Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus mandshurica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Nutritional Treatments
2.2. Sample Harvesting and Determination of Dry Weight and Leaf Area
2.3. Determination of the Content and Amount of C, N and P
2.4. Determination of Physiological Traits
2.5. Determination of Enzyme Activities
2.6. Determination of Gene Transcripts Involved in N and P Assimilation
2.7. Statistical Analysis and Graphic Production
3. Results
3.1. Dry Weight, DRS, Foliar Area and Chlorophyll Content
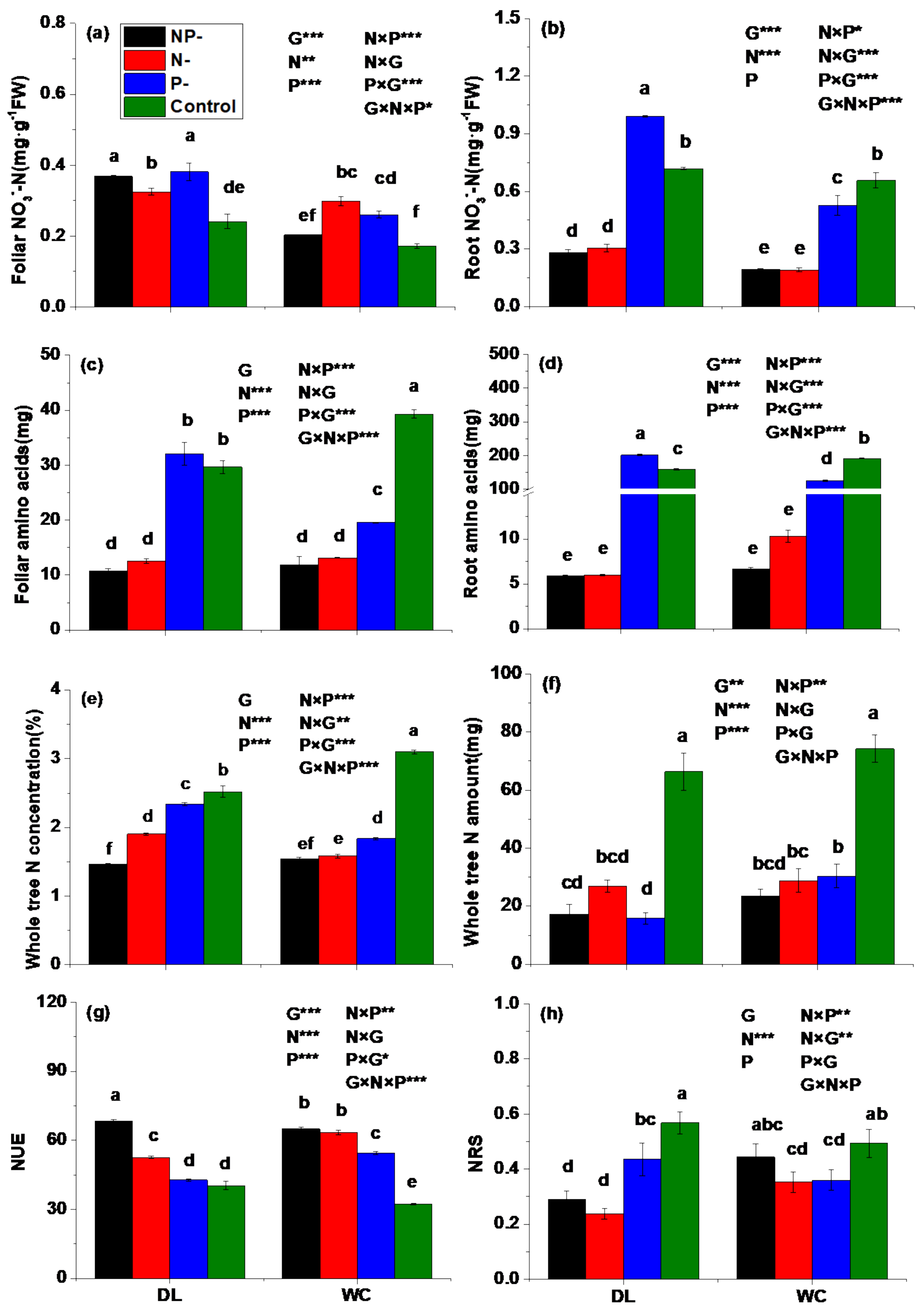
3.2. Nitrogen Status
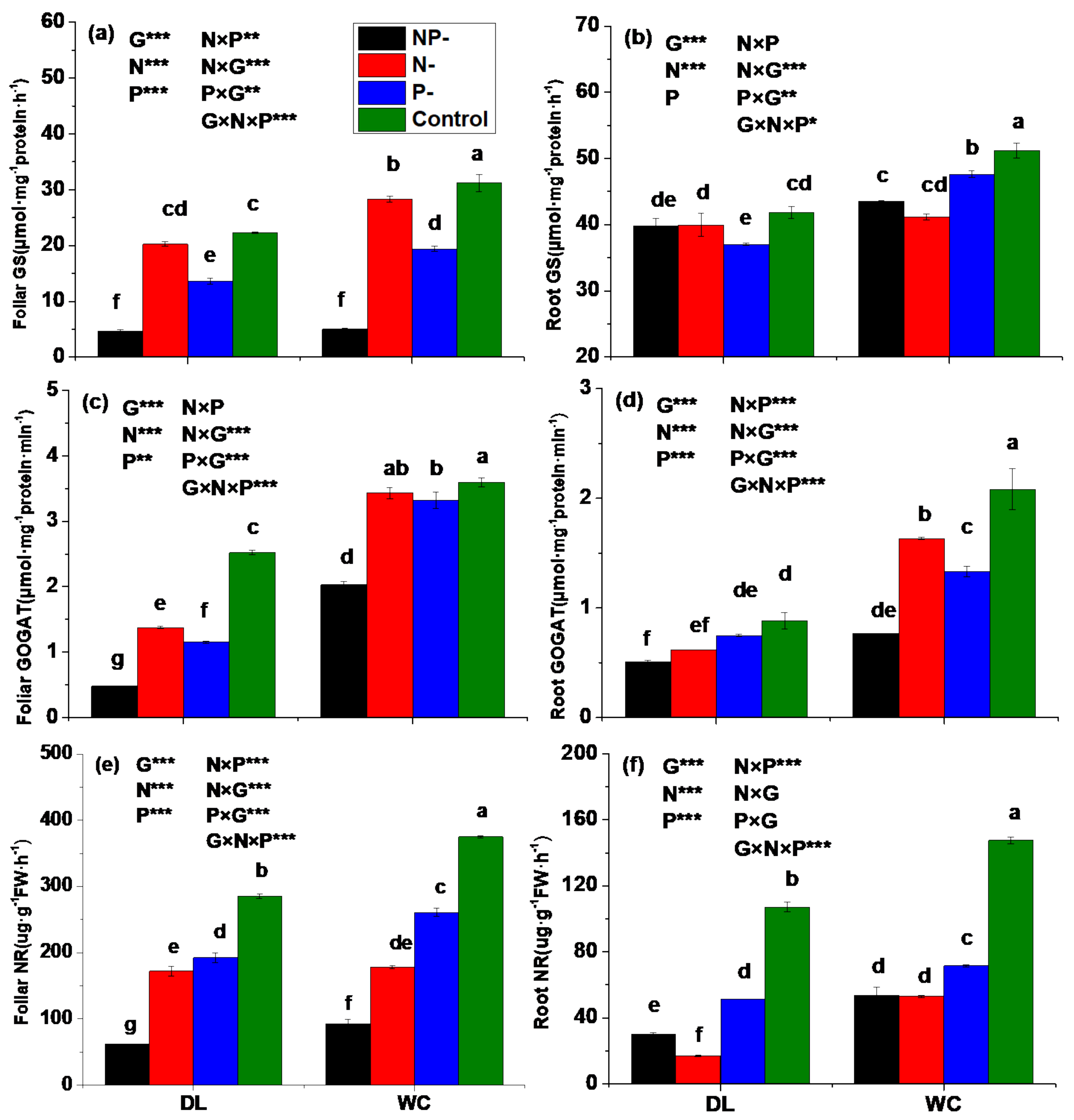
3.3. Activities of Enzymes Involved in N Assimilation
3.4. Phosphorus Status
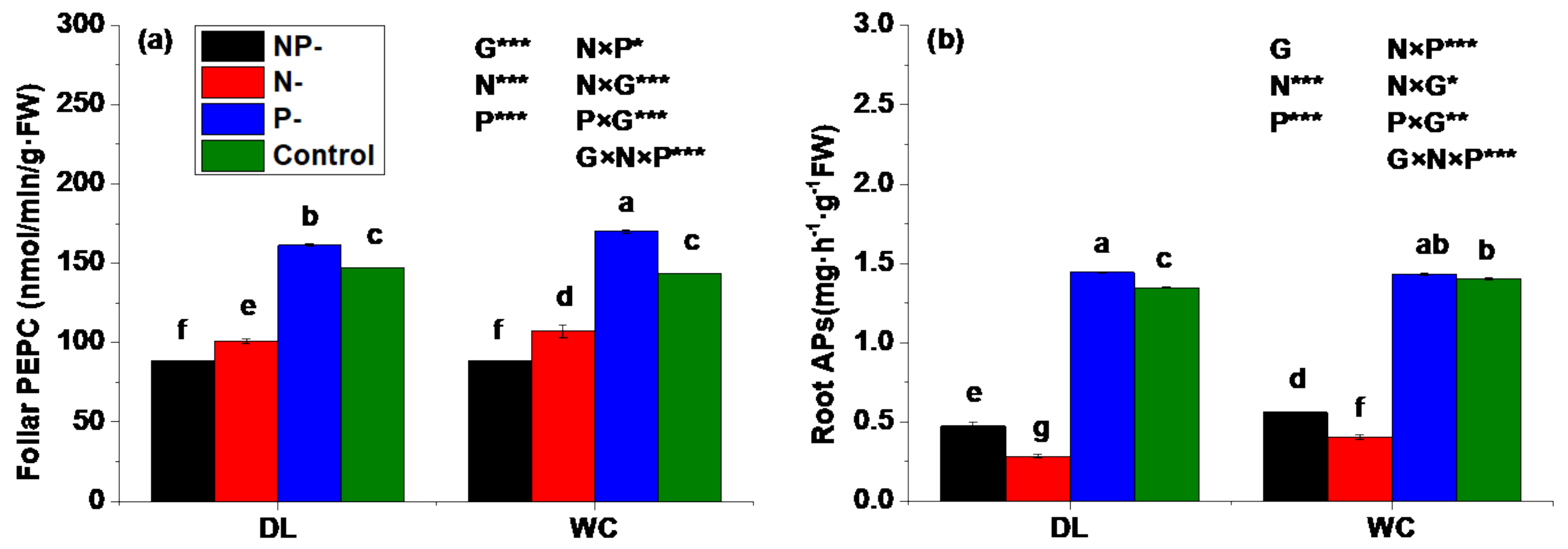
3.5. Activities of Enzymes Involved in P Assimilation
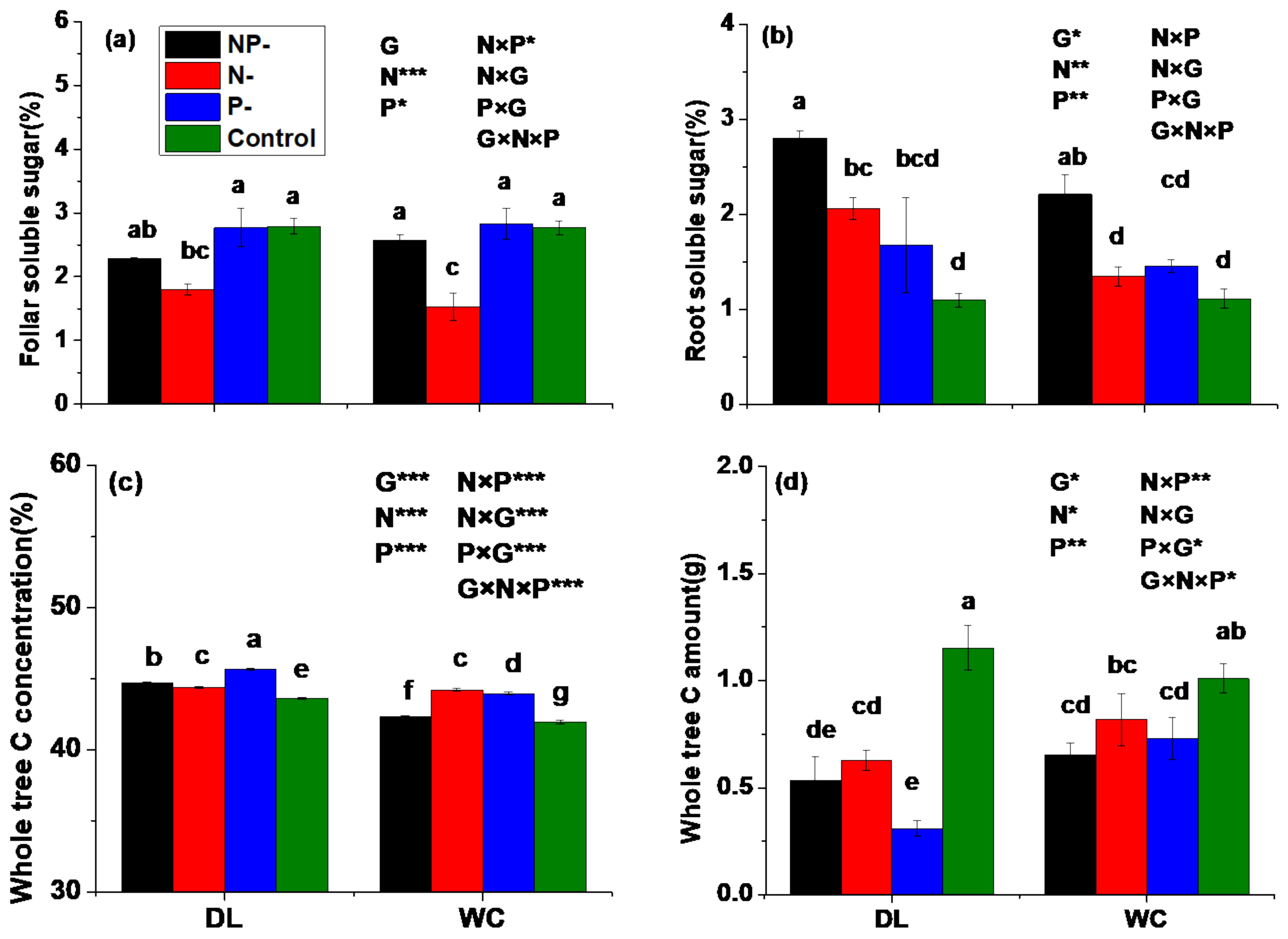
3.6. Carbon Status
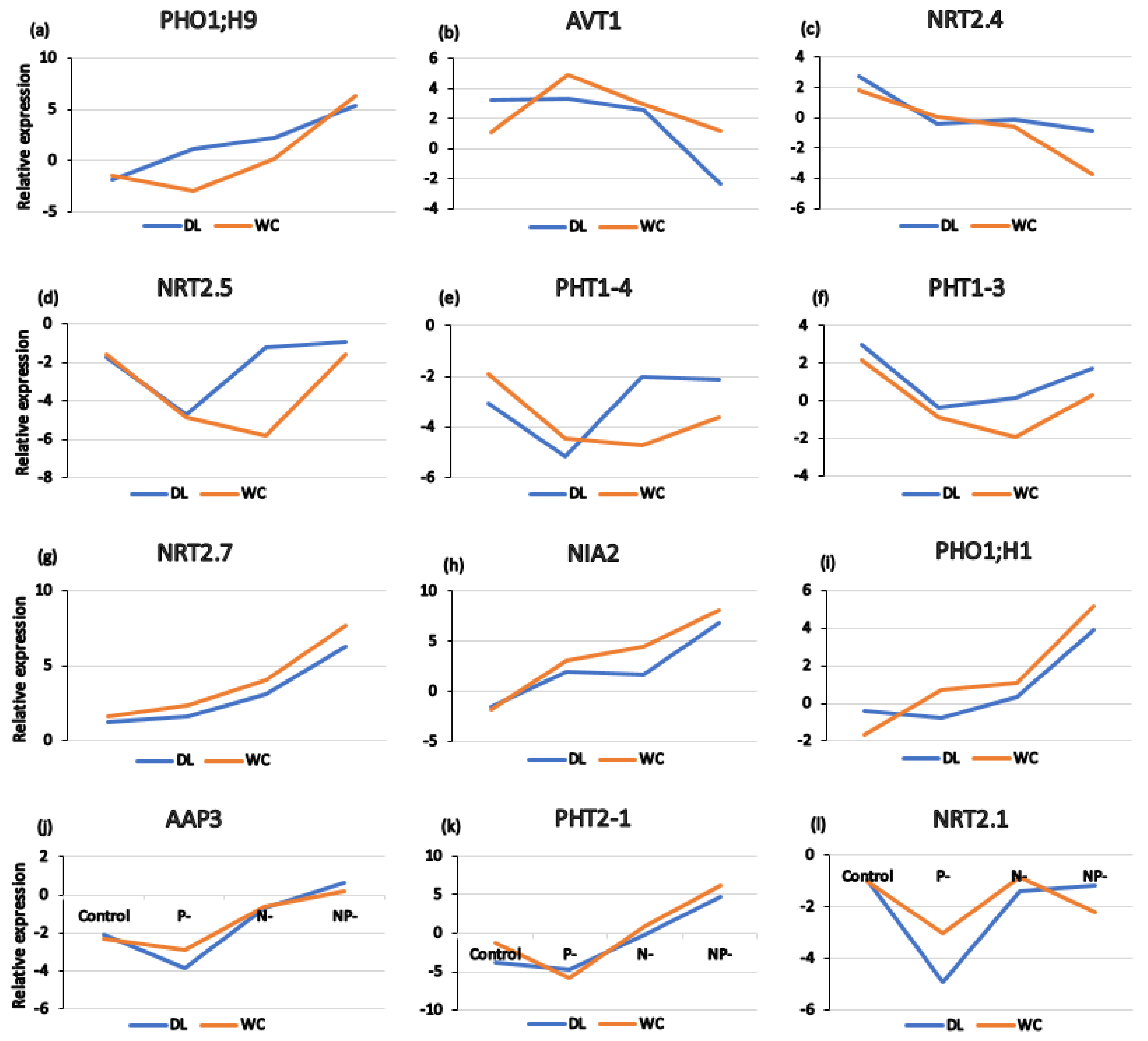
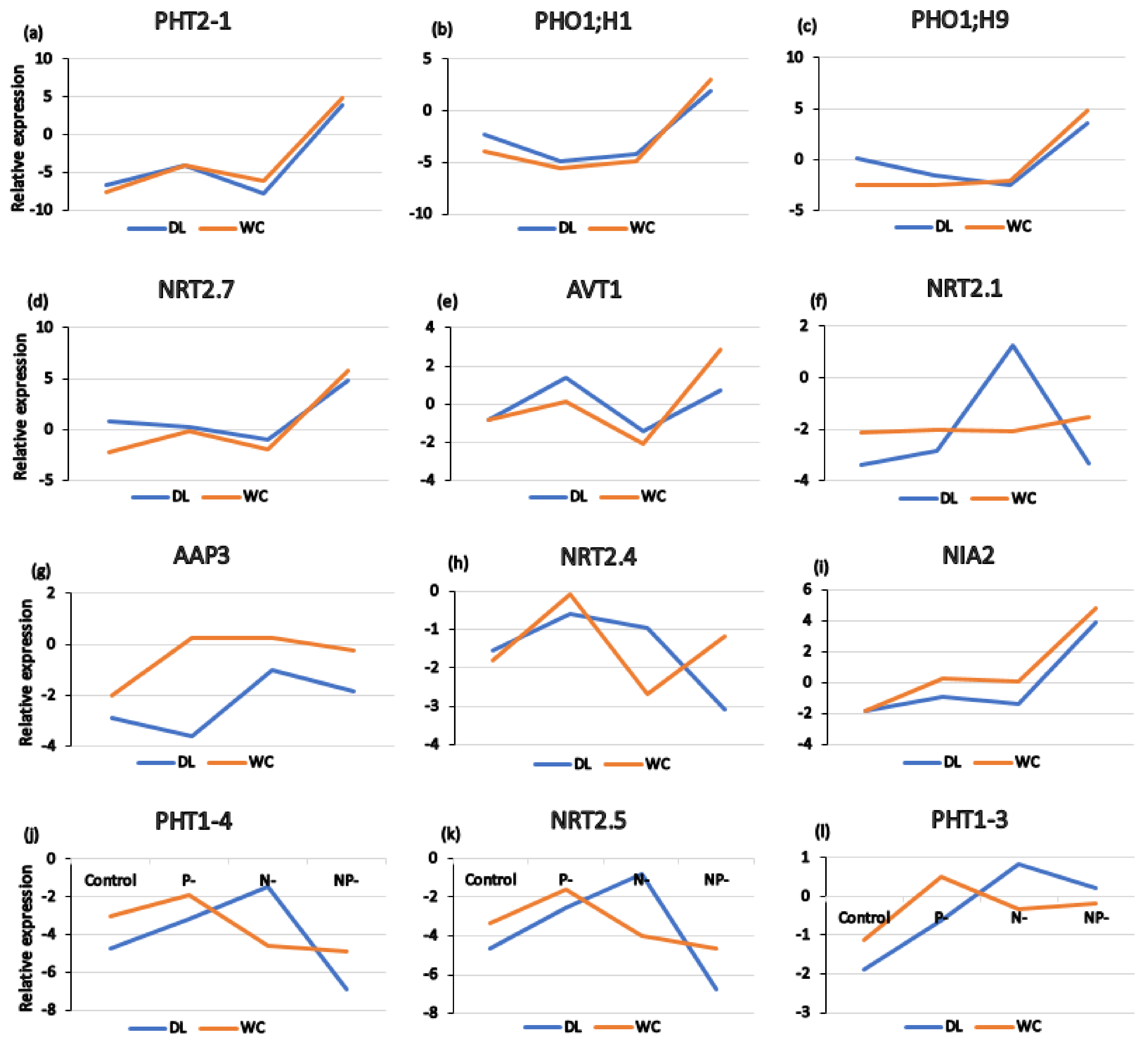
3.7. Changes in Transcript Levels of Key Genes Involved in P/N Assimilation
4. Discussion
4.1. Effects of N- and P- on the Growth, Physiology and Transcript Levels of N and P Assimilation Genes of F. mandshurica
4.2. Relationship between the Growth Rate and Tolerance to N- and P- for F. mandshurica
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fleischer, K.; Rammig, A.; De Kauwe, M.G.; Walker, A.P.; Domingues, T.F.; Fuchslueger, L.; Garcia, S.; Goll, D.S.; Grandis, A.; Jiang, M.; et al. Amazon forest response to CO2 fertilization dependent on plant phosphorus acquisition. Nat. Geosci. 2019, 12, 736–741. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Phosphorus addition enhances loss of nitrogen in a phosphorus-poor soil. Soil Biol. Biochem. 2015, 82, 99–106. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, M.; Luo, J.; Cao, X.; Qu, L.; Gai, Y.; Jiang, X.; Liu, T.; Bai, H.; Janz, D.; et al. N fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow- and fast-growing Populus species. J. Exp. Bot. 2012, 63, 6173–6185. [Google Scholar] [CrossRef]
- Okamoto, M.; Vidmar, J.J.; Glass, A.D.M. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana responses to nitrate provision. Plant Cell Physiol. 2003, 44, 304–317. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, A.D. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007, 143, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Brehaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H.; et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 2012, 24, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Okamoto, M.; Xing, X.; Crawford, N.M. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003, 132, 556–567. [Google Scholar] [CrossRef] [Green Version]
- David, L.C.; Dechorgnat, J.; Berquin, P.; Routaboul, J.M.; Debeaujon, I.; Daniel-Vedele, F.; Ferrario-Méry, S. Proanthocyanidin oxidation of Arabidopsis seeds is altered in mutant of the high-affinity nitrate transporter NRT2.7. J. Exp. Bot. 2014, 65, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Chopin, F.; Orsel, M.; Dorbe, M.F.; Chardon, F.; Truong, H.N.; Miller, A.J.; Krapp, A.; Daniel-Vedele, F. The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 2007, 19, 1590–1602. [Google Scholar] [CrossRef] [Green Version]
- Kotur, Z.; Glass, A.D. A 150 kDa plasma membrane complex of AtNRT2.5 and AtNAR2.1 is the major contributor to constitutive high-affinity nitrate influx in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 1490–1502. [Google Scholar] [CrossRef]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. Cell Mol. Biol. 2014, 80, 230–241. [Google Scholar] [CrossRef]
- Wei, J.; Zheng, Y.; Feng, H.; Qu, H.; Fan, X.; Yamaji, N.; Ma, J.F.; Xu, G. OsNRT2.4 encodes a dual-affinity nitrate transporter and functions in nitrate-regulated root growth and nitrate distribution in rice. J. Exp. Bot. 2018, 69, 1095–1107. [Google Scholar] [CrossRef]
- Fine, K.E.; Smith, M.W.; Cole, J.C. Partitioning of nitrogen, phosphorus, and potassium in redbud trees. Sci. Hortic. 2013, 152, 1–8. [Google Scholar] [CrossRef]
- Fam, R.R.S.; Hiong, K.C.; Choo, C.Y.L.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Molecular characterization of a novel algal glutamine synthetase (GS) and an algal glutamate synthase (GOGAT) from the colorful outer mantle of the giant clam, Tridacna squamosa, and the putative GS-GOGAT cycle in its symbiotic zooxanthellae. Gene 2018, 656, 40–52. [Google Scholar] [CrossRef]
- Gan, H.; Jiao, Y.; Jia, J.; Wang, X.; Li, H.; Shi, W.; Peng, C.; Polle, A.; Luo, Z.B. Phosphorus and nitrogen physiology of two contrasting poplar genotypes when exposed to phosphorus and/or nitrogen starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Okumoto, S.; Koch, W.; Tegeder, M.; Fischer, W.N.; Biehl, A.; Leister, D.; Stierhof, Y.D.; Frommer, W.B. Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP3. J. Exp. Bot. 2004, 55, 2155–2168. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [Green Version]
- Fäth, J.; Kohlpaintner, M.; Blum, U.; Göttlein, A.; Mellert, K.H. Assessing phosphorus nutrition of the main European tree species by simple soil extraction methods. For. Ecol. Manag. 2019, 432, 895–901. [Google Scholar] [CrossRef]
- Brown, K.R.; Courtin, P.J. Can phosphorus additions increase long-term growth and survival of red alder (Alnus rubra Bong.) on periodically dry sites? For. Ecol. Manag. 2018, 430, 545–557. [Google Scholar] [CrossRef]
- Huang, C.Y.; Shirley, N.; Genc, Y.; Shi, B.; Langridge, P. Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiol. 2011, 156, 1217–1229. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. Cell Mol. Biol. 2004, 39, 629–642. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Pierangelini, M.; Shearman, L.A.; Gleadow, R.; Beardall, J. Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J. Appl. Phycol. 2015, 28, 1509–1520. [Google Scholar] [CrossRef]
- Wang, Y.; Ribot, C.; Rezzonico, E.; Poirier, Y. Structure and expression profile of the Arabidopsis PHO1 gene family indicates a broad role in inorganic phosphate homeostasis. Plant Physiol. 2004, 135, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, A.S.; Varadarajan, D.K.; Mukatira, U.T.; D’Urzo, M.P.; Damsz, B.; Raghothama, K.G. Regulated expression of Arabidopsis phosphate transporters. Plant Physiol. 2002, 130, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Versaw, W.K.; Harrison, M.J. A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 2002, 14, 1751–1766. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Chen, X.; Gao, Y.; Hong, L.; Chen, Y. Alteration in root morphological and physiological traits of two maize cultivars in response to phosphorus deficiency. Rhizosphere 2020, 14, 100201. [Google Scholar] [CrossRef]
- Fang, X.-M.; Zhang, X.-L.; Chen, F.-S.; Zong, Y.-Y.; Bu, W.-S.; Wan, S.-Z.; Luo, Y.; Wang, H. Phosphorus addition alters the response of soil organic carbon decomposition to nitrogen deposition in a subtropical forest. Soil Biol. Biochem. 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Penaloza, E.; Munoz, G.; Salvo-Garrido, H.; Silva, H.; Corcuera, L.J. Phosphate deficiency regulates phosphoenolpyruvate carboxylase expression in proteoid root clusters of white lupin. J. Exp. Bot. 2005, 56, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Cheng, L. Foliar Urea Application in the Fall Affects Both Nitrogen and Carbon Storage in Young ‘Concord’ Grapevines Grown under a Wide Range of Nitrogen Supply. J. Am. Soc. Hortic. Sci. 2004, 129, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Fuchigami, L.H. Growth of young apple trees in relation to reserve nitrogen and carbohydrates. Tree Physiol. 2002, 22, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Wu, B.; Gao, Y.; Wu, J.; Niu, J.; Xie, Y.; Cui, Z.; Zhang, Z. Effects of nitrogen and phosphorus on the regulation of nonstructural carbohydrate accumulation, translocation and the yield formation of oilseed flax. Field Crop. Res. 2018, 219, 229–241. [Google Scholar] [CrossRef]
- Sanchez-Calderon, L.; Lopez-Bucio, J.; Chacon-Lopez, A.; Gutierrez-Ortega, A.; Hernandez-Abreu, E.; Herrera-Estrella, L. Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol. 2006, 140, 879–889. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Wang, Z.; Li, X.; Sun, Y.; Zhang, X.; Liang, A. N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 2010, 333, 325–336. [Google Scholar] [CrossRef]
- Xin, J.S.; Zheng, L.; Huang, Y.R.; Pan, Y.D.; Lu, G.J. NYT 2419-2013 Determination of Total Nitrogen in Plant, Automatic Kjeldahl Apparatus Method; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2014.
- Bao, S.D. (Ed.) Soil Agrochemical Analysis: Determination of Phosphorus in Plants; China Agriculture Press: Beijing, China, 1999; pp. 268–270. [Google Scholar]
- Yang, J.H. (Ed.) Soil Agrochemical Analysis and Environmental Monitoring; China Land Press: Beijing, China, 2008. [Google Scholar]
- Wu, Y.-W.; Li, Q.; Jin, R.; Chen, W.; Liu, X.-L.; Kong, F.-L.; Ke, Y.-P.; Shi, H.-C.; Yuan, J.-C. Effect of low-nitrogen stress on photosynthesis and chlorophyll fluorescence characteristics of maize cultivars with different low-nitrogen tolerances. J. Integr. Agric. 2019, 18, 1246–1256. [Google Scholar] [CrossRef]
- Hao, Y.-Q.; Lu, G.-Q.; Wang, L.-H.; Wang, C.-L.; Guo, H.-M.; Li, Y.-F.; Cheng, H.-M. Overexpression of AmDUF1517 enhanced tolerance to salinity, drought, and cold stress in transgenic cotton. J. Integr. Agric. 2018, 17, 2204–2214. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 2008, 6, 71–80. [Google Scholar] [CrossRef]
- Bradrord, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rosen, H. A Modified Ninhydrin Calorimetric Analysis for Amino Acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef]
- Turnao, F.J.; Muhitch, M.J. Differential accumulation of ferredoxin- and NADH-dependent glutamate synthase activities, peptides, and transcripts in developing soybean seedlings in response to light, nitrogen, and nodulation. Physiol. Plant. 1999, 107, 407–418. [Google Scholar] [CrossRef]
- Brugière, N.; Dubois, F.; Limami, A.M.; Lelandais, M.; Roux, Y.; Sangwan, R.S.; Hirel, B. Glutamine Synthetase in the Phloem Plays a Major Role in Controlling Proline Production. Plant Cell 1999, 11, 1995–2011. [Google Scholar] [CrossRef] [Green Version]
- Guiz, C.; Hirel, B.; Shedlofsky, G.; Gadal, P. Occurrence and influence of light on the relative proportions of two glutamine sythetases in rice leaves. Plant Sci. Lett. 1979, 15, 271–277. [Google Scholar] [CrossRef]
- Wang, J.; Hui, D.; Lu, H.; Wang, F.; Liu, N.; Sun, Z.; Ren, H. Main and interactive effects of increased precipitation and nitrogen addition on growth, morphology, and nutrition of Cinnamomum burmanni seedlings in a tropical forest. Glob. Ecol. Conserv. 2019, 20, e00734. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.-H. Nitrogen addition effects on tree growth and soil properties mediated by soil phosphorus availability and tree species identity. For. Ecol. Manag. 2019, 449, 117478. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 2013, 64, 4207–4224. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Lei, Y.; Zhang, S. Differences in resistance to nitrogen and phosphorus deficiencies explain male-biased populations of poplar in nutrient-deficient habitats. J. Proteom. 2018, 178, 123–127. [Google Scholar] [CrossRef]
- Netzer, F.; Pozzi, L.; Dubbert, D.; Herschbach, C. Improved photosynthesis and growth of poplar during nitrogen fertilization is accompanied by phosphorus depletion that indicates phosphorus remobilization from older stem tissues. Environ. Exp. Bot. 2019, 162, 421–432. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Zhao, Q.; Sun, Q.-Y.; Liu, L.; Zeng, D.-H. Nitrogen addition elevated autumn phosphorus retranslocation of living needles but not resorption in a nutrient-poor Pinus sylvestris var. Mongolica plantation. For. Ecol. Manag. 2020, 468, 118174. [Google Scholar] [CrossRef]
- Gu, C.; Song, A.; Zhang, X.; Wang, H.; Li, T.; Chen, Y.; Jiang, J.; Chen, F.; Chen, S. Cloning of chrysanthemum high-affinity nitrate transporter family (CmNRT2) and characterization of CmNRT2.1. Sci. Rep. 2016, 6, 23462. [Google Scholar] [CrossRef] [Green Version]
- Talkner, U.; Meiwes, K.J.; Potočić, N.; Seletković, I.; Cools, N.; De Vos, B.; Rautio, P. Phosphorus nutrition of beech (Fagus sylvatica L.) is decreasing in Europe. Ann. For. Sci. 2015, 72, 919–928. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The Mineral Nutrition of Higher Plants. Ann. Rev. Plant Physol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Chen, Y.; Nguyen, T.H.N.; Qin, J.; Jiao, Y.; Li, Z.; Ding, S.; Lu, Y.; Liu, Q.; Luo, Z.-B. Phosphorus assimilation of Chinese fir from two provenances during acclimation to changing phosphorus availability. Environ. Exp. Bot. 2018, 153, 21–34. [Google Scholar] [CrossRef]
- Stefanovic, A.; Ribot, C.; Rouached, H.; Wang, Y.; Chong, J.; Belbahri, L.; Delessert, S.; Poirier, Y. Members of the PHO1 gene family show limited functional redundancy in phosphate transfer to the shoot, and are regulated by phosphate deficiency via distinct pathways. Plant J. Cell Mol. Biol. 2007, 50, 982–994. [Google Scholar] [CrossRef]
- You, C.; Wu, F.; Yang, W.; Xu, Z.; Tan, B.; Zhang, L.; Yue, K.; Ni, X.; Li, H.; Chang, C.; et al. Does foliar nutrient resorption regulate the coupled relationship between nitrogen and phosphorus in plant leaves in response to nitrogen deposition? Sci. Total Environ. 2018, 645, 733–742. [Google Scholar] [CrossRef]
- Li, J.; Guo, Q.; Zhang, J.; Korpelainen, H.; Li, C. Effects of nitrogen and phosphorus supply on growth and physiological traits of two Larix species. Environ. Exp. Bot. 2016, 130, 206–215. [Google Scholar] [CrossRef]
- Almeida, J.P.; Rosenstock, N.P.; Forsmark, B.; Bergh, J.; Wallander, H. Ectomycorrhizal community composition and function in a spruce forest transitioning between nitrogen and phosphorus limitation. Fungal Ecol. 2019, 40, 20–31. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.; Li, D.; Mao, Q.; Zhang, W.; Mo, J. Responses of soil phosphorus availability to nitrogen addition in a legume and a non-legume plantation. Geoderma 2018, 322, 12–18. [Google Scholar] [CrossRef]
- Du, E.; de Vries, W. Nitrogen-induced new net primary production and carbon sequestration in global forests. Environ. Pollut. 2018, 242 Pt B, 1476–1487. [Google Scholar] [CrossRef]
- Linkohr, B.I.; Williamson, L.C.; Fitter, A.H.; Leyser, H.M. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. Cell Mol. Biol. 2002, 29, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, C.P.; Stone, C.U.; Allan, D.L. Phosphorus acquisition and use critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wu, G.; Guo, Q.; Korpelainen, H.; Li, C. Fast-growing Larix kaempferi suffers under nutrient imbalance caused by phosphorus fertilization in larch plantation soil. For. Ecol. Manag. 2018, 417, 49–62. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, X.; Liu, Z.; Lv, Y.; Song, T.; Cui, J.; Chen, T.; Li, J.; Zeng, F.; Zhan, Y. Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus mandshurica. Forests 2021, 12, 1760. https://doi.org/10.3390/f12121760
Zhao X, Zhang X, Liu Z, Lv Y, Song T, Cui J, Chen T, Li J, Zeng F, Zhan Y. Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus mandshurica. Forests. 2021; 12(12):1760. https://doi.org/10.3390/f12121760
Chicago/Turabian StyleZhao, Xingtang, Xu Zhang, Zhang Liu, Yipin Lv, Tingting Song, Jinghong Cui, Tianchi Chen, Jianxia Li, Fansuo Zeng, and Yaguang Zhan. 2021. "Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus mandshurica" Forests 12, no. 12: 1760. https://doi.org/10.3390/f12121760
APA StyleZhao, X., Zhang, X., Liu, Z., Lv, Y., Song, T., Cui, J., Chen, T., Li, J., Zeng, F., & Zhan, Y. (2021). Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus mandshurica. Forests, 12(12), 1760. https://doi.org/10.3390/f12121760






