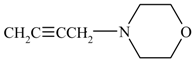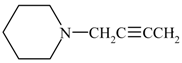Introduction
Thiadiazoles, oxadiazoles and triazoles are five–membered rings associated with diverse biological and pharmacological properties [
1]. For example, triazole derivatives are active as antibacterial, antiviral and insecticidal agents [
2]. Substituted 1,2,4-triazoles have been associated with activities such as anti-inflammatory and diuretic agents and as plant grown regulators [
3]. Esters of 1,1- cyclopropane dicarboxylic acid are important insecticidal agents [
4]. In continuation of our interest in the chemistry of 1,1-cyclopropane dicarboxylic acid, thiadiazole and 1,2,4-triazole derivatives of this acid have been prepared by conventional synthetic techniques.
Results and Discussion
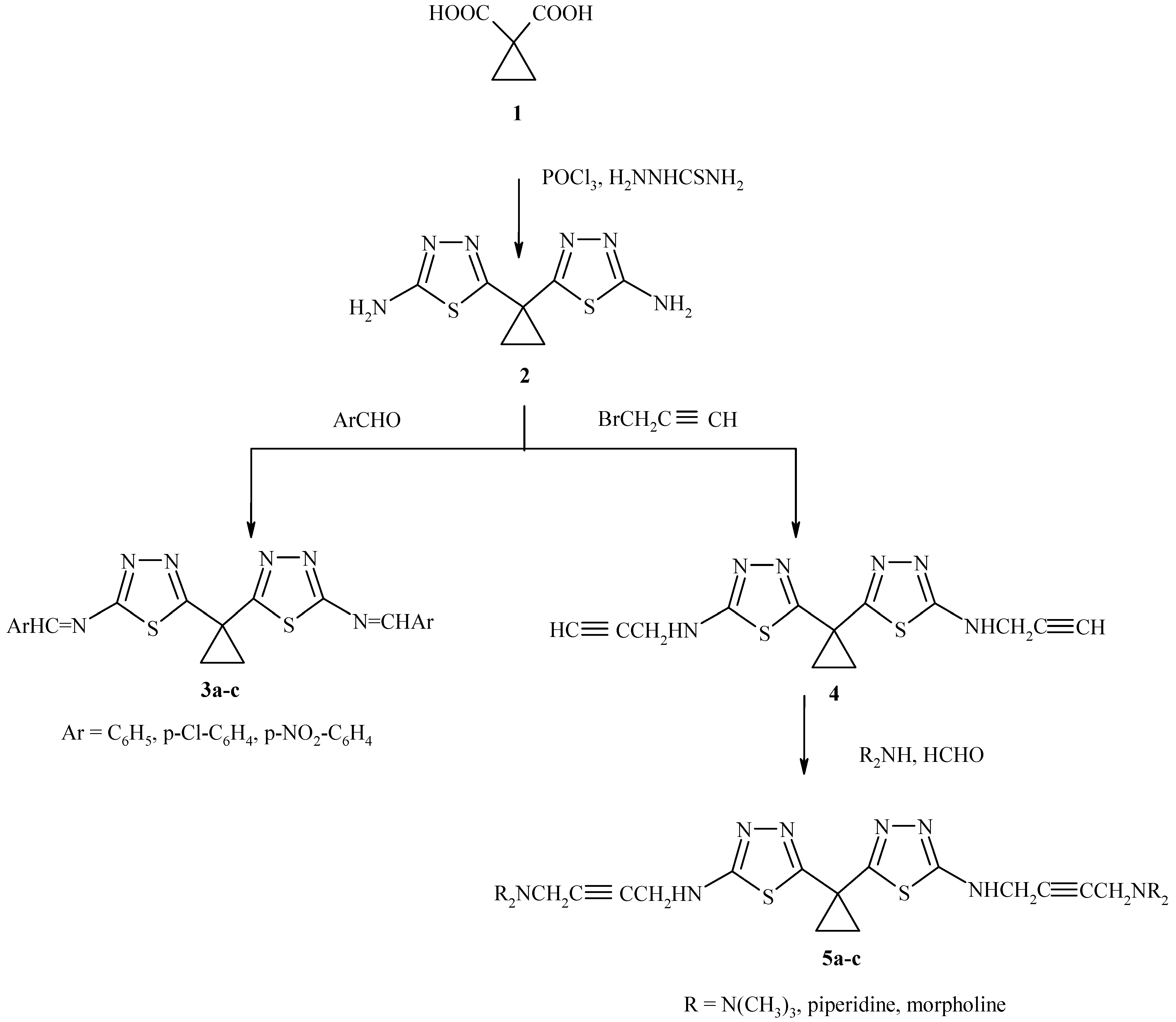
1,1-Cyclopropane dicarboxylic acid was obtained from diethylmalonate and 1,2-dibromoethane. When the acid was treated with thiosemicarbazide and phosphorous oxychloride (
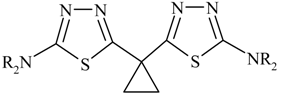
Scheme 1), 1,1- bis(2-amino-1,3,4-thiadiazol-5-yl) cyclopropane (
2) was obtained in good yield. The IR spectrum indicated the presence of a C=N function (1605 cm
-1) and the NMR spectrum showed a singlet at δ 3.29 ppm due to the NH
2 protons and a multiplet at 1.08 – 1.51 ppm for the cyclopropane ring protons.
Reaction of the aminothiadiazole
2 with aromatic aldehydes produced new Schiff bases
3a-c in high yield (
Scheme 1,
Table 1). Propynylation of
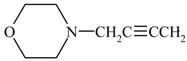
2 with propargyl chloride, followed by reaction with formaldehyde and secondary amines afforded the Mannich bases
5a-c (
Scheme 1,
Table 1).
Table 1.
Properties of 1,1- bis-(1,3,4- thiadiazolyl) cyclopropane derivatives.
Table 1.
Properties of 1,1- bis-(1,3,4- thiadiazolyl) cyclopropane derivatives.
| Comp. No. | R | mp0C | Yield % | Spectral data | IR cm-1 |
|---|
| UV, λmax (EtOH) |
|---|
| 2 | H | 214-217 | 38 | 290 | 1605 (C=N), 3165, 3280 (NH2), 660 (C-S-C) |
| 3b | =CHC6H4-Cl-p | 201-204 | 45 | 293 | 1630 (C=N), 2940-2980 (C-H), 3020 (C-HAr), 830 (C-Cl) |
| 3c | =CH-C6H4NO2-p | 199-201 | 40 | 263 | 1620 (C=N), 2900-2920 (C-H), 3025 (C-HAr), 1345,1520 (NO2) |
| 4 | CH2C ≡ CH | 243-246 | 60 | 260 | 1620 (C=N), 3130 (N-H), 2120 (C≡C), 3240 (≡C-H), 1180 (C-N), 760 (C-S-C), |
| 5a | CH2C≡C-CH2N(CH3)2 | 230-232 | 48 | 298 | 1620 (C=N), 3190 (N-H), 1220 (C-N) |
| 5b | ![Molecules 10 01153 i002]() | 237-298 | 54 | 300 | 1610 (C=N), 3250 (NH), 2125 (C≡C), 650 (C-S -C) |
| 5c | ![Molecules 10 01153 i003]() | 233-235 | 50 | 345 | 1610 (C=N), 3250 (N-H), 1180 (C-N) |
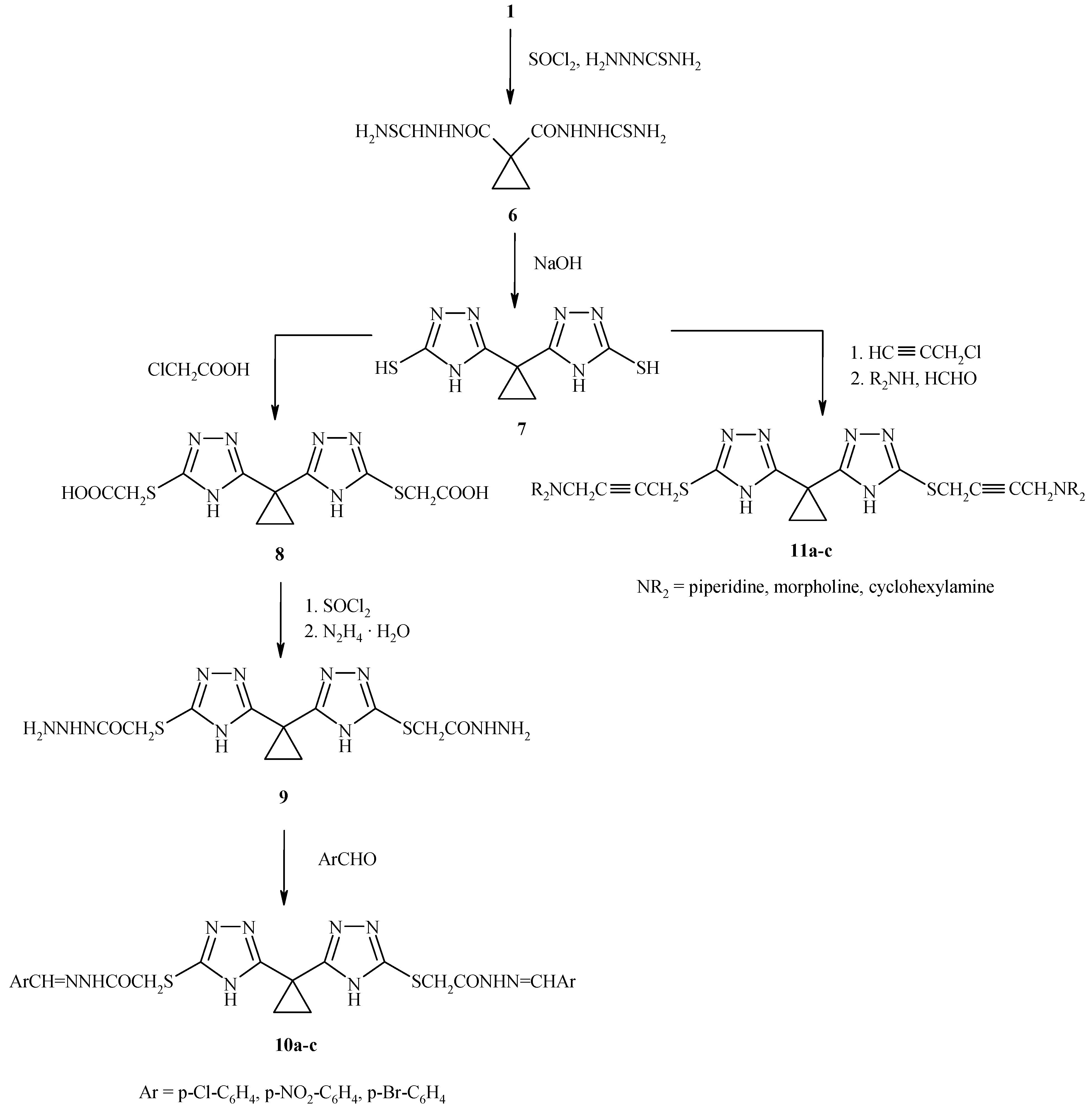
On the other hand, oxidative cyclization of the 1,1-bis-cyclopropane dicarboxylic acid thiosemicarbazide
6 with aqueous sodium hydroxide (
Scheme 2) afforded 1,1-bis(3-thio-4H-1,2,4- triazol-5-yl) cyclopropane (
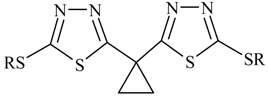
7), which exists in two tautomeric forms [
5,
6], the thiol
7a and the thione
7b, as indicated by the presence of the characteristic S-H stretching at 2620 cm
-1 (
7a) or the C=S stretching at 1220 cm
-1 (
7b), in addition to the absorption bands at 1615 cm
-1 for the C=N stretching and 3120 cm
-1 for N-H stretch. The NMR spectrum of
7 shows two singlets at 3.7 ppm and 3.8 ppm for the N-H [
6] and S-H [
8] protons of the triazole ring, respectively, and a multiplet at 0.9 – 1.1 ppm for the cyclopropane ring protons [
9].
Reaction of
7 with chloroacetic acid yielded
8 which on treatment with thionyl chloride and hydrazine hydrate afforded the acid hydrazide
9. Condensation with aromatic aldehydes in absolute ethanol gave the Schiff bases
10a-c. Introduction of a propynylic function by reaction with propargyl chloride in alkaline medium followed by the Mannich reaction with paraformaldehyde and secondary amines gave the Mannich bases
11a-c (
Scheme 2,
Table 2).
The structures of all derivatives of the thiotriazole 7 were proven on the basis of melting points (mp), thin layer chromatography (TLC) and spectral data. The IR spectra of compound 9 exhibited a C=O stretching vibration near 1675 cm-1 and NH,NH2 stretching vibrations at 3180–3200 cm-1 .
The Schiff bases
10a-c (
Table 2) display in their IR carbonyl and azomethine absorptions near 1680 cm
-1 and 1625 cm
-1, respectively, in addition to aromatic C=C at 1600 cm
-1 and =C-H at 3080 cm
-1 . The formation of the Mannich bases
11 was confirmed by the presence of a weak absorption near 2120 cm
-1 for C ≡ C and at 1260-1280 due to C-N stretching.
Table 2.
Properties of 1,1- bis (3- thiol -4H–1,2,4-triazol- 5–yl) cyclopropanes.
Table 2.
Properties of 1,1- bis (3- thiol -4H–1,2,4-triazol- 5–yl) cyclopropanes.
| Comp. No. | R | Mp°C | Yield % | Spectral data | IR cm-1 |
|---|
| UV, λmax (EtOH) |
|---|
| 7 | H | 270 | 58 | 223.5 | 2860 (C-H), 3120 (N-H), 1615 (C=N), 2620 (S-H), 1220 (C=S). |
| 8 | HOOC-CH2 | 237-39 | 45 | 290 | 2970, 2910 (C-H), 3500-3000 (COOH), 1630 (C=N), 1670 (C=O), 770 (C-S-C) . |
| 9 | H2NNHCOCH2 | 217-20 | 48 | 305.5 | 2990, 2920 (C-H), 1640 (C=O), 1610 (C=N), 3180 (N-H), 3200 (NH2) . |
| 10a | p-ClC6H4CH=NNHCOCH2 | 203-05 | 50 | 297.5 | 3200 (N-H), 3080 (C-Har), 2890, 2930 (C-Hal), 1600 (C=N), 1665 (C=O), 790 (C-Cl) . |
| 10b | p-NO2C6H4CH=NNHCOCH2 | 209-211 | 48 | 308 | 3150 (N-H), 3020 (C-Har), 2920 (C-Hal), 1610 (C=N), 1665 (C=O), 1540,1325 (C-NO2) |
| 10c | p-BrC6H4CH=NNHCOCH2 | 215-17 | 46 | 315 | 3220 (N-H), 3100 (C-Har), 2970 (C-Hal), 1630 (C=N), 1670 (C=O), 650 (C-Br) . |
| 11a | ![Molecules 10 01153 i005]() | 249-50 | 48 | 237 | 3150 (N-H), 2120 (C ≡C,w), 1605 (C=N), 1275 (C-N) . |
| 11b | ![Molecules 10 01153 i006]() | 256-57 | 50 | 302 | 3200 (N-H), 2110 (C ≡C,w), 1610 (C=N), 1260 (C-N) . |
| 11c | (C6H11)2NCH2C ≡ CCH2 | 259-60 | 47 | 301 | 3165 (N-H), 2115 (C ≡C,w), 1610 (C=N), 1280 (C-N) . |
Experimental
General
Melting points were determined in open capillary tubes on a Gallenkamp melting point apparatus and are uncorrected. The IR spectra were recorded by KBr discs with a Pye-Unicam SP3-100 spectrometer. UV spectra were recorded with Hitachi 2000 spectrophotomer.
1H-NMR spectra were recorded on a Hitachi – Perkin- Elmer 60 – MHz NMR spectrometer in CDCl
3 – DMSO-d
6 with TMS as an internal standard. Elemental analyses were done on a Carlo- Erba Analyzer type 1106. Starting chemical compounds were obtained from Fluka or Aldrich. Characterization data of the products is given in
Table 1 and
Table 2.
Preparation of 1,1-Bis (2-amino-1,3,4-thiadiazol-5-yl) cyclopropane (2).
A mixture of 1,1-cyclopropane dicarboxylic acid [
10]) (0.01 mole), thiosemicarbazide (0.02 mole) and phosphorous oxychloride (5 mL) was gently refluxed for 30 minutes. After cooling water (10 mL) was added and the reaction mixture was refluxed for four hours and filtered. The solution was neutralized with KOH and the precipitate was filtered and recrystallized from ethanol.
Preparation of compounds 3a-c.
The corresponding aryl aldehyde (0.1 mole) was added to a stirred solution of compound 2 (0.1 mole) in absolute ethanol (30 mL) and the mixture was refluxed for 2 hours. After cooling the mixture was filtered and the solid recrystallized for methanol.
Preparation of 1,1- bis(3-aminoprop-2-ynyl-1,3,4–thiadiazol-5-yl) cyclopropane (4) .
Propargyl bromide (0.02 mole) was added dropwise to a stirred solution of compound 2 (0.01 mole) and triethylamine (0.01 mole) in ethanol (25 mL). The mixture was refluxed for 2 hours then the solvent was evaporated and the product was collected and crystallized from ethanol-water.
Preparation of Mannich bases 5a–c.
To a solution of compound 4 (0.025 mole) in dry dioxane (50 mL) was added paraformaldehyde (0.05 mole), the appropriate secondary amine (0.05 mole) and catalytic amount (0.05 g) of cuprous chloride. The reaction mixture was heated for 2 hours at 900C. After cooling the mixture was filtered and poured on to ice-water and the precipitite was filtered and crystallized from chloroform.
Preparation of 1,1-cyclopropane dicarboxylic acid thiosemicarbazide (6).
A mixture of compound 1 (0.01 mole) and thionyl chloride (10 mL) was refluxed gently for 7 hours. Excess thionyl chloride was removed under vaccum to give red-brown oil of the acid chloride which was dissolved in dry benzene (25 mL) and thiosemicarbazide (0.02 mole) was added. The reaction mixture was refluxed for 2-3 hours. The product was filtered and recrystallized from ethanol .
Preparation of 1,1-bis(3-thio-1,2,4-triazol-5-yl) cyclopropane (7).
A stirring mixture of 6 (0.01 mole) and sodium hydroxide (0.01 mole, as 4% solution) was refluxed for 4 hours. After cooling the solution was decolorized with activated carbon and filtered. The filtrate was acidified with hydrochloric acid and the precipitate was filtered and recrystallized from ethanol – water.
Preparation of compound 8.
To a stirring solution of α–chloroacetic acid (0.01 mole ) in 10% sodium hydroxide (10 ml) was added a solution of compound 7 in 10% sodium hydroxide (10 ml) .The reaction mixture was refluxed for 3 hours . After cooling the solution was acidified with coc. HCl and the product was collected and recrystallized from ethanol.
Preparation of compound 9.
A mixture of compound 8 (0.002 mole) and thionyl chloride (8 mL) was refluxed for 2 hours. Excess thionyl chloride was removed in vacuo and the resulting acid chloride was crystallized from methanol. This was dissolved in pyridine (10 mL) and hydrazine hydrate (2 mL) was added dropwise with cooling. The reaction mixture was stirred overnight at room temperature then it was heated for 2 hours at 80 °C. Excess pyridine was removed in vacuo and product 9 was crystallized from ethanol- water
Preparation of Schiff bases 10 a-c.
These compounds were prepared following the same procedure used in preparation of compounds 3a –c.
Preparation of Mannich bases 11a-c.
These compounds were prepared by the same procedure used for compounds 5a-c.