Utilization of Greenhouse Gases for Syngas Production by Dry Reforming Process Using Reduced BaNiO3 Perovskite as a Catalyst
Abstract
:1. Introduction
2. Materials and Methods
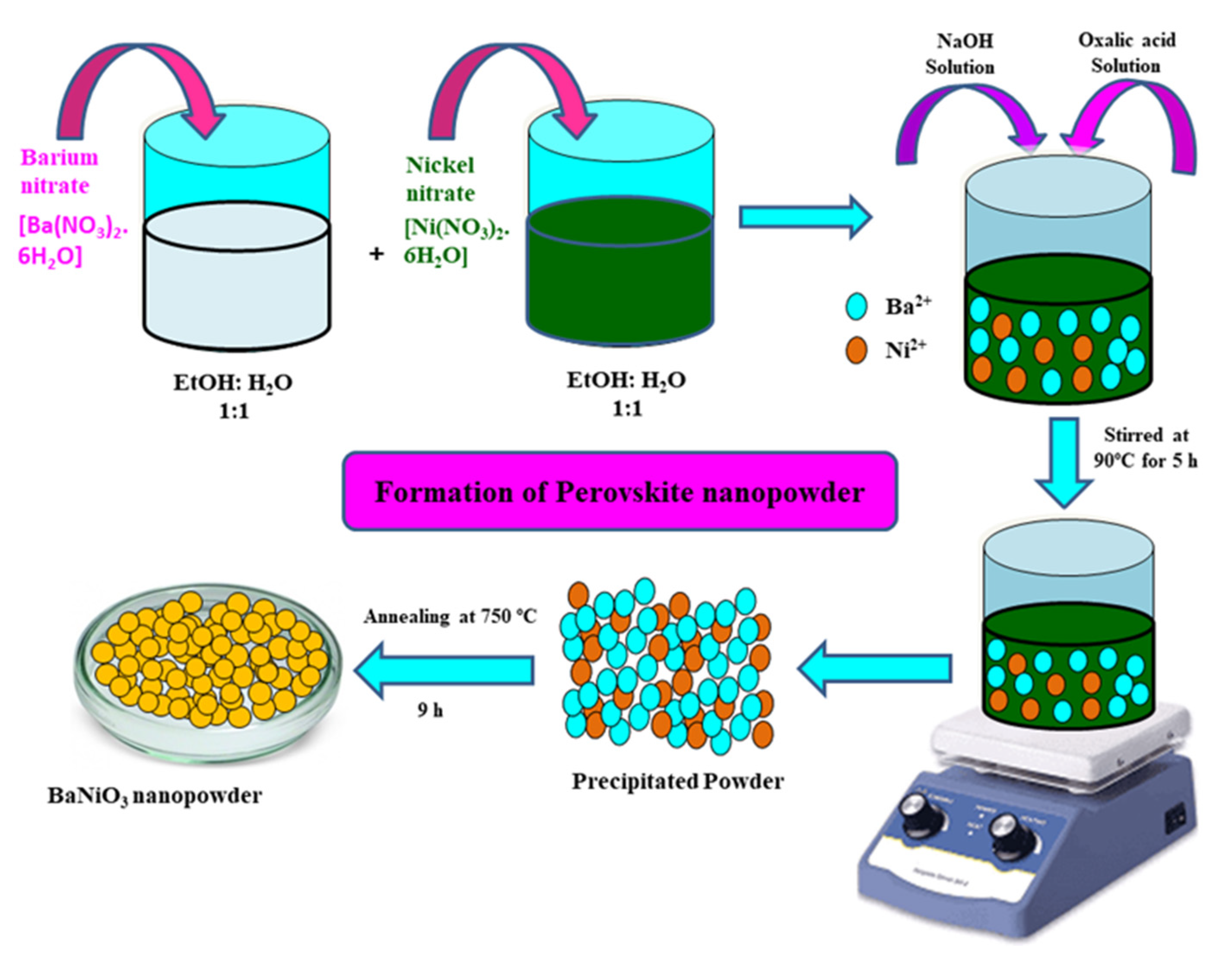
2.1. Preparation of Perovskite BaNiO3
2.2. Characterizations
2.3. Catalytic Activity Measurements
3. Results and Discussion
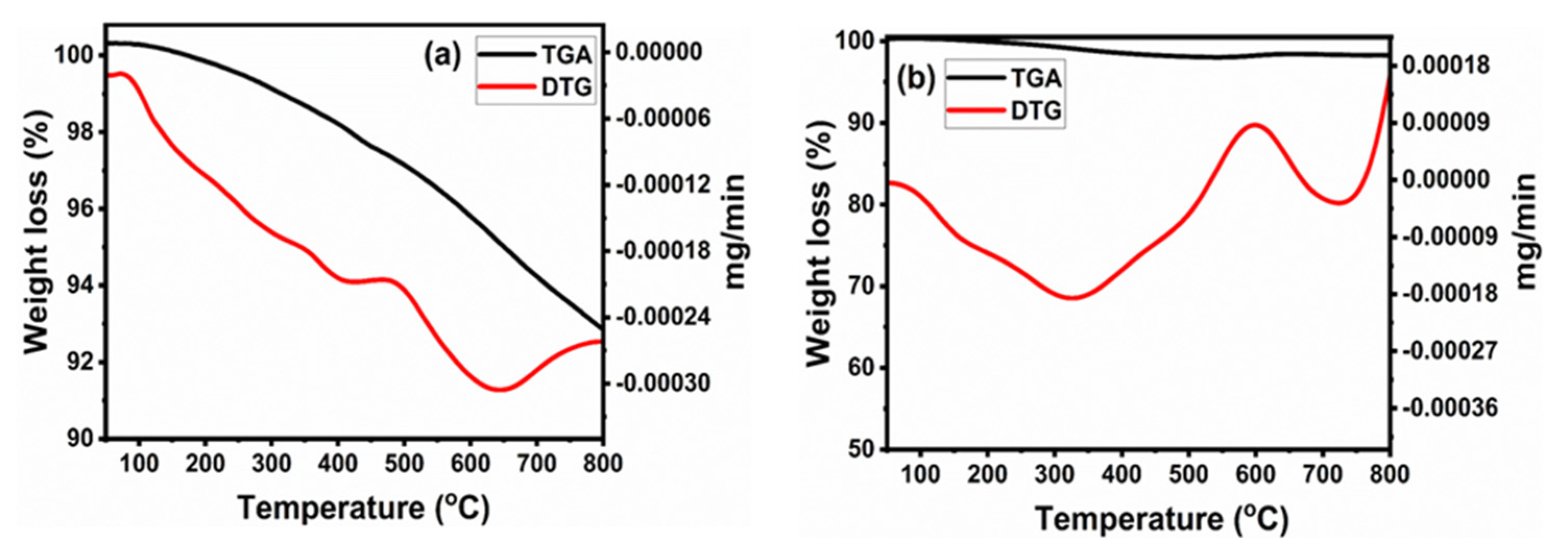
3.1. Thermal Analysis (TG-DTG)
3.2. Fourier-Transform Infrared Spectroscopy Analysis (FT-IR)
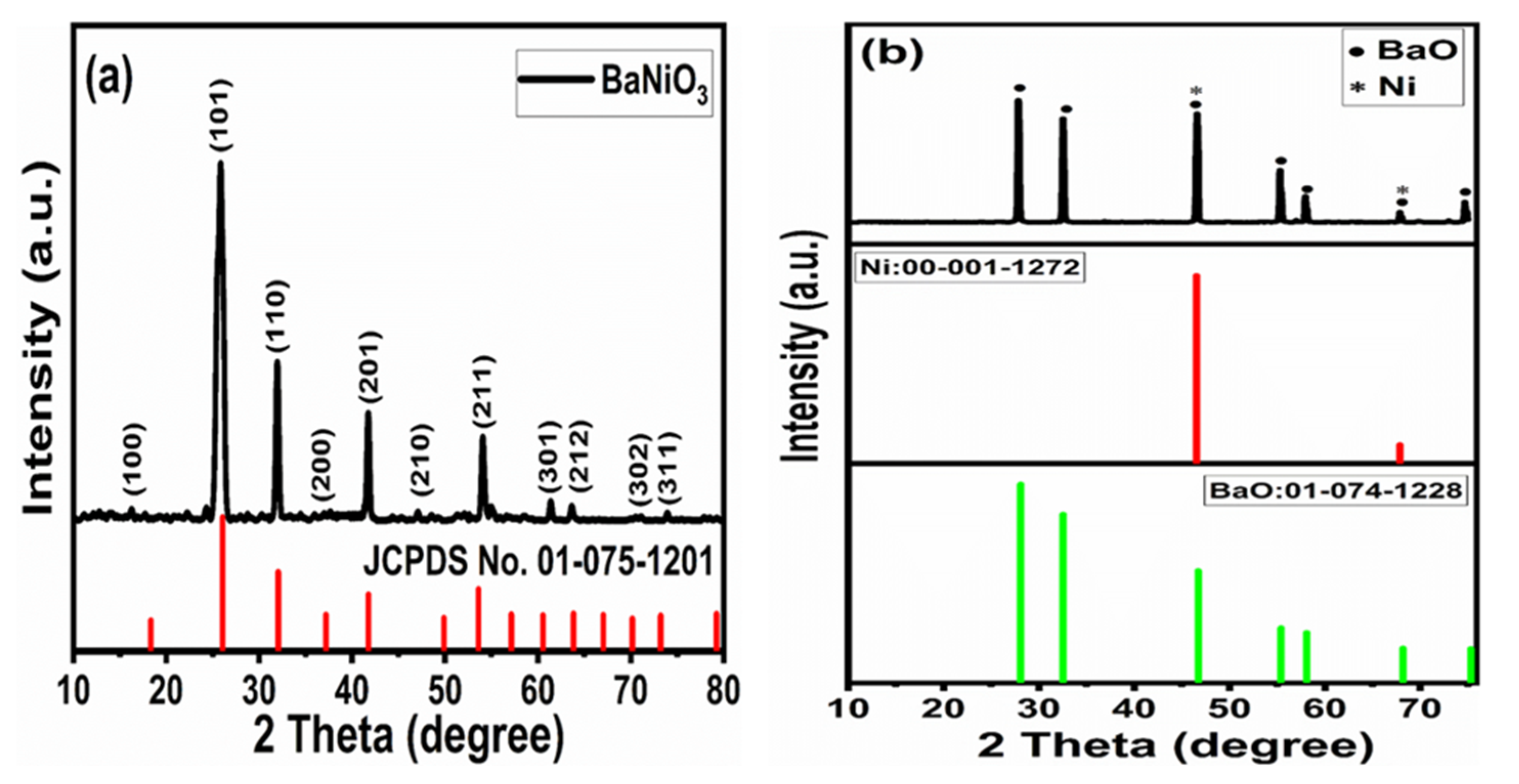
3.3. X-ray Diffraction Analysis (XRD)
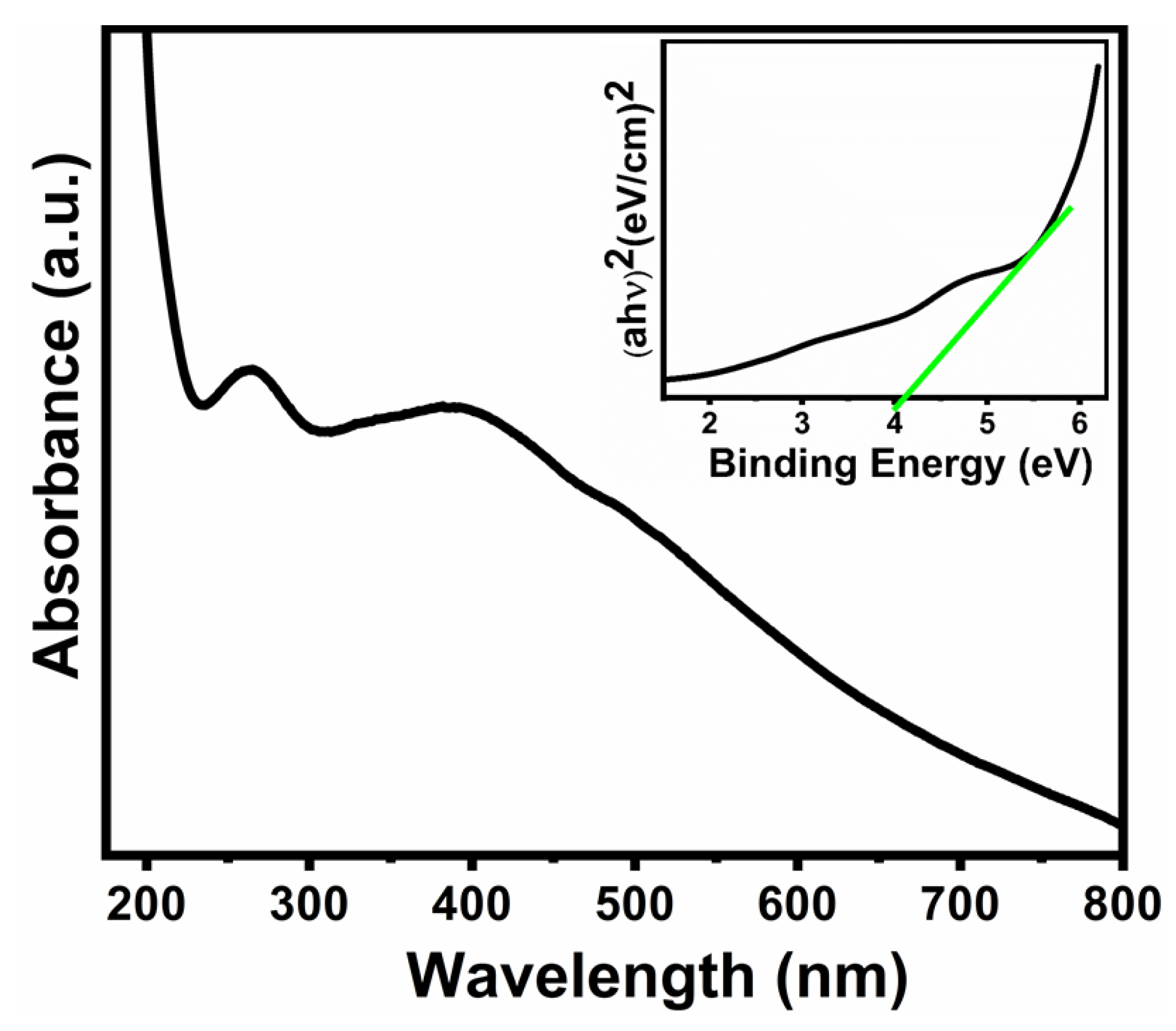
3.4. UV–Vis Spectroscopic Study
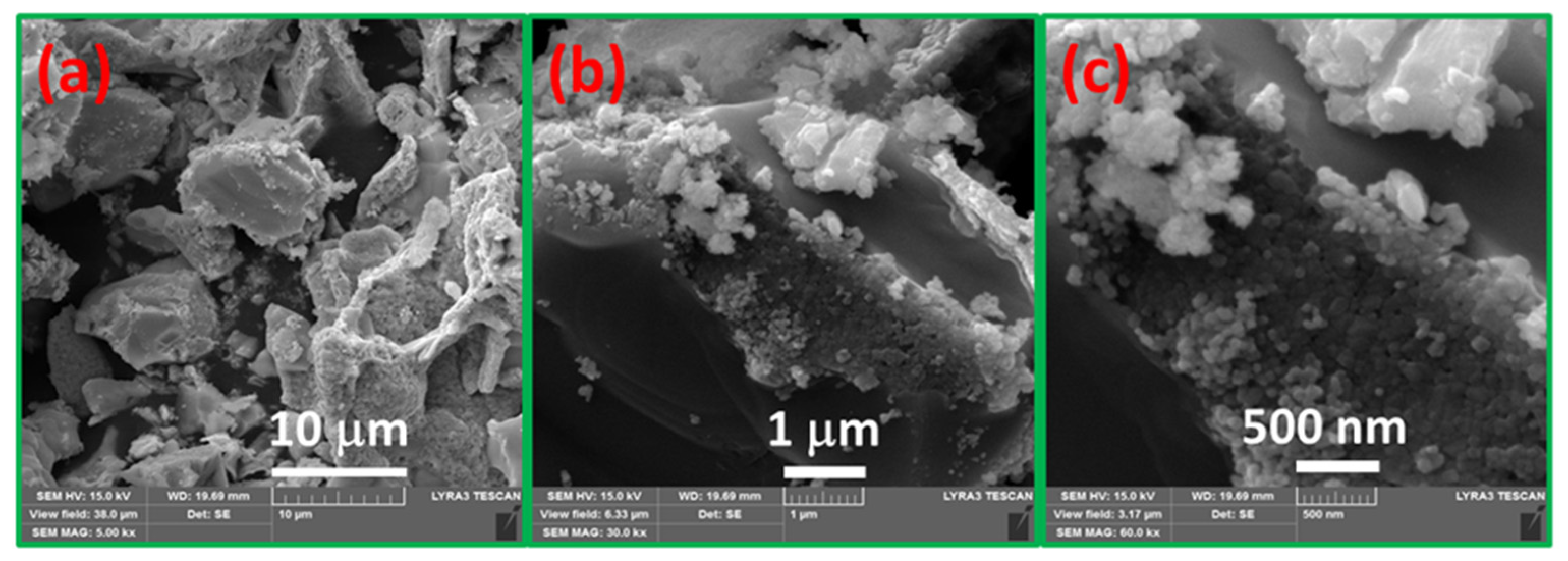
3.5. Morphological Analysis
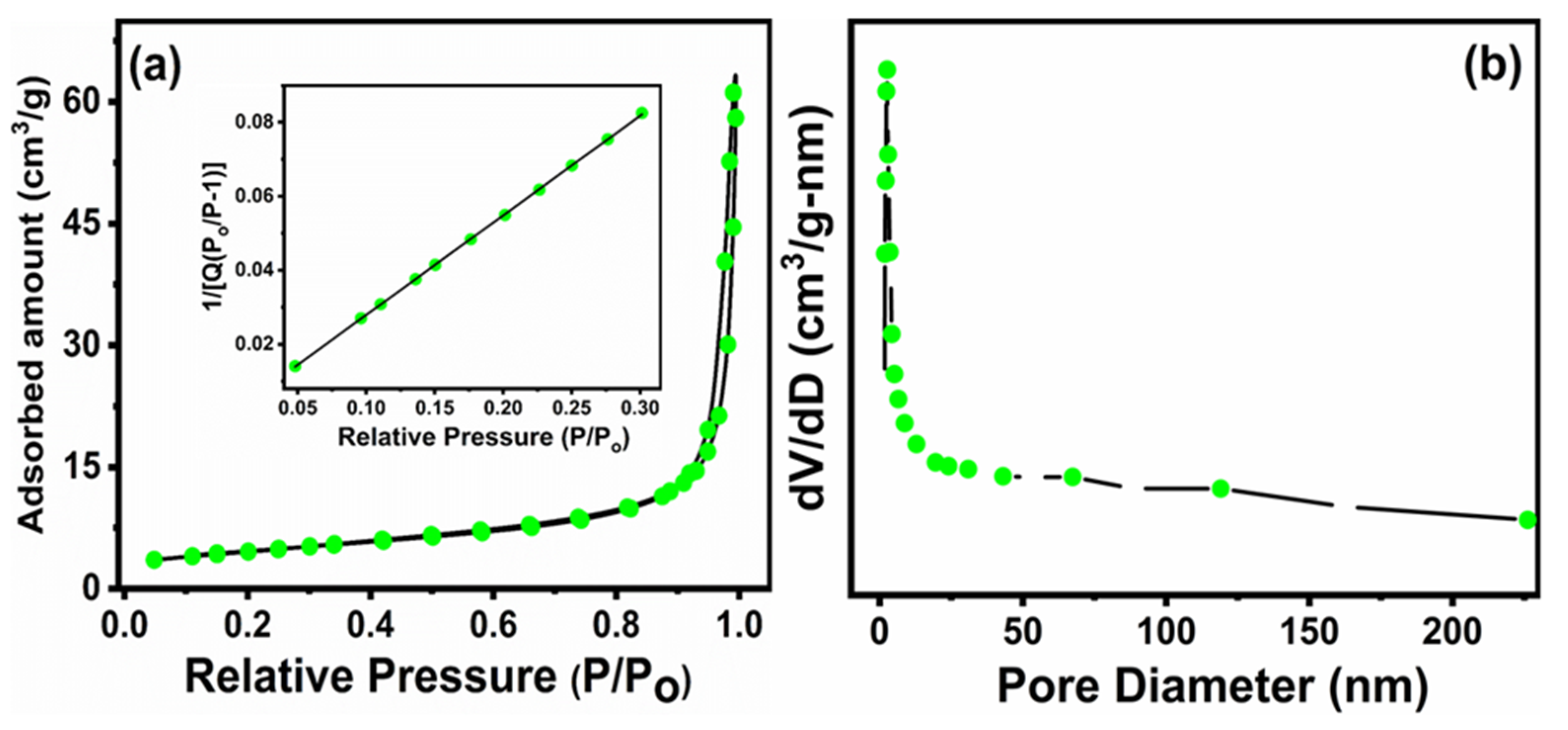
3.6. Textural Analysis (BET Surface Area)
3.7. X-ray Photoelectron Spectroscopy (XPS)
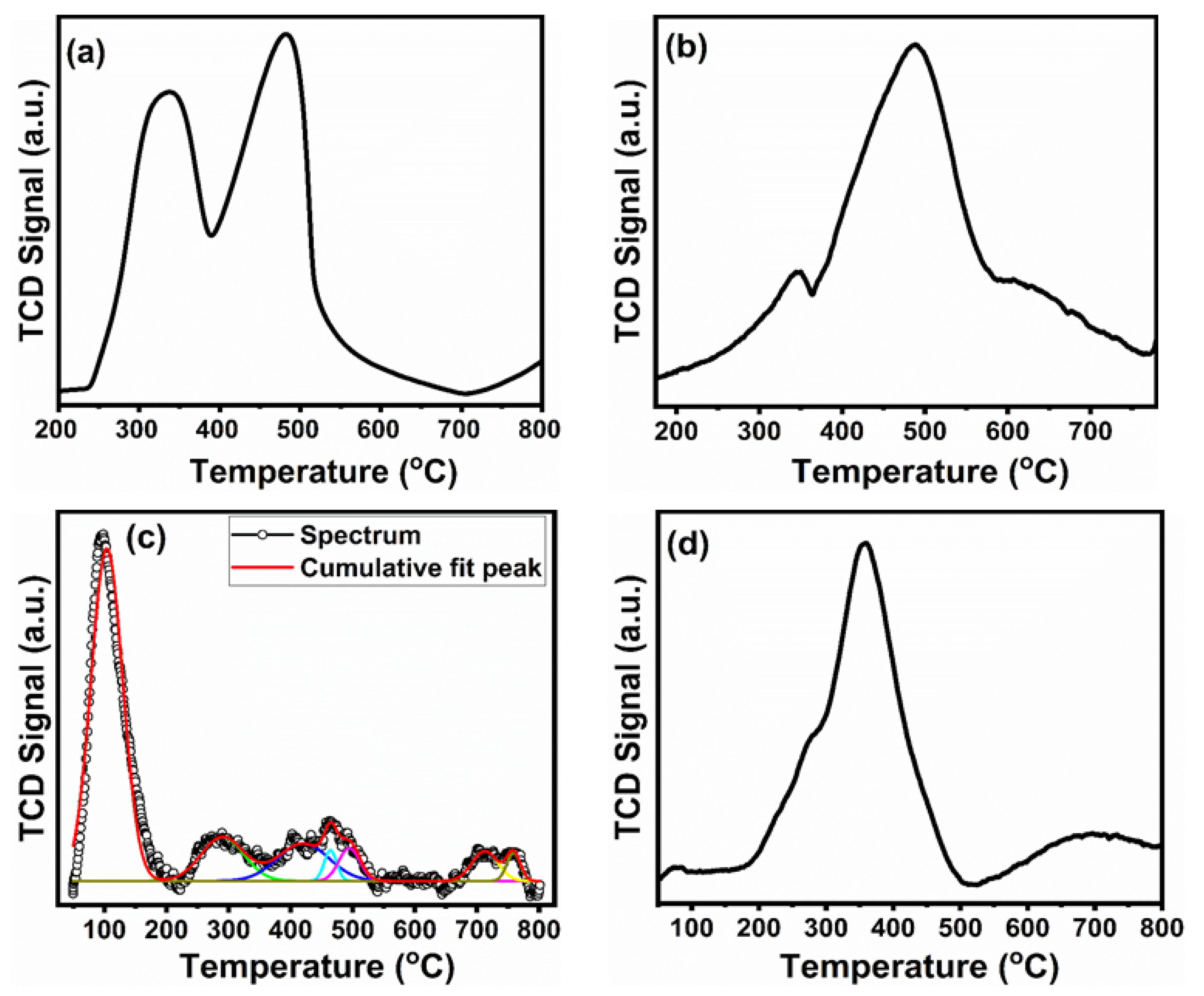
3.8. Temperature-Programmed Reactions (H2-TPR, O2-TPO, CO2-TPD)
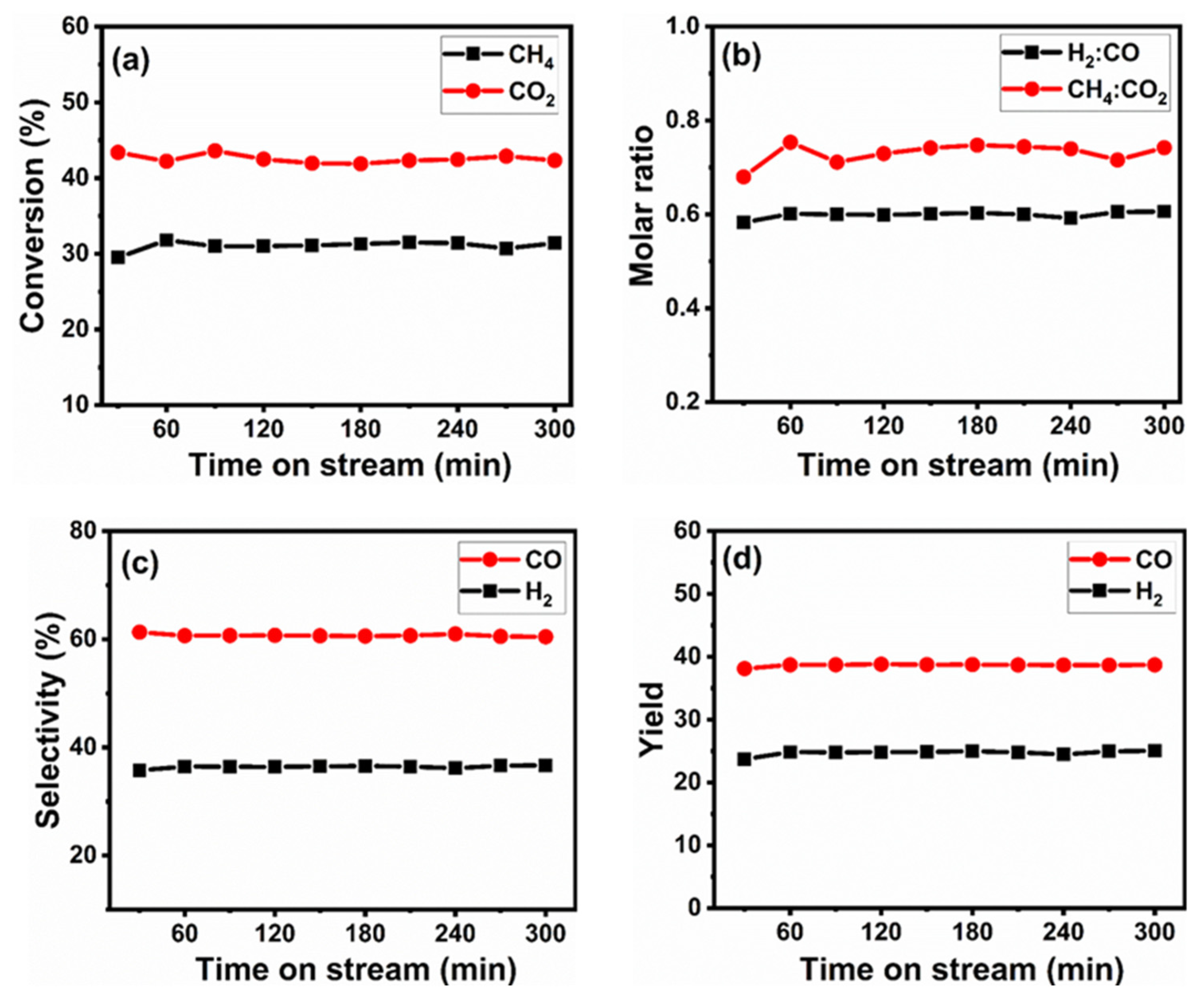
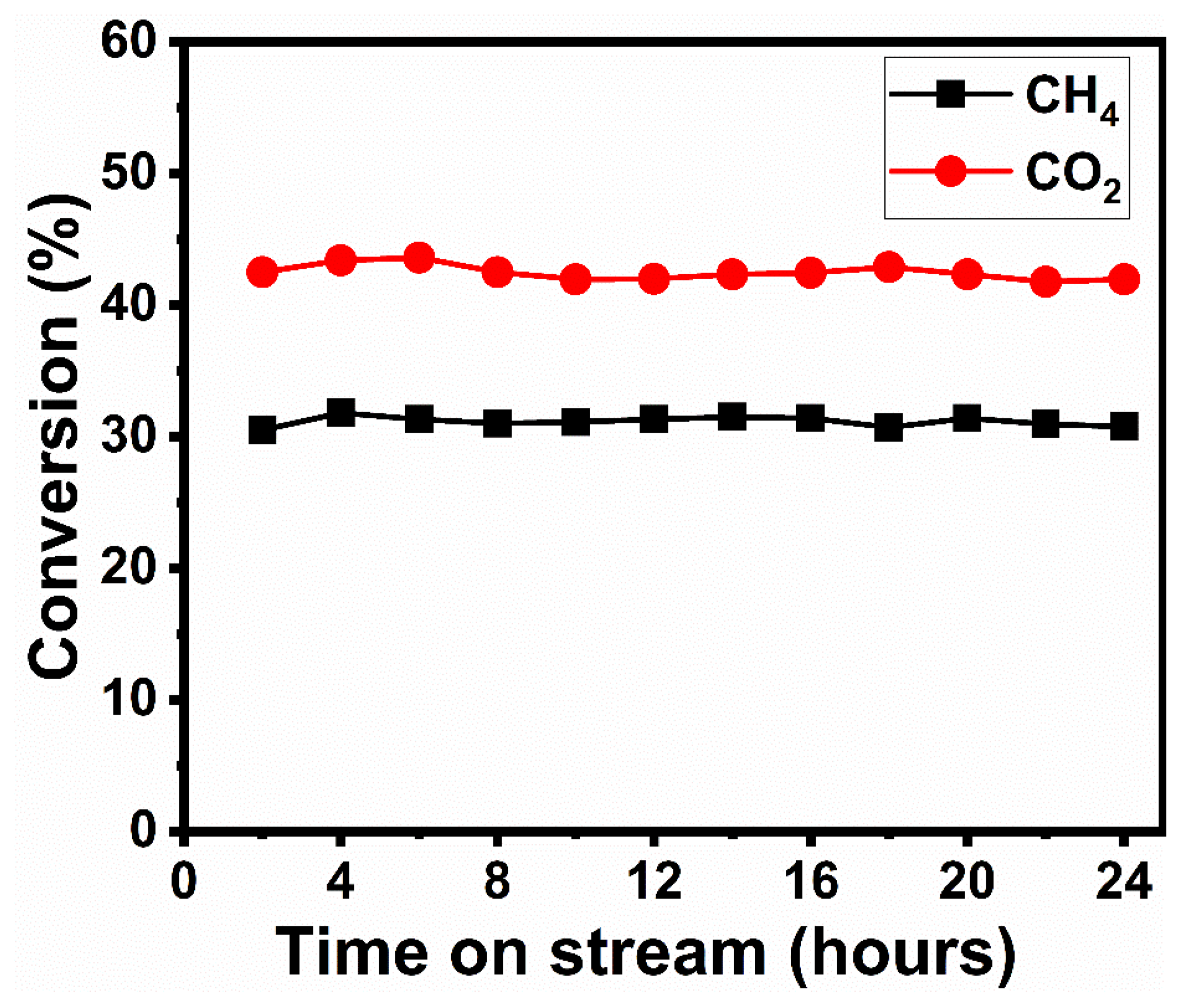
4. Catalytic Performance of Reduced Perovskite Precursor (r-BNO)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, D.; Nwaoha, C.; Towler, B. Gas-to-liquids (GTL): A review of an industry offering several routes for monetizing natural gas. J. Nat. Gas Sci. Eng. 2012, 9, 196–208. [Google Scholar] [CrossRef]
- Yagi, F.; Hodoshima, S.; Wakamatsu, S.; Kanai, R.; Kawazuishi, K.; Suehiro, Y.; Shimura, M. Development of CO2 reforming technology. Stud. Surf. Sci. Catal. 2007, 67, 385–390. [Google Scholar] [CrossRef]
- Bockris, J.O.M. The origin of ideas on a Hydrogen Economy and its solution to the decay of the environment. Int. J. Hydrogen Energy 2002, 27, 731–740. [Google Scholar] [CrossRef]
- Ueda, K.; Hirata, Y.; Sameshima, S.; Shimonosono, T.; Yamaji, K. Formation of hydrogen from the CO–H2O system using porous Gd-doped ceria electrochemical cell with MnO cathode and Fe3O4 anode. J. Asian Ceram. Soc. 2015, 3, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zhang, H.-B.; Lin, G.-D.; Tsai, K.-R. Development of coking-resistant Ni-based catalyst for partial oxidation and CO2-reforming of methane to syngas. Appl. Catal. A Gen. 1998, 166, 343–350. [Google Scholar] [CrossRef]
- Bjørgen, M.; Kolboe, S. The conversion of methanol to hydrocarbons over dealuminated zeolite H-beta. Appl. Catal. A Gen. 2002, 225, 285–290. [Google Scholar] [CrossRef]
- Hartadi, Y.; Behm, R.J.; Widmann, D. Competition of CO and H2 for Active Oxygen Species during the Preferential CO Oxidation (PROX) on Au/TiO2 Catalysts. Catalysts 2016, 6, 21. [Google Scholar] [CrossRef]
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.; Mirzaei, M.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, Q.; Li, S.; Yang, G.; Huang, N. B-site cobalt-doped perovskite oxide BaNiO3 oxygen sorbents for performance improvement of oxygen enriched gas production. New J. Chem. 2020, 44, 6003–6009. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Guczi, L.; Koppány, Z.; Sarma, K.V.; Borkó, L.; Kiricsi, I. Structure and catalytic activity of Co-based bimetallic systems in NaY zeolite: Low temperature methane activation. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; Volume 105, pp. 861–868. [Google Scholar] [CrossRef]
- Nam, J.W.; Chae, H.; Lee, S.H.; Jung, H.; Lee, K.Y. Methane dry reforming over well-dispersed Ni catalyst prepared from perovskite-type mixed oxides. Stud. Surf. Sci. Catal. 1998, 119, 843–848. [Google Scholar] [CrossRef]
- Zhang, R.-J.; Xia, G.-F.; Li, M.-F.; Wu, Y.; Nie, H.; Li, D.-D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Richardson, J.; Paripatyadar, S. Carbon dioxide reforming of methane with supported rhodium. Appl. Catal. 1990, 61, 293–309. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nat. Cell Biol. 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Solymosi, F.; Kutsan, G.; Erdohelyi, A. Catalytic reaction of CH4 with CO2 over alumina-supported Pt metals. Catal. Lett. 1991, 11, 149–156. [Google Scholar] [CrossRef]
- Wang, L.; Hu, R.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Zhang, Z. Encapsulated Ni@La2O3/SiO2 Catalyst with a One-Pot Method for the Dry Reforming of Methane. Catalysts 2019, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Verykios, X.E. Carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 catalysts. Appl. Catal. A Gen. 1996, 138, 109–133. [Google Scholar] [CrossRef]
- Hayakawa, T.; Suzuki, S.; Nakamura, J.; Uchijima, T.; Hamakawa, S.; Suzuki, K.; Shishido, T.; Takehira, K. CO2 reforming of CH4 over Ni/perovskite catalysts prepared by solid phase crystallization method. Appl. Catal. A Gen. 1999, 183, 273–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E. A stable and active nickel-based catalyst for carbon dioxide reforming of methane to synthesis gas. J. Chem. Soc. Chem. Commun. 1995, 71–72. [Google Scholar] [CrossRef]
- Shiozaki, R.; Andersen, A.G.; Hayakawa, T.; Hamakawa, S.; Suzuki, K.; Shimizu, M.; Takehira, K. Sustainable Ni/BaTiO3 catalysts for partial oxidation of methane to synthesis gas. Stud. Surf. Sci. Catal. 1997, 110, 701–710. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Valderrama, G.; Meneses-Jacome, A.; Martinez, F.; Barrault, J.; Tatibouët, J. Pulse study of CO2 reforming of methane over LaNiO3. Appl. Catal. A Gen. 2003, 248, 143–151. [Google Scholar] [CrossRef]
- Lago, R.; Bini, G.; Peña, M.A.; Fierro, J. Partial Oxidation of Methane to Synthesis Gas Using LnCoO3 Perovskites as Catalyst Precursors. J. Catal. 1997, 167, 198–209. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Kinetic study of the catalytic reforming of methane with carbon dioxide to synthesis gas over Ni/La2O3 catalyst. Catal. Today 2001, 64, 83–90. [Google Scholar] [CrossRef]
- Verykios, X.E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int. J. Hydrogen Energy 2003, 28, 1045–1063. [Google Scholar] [CrossRef]
- Slagtern, Å.; Olsbye, U. Partial oxidation of methane to synthesis gas using La-M-O catalysts. Appl. Catal. A Gen. 1994, 110, 99–108. [Google Scholar] [CrossRef]
- Tao, K.; Shi, L.; Ma, Q.; Wang, D.; Zeng, C.; Kong, C.; Wu, M.; Chen, L.; Zhou, S.; Hu, Y.; et al. Methane reforming with carbon dioxide over mesoporous nickel–alumina composite catalyst. Chem. Eng. J. 2013, 221, 25–31. [Google Scholar] [CrossRef]
- Provendier, H.; Petit, C.; Estournes, C.; Libs, S.; Kiennemann, A. Stabilisation of active nickel catalysts in partial oxidation of methane to synthesis gas by iron addition. Appl. Catal. A Gen. 1999, 180, 163–173. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.; Sahebdelfar, S.; Bai, P.; Liu, X.; Yan, Z.-F. CO2 reforming of CH4 over nanocrystalline zirconia-supported nickel catalysts. Appl. Catal. B Environ. 2008, 77, 346–354. [Google Scholar] [CrossRef]
- Tangwiwat, S.; Milne, S.J. Barium titanate sols prepared by a diol-based sol–gel route. J. Non-Cryst. Solids 2005, 351, 976–980. [Google Scholar] [CrossRef]
- Nikoofar, K.; Haghighi, M.; Khademi, Z. Preparation, characterization, and catalytic application of metallic nanocrystalline MgAl2O4 in the synthesis of 3-hydroxy-3-indolyl-indolin-2-ones, symmetrical and unsymmetrical 3,3′-bis(indolyl)indolin-2-ones, and 3,3′-bis(indolyl)methanes. Arab. J. Chem. 2019, 12, 3776–3784. [Google Scholar] [CrossRef] [Green Version]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Oluwafemi, O.S. Comparative Catalytic Properties of Ni(OH) and NiO Nano-particles Towards the Degradation of Nitrite (NO2) and Nitric Oxide (NO). Int. J. Electrochem. Sci. 2014, 9, 3008–3021. [Google Scholar]
- Gottschall, R.; Schöllhorn, R.; Muhler, M.; Jansen, N.; Walcher, D.; Gütlich, P. Electronic State of Nickel in Barium Nickel Oxide, BaNiO3. Inorg. Chem. 1998, 37, 1513–1518. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials; Wiley: London, UK, 1962. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Israr, M.Q.; Hamad, A.B.A.; El Desouky, F.G.; Salem, M.A.; Battisha, I.K. Determination of A.C. Conductivity of Nano-Composite Perovskite Ba1−x−ySrxTiFeyO3 Prepared by the Sol-Gel Technique. J. Cryst. Process. Technol. 2012, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced Photocatalytic Activity and Ferromagnetism in Gd Doped BiFeO3 Nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Yang, M.; He, J. Fine tuning of the morphology of copper oxide nanostructures and their application in ambient degradation of methylene blue. J. Colloid Interface Sci. 2011, 355, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhong, P.; Zhu, J.J. Preparation of Highly Active and Stable Perovskite-like Catalyst by Combustion Method: Effect of Complex. Catal. Lett. 2009, 131, 672–675. [Google Scholar] [CrossRef]
- Eltejaei, H.; Bozorgzadeh, H.R.; Towfighi, J.; Omidkhah, M.R.; Rezaei, M.; Zanganeh, R.; Zamaniyan, A.; Ghalam, A.Z. Methane dry reforming on Ni/Ce0.75Zr0.25O2–MgAl2O4 and Ni/Ce0.75Zr0.25O2–γ-alumina: Effects of support composition and water addition. Int. J. Hydrogen Energy 2012, 37, 4107–4118. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 Nanowire@MnO2 Ultrathin Nanosheet Core/Shell Arrays: A New Class of High-Performance Pseudocapacitive Materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-W.; Yin, Y.-M.; Lu, J.; Zhang, C.; Minh, N.Q.; Ma, Z.-F. Structure and Properties of Novel Cobalt-Free Oxides NdxSr1–xFe0.8Cu0.2O3−δ (0.3 ≤ x ≤ 0.7) as Cathodes of Intermediate Temperature Solid Oxide Fuel Cells. J. Phys. Chem. C 2014, 118, 13357–13368. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology Dependence of Catalytic Properties of Ni/CeO2 Nanostructures for Carbon Dioxide Reforming of Methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, P.; Sharma, A. Free-standing NiV2S4 nanosheet arrays on a 3D Ni framework via an anion exchange reaction as a novel electrode for asymmetric supercapacitor applications. J. Mater. Chem. A 2016, 4, 17512–17520. [Google Scholar] [CrossRef]
- Lin, J.; Zhong, Z.; Wang, H.; Zheng, X.; Wang, Y.; Qi, J.; Cao, J.; Fei, W.; Huang, Y.; Feng, J. Rational constructing free-standing Se doped nickel-cobalt sulfides nanotubes as battery-type electrode for high-performance supercapattery. J. Power Sources 2018, 407, 6–13. [Google Scholar] [CrossRef]
- Miot, C.; Husson, E.; Proust, C.; Erre, R.; Coutures, J. X-ray Photoelectron Spectroscopy Characterization of Barium Titanate Ceramics Prepared by the Citric Route: Residual Carbon Study. J. Mater. Res. 1997, 12, 2388–2392. [Google Scholar] [CrossRef]
- Fierro, J.; Tejuca, L. Non-stoichiometric surface behaviour of LaMO3 oxides as evidenced by XPS. Appl. Surf. Sci. 1987, 27, 453–457. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G. XPS and TPD probe techniques for the study of LaNiO3 perovskite oxide. Thermochim. Acta 1989, 147, 361–375. [Google Scholar] [CrossRef]
- De Lima, S.M.; Assaf, J. Ni–Fe Catalysts Based on Perovskite-type Oxides for Dry Reforming of Methane to Syngas. Catal. Lett. 2006, 108, 63–70. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Hydrogen production by methane tri-reforming process over Ni–ceria catalysts: Effect of La-doping. Appl. Catal. B Environ. 2011, 104, 64–73. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.-S.; Jung, U.H.; Seo, D.J.; Seo, Y.-S.; Yoon, W.L. Combined H2O and CO2 reforming of CH4 over nano-sized Ni/MgO-Al2O3 catalysts for synthesis gas production for gas to liquid (GTL): Effect of Mg/Al mixed ratio on coke formation. Catal. Today 2009, 146, 166–171. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zheng, X. The deposition of coke from methane on a Ni/MgAl2O4 catalyst. Carbon 2007, 45, 1314–1321. [Google Scholar] [CrossRef]
- Wu, Y.; Kawaguchi, O.; Matsuda, T. Catalytic Reforming of Methane with Carbon Dioxide on LaBO3 (B = Co, Ni, Fe, Cr) Catalysts. Bull. Chem. Soc. Jpn. 1998, 71, 563–572. [Google Scholar] [CrossRef]
- Dupeyrat, C.B.; Gallego, G.A.S.; Mondragon, F. CO2 reforming of methane over LaNiO3 as precursor material. Catal. Today 2005, 107–108, 474–480. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K. Influence of reduction temperature on the catalytic behavior of Co/TiO2 catalysts for CH4/CO2 reforming and its relation with titania bulk crystal structure. J. Catal. 2005, 230, 75–85. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Wahab, R.; Manoharadas, S.; Alrayes, B.F.; Alharthi, F. Utilization of Greenhouse Gases for Syngas Production by Dry Reforming Process Using Reduced BaNiO3 Perovskite as a Catalyst. Sustainability 2021, 13, 13855. https://doi.org/10.3390/su132413855
Ahmad N, Wahab R, Manoharadas S, Alrayes BF, Alharthi F. Utilization of Greenhouse Gases for Syngas Production by Dry Reforming Process Using Reduced BaNiO3 Perovskite as a Catalyst. Sustainability. 2021; 13(24):13855. https://doi.org/10.3390/su132413855
Chicago/Turabian StyleAhmad, Naushad, Rizwan Wahab, Salim Manoharadas, Basel F. Alrayes, and Fahad Alharthi. 2021. "Utilization of Greenhouse Gases for Syngas Production by Dry Reforming Process Using Reduced BaNiO3 Perovskite as a Catalyst" Sustainability 13, no. 24: 13855. https://doi.org/10.3390/su132413855





