CrossRef]
- Boykin, D. W.; Kumar, A.; Hall, J. E.; Bender, B. C.; Tidwell, R. R. Anti-pneumocystis activity of bis-amidoximes and bis-o-alkylamidoximes prodrugs. Bioorg Med. Chem. Lett. 1996, 6, 3017–3020. [Google Scholar] [CrossRef]
- Berger, B. J.; Lombardy, R. J.; Marbury, G. D.; Bell, C. A.; Dykstra, C. C.; Hall, J. E.; Tidwell, R. R. Metabolic N-hydroxylation of pentamidine in vitro. Antimicrob. Agents Chemother. 1990, 34, 1678–1684. [Google Scholar] [CrossRef]
- Clement, B.; Lomb, R.; Moller, W. Isolation and Characterization of the Protein Components of the Liver Microsomal O2-insensitive NADH-Benzamidoxime Reductase. J. Biol. Chem. 1997, 272, 19615–19620. [Google Scholar] [CrossRef]
- Outtara, M.; Wein, S.; Calas, M.; Hoang, Y. V.; Vial, H.; Escale, R. Synthesis and antimalarial activity of new 1,12-bis- (N,N)-acetamidinyl)dodecane derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 593–596. [Google Scholar] [CrossRef]
- Cerami, C.; Frevert, U.; Sinnis, P.; Takacs, B.; Clavijo, P.; Santos, M. J.; Nussenzweig, V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992, 70, 1021–1033. [Google Scholar] [CrossRef]
- Ding, Z. M.; Cristiano, R. J.; Roth, J. A.; Takacs, B.; Kuo, M. T. Malarial circumsporozoite protein is a novel gene delivery vehicle to primary hepatocyte cultures and cultured. J. Biol. Chem. 1995, 270, 3667–3676. [Google Scholar] [CrossRef]
- Lin-Lee, Y-C.; Nakamura, S.; Gandhi, V.; Curley, S. A.; Stüber, D.; Burkot, T. R.; Kuo, M. T. Prolonged stability and sustained prodrug cell killing activity using receptor-mediated delivery of malarial circumsporozoite-cytosine deaminase fusion protein into liver cancer cells. Mol. Cancer Ther. 2002, 1, 461–467. [Google Scholar] [CrossRef]
- Nussenzweig, V. Malaria sporozoites and chylomicron remnants compete for binding sites in the liver. Behring Inst. Mitt. 1997, 99, 85–89. [Google Scholar]
- Parise Filho, R.; Menezes, C.M.S.; Pinto, P.L.S.; Paula, G.A.; Brandt, C.A.; Siveira, M.A.B. Design, synthesis, and in vivo evaluation of oxamniquine methacrylate and acrylamide prodrugs. Bioorg. Med. Chem. 2007, 15, 1229–1236. [Google Scholar] [CrossRef]
- Almeida, A.E.; Ferreira, A.G.; Crespi, M.S.; Andrade, Z.A.; Man-Chin, C. Synthesis and thermal study of prodrug of oxamniquine. J. Therm. Anal. Cal. 2006, 83, 277–281. [Google Scholar] [CrossRef]
- El-Hamouly, W.; Pica-Mattocia, L.; Cioli, D.; Schwartz, H.M.; Archer, S. Studies on some derivatives of oxamniquine. J. Med. Chem. 1988, 31, 1629–1631. [Google Scholar] [CrossRef]
- Shaohong, L.; Kumagai, T.; Qinghua, A.; Xiaolan, Y.; Ohmae, H.; Yabu, Y.; Siwen, L.; Liyong, W.; Maruyama, H.; Ohta, N. Evaluation of the anthelmintic effects of artesunate against experimental Schistosoma mansoni infection in mice using different treatment protocols. Parasitol. Int. 2006, 55, 63–68. [Google Scholar] [CrossRef]
- Utzinger, J.; Chollet, J.; Tu, Z.; Shuhua, X.; Tanner, M. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Trans. R. Soc. Trop. Med. Hy. 2002, 96, 318–323. [Google Scholar] [CrossRef]
- Jiraungkoorskul, W.; Sahaphong, S.; Sobhon, P.; Riengrojpitak, S.; Kangwanrangsan, N. Effects of praziquantel and artesunate on the tegument of adult Schistosoma mekongi harboured in mice. Parasitol. Int. 2005, 54, 177–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Scorpio, A.; Nikaido, H. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 1999, 181, 2044–9. [Google Scholar]
- Zhang, Y. The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 529–64. [Google Scholar] [CrossRef]
- Scorpio, A.; Zhang, Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 1996, 2, 662–667. [Google Scholar] [CrossRef]
- Hanoulle, X.; Wieruszeski, J-M.; Rousselot-Pailley, P.; Landrieu, I.; Locht, C.; Lippens, G.; Baulard, A. R. Selective intracellular accumulation of the major metabolite issued from the activation of the prodrug ethionamide in mycobacteria. J. Antimicrob. Chemother. 2006, 58, 768–772. [Google Scholar] [CrossRef]
- DeBarber, A. E.; Mdluli, K.; Bosman, M.; Bekker, L. G.; Barry III, C. E. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 9677–9682. [Google Scholar]
- Zhang, Y.; Heym, B.; Allen, B.; Young, D.; Cole, S. The catalase peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 1992, 358, 591–593. [Google Scholar] [CrossRef]
- Heym, B.; Zhang, Y.; Poulet, S.; Young, D.; Cole, S. T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J. Bacteriol. 1993, 175, 4255–4259. [Google Scholar]
- Ghiladi, R. A.; Medzihardsky, K. F.; Rusnak, F. M.; de Montellano, P. R. O. Correlation between isoniazid resistance and superoxide reactivity in mycobacterium tuberculosis katg. J. Am. Chem. Soc. 2005, 127, 13428–13442. [Google Scholar]
- Vannelli, T. A.; Dykman, A.; de Montellano, P. R. O. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J. Biol. Chem. 2002, 277, 12824–12829. [Google Scholar]
- Fraaije, M. W.; Kamerbeek, N. M.; Heidekamp, A. J.; Fortin, R.; Janssen, D. B. The prodrug activator etaa from Mycobacterium tuberculosis is a baeyer-villiger monooxygenase. J. Biol. Chem. 2004, 279, 3354–3360. [Google Scholar]
- Baulard, A. R.; Betts, J. C.; Engohang-Ndong, J.; Quan, S.; McAdam, R. A.; Brennan, P. J.; Locht, C.; Besra, G. S. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 2000, 275, 28326–28331. [Google Scholar]
- Phetsuksiri, B.; Baulard, A. R.; Cooper, A. M.; Minnikin, D. E.; Douglas, J. D.; Besra, G. S.; Brennan, P. J. Antimycobacterial Activities of Isoxyl and New Derivatives through the Inhibition of Mycolic Acid Synthesis. Antimicrob. Agents Chemother. 1999, 43, 1042–1051. [Google Scholar]
- Qian, L; de Montellano, P. R. O. Oxidative activation of thiacetazone by the mycobacterium tuberculosis flavin monooxygenase etaa and human fmo1 and fmo3. Chem. Res. Toxicol. 2006, 19, 443–449. [Google Scholar] [CrossRef]
- Kathleen, P. Martindale: The Complete Drug Reference, 32nd Edn. ed; Pharmaceutical Press: Chicago, USA, 1999; p. 151. [Google Scholar]
- Byrd, R. B.; Horn, B. R.; Solomon, D. A.; Griggs, G. W. Toxic effects of isoniazid in tuberculosis chemoprophylaxis. Role of biochemical monitoring in 1,000 patients. JAMA. 1979, 241, 1239–1241. [Google Scholar] [CrossRef]
- Miller, R. R.; Porter, J.; Greenblatt, D. J. Clinical importance of the interaction of phenytoin and isoniazid: a report from the Boston Collaborative Drug Surveillance Program. Chest. 1979, 75, 356–8. [Google Scholar] [CrossRef]
- Prateek, J. R.; Jaim, K.; Ravichandran, V.; Agrawal, R. K. Synthesis and evaluation of mutual prodrugs of isoniazid, p-amino salicylic acid and ethambutol. Arkivoc 2007, 1, 105–118. [Google Scholar]
- Wayne, L. G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 908–914. [Google Scholar] [CrossRef]
- Wayne, L. G.; Hayes, L. G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 1996, 64, 2062–2069. [Google Scholar]
- Samuelson, J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999, 43, 1533–1541. [Google Scholar]
- Brooks, J. V.; Furney, S. K.; Orme, I. M. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 1999, 43, 1285–1288. [Google Scholar]
- Lenaerts, A. J.; Gruppo, V.; Marietta, K. S.; Johnson, C. M.; Driscoll, D. K.; Tompkins, N. M.; Rose, J. D.; Reynolds, R. C.; Orme, I. M. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 2005, 49, 2294–2301. [Google Scholar] [CrossRef]
- Manjunatha, U. H.; Boshoff, H.; Dowd, C. S.; Zhang, L.; Albert, T. J.; Norton, J. E.; Daniels, L.; Dick, T.; Pang, S. S.; Barry, C. E. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2006, 103, 431–436. [Google Scholar] [CrossRef]
- Stover, C. K.; Warrener, P.; Vandevanter, D. R.; Sherman, D. R.; Arain, T. M.; Langhorne, M. H.; Anderson, S. W.; Towell, J. A.; Yuan, Y.; McMurray, D. N.; Kreiswirth, B. N.; Barry, C. E.; Baker, W. R. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 2000, 405, 962–966. [Google Scholar] [CrossRef]
- Papadopoulou, M. V.; Bloomer, W. D.; McNeil, M. R. NLCQ-1 and NLCQ-2, two new agents with activity against dormant Mycobacterium tuberculosis. Int. J. Antimicrob. Agents. 2007, 29, 724–727. [Google Scholar] [CrossRef]
- Papadopoulou, M. V.; Ji, M.; Rao, M. K.; Bloomer, W. D. 4-[3-(2-Nitro-1-imidazolyl)-propylamino]-7-chloroquinoline hydrochloride (NLCQ-1), a novel bioreductive compound as a hypoxia-selective cytotoxin. Oncol. Res. 2000, 12, 185–192. [Google Scholar]
- Papadopoulou, M. V.; Ji, M.; Rao, M. K.; Bloomer, W. D. Reductive metabolism of the nitroimidazole-based hypoxia-selective cytotoxin NLCQ-1 (NSC 709257). Oncol. Res. 2003, 14, 21–29. [Google Scholar]
- Sriram, D.; Yogeeswari, P. Towards the design and development of agents with broad spectrum chemotherapeutic properties for the effective treatment of HIV / AIDS. Curr. Med. Chem. 2003, 10, 1909–1915. [Google Scholar]
- Sriram, D.; Yogeeswari, P.; Narasimharaghavan, S.; Bai, T. R. Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Bioorg. Med. Chem. Lett. 2004, 14, 1085–1087. [Google Scholar] [CrossRef]
- Sriram, D.; Bal, T. R.; Yogeeswari, P. Design, synthesis and biological evaluation of novel non-nucleoside HIV-1 reverse transcriptase inhibitors with broad-spectrum chemotherapeutic properties. Bioorg. Med. Chem. 2004, 12, 5865–5873. [Google Scholar] [CrossRef]
- Sriram, D.; Srichakravarthy, N.; Bal, T. R.; Yogeeswari, P. Dossier: HIV/AIDS: New approaches in chemotherapy and Immunotherapy. Synthesis of zidovudine prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Biomed. Pharmacother. 2005, 59, 452–455. [Google Scholar]
- Sriram, D.; Yogeeswari, P.; Gopal, G. Synthesis, anti-HIV and antitubercular activities of lamivudine prodrugs. Eur. J. Med. Chem. 2005, 40, 1373–1376. [Google Scholar] [CrossRef]
- Steinberg, T. H. Cellular transport of drugs. Clin. Infect. Dis. 1994, 19, 916–921. [Google Scholar] [CrossRef]
- Donowitz, G. R. Tissue-directed antibiotics and intracellular parasites: complex interaction of phagocytes, pathogens, and drugs. Clin. Infect. Dis. 1994, 19, 926–930. [Google Scholar] [CrossRef]
- Gordon, S.; Rabinowitz, S. Macrophages as targets for drug delivery. Adv. Drug Deliv. Rev. 1989, 4, 27–47. [Google Scholar] [CrossRef]
- Roseeuw, E.; Coessens, V.; Balazuc, A-M.; Lagranderie, M.; Chavarot, P.; Pessina, A.; Néri, M. G.; Schacht, E.; Marchal, G.; Domurado, D. Synthesis, Degradation, and Antimicrobial Properties of Targeted Macromolecular Prodrugs of Norfloxacin. Antimicrob. Agents Chemother. 2003, 47, 3435–3441. [Google Scholar] [CrossRef]
- Roseeuw, E.; Coessens, V.; Schat, E.; Vrooman, B.; Domurado, D.; Marchal, G. Polymeric prodrugs of antibiotics with improved efficiency. J. Mater. Sci. Mater. Med. 1999, 10, 743–746. [Google Scholar] [CrossRef]
- Aubrée-Lecat, A.; Duban, M-C.; Demignot, S.; Domurado, M.; Fournié, P.; Domurado, D. Influence of barrier-crossing limitations on the amount of macromolecular drug taken up by its target. J. Pharmacokinet. Biopharm. 1993, 21, 75–98. [Google Scholar] [CrossRef]
- Balazuc, A. M.; Lagranderie, M.; Chavarot, P.; Pescher, P.; Roseeuw, E.; Schacht, E.; Domurado, D.; Marchal, G. In vivo efficiency of targeted norfloxacin against persistent, isoniazid-insensitive, Mycobacterium bovis BCG present in the physiologically hypoxic mouse liver. Microbes Infect. 2005, 7, 969–975. [Google Scholar] [CrossRef]
- Silva, M.; Lara, A. S.; Leite, C. Q. F.; Ferreira, E. I. Potential tuberculostatic agents: micelle-forming copolymer poly(ethyleneglycol)-poly(aspartic acid) prodrug with isoniazid. Arch. Pharm. Pharm. Med. Chem. 2001, 334, 189–193. [Google Scholar] [CrossRef]
- Silva, M.; Ricelli, N. L.; Seoud, O. E.; Valentim, C. S.; Ferreira, A.; Sato, D. N.; Leite, C. Q. F.; Ferreira, E. I. Potential Tuberculostatic Agent: Micelle-forming Pyrazinamide Prodrug. Arch. Pharm. Chem. Life Sci. 2006, 339, 283–290. [Google Scholar] [CrossRef]
- Yokoyama, M.; Miyauchi, M.; Yamada, N.; Okano, T.; Sakurai, Y.; Kataoka, K.; Inoue, S. Polymer micelles as novel drug carriers: adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. In Advances in Drug Delivery Systems; Anderson, J. M., Kim, S. W., Knutson, K., Eds.; Elsevier: Amsterdam, 1990; Vol. 4, pp. 269–278. [Google Scholar]
- Nakanishi, T.; Fukushima, S.; Okamoto, K.; Suzuki, M.; Matsumura, Y.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Development of the polymer micelle carrier system for doxorubicin. J. Control Release. 2001, 74, 295–302. [Google Scholar] [CrossRef]
- Rando, D. G.; Brandt, C. A.; Ferreira, E. I. Use of N-methylene phosphonic chitosan to obtain an isoniazid prodrug. Rev. Bras. Ciên. Farm. 2004, 40, 335–344. [Google Scholar]
- Shibata, Y.; Metzger, W. J.; Myrvik, Q. N. Chitin particle-induced cell- mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 1997, 159, 2462–2467. [Google Scholar]
- Heras, A,; Rodríguez, N.M.; Ramos, V.M.; Agulló, E. N-methylene phosphonic chitosan: a novel soluble derivative. Carbohydr. Polym. 2001, 44, 1–8. [Google Scholar] [CrossRef]
- Jones, R. N.; Ross, J. E.; Fritsche, T. R.; Sader, H. S. J. Oxazolidinone susceptibility patterns in 2004: report from the Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) program assessing isolates from 16 nations. Antimicrob. Chemother. 2006, 57, 279–287. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Ochoa-Felix, E. Y.; Gonzalez, G.; Tijerina, R.; Choi, S. H.; Welsh, O. In vitro activities of new quinolones and oxazolidinones against Actinomadura madurae. Antimicrob. Agents Chemother. 2004, 48, 1037–1039. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Castro-Garza, J.; Rendon, A.; Ocampo-Candiani, J.; Welsh, O.; Choi, S. H.; Blackwood, K.; Molina-Torres, C. In vitro susceptibility of Mycobacterium tuberculosis clinical isolates to garenoxacin and DA-7867. Antimicrob. Agents Chemother. 2005, 49, 4351–4353. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Gonzalez, E.; Choi, S. H.; Welsh, O. In vitro activities of new antimicrobials against Nocardia brasiliensis. Antimicrob. Agents Chemother. 2004, 48, 602–604. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Welsh, O.; Said-Fernandez, S.; Lozano-Garza, G.; Tavarez-Alejandro, R. E.; Vera-Cabrera, L. In vitro and in vivo activities of antimicrobials against Nocardia brasiliensis. Antimicrob. Agents Chemother. 2004, 48, 832–837. [Google Scholar] [CrossRef]
- Fortun, J.; Martin-Davila, P.; Navas, E.; Perez-Elias, M. J.; Cobo, J.; Tato, M.; De la Pedrosa, E. G.; Gomez-Mampaso, E.; Moreno, S. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 2005, 56, 180–185. [Google Scholar] [CrossRef]
- Shinabarger, D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin. Investig. Drugs. 1999, 8, 1195–1202. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Gonzalez, E.; Rendon, A.; Ocampo-Candiani, J.; Welsh, O.; Velazquez-Moreno, V. M.; Choi, S. H.; Molina-Torres, C. In Vitro Activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrob. Agents Chemother. 2006, 50, 3170–3172. [Google Scholar] [CrossRef]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef]
- Kruh, J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol. Cell Biochem. 1982, 42, 65–82. [Google Scholar]
- Nudelman, A.; Gnizi, E.; Katz, Y.; Azulai, R.; Cohen-Ohana, M.; Zhuk, R.; Sampson, S.R.; Langzam, L.; Fibach, E.; Prus, E.; Pugach, V.; Rephaeli, A. Prodrugs of butyric acid. Novel derivatives possessing increased aqueous solubility and potential for treating cancer and blood diseases. Eur. J. Med. Chem. 2001, 36, 63–74. [Google Scholar] [CrossRef]
- Dover, G.J.; Brusilow, S.; Samid, D. Increased fetal hemoglobin in patients receiving sodium 4-Phenylbutyrate. New Engl. J. Med. 1992, 327, 569–570. [Google Scholar]
- Charache, S.; Terrin, M.L.; Moore, R.D.; Dover, G.J.; Barton, F.B.; Eckert, S.V.; McMahon, R.P.; Bonds, D.R. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N. Engl. J. Med. 1995, 332, 1317–1322. [Google Scholar] [CrossRef]
- Atweh, G.F.; Sutton, M.; Nassif, I.; Boosalis, V.; Dover, G.J.; Wallenstei, S.; Wright, E.; McMahon, L.; Stamatoyannopoulos, G.; Faller, D.V.; Perrine, S.P. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood 1999, 93, 1790–1797. [Google Scholar]
- Safo, M.K.; Abdulmalik, O.; Danso-Danquat, R.; Burnett, J.C.; Nokuri, S.; Joshi, G.S.; Musayev, F.N.; Asakura, T.; Abraham, D.J. Structural basis for the potent antisickiling effects of a novel class of five membered heterocyclic aldehydic compounds. J. Med. Chem. 2004, 47, 4665–4676. [Google Scholar] [CrossRef]
- Zaugg, R.H.; Walder, J.A.; Klotz, I.M. Schiff base adducts of hemoglobin. Modifications that inhibit erythrocyte sickling. J. Biol. Chem. 1977, 252, 8542–8548. [Google Scholar]
- Abraham, D.J.; Mehanna, A.S.; Wireko, F.C.; Whitney, J.; Thomas, R.P.; Orringer, E.P. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991, 77, 1334–1341. [Google Scholar] [Green Version]
- Safo, M,K.; Danso-Danquat, R.; Joshi, G.S.; Abraham, D.J. Antisickling agents. US2005/ 0209199 A1, 2005. [Google Scholar]
- Zhang, C.; Li, X.; Lian, L.; Chen, Q.; Abdulmalik, O.; Vassilev, V.; Lai, C.S.; Asakura, T. Anti-sickling effect of MX-1520, a prodrug of vanillin: an in vivo study using rodents. Br. J. Haematol. 2004, 125, 788–795. [Google Scholar] [CrossRef]
- Yarbro, J.M. Mechanism of action oh hydroxyurea. Semin. Oncol. 1992, 19, 1–10. [Google Scholar]
- Hanft, V.N.; Fruchtman, S.R.; Pickens, C.V.; Rosse, W.F.; Howard, T.A.; Ware, R.E. Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood 2000, 95, 3589–3593. [Google Scholar]
- Steinberg, M.H.; Barton, F.; Castro, O.; Pegelow, C.H.; Balas, S.K.; Kutlar, A.; Orringer, E.; Bellevie, R.; Olivieri, N.; Eckman, J.; Varma, M.; Ramirez, G.; Adler, B.; Smith, W.; Carlos, T.; Ataga, K.; Decastro, L.; Bjelow, C.; Sauntharajah, Y.; Telfer, M.; Bonds, D.; Terrin, M. Effect Of Hydroxyurea On Mortality And Morbidity In Adult Sickle Cell anemia: risks and benefits up to nine years of treatment. JAMA 2003, 289, 1645–1651. [Google Scholar] [CrossRef]
- Charache, S.; Barton, F.B.; Moore, R.D.; Terrin, M.L.; Steinberg, M.H.; Dover, G.J.; Ballas, S.K.; Mcmahon, R.P.; Castro, O.; Orringer, E.P. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive "switching" agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine 1996, 75, 300–326. [Google Scholar] [CrossRef]
- Space, S.L.; Lane, P.A.; Pickett, C.K.; Weil, J.L. nitric oxide attenuates normal and sickle red blood cell adherence to pulmonary endothelium. Am. J. Hematol. 2000, 63, 200–204. [Google Scholar] [CrossRef]
- Conran, N.; Oresco-Santos, C.; Acosta, H.C.; Fattori, A.; Saad, S.T.; Costa, F.F. Increased soluble guanylate cyclase activity in the red blood cells of sickle cell patients. Br. J. Haematol. 2004, 124, 547–554. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Takemura, S.; Hai, S.; Suehiro, S.; Okada, S.; Funae, Y. Nicorandil Elevates Tissue cGMP Levels in a Nitric-Oxide-Independent Manner. J. Pharmacol. Sci. 2007, 103, 33–39. [Google Scholar] [CrossRef]
- Cokic, V.P.; Smith, R.D.; beleslin-cokic, B.B.; Njoroge, J.M.; Miller, J.L.; Gladwin, M.T.; Schechter, A.N. Hydroxyurea induces fetal hemoglobin by the nitric oxide–dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 2003, 111, 231–239. [Google Scholar] [CrossRef]
- Unpublished results
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.