Extracellular Matrix and the Production of Cultured Meat
Abstract
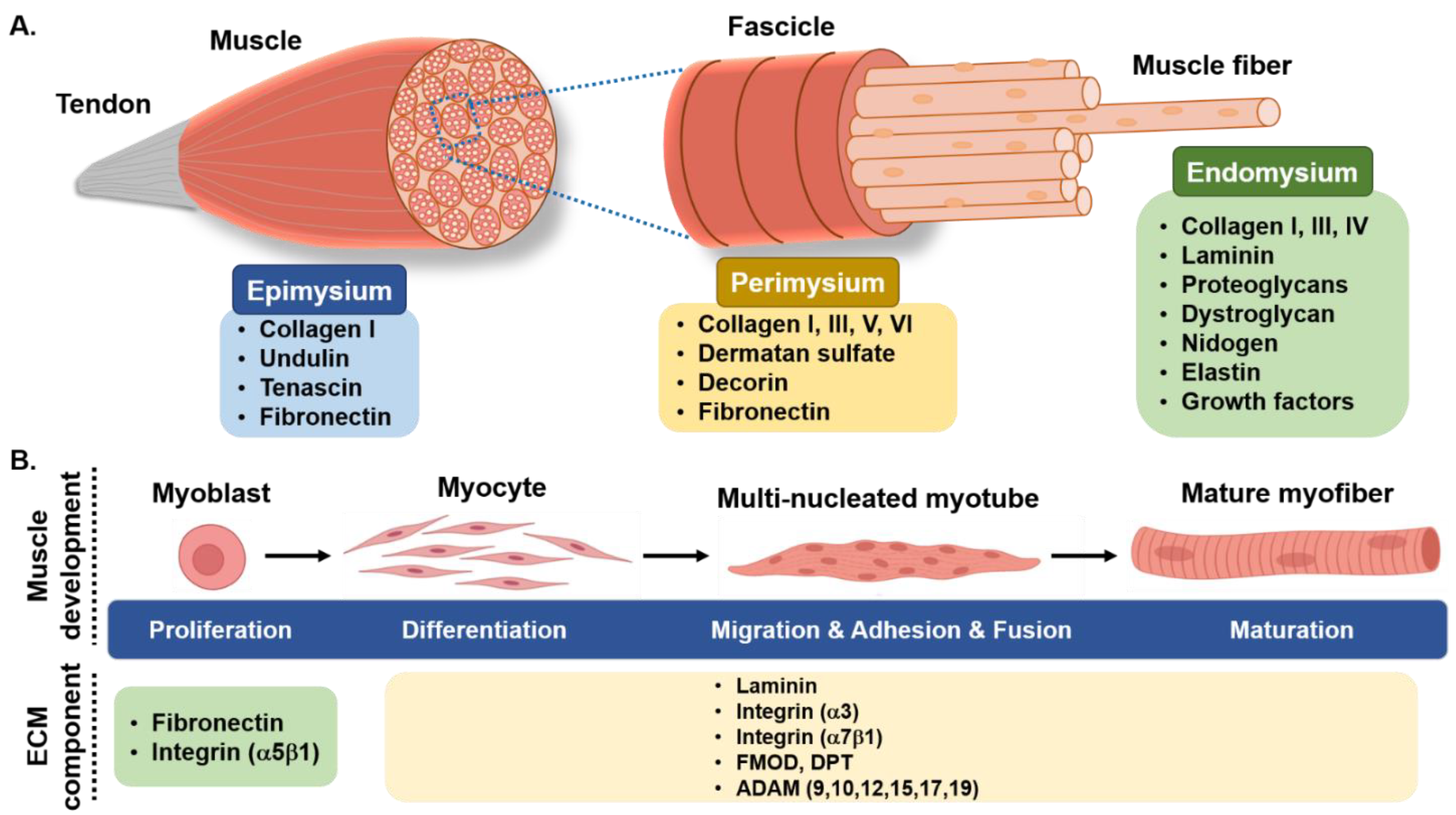
:1. Introduction
2. Skeletal Muscle Myogenesis and Its Regulation
3. Skeletal Muscle Extracellular Matrix
3.1. Collagen
| Protein Name | Function in Skeletal Muscle | Reference | |
|---|---|---|---|
| Collagen | collagen I | collagen I, III, V, and XI form collagen fiber in the SM. collagen I promotes myoblast proliferation and migration while inhibiting myogenic differentiation | [27,29,38] |
| collagen III | |||
| collagen V | |||
| collagen XI | |||
| collagen IV | promotes the regeneration of SM | ||
| collagen VI | maintains the physiological function of SM | ||
| collagen XII and XIV | localized primarily to perimysium and connect fibrillar collagen to other ECM constituents | ||
| collagen XV and XVIII | both collagen XV and XVIII bind growth factors | ||
| collagen XV provides mechanical support between cells and the ECM in SM fibers and microvessels. | |||
| have the ability to bind growth factors and contribute to the interaction of the basement membrane (BM) to other BM glycoproteins and endomysium | |||
| Proteoglycans | decorin | regulator of type I collagen fibrillogenesis in SM | [27] |
| perlecan | found in the BM and has a transient increase in expression throughout muscle differentiation | [39] | |
| fibromodulin | regulator of myostatin during myoblast differentiation | [15,25,40,41] | |
| regulates collagen fibrillogenesis | |||
| glycosaminoglycan | promotes myoblast proliferation and differentiation | [42] | |
| biglycan | binds with α- and γ-sarcoglycan of the dystrophin-glycoprotein complex | [43] | |
| syndecan-1,2,3 and 4 | downregulated during the muscle differentiation | [44] | |
| Nidogen/entactin | supports cross-links between collagen IV and laminin | [45] | |
| Dermatopontin | increases cell adhesion, decreases proliferation, and indorses the myoblast differentiation in C2C12 cells | [31] | |
| Fibronectin | endorses myoblast adhesion and proliferation | [46] | |
| inhibits differentiation and contributes to fibrillogenesis of collagen | |||
| Laminin | situated in the basal lamina of muscle fibers, promotes integrin expression and activation | [29] | |
| promotes cell proliferation, adhesion, and differentiation | |||
| Dystrophin and dystroglycan | important links between cytoskeleton and ECM | [29] | |
| maintain the integrity of cell membrane | |||
| Integrins (α3β1, α6β1, α6β4 and α7β1) | serve as laminin receptors with a high degree of selectivity | [12,47] | |
3.2. Integrin
3.3. Decorin and Biglycan
3.4. Dermatopontin
3.5. Fibromodulin
3.6. Fibronectin
3.7. Glycosaminoglycan
3.8. Laminin
3.9. Dystrophin
4. Co-Culture of Adipose and Muscle Tissue
5. Non-Animal Sourced Scaffolds and the Production of Cultured Meat
| Microcarrier/ECM Components | Source | Edibility | Biodegradability | Cost | Reference | |
|---|---|---|---|---|---|---|
| Collagens | recombinant collagen | Yes | Yes | Low | [87] | |
| Gelatin | fish species (salmon) | Yes | NA | Low | [88] | |
| Cellulose scaffold | apple hypanthium (decellularized) | Yes | NA | Low | [90] | |
| Recombinant silk | combined silkworm silk and FN-4RC | Yes | Low | [98,99] | ||
| Polysaccharides | Hyaluronic acid | plants | Yes | NA | Low | [92] |
| Alginate | 0 | |||||
| Agar | algae | |||||
| Decellularized materials | plant-derived fungal-derived (chitin) decellularized spinach | Yes Yes Yes | Yes | Low | [94] | |
| Bacterial cellulose | bacteria | Yes | Yes | Low | [96,97] | |
6. Recombinant Collagen as a Scaffold for Cultured Meat
7. Challenges and Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [Green Version]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO, Rome/OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Fayaz, H. Prospectus of cultured meat—Advancing meat alternatives. J. Food Sci. Technol. 2010, 48, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A.; Bhat, H.F. Technological, Regulatory, and Ethical Aspects ofIn VitroMeat: A Future Slaughter-Free Harvest. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1192–1208. [Google Scholar] [CrossRef] [Green Version]
- Bryant, C.; Van Nek, L.; Rolland, N.C.M. European Markets for Cultured Meat: A Comparison of Germany and France. Foods 2020, 9, 1152. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Baqir, S.; Faye, B.; Purchas, R. Cultured meat from muscle stem cells: A review of challenges and prospects. J. Integr. Agric. 2015, 14, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. eLife 2020, 9, 60445. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Yue, B. Biology of the Extracellular Matrix. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Ahmad, K.; Shaikh, S.; Ahmad, S.S.; Lee, E.J.; Choi, I. Cross-Talk Between Extracellular Matrix and Skeletal Muscle: Implications for Myopathies. Front. Pharmacol. 2020, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.-J.; Ahmad, K.; Pathak, S.; Lee, S.; Baig, M.; Jeong, J.-H.; Doh, K.-O.; Lee, D.-M.; Choi, I. Identification of Novel FNIN2 and FNIN3 Fibronectin-Derived Peptides That Promote Cell Adhesion, Proliferation and Differentiation in Primary Cells and Stem Cells. Int. J. Mol. Sci. 2021, 22, 3042. [Google Scholar] [CrossRef]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ahmad, K.; Malik, A.; Rabbani, G.; Kim, T.; Lee, I.; Lee, Y.H.; Park, S.; et al. Fibromodulin and regulation of the intricate balance between myoblast differentiation to myocytes or adipocyte-like cells. FASEB J. 2018, 32, 768–781. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Lee, E.; Ahmad, K.; Ahmad, S.-S.; Chun, H.; Lim, J.; Lee, Y.; Choi, I. Cell Types Used for Cultured Meat Production and the Importance of Myokines. Foods 2021, 10, 2318. [Google Scholar] [CrossRef]
- Verbruggen, S.; Luining, D.; Van Essen, A.; Post, M.J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2017, 70, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Zammit, P.; Beauchamp, J. The skeletal muscle satellite cell: Stem cell or son of stem cell? Differentiation 2001, 68, 193–204. [Google Scholar] [CrossRef]
- Baig, M.H.; Rashid, I.; Srivastava, P.; Ahmad, K.; Jan, A.T.; Rabbani, G.; Choi, D.; Barreto, G.E.; Ashraf, G.M.; Lee, E.J.; et al. NeuroMuscleDB: A Database of Genes Associated with Muscle Development, Neuromuscular Diseases, Ageing, and Neurodegeneration. Mol. Neurobiol. 2019, 56, 5835–5843. [Google Scholar] [CrossRef]
- Relaix, F.; Rocancourt, D.; Mansouri, A.; Buckingham, M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nat. Cell Biol. 2005, 435, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Chargé, S.B.P.; Rudnicki, M. Cellular and Molecular Regulation of Muscle Regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Kuang, S.; Gillespie, M.A.; Rudnicki, M.A. Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell Stem Cell 2008, 2, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Lee, H.J.; Kamli, M.R.; Pokharel, S.; Bhat, A.R.; Lee, Y.-H.; Choi, B.-H.; Chun, T.; Kang, S.W.; Kim, J.W.; et al. Depot-specific gene expression profiles during differentiation and transdifferentiation of bovine muscle satellite cells, and differentiation of preadipocytes. Genomics 2012, 100, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Bajracharya, P.; Lee, D.-M.; Kang, S.W.; Lee, Y.S.; Lee, H.-J.; Hong, S.K.; Chang, J.; Kim, J.W.; Schnabel, R.D.; et al. Gene expression profiles during differentiation and transdifferentiation of bovine myogenic satellite cells. Genes Genom. 2012, 34, 133–148. [Google Scholar] [CrossRef]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ashraf, J.M.; Nahm, S.; Kim, Y.; Park, S.; Choi, I. Fibromodulin: A master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J. 2016, 30, 2708–2719. [Google Scholar] [CrossRef]
- Ahmad, S.; Jan, A.T.; Baig, M.H.; Lee, E.J.; Choi, I. Matrix gla protein: An extracellular matrix protein regulates myostatin expression in the muscle developmental program. Life Sci. 2017, 172, 55–63. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [Green Version]
- DiMaio, T. This Scientist is Developing New Cell Lines for Slaughter-Free Meat. The Good Food Institute. 2019. Available online: https://gfi.org/blog/gareth-sullivan-cell-lines-research-grant/ (accessed on 13 September 2021).
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Thorsteinsdóttir, S.; Deries, M.; Cachaço, A.S.; Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 2011, 354, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.-G.; Lee, E.J.; Choi, I. Dermatopontin in Skeletal Muscle Extracellular Matrix Regulates Myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Unamuno, X.; Gómez-Ambrosi, J.; Ramírez, B.; Rodríguez, A.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; Frühbeck, G.; et al. Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity. J. Clin. Med. 2020, 9, 1069. [Google Scholar] [CrossRef] [Green Version]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle tissue engineering in fibrous gelatin: Implications for meat analogs. npj Sci. Food 2019, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Eklund, L.; Piuhola, J.; Komulainen, J.; Sormunen, R.; Ongvarrasopone, C.; Fässler, R.; Muona, A.; Ilves, M.; Ruskoaho, H.; Takala, T.E. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1194–1199. [Google Scholar] [CrossRef]
- Zoeller, J.J.; McQuillan, A.; Whitelock, J.; Ho, S.-Y.; Iozzo, R.V. A central function for perlecan in skeletal muscle and cardiovascular development. J. Cell Biol. 2008, 181, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Oldberg, A.; Chakravarti, S.; Birk, D.E. Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev. Dyn. 2010, 239, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.T.; Lee, E.J.; Choi, I. Fibromodulin: A regulatory molecule maintaining cellular architecture for normal cellular function. Int. J. Biochem. Cell Biol. 2016, 80, 66–70. [Google Scholar] [CrossRef]
- Rønning, S.B.; Pedersen, M.E.; Andersen, P.V.; Hollung, K. The combination of glycosaminoglycans and fibrous proteins improves cell proliferation and early differentiation of bovine primary skeletal muscle cells. Differentiation 2013, 86, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Nastase, M.V.; Young, M.F.; Schaefer, L. Biglycan. J. Histochem. Cytochem. 2012, 60, 963–975. [Google Scholar] [CrossRef]
- Brandan, E.; Gutierrez, J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013, 32, 289–297. [Google Scholar] [CrossRef]
- Grzelkowska-Kowalczyk, K. The Importance of Extracellular Matrix in Skeletal Muscle Development and Function. In Composition and Function of the Extracellular Matrix in the Human Body; Books on Demand: Rijeka, Croatia, 2016; pp. 3–24. [Google Scholar]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2009, 339, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Gullberg, D.; Velling, T.; Lohikangas, L.; Tiger, C.F. Integrins during muscle development and in muscular dystrophies. Front. Biosci. 1998, 3, d1039–d1050. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.; Lee, E.J.; Shaikh, S.; Kumar, A.; Rao, K.M.; Park, S.-Y.; Jin, J.O.; Han, S.S.; Choi, I. Targeting integrins for cancer management using nanotherapeutic approaches: Recent advances and challenges. Semin. Cancer Biol. 2021, 69, 325–336. [Google Scholar] [CrossRef]
- Svensson, L.; Heineg, D.; Oldberg, A. Decorin-binding Sites for Collagen Type I Are Mainly Located in Leucine-rich Repeats 4–5. J. Biol. Chem. 1995, 270, 20712–20716. [Google Scholar] [CrossRef] [Green Version]
- Pringle, G.A.; Dodd, C.M. Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. J. Histochem. Cytochem. 1990, 38, 1405–1411. [Google Scholar] [CrossRef]
- Vogel, K.G.; Koob, T.J.; Fisher, L.W. Characterization and interactions of a fragment of the core protein of the small proteoglycan (PGII) from bovine tendon. Biochem. Biophys. Res. Commun. 1987, 148, 658–663. [Google Scholar] [CrossRef]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Corsi, A.; Xu, T.; Chen, X.-D.; Boyde, A.; Liang, J.; Mankani, M.; Sommer, B.; Iozzo, R.; Eichstetter, I.; Robey, P.; et al. Phenotypic Effects of Biglycan Deficiency Are Linked to Collagen Fibril Abnormalities, Are Synergized by Decorin Deficiency, and Mimic Ehlers-Danlos-Like Changes in Bone and Other Connective Tissues. J. Bone Miner. Res. 2002, 17, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Mercado, M.L.; Amenta, A.R.; Hagiwara, H.; Rafii, M.S.; Lechner, B.E.; Owens, R.T.; McQuillan, D.J.; Froehner, S.C.; Fallon, J.R. Biglycan regulates the expression and sarcolemmal localization of dystrobrevin, syntrophin, and nNOS. FASEB J. 2006, 20, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, O.; Fujiwara, S. Dermatopontin, a Novel Player in the Biology of the Extracellular Matrix. Connect. Tissue Res. 2006, 47, 177–189. [Google Scholar] [CrossRef]
- Liu, X.; Meng, L.; Shi, Q.; Liu, S.; Cui, C.; Hu, S.; Wei, Y. Dermatopontin promotes adhesion, spreading and migration of cardiac fibroblasts in vitro. Matrix Biol. 2013, 32, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, O.; Hozumi, K.; Katagiri, F.; Takahashi, N.; Sumiyoshi, H.; Matsuo, N.; Yoshioka, H.; Nomizu, M.; Fujiwara, S. Dermatopontin Promotes Epidermal Keratinocyte Adhesion via α3β1 Integrin and a Proteoglycan Receptor. Biochemistry 2010, 49, 147–155. [Google Scholar] [CrossRef]
- Okamoto, O.; Fujiwara, S.; Abe, M.; Sato, Y. Dermatopontin interacts with transforming growth factor beta and enhances its biological activity. Biochem. J. 1999, 337, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Okamoto, O.; Ishikawa, K.; Sumiyoshi, H.; Matsuo, N.; Yoshioka, H.; Nomizu, M.; Shimada, T.; Fujiwara, S. Dermatopontin Interacts with Fibronectin, Promotes Fibronectin Fibril Formation, and Enhances Cell Adhesion. J. Biol. Chem. 2011, 286, 14861–14869. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Cui, C.; Han, S.; Chen, Y.; Zhao, J.; He, H.; Li, D.; Zhu, Q. Fibromodulin Modulates Chicken Skeletal Muscle Development via the Transforming Growth Factor-β Signaling Pathway. Animals 2020, 10, 1477. [Google Scholar] [CrossRef]
- Lee, E.J.; Nam, J.H.; Choi, I. Fibromodulin modulates myoblast differentiation by controlling calcium channel. Biochem. Biophys. Res. Commun. 2018, 503, 580–585. [Google Scholar] [CrossRef]
- Maurer, L.M.; Ma, W.; Mosher, D.F. Dynamic structure of plasma fibronectin. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Reheman, A.; Spring, C.M.; Kalantari, J.; Marshall, A.H.; Wolberg, A.S.; Gross, P.L.; Weitz, J.I.; Rand, M.L.; Mosher, D.F.; et al. Plasma fibronectin supports hemostasis and regulates thrombosis. J. Clin. Investig. 2014, 124, 4281–4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamkun, J.W.; Hynes, R.O. Plasma fibronectin is synthesized and secreted by hepatocytes. J. Biol. Chem. 1983, 258, 4641–4647. [Google Scholar] [CrossRef]
- Moretti, F.A.; Chauhan, A.; Iaconcig, A.; Porro, F.; Baralle, F.E.; Muro, A.F. A Major Fraction of Fibronectin Present in the Extracellular Matrix of Tissues Is Plasma-derived. J. Biol. Chem. 2007, 282, 28057–28062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of Fibronectin Extracellular Matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [Green Version]
- Früh, S.M.; Schoen, I.; Ries, J.; Vogel, V. Molecular architecture of native fibronectin fibrils. Nat. Commun. 2015, 6, 7275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruoslahti, E. Fibronectin. J. Oral Pathol. Med. 1981, 10, 3–13. [Google Scholar] [CrossRef]
- Pisconti, A.; Bernet, J.D.; Olwin, B.B. Syndecans in skeletal muscle development, regeneration and homeostasis. Muscle Ligaments Tendons J. 2012, 2, 1–9. [Google Scholar]
- Bi, Y.; Patra, P.; Faezipour, M. Structure of collagen-glycosaminoglycan matrix and the influence to its integrity and stability. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 3949–3952. [Google Scholar]
- Beachley, V.; Ma, G.; Papadimitriou, C.; Gibson, M.; Corvelli, M.; Elisseeff, J. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. J. Biomed. Mater. Res. Part A 2018, 106, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Weber, I.T.; Harrison, R.; Iozzo, R.V. Model Structure of Decorin and Implications for Collagen Fibrillogenesis. J. Biol. Chem. 1996, 271, 31767–31770. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.L.; Steiner, M.; Witkos, T.; Egerer, J.; Busse, B.; Mizumoto, S.; Pestka, J.M.; Zhang, H.; Hausser, I.; Khayal, L.A.; et al. Impaired proteoglycan glycosylation, elevated TGF-β signaling, and abnormal osteoblast differentiation as the basis for bone fragility in a mouse model for gerodermia osteodysplastica. PLoS Genet. 2018, 14, e1007242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Xia, W.; Lei, D.; Voorhees, J.J.; Fisher, G.J. Age-dependent alterations of decorin glycosaminoglycans in human skin. Sci. Rep. 2013, 3, srep02422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, T. Role of extracellular matrix in development of skeletal muscle and postmortem aging of meat. Meat Sci. 2015, 109, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, E.W.; Gradall, K.S. Basal lamina molecules are concentrated in myogenic regions of the mouse limb bud. Zeitschrift für Anatomie und Entwicklungsgeschichte 1998, 198, 481–486. [Google Scholar] [CrossRef]
- Yap, L.; Tay, H.G.; Nguyen, M.T.; Tjin, M.S.; Tryggvason, K. Laminins in Cellular Differentiation. Trends Cell Biol. 2019, 29, 987–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, K.; De Lisio, M.; Huntsman, H.D.; Pincu, Y.; Mahmassani, Z.; Miller, M.; Olatunbosun, D.; Jensen, T.; Boppart, M.D. Laminin-111 Improves Skeletal Muscle Stem Cell Quantity and Function Following Eccentric Exercise. STEM CELLS Transl. Med. 2014, 3, 1013–1022. [Google Scholar] [CrossRef]
- Pradhan, B.S.; Prószyński, T.J. A Role for Caveolin-3 in the Pathogenesis of Muscular Dystrophies. Int. J. Mol. Sci. 2020, 21, 8736. [Google Scholar] [CrossRef]
- Haenggi, T.; Fritschy, J.-M. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue. Cell. Mol. Life Sci. 2006, 63, 1614–1631. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Kim, D.; Soundharrajan, I.; Hwang, I.; Choi, K.C. Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment. Biology 2020, 10, 6. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2021, 236, 2393–2412. [Google Scholar] [CrossRef]
- Bi, P.; Kuang, S. MEAT SCIENCE AND MUSCLE BIOLOGY SYMPOSIUM: Stem cell niche and postnatal muscle growth 1,2. J. Anim. Sci. 2012, 90, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plunkett, N.; O’Brien, F.J. Bioreactors in tissue engineering. Technol. Health Care 2011, 19, 55–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguín, Y.; Sánchez, E.; Acevedo, C.A. Edible Scaffolds Based on Non-Mammalian Biopolymers for Myoblast Growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [Green Version]
- Patricio, T.; Glória, A.; Bártolo, P. Mechanical and biological behaviour of PCL and PCL/PLA scaffolds for tissue engineering applications. Chem. Eng. 2013, 32, 1645–1650. [Google Scholar]
- Jin, M.M.; Shi, M.J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized spinach: An edible scaffold for laboratory-grown meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Bin Ariff, A.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterial 2017, 7, 257. [Google Scholar] [CrossRef] [Green Version]
- Garred, P.; Brygge, K.; Sörensen, C.H.; Madsen, H.O.; Thiel, S.; Svejgaard, A. Mannan-binding protein-levels in plasma and upper-airways secretions and frequency of genotypes in children with recurrence of otitis media. Clin. Exp. Immunol. 2008, 94, 99–104. [Google Scholar] [CrossRef]
- Widhe, M.; Bysell, H.; Nystedt, S.; Schenning, I.; Malmsten, M.; Johansson, J.; Rising, A.; Hedhammar, M. Recombinant spider silk as matrices for cell culture. Biomaterials 2010, 31, 9575–9585. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.-U.; Thatikonda, N.; Naidu, V.; Hedhammar, M.; Mandal, B.B. Silkworm Silk Scaffolds Functionalized with Recombinant Spider Silk Containing a Fibronectin Motif Promotes Healing of Full-Thickness Burn Wounds. ACS Biomater. Sci. Eng. 2019, 5, 4634–4645. [Google Scholar] [CrossRef]
- Ruggiero, F.; Exposito, J.-Y.; Bournat, P.; Gruber, V.; Perret, S.; Comte, J.; Olagnier, B.; Garrone, R.; Theisen, M. Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett. 2000, 469, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineerin 2020, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valorizat. 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesik-Brodacka, M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staunton, D.; Millard, C.J.; Aricescu, A.R.; Campbell, I.D. Preparation of recombinant fibronectin fragments for functional and structural studies. Methods Mol. Biol. 2009, 522, 73–99. [Google Scholar] [PubMed]
- Pakkanen, O.; Hämäläinen, E.-R.; Kivirikko, K.I.; Myllyharju, J. Assembly of stable human type I and III collagen molecules from hydroxylated recombinant chains in the yeast Pichia pastoris: Effect of an engineered C-terminal oligomerization domain foldon. J. Biol. Chem. 2003, 278, 32478–32483. [Google Scholar] [CrossRef] [Green Version]
- Que, R.; Mohraz, A.; Da Silva, N.A.; Wang, S.-W. Expanding Functionality of Recombinant Human Collagen Through Engineered Non-Native Cysteines. Biomacromolecules 2014, 15, 3540–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, B.; Liu, W.; Pushparaj, K.; Park, S. The Epic of In Vitro Meat Production—A Fiction into Reality. Foods 2021, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, K.; Lim, J.-H.; Lee, E.-J.; Chun, H.-J.; Ali, S.; Ahmad, S.S.; Shaikh, S.; Choi, I. Extracellular Matrix and the Production of Cultured Meat. Foods 2021, 10, 3116. https://doi.org/10.3390/foods10123116
Ahmad K, Lim J-H, Lee E-J, Chun H-J, Ali S, Ahmad SS, Shaikh S, Choi I. Extracellular Matrix and the Production of Cultured Meat. Foods. 2021; 10(12):3116. https://doi.org/10.3390/foods10123116
Chicago/Turabian StyleAhmad, Khurshid, Jeong-Ho Lim, Eun-Ju Lee, Hee-Jin Chun, Shahid Ali, Syed Sayeed Ahmad, Sibhghatulla Shaikh, and Inho Choi. 2021. "Extracellular Matrix and the Production of Cultured Meat" Foods 10, no. 12: 3116. https://doi.org/10.3390/foods10123116
APA StyleAhmad, K., Lim, J.-H., Lee, E.-J., Chun, H.-J., Ali, S., Ahmad, S. S., Shaikh, S., & Choi, I. (2021). Extracellular Matrix and the Production of Cultured Meat. Foods, 10(12), 3116. https://doi.org/10.3390/foods10123116








