Regioselective Synthesis of Vinylic Derivatives of Common Monosccarides Through Their Activated Stannylene Acetal Intermediates
Abstract
:Introduction
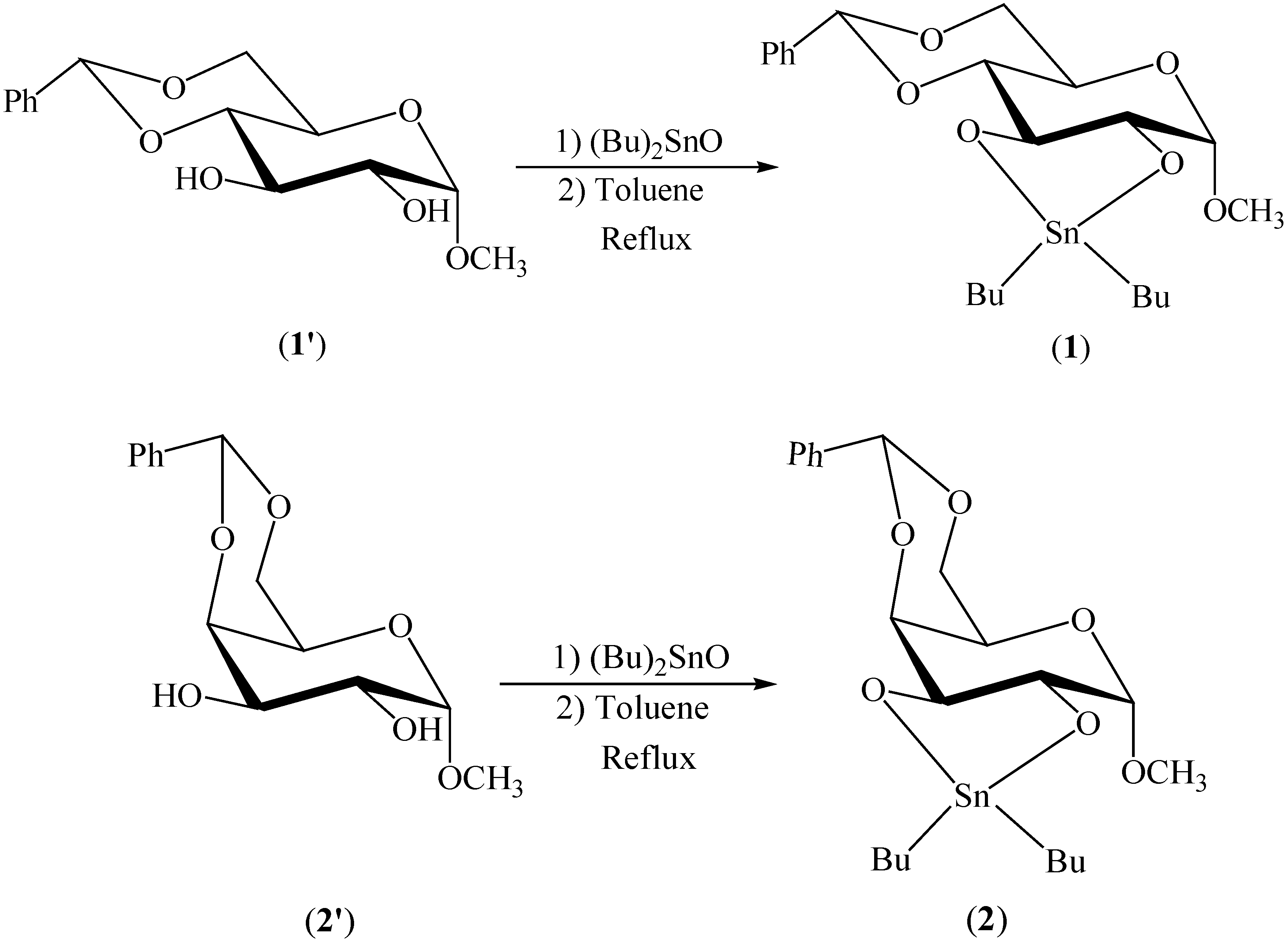
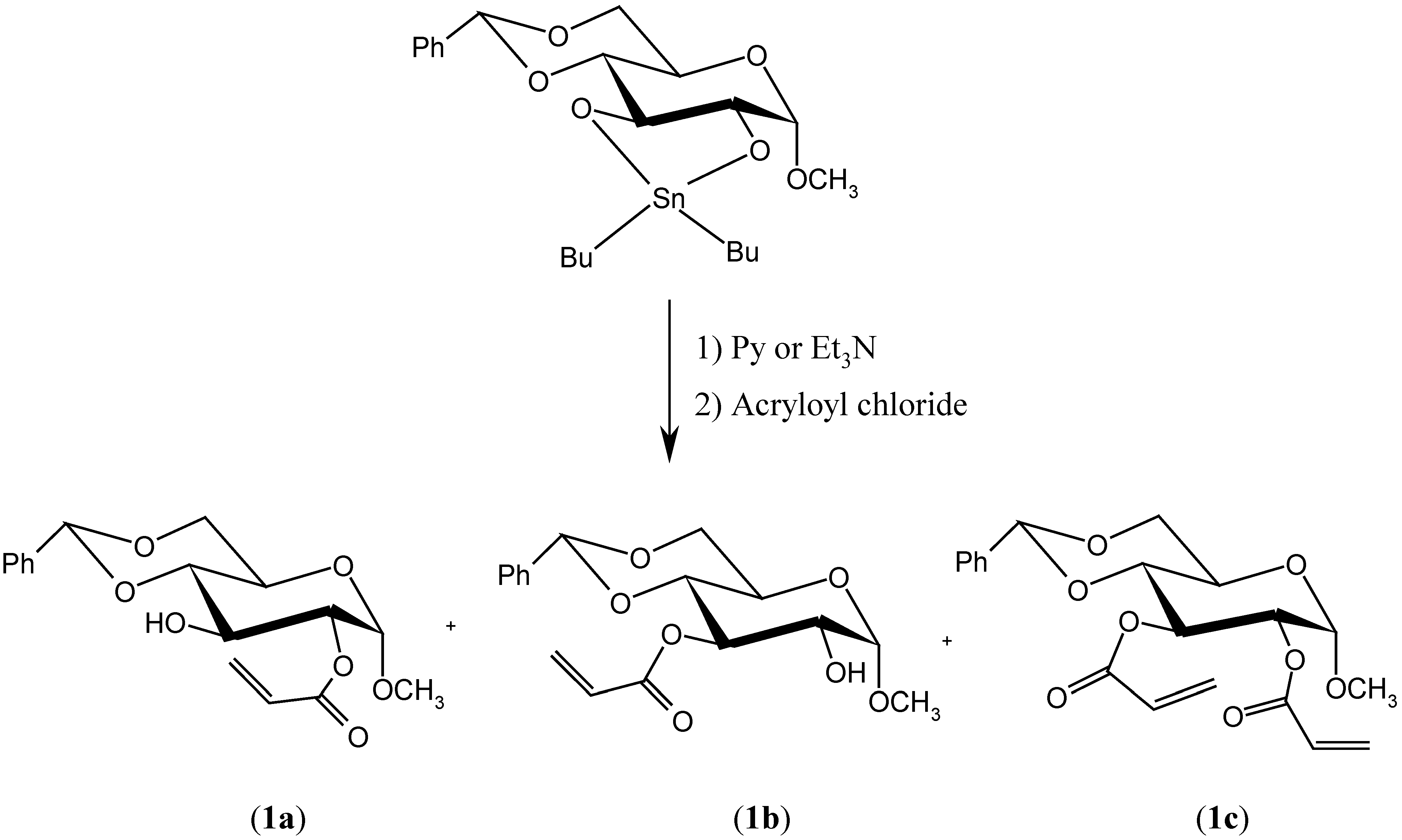
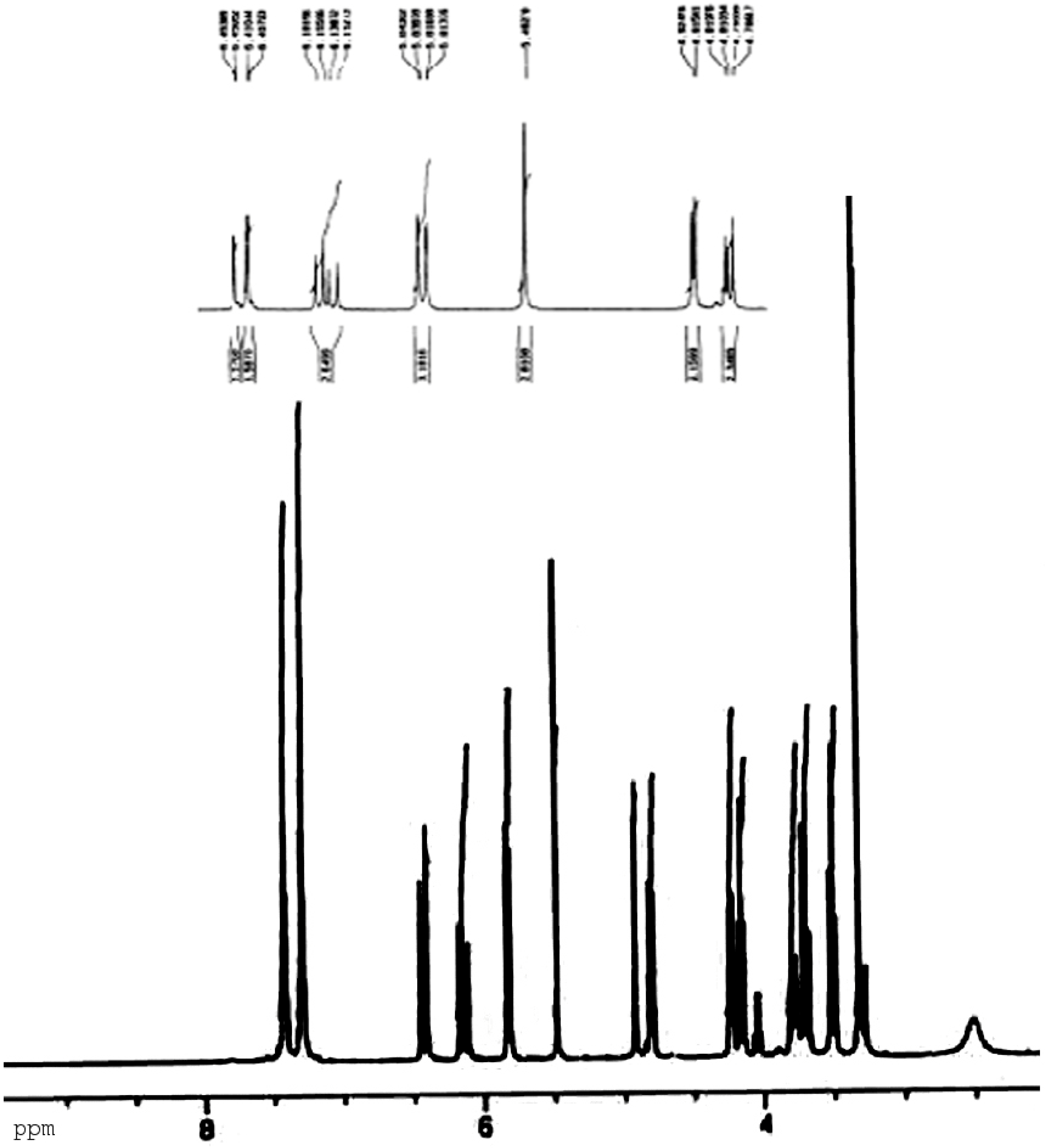
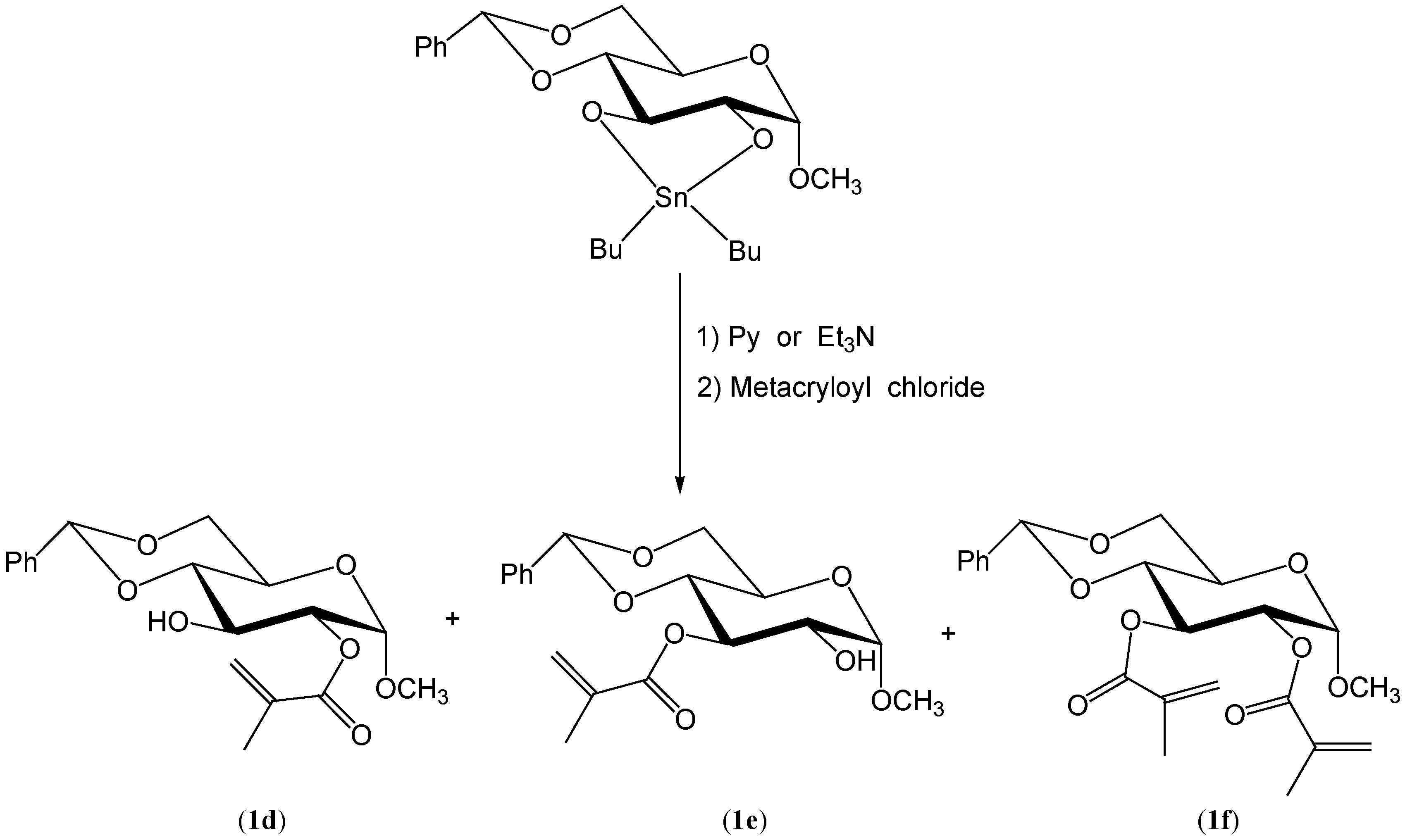
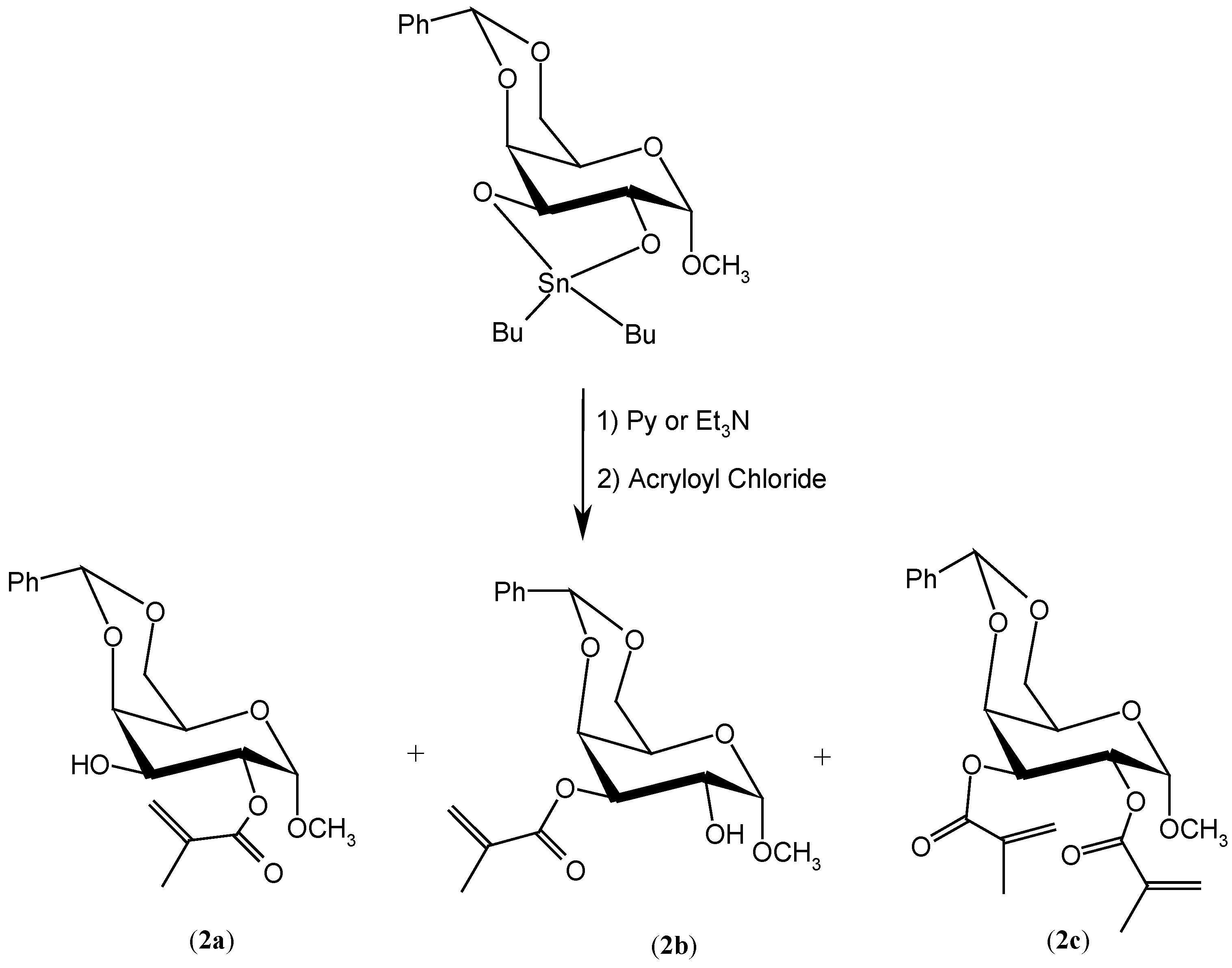
Results and Discussion
Conclusions
Experimental
General
General method for preparation of dibutystannylene acetals 1 and 2:
General workup procedure for the reactions described below.
Preparation of methyl 4,6-O-benzylidene-2-O-acryloyl-α-D-glucopyranoside (1a), methyl 4,6-O-benzylidene-3-O-acryloyl-α-D-glucopyranoside (1b) and methyl 4,6-O-benzylidene-2,3-O-diacryloyl-α-D-glucopyranoside (1c)
Preparation of methyl 4,6-O-benzylidene-2-O-metacryloyl-α-D-glucopyranoside (1d), methyl 4,6-O-benzylidene-3-O-metacryloyl-α-D-glucopyranoside (1e) and methyl 4,6-O-benzylidene-2,3-O-dimetacryloyl-α-D-glucopyranoside (1f).
Preparation of methyl 4,6-O-benzylidene-2-O-metacryloyl-α-D-galactopyranoside (2a)
Acknowledgments
References
- Kaji, E.; Harita, N. Stannyleneacetal-mediated regioselective open glycosylation of methyl 4,6-O-benzylidene-β-D-galactopyranoside and methyl-α-L-ramnopyranoside. Tetrahedron Lett. 2000, 41, 53–56. [Google Scholar] [CrossRef]
- Therisod, M.; Klibanov, A. Regioselective acylation of secondary hydroxyl groups in sugars catalyzed by lipases in organic solvents. J. Am. Chem. Soc. 1987, 109, 3977–3981. [Google Scholar] [CrossRef]
- Jenkins, D. J.; Potter, B. V. L. On the selectivity of stannylene-mediated alkylation and esterification of methyl 4,6-O-benzylidene-α-D-glucopyranoside. Carbohydr. Res. 1994, 265, 145–149. [Google Scholar] [CrossRef]
- Martin, B. D.; Ampofo, S. A.; Dordick, J. S. Biocatalytic synthesis of sugar-containing poly(acrylate)-based hydrogels. Macromolecules 1992, 25, 7081–7086. [Google Scholar] [CrossRef]
- Barili, P. L.; Berti, G.; Catelani, G.; Cini, C.; Andrea, F.; Mastrorilli, E. 4,6-O-Benzylidene-α-D-glucopyranoside and its sodium salt: new data on their preparation and properties. Carbohydr. Res. 1995, 287, 43–57. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Fan, H.; Hiraguri, Y.; Kurane, R. Biodegradation of a sugar branched polymer consisting of sugar, fatty acid and poly(vinyl alcohol). Macromolecules 2000, 33, 1636–1639. [Google Scholar] [CrossRef]
- Osborn, H. M. I.; Brome, V. A Regioselective C-3-O-acylation and O-methylation of 4,6-O-benzylidene-β-D-gluco- and galacto- pyranosides displaying a range of anomeric substituents. Carbohydr. Res. 2001, 332, 157–166. [Google Scholar] [CrossRef]
- Klein, J.; Herzog, D.; Hajibegli, A. Poly (vinyl saccharide)s, 1. Emulsion polymerization of poly(methacryloylglucose). Makromol. Chem., Rapid Commun. 1985, 6, 675–678. [Google Scholar] [CrossRef]
- Dasgupta, F.; Garegg, P. J. Monoalkylation of tributyltin activated methyl 4,6-O-benzylidene-α-D-gluco- and -galacto- pyranosides. Synthesis 1994, 1121–1123. [Google Scholar] [CrossRef]
- Andersson, L.; Kenne, L. Synthesis and NMR studies of methyl 3-O-[9R0- and (S)-1-carboxyethyl]-α-D-gluco-, galacto- and manno- pyranosides. Carbohydr. Res. 1998, 313, 157–164. [Google Scholar] [CrossRef]
- Nishida, Y.; Thiem, J. Unexpected formation of a 4,6-O-propylidene derivative from galactose and allyl alcohol in the presence of amberlystR15. J. Carbohydr. Res. 1994, 13, 1059–1063. [Google Scholar] [CrossRef]
- Loykulnant, S.; Hirao, A. Synthesis and characterization of well-defined chain-end- and in-chain-functionalized polystyrenes with a definite number of D-glucose and/or D-galactose. Macromolecules 2001, 34, 8434–8445. [Google Scholar] [CrossRef]
- Kitazawa, S.; Okumura, M.; Kinomura, K.; Sakakibara, T. Syntheses and proprieties of novel vinyl monomers bearing a glycoside residue. Chem. Lett. 1990, 1733–1736. [Google Scholar] [CrossRef]
- Wang, Q.; Dordick, J. S.; Linhardt, R. Synthesis and application of carbohydrate-containing polymers. J. Chem. Mater. 2002, 14, 3232–3244. [Google Scholar] [CrossRef]
- Grindley, T. B.; Thangarasa, R. The structure and reactions of stannylene acetals from carbohydrate-derived trans-diols. Can. J. Chem. 1990, 68, 1007–1019. [Google Scholar] [CrossRef]
- Grindley, T. B.; Namazi, H. The regioselective synthesis of non-glycosidically linked oligosaccharides. Tetrahedron Lett. 1996, 37, 991–996. [Google Scholar] [CrossRef]
- Kong, X.; Grindley, T. B. Control of regioselectivity in reactions of dialkylstannylene acetals. Can. J. Chem. 1994, 72, 2405–2415. [Google Scholar] [CrossRef]
- Garcia-Martin, M. G.; Jimenez-Hidalgo, C.; Al-Kass, S. S. J.; Caraballo, I.; Galibia, J. A. Synthesis and characterization of some new homo- and co-poly(vinylsccharides). Some preliminary studies as drug delivery. Polymer 2000, 41, 821–826. [Google Scholar] [CrossRef]
- Namazi, H.; Grindley, T. B. A novel approach to carbohydrate clusters: the regioselective synthesis of non- glycosidically linked oligosaccharides. Can. J. Chem. 1997, 75, 983–995. [Google Scholar] [CrossRef]
- Yoshida, T. Synthesis of polysaccharides having specific biological activities. Prog. Polym. Sci. 2001, 26, 379–441. [Google Scholar] [CrossRef]
- Evans, M. E. Methyl 4,6-O-benzylidene-α-and- β-D-glucosides. Carbohydr. Res. 1972, 21, 473–475. [Google Scholar] [CrossRef]
- Dubber, M.; Lindhorst, T. K. Synthesis of octopus: core molecules for the construction of glycoclusters and carbohydrate-centered dendrimers. Carbohydr. Res. 1999, 310, 35–41. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Namazi, H.; Sharifzadeh, R. Regioselective Synthesis of Vinylic Derivatives of Common Monosccarides Through Their Activated Stannylene Acetal Intermediates. Molecules 2005, 10, 772-782. https://doi.org/10.3390/10070772
Namazi H, Sharifzadeh R. Regioselective Synthesis of Vinylic Derivatives of Common Monosccarides Through Their Activated Stannylene Acetal Intermediates. Molecules. 2005; 10(7):772-782. https://doi.org/10.3390/10070772
Chicago/Turabian StyleNamazi, H., and R. Sharifzadeh. 2005. "Regioselective Synthesis of Vinylic Derivatives of Common Monosccarides Through Their Activated Stannylene Acetal Intermediates" Molecules 10, no. 7: 772-782. https://doi.org/10.3390/10070772
APA StyleNamazi, H., & Sharifzadeh, R. (2005). Regioselective Synthesis of Vinylic Derivatives of Common Monosccarides Through Their Activated Stannylene Acetal Intermediates. Molecules, 10(7), 772-782. https://doi.org/10.3390/10070772



