Intercropping Short Rotation Timber Species with Teak: Enabling Smallholder Silviculture Practices
Abstract
:1. Introduction
2. Materials and Methods
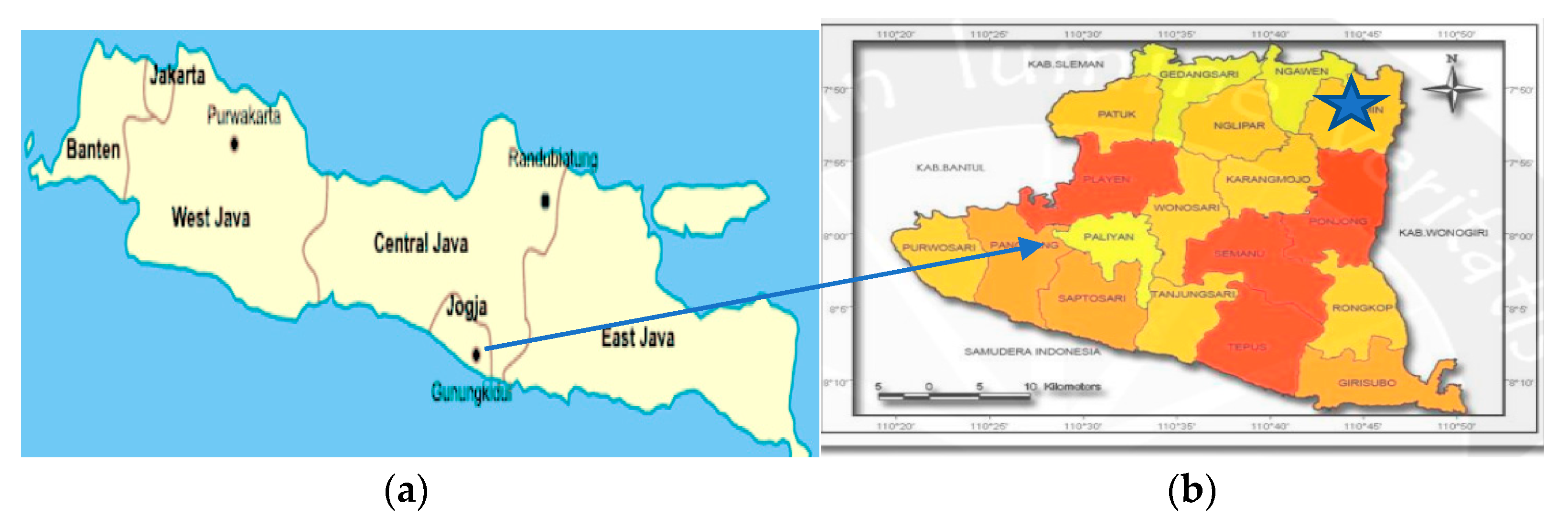
2.1. Site and Soil Characteristics
2.2. Germplasm Procurement, Trial Establishment and Trial Design
2.3. Data Collection and Analysis
3. Results
4. Discussion
4.1. Smallholder Teak System in Gunungkidul, Yogyakarta
4.2. Enabling Smallholder Silviculture Practices
4.3. Early Growth of Four Fast-Growing Species as Companion Crops for Teak
4.3.1. Adaptability and Survival Rate of Four Fast-Growing Tree Species
4.3.2. Growth Comparison of Four-Fast Growing Tree Species at Other Sites
4.4. Mixed Planting Designs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertomeu, M.; Giménez, J.C. Improving Adoptability of Farm Forestry in the Philippine Uplands: A Linear Programming Model. Agrorestry Syst. 2006, 68, 81–91. [Google Scholar] [CrossRef]
- Perdana, A.; Roshetko, J.M.; Kurniawan, I. Forces of competition: Smallholding teak producers in Indonesia. Int. For. Rev. 2012, 14, 238–248. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Dianarto, A. Tree seed procurement–diffusion pathways in Wonogiri and Ponorogo, Java. Small-Scale For. 2008, 7, 333–352. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Mulawarman, P.P. Gmelina arborea—A viable species for smallholder tree farming in Indonesia. New For. 2004, 28, 207–215. [Google Scholar] [CrossRef]
- Bartlett, A.G. Fostering a Systems-Based Agroforestry Research for Development. Agric. Sci. 2019, 30/31, 20–30. [Google Scholar]
- Garrity, D.P. Agroforestry and the Achievement of the Millennium Development Goals. Agrofor. Syst. 2004, 61, 5–17. [Google Scholar] [CrossRef]
- Snelder, D.J.; Lasco, R.D. Smallholder Tree Growing in South and Southeast Asia. In Smallholder Tree Growing for Rural Development and Environmental Services, Lessons from Asia; Advances in Agroforestry; Springer: Dordrecht, The Netherlands, 2008; Volume 5, pp. 11–12. ISBN 978-1-4020-8261-0. [Google Scholar]
- German, L.A.; Kidane, B.; Shemdoe, R. Social and Environmental Trade-Offs in Tree Species Selection: A Methodology for Identifying Niche Incompatibilities in Agroforestry. Environ. Dev. Sustain. 2006, 8, 535–552. [Google Scholar] [CrossRef]
- Idol, T.; Haggar, J.; Cox, L. Ecosystem Services from Smallholder Forestry and Agroforestry in the Tropics; Springer: Dordrecht, The Netherlands, 2011; Volume 1, ISBN 978-94-007-1308-6. [Google Scholar]
- Calvo-Alvarado, J.C.; Arias, D.; Richter, D.D. Early Growth Performance of Native and Introduced Fast Growing Tree Species in Wet to Sub-Humid Climates of the Southern Region of Costa Rica. Ecol. Manag. 2002, 242, 227–235. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of Degraded Tropical Forest Landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D.; Brown, C. Teak: A Global Overview. Unasylva-FAO 2000, 51, 3–13. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.483.8815&rep=rep1&type=pdf (accessed on 5 May 2021).
- Singh, S.; Das, R.; Krishnamurty, R. Teak in Mixed Plantations: An Appraisal of Productivity, Compatibility and Ecological Sustainability. Biremediation Biodivers. Bioavailab. 2010, 4, 8–14. [Google Scholar]
- Dendang, B.; Sudomo, A. Growth performance of Falcataria moluccana in the mixed cropping pattern and its severity from Gall-rust disease: A case study in Ciamis, West Java. In Proceedings of the IOP Conference Series: Earth and Environmental Science, IPB Convention Center Indonesia; IOP Publishing: Bristol, UK, 2020; Volume 533, p. 012044. [Google Scholar]
- Simons, A.J.; Leakey, R.R.B. Tree domestication in tropical agroforestry. Agrofor. Syst. 2004, 61–62, 167–181. [Google Scholar] [CrossRef]
- Junaedi, A.; Mindawati, N.; Rochmayanto, Y. Early Growth of Jabon (Anthocephalus Cadamba Miq) in a Drained Peatland of Pelalawan, Riau. IJFR 2021, 8, 59–72. [Google Scholar] [CrossRef]
- Hardiyanto, E.B. Beberapa Isu Silvikultur Dalam Pengembangan Hutan Tanaman. In Makalah Seminar Peningkatan Produktivitas Hutan; Fakultas Kehutanan UGM: Yogyakarta, Indonesia, 2005. [Google Scholar]
- Marquez, C.; Aguilos, R.; Bacsal, R.; Adornado, H.; Aguilos, M. Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines. Forests 2021, 12, 909. [Google Scholar] [CrossRef]
- Naiem, M. Pengembangan Spesies Non-Acacia Mangium Untuk Hutan Tanaman Buku Pembangunan Hutan Tanaman Acacia Mangium. In Hutan Tanaman; PT Musi Hutan Persada: Palembang, Indonesia, 2004. [Google Scholar]
- Kindt, R.; Lillesø, J.P.B.; Mbora, A.; Wambugu, C.; Frost, W.; Beniest, J.; Aithal, A.T.; Awimbo, J.; Rao, S.H.; Holding-Anyonge, C.; et al. Tree Seeds for Farmers: A Toolkit and Reference Source; World Agroforestry Centre: Nairoby, Kenya, 2006. [Google Scholar]
- Vigulu, V.; Blumfield, T.J.; Reverchon, F.; Bai, S.H.; Xu, Z. Nitrogen and carbon cycling associated with litterfall production in monoculture teak and mixed species teak and flueggea stands. J. Soils Sediments 2019, 19, 1672–1684. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Rohadi, D.; Perdana, A.; Sabastian, G.; Nuryartono, N.; Pramono, A.A.; Widyani, N.; Manalu, P.; Fauzi, M.A.; Sumardamto, P. Teak agroforestry systems for livelihood enhancement, industrial timber production, and environmental rehabilitation. For. Trees Livelihoods 2013, 22, 241–256. [Google Scholar] [CrossRef]
- Roshetko, J.M.; Manurung, G.E. Smallholder teak production systems in Gunungkidul, Indonesia. In Proceedings of the Agroforestry—The Future of Global Land Use; Winrock International, Nairobi, Kenya, 24–28 August 2009. [Google Scholar]
- Sudomo, A.; Maharani, D. Aplication of Plant Diversity through Coplex Agrofresty on Three Land Use System. In Advant in Environement Research; Nova Publisher: Washington, DC, USA, 2019; pp. 47–75. [Google Scholar]
- Kanninen, M.; Pérez, D.; Montero, M.; Víquez, E. Intensity and Timing of the First Thinning of Tectona Grandis Plantations in Costa Rica: Results of a Thinning Trial. For. Ecol. Manag. 2004, 203, 89–99. [Google Scholar] [CrossRef]
- Sabastian, G.E.; Yumn, A.; Roshetko, J.M.; Manalu, P.; Martini, E.; Perdana, A. Adoption of silvicultural practices in smallholder timber and NTFPs production systems in Indonesia. Agrofor. Syst. 2017, 93, 607–620. [Google Scholar] [CrossRef]
- Kallio, M.; Kanninen, M.; Rohadi, D. Farmers’ Tree Planting Activity in Indonesia—Case Studies in the Provinces of Central Java, Riau, and South Kalimantan. For. Trees Livelihoods 2011, 20, 191–209. [Google Scholar] [CrossRef]
- Riyanto, H.D.; Pamungkas, B. Model pertumbuhan tegakan hutan tanaman sengon untuk pengelolaan hutan. Tekno Hutan Tanam. 2010, 3, 113–120. [Google Scholar]
- Indrajaya, Y.; Siarudin, M. Daur Finansial Hutan Rakyat Jabon Di Kecamatan Pakenjeng, Kabupaten Garut, Jawa Barat. J. Penelit. Hutan Tanam. 2013, 10, 201–211. [Google Scholar] [CrossRef]
- Krisnawati, H.; Kallio, M.; Kanninen, M. Antohocephalus Cadamba: Ekologi, Silvikultur, Produktivitas; CIFOR: Bogor, Indonesia, 2011. [Google Scholar]
- Indrajaya, Y.; Siarudin, M. Daur Tebang Optimal Hutan Rakyat Gmelina. J. Penelit. Sos. Ekon. Kehutan. 2015, 12, 111–119. [Google Scholar] [CrossRef]
- Indrajaya, Y.; Astana, S. Daur Optimal Gmelina Pada Proyek Karbon Dan Aforestasi. J. Sosek Kehutan. 2016, 7, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Mindawati, N.; Pratiwi, P. Kajian Penetapan Daur Optimal Hutan Tanaman Acacia Mangium Ditinjau Dari Kesuburan Tanah. J. Penelit. Hutan Tanam. 2008, 5, 109–118. [Google Scholar] [CrossRef]
- Permana, D. Penentuan Daur Optimum Kelas Perusahaan Acacia Mangium Willd. di Kesatuan Pemangkuan Hutan Bogor Perum Perhutani Unit III Jawa Barat Dan Banten; IPB Bogor: Bogor, Indonesia, 2006. [Google Scholar]
- Krisnawati, H.; Varis, E.; Kallio, M.H.; Kanninen, M. Paraserianthes falcataria (L.) Nielsen: Ecology, Silviculture and Productivity; CIFOR: Bogor, Indonesia, 2011. [Google Scholar]
- Kurnia, N.; Jumadi, O.; Hiola, S.F. Atlas Tumbuhan Sulawesi Selatan; Jurusan Bioliogi FMIPA UNM: South Sulawesi Province, Indonesia, 2014. [Google Scholar]
- Krisnawati, H.; Kallio, M.; Kanninen, M. Acacia Mangium Willd. Ekologi, Silvikultur Dan Produktivitas; CIFOR: Bogor, Indonesia, 2011. [Google Scholar]
- Hadijah, M.H. Pengaruh perbedaan suhu awal air rendaman dan lama perendaman terhadap perkecambahan benih gmelina (Gmelina arborea Roxb.). J. Ilm. Agribisnis Dan Perikan. 2013, 6, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Sukajadi Sekelumit Tentang Tanaman Gmelina. In Duta Rimba Perhutani; 1992; Volume 18, pp. 45–50. Available online: http://digilib.unhas.ac.id/uploaded_files/temporary/DigitalCollection/MGUwODlkMTcyZjkzM2E2ZmM4M2YyNzMyODM1NTZkNTczNzZjZjYxYg==.pdf (accessed on 5 May 2021).
- Wormald, T.J. Mixed and Pure Forest Plantations in the Tropics and Subtropics; FAO: Rome, Italy, 1992; Available online: https://www.fao.org/3/ap421e/ap421e.pdf (accessed on 1 May 2021).
- Balooni, K. Teak Invesmenet Programs an Indian Perspective. Unasylva 2000, 51, 201. [Google Scholar]
- Anonim. Kabupaten Gunungkidul Dalam Angka 2021; Gunungkidul: Yogyakarta, Indonesia, 2021; ISBN 34030.2101. [Google Scholar]
- Anonim. Kabupaten Gunungkidul Dalam Angka 2020; BPS Gunungkidul: Yogyakarta, Indonesia, 2020; ISBN 34030.2003. [Google Scholar]
- Anonim. Profil Daerah Kabupaten Gunungkidul 2012; Bappeda Gunungkidul: Yogyakarta, Indonesia, 2012. [Google Scholar]
- Budiadi, W.; Ishii, H. Response of a Clonal Teak Plantation to Thinning and Pruning in Java, Indonesia. J. Trop. For. Sci. 2017, 29, 44–53. [Google Scholar]
- Seo, J.-W.; Kim, H.; Chun, J.-H.; Mansur, I.; Lee, C.-B. Silvicultural practice and growth of the jabon tree(Anthocephalus cadamba Miq.) in community forests of West Java, Indonesia. J. Agric. Life Sci. 2015, 49, 81–93. [Google Scholar] [CrossRef]
- Anonim Standar Operasional Prosedure Silvikultur Intensif Jati Perhutani Jati Perhutani Plus, 1st ed.; Perhutani: Cepu Jawa, Indonesia, 2019; Volume 1.
- Sudomo, A.; Yamin Mile, M. Uji Lima Sumeber Benih Sengon Dengan Pemberian Pupuk Kandang. Pemuliiaan Pohon 2007, 1, 5–24. [Google Scholar]
- Arsa, R.D. Pendugaan Volume Batang Bebas Cabang Pohon Jati Menggunakan Persamaan Taper di KPH Kendal Perum Perhutani Unit I Jawa Tengah, Fakultas Kehutanan UGM Yogyakarta; Tidak Dipublikasikan: Yogyakarta, Indonesia, 2008. [Google Scholar]
- Gasversz, V. Metode Perancangan Percobaan Untuk Ilmu-Ilmu Biologi, Pertanian. Bandung; Armico: Bandung, Indonesia, 1991. [Google Scholar]
- Sastrasupadi, A. Rancangan Percobaan Praktis Bidang Pertanian; Kanisius: Yogyakarta, Indonesia, 2000. [Google Scholar]
- Enters, T. Site, Technology and Productivity of Teak Plantation in Solutheast Asia; Unasylva: India, Indonesia, 2000; pp. 55–61. [Google Scholar]
- Wiyono, W.; Lestari, P.; Hidayat, R.; Oktalina, S.N.; Utomo, S.; Prasetyo, E.; Ngadianto, A.; Nugroho, P. Penerapan Teknik Silvikultur Intensif Pada Pengelolaan Hutan Rakyat di Kabupaten Gunungkidul. J. Pengabdi. Dan Pengemb. Masy. 2018, 1, 57–70. [Google Scholar] [CrossRef]
- Rohadi, D.; Kallio, M.; Krisnawati, H.; Manalu, P. Economic incentives and household perceptions on smallholder timber plantations: Lessons from case studies in Indonesia. In Proceedings of the Montpellier Conference, Montpellier, France, 24–26 March 2010. [Google Scholar]
- Pérez, D.; Kanninen, M. Effect of thinning on stem form and wood characteristics of teak (Tectona grandis) in a humid tropical site in Costa Rica. Silva Fenn. CIFOR Bogor Indones. 2005, 39, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Seta, G.W.; Widiyatno Hidayati, F.; Na’iem, M. Impact of Thinning and Pruning on Tree Growth, Stress Wave Velocity, and Pilodyn Penetration Response of Clonal Teak (Tectona Grandis) Plantation. For. Sci. Technol. 2021, 17, 57–66. [Google Scholar]
- Vigulu, V.; Blumfield, T.; Reverchon, F.; Hosseini Bai, S.; Xu, Z. Growth and yield of 5 years old teak and flueggea in single and mixed species forestry systems in the Solomon Islands. New For. 2019, 50, 629–642. [Google Scholar] [CrossRef]
- Pramono, A.A.; Fauzi, M.A.; Widyani, N.; Heriansyah, I.; Roshetko, J.M. Panduan Pengelolaan Hutan Jati Rakyat; 2010; ISBN 9786028693196. Available online: https://www.cifor.org/publications/pdf_files/Books/BCIFOR1001.pdf (accessed on 5 May 2021).
- Tondjo, K.; Brancheriau, L.; Sabatier, S.-A.; Kokutse, A.D.; Akossou, A.; Kokou, K.; Fourcaud, T. Is the variability of key wood properties linked with the variability of key architectural traits? Case of planted Teak in Togo regarding thinning and provenance. Ann. For. Sci. 2015, 72, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Peter, A.O.; Oluwafemi, O.A. Interim Crown Ratio Model for a MIxed Teak and Gmelina Arborea Stand in University of Ibadan Nigeria. Res. J. For. 2008, 2, 34–42. [Google Scholar]
- Víquez, E.; Pérez, D. Effect of pruning on tree growth, yield, and wood properties of Tectona grandis plantations in Costa Rica. Silva Fenn. 2005, 39, 381. [Google Scholar] [CrossRef]
- Acosta, F.C.; Silva, I.M.; Garcia, M.L.; de Melo, R.R. Productivity and costs of harvester cutting of teak trees for thinning. Floresta E Ambiente 2021, 28, e20200002. [Google Scholar] [CrossRef]
- Nirawati, N.B.; Putranto, B. Evaluasi Keberhasilan Pertumbuhan Tanaman pada Kegiatan Rehabilitasi Hutan dan Lahan (GNRHL) di Taman nasional Bantimurung Bulusaraung (Studi Kegiatan GNRHL Tahun 2003–2007). J. Sains Tekmologi 2013, 13, 175–183. [Google Scholar]
- Engelbrecht, B.M.J.; Comita, L.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef]
- Poorter, L.; Markesteijn, L. Seedling traits determine drought tolerance of tropical tree species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Dewanti, D. Ekologi Tanaman. Bandung; SInar Baru: Bandung, Indonesia, 2000. [Google Scholar]
- Martawijaya, A. Barly Sifat Dan Kegunaan Kayu Gmelina Arborea Roxb.; Puslit Sosek Kehutanan: Bogor, Indonesia, 1995. [Google Scholar]
- Alrasyid, H.; Widiarti, A. Teknik Penanaman Dan Pemungutan Hasil Gmelina Arborea; Pusat Penelitian dan Pengembangan Hutan: Bogor, Indonesia, 1992. [Google Scholar]
- Munawaroh, K. Panjang Dan Kedalaman Akar Lateral Gmelina (Gmelina Arborea Roxb.) Pada Beberapa Pola Agroforestri di Desa Sekarwangi, Kecamatan Malangbong, Kabupaten Garut; IPB University: Bogor, Indonesia, 2012; Available online: https://adoc.pub/panjang-dan-kedalaman-akar-lateral-gmelina.html (accessed on 5 May 2021).
- Sumarna, S.H. Sukses Budidaya 9 Jenis Kayu Penghasil Rupiah; Cable Book: Klaten, Indonesia, 2012. [Google Scholar]
- MacDicken, K.G. Selection and Management of Nitrogen-Fixing Trees; Winrock International Institute for Agricultural Development: Little Rock, AR, USA, 1994; ISBN 0-933595-86-7. [Google Scholar]
- Hardie, M.; Akhmad, N.; Mohammed, C.; Mendham, D.; Corkrey, R.; Gafur, A.; Siregar, S. Siregar Role of Site in the Mortality and Production of Acacia Mangium Plantations in Indonesia. South For. 2010, 80, 37–50. [Google Scholar] [CrossRef]
- Latib, A.A.; Aini, S.N.; Hazandy, A.H.; Kamis, A. 18 Month-Old Growth Performance of Four Selected Acacia Species Provenance Trial. Malays. For. 2007, 70, 1–11. [Google Scholar]
- Arisman, H. Sustainable Acacia Plantations: A Case of Short-Rotation Plantation at PT. Musi Hutan Persada. In Advances in Genetic Improvement of Tropical Tree Species; Badan Penelitian dan Pengembangan Kehutanan dan Japan International Cooperation Agency: Yogyakarta, Indonesia, 2002; pp. 9–13. [Google Scholar]
- Sudrajat, D.J.; Siregar, I.Z.; Khumaida, N.; Siregar, U.J.; Mansur, I. Adaptability of White Jabon (Anthocephalus Cadamba MIQ.) Seedling from 12 Populations to Drought and Waterlogging. Agrivita 2012, 37, 130–143. [Google Scholar] [CrossRef]
- Scale, S.; Owners, F. Domestication of Lesser Known Tropical Species Neolamarckia Cadamba among the My Presentation; 2012, pp. 24–27. Available online: https://businessdocbox.com/Forestry/99260486-Domestication-of-lesser-known-tropical-species-neolamarckia-cadamba-among-the-small-scale-forest-owners.html (accessed on 5 May 2021).
- Otsamo, A. Early Effects of Four Fast-Growing Tree Species and Their Planting Density on Ground Vegetation in Imperata Grasslands. New For. 2002, 23, 1–17. [Google Scholar] [CrossRef]
- Soerianegara, I.; Lemmens, R.H.M.J. Plant Resources of South East Asia. In Timber Trees: Major Conunercial Timbers; 1993; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1756-1051.1998.tb01861.x (accessed on 1 May 2021).
- Ishemat, S. Factors Which Determine The Succes Of Regreening In Gunungkidul, Central Java. J. Trop. For. Sci. 1994, 7, 64–75. [Google Scholar]
- van Breugel, M.; Hall, J.S.; Craven, D.J.; Gregoire, T.G.; Park, A.; Dent, D.H.; Wishnie, M.H.; Mariscal, E.; Deago, J.; Ibarra, D.; et al. Early Growth and Survival of 49 Tropical Tree Species across Sites Differing in Soil Fertility and Rainfall in Panama. Ecol. Manag. 2011, 261, 1580–1589. [Google Scholar] [CrossRef]
- Adlard, P.G. Research Strategy for Monitoring Tree Growth and Site Change. In Growth and Water Use of Forest Plantations; John Wiley and Sons, Inc.: New York, NY, USA, 1992; pp. 48–62. [Google Scholar]
- Junaedi, A. Growth Performance Of Three Native Tree Species For Pulpwood Plantation in Drained Peatland of Pelalawan District, Riau Growth Performance of Three Native Tree Species For Pulpwood. IJFR 2018, 5, 119–132. [Google Scholar]
- Knaofmone, A. Pengaruh Konsentrasi Dan Dosis Pupuk Organik Cair Terhadap Pertumbuhan Bibit Sengon Laut (Paraserianthes falcataria L.). J. Pertan. Konserv. Lahan Kering 2016, 1, 90–92. [Google Scholar] [CrossRef]
- Djogo, A.P. Use of Albizia and Paraserianthes Species in Small-Scale Farming Systems in Indonesia. In Proceedings of the International Workshop on Albizia and Paraserianthes Species, Bislig, Surigao del Sur, Philippines, 13–19 November 1994; Zabala, N., Ed.; Winrock International: Little Rock, AR, USA, 1994; pp. 27–36. [Google Scholar]
- Abdulah, L.; Nina Mindawati, A.; Kosasih, A.S.; Darwo, D. Evaluasi Pertumbuhan Awal Jabon (Neolamarckia Cadamba Roxb at Private Forest. J. Penelit. Hutan Tanam. 2013, 10, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Chaerani, N.; Sudrajat, D.J.; Siregar, I.Z.; Siregar, U.J. Growth Performance and Wood Quality of White Jabon (Neolamarckia Cadamba) Progeny Testing at Parung Panjang, Bogor, Inodonesia. Biodiversitas 2019, 20, 2295–2301. [Google Scholar] [CrossRef]
- Junaedi, A. Pertumbuhan Jabon (Anthocephalus Cadamba Miq.) Pada Lahan Marginal Berjenis Tanah Ultisol Di Riau. J. Pemuliaan Tanam. Hutan 2018, 12, 51–63. [Google Scholar] [CrossRef]
- Chen, S.X.; Wu, Z.H.; Li, Z.H.; Xie, Y.J.; Li, T.H.; Zhou, Q.Y.; Arnold, R. Selection of Species for Solid Wood Production in Southern China. J. Trop. For. Sci. 2010, 22, 308–316. [Google Scholar]
- Setiadi, D.; Susanto, M.; Baskorowati, L.; Pudjiono, S. Genetic variation of Gmelina arborea Roxb in Trenggalek, East Java. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bogor, Indonesia, 2021. [Google Scholar]
- Sandalayuk, D. Analisis Pertumbuhan Gmelina (Gmelina Alborea.Roxb) Dan Mahoni (Swietenia Magrophylla.King) di Gorontalo. J. For. Res. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Rohandi, A.; Gunawan, G. Ketahanan Sengon Provenan Papua Umur 2 Tahun Terhadap Karat Tumor Pada Uji Resistensi di Ciamis, Jawa Barat. J. Agroforestri Indones. 2019, 2, 37–50. [Google Scholar] [CrossRef]
- Kurinobu, S.; Prehatin, D.; Mohanmad, N.; Matsune, K.; Chigira, O. A Provisional Growth Model with a Size—Density Relationship for a Plantation of Paraserianthes Falcataria Derived from Measurements Taken over 2 Years in Pare, Indonesia. J. For. Res. 2007, 12, 230–236. [Google Scholar] [CrossRef]
- Saravanan, S. Constraints Faced by the Farmers in Adoption of Gmelina Arborea—A Case Study in Tamil Nadu. Indian J. Hill Farming 2012, 25, 13–16. [Google Scholar]
- Lauredsen, E.B. Gmelina Arborea Linn. Danida Forest Seed Centre; Seed Leaflet: Hunlebaek, Denmark, 1986; pp. 6–31. [Google Scholar]
- Hamilton, P.C.; Chandler, L.R.; Brodie, A.W.; Cornelius, J.P. A Financial Analysis of a Small Scale Gmelina Arborea Roxb. Improvement Program in Costa Rica. New For. 1998, 16, 89–99. [Google Scholar] [CrossRef]
- Zaremba, L.S.; Smoleński, W.H. Optimal portfolio choice under a liability constraint. Ann. Oper. Res. 2000, 97, 131–141. [Google Scholar] [CrossRef]
- Afolabi, J.; Abiodun, F.; Ojo, P.; Ogunwande, O. Influence of Watering Regimes and Bamboo Biochar on the Growth. Ethiop. J. Environ. Stud. Manag. 2021, 14, 515–529. [Google Scholar]
- Lamb, A.F. Fast Growing Timber Trees of the Low Land Tropics (Gmelina Arborea); Commonwealth Forestry Institute, University Oxford: Oxford, UK, 1968. [Google Scholar]
- Faboya, I.O.; Adebola, S.I.; Awotoye, O.O. Assessment of Decomposition Rate and Soil Nutrient Status under Different Woody Species Combination in a Tree Plantation. Agricutlture For. Fish. 2015, 4, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Wahyudi, I.; Sinaga, D.K.D.; Jasni, L.B. Pengaruh Jarak Tanam Terhadap Pertumbuhan Pohon dan Beberapa Sifat Fisis-Mekanis Kayu Jati Cepat Tumbuh. J. Ilmu Pertan. Indones. JIPI 2014, 19, 7. [Google Scholar]
- Sudomo, M.F.A.; Hardiwinoto, S.; Indrioko, S.; Prehaten, D.; Wibowo, A. Respon Pertumbuhan Tanaman Jati Plus Perhutani Umur 11 Tahun Terhadap Intensitas Penjarangan Dan Tumpang Sari (Studi Kasus Di Bkph Begal Kph Ngawi, Perhutani Jawa Timur). J. Pemuliaan Tanam. Hutan 2021, 15, 13–23. [Google Scholar] [CrossRef]
- Nichols, J.D.; Bristow, M.; Vanclay, J.K. Mixed-species plantations: Prospects and challenges. For. Ecol. Manag. 2006, 233, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Roshetko, J.M. Karakteristik Sosio Ekonomi Rumah Tangga Petani Dan Pengaruhnya Terhadap Pilihan Berusahatani Tanaman Pohon-Pohonan Oleh Petani di Kecamatan Pakuan Ratu, Kabupaten Way Kanan Dan Kecamatan Muara Sungkai, Kabupaten Lampung Utara, Propinsi Lampung; ICRAF: Bogor, Indonesia, 2002. [Google Scholar]


| Site × Species | Soil Texture | pH (1:5) | C-Organic Material | N Total | K Available | P2O5 Potencial Available | Rocky Percentage | Slope | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Dust (%) | Clay (%) | (1:5) | (%) | (%) | ppm | mg/100 g | (%) | (%) | |
| B1P1 | 22 | 37 | 41 | 6.05 | 1.00 (l) | 0.07 (vl) | 138 (vh) | 20 (l) | 20 (vl) | 0–5 |
| B1P2 | 35 | 26 | 39 | 6.34 | 1.11 (l) | 0.08 (vl) | 169 (vh) | 21 (m) | ||
| B1P3 | 43 | 25 | 32 | 6.15 | 1.48 (l) | 0.07 (vl) | 108 (vh) | 20 (l) | ||
| B1P4 | 40 | 28 | 32 | 6.15 | 0.92 (vl) | 0.06 (vl) | 69 (vh) | 20 (l) | ||
| B2P1 | 52 | 27 | 21 | 6.11 | 1.1 (l) | 0.06 (vl) | 373 (vh) | 32 (m) | 30 (l) | 5–10 |
| B2P2 | 34 | 35 | 31 | 6.20 | 1.26 (l) | 0.08 (vl) | 354 (vh) | 39 (m) | ||
| B2P3 | 41 | 35 | 24 | 6.29 | 1.13 (l) | 0.06 (vl) | 263 (vh) | 25 (m) | ||
| B2P4 | 44 | 28 | 28 | 6.54 | 0.79 (vl) | 0.07 (vl) | 211 (vh | 23 (m) | ||
| B3P1 | 33 | 17 | 50 | 6.09 | 0.9 (vl) | 0.07 (vl) | 172 (vh) | 28 (m) | 20 (l) | 0–5 |
| B3P2 | 42 | 39 | 19 | 6.15 | 2.05 (m) | 0.17 (l) | 796 (vh) | 39 (m) | ||
| B3P3 | 40 | 39 | 21 | 5.87 | 1.52 (l) | 0.1 (l) | 383 (vh) | 23 (m) | ||
| B3P4 | 22 | 46 | 32 | 6.25 | 1.41 (l) | 0.11 (l) | 480 (vh) | 30 (m) | ||
| B4P1 | 30 | 43 | 27 | 6.15 | 1.39 (l) | 0.09(vl) | 627 (vh) | 33 (m) | (90) (vh) | 30%–50% |
| B4P2 | 18 | 45 | 37 | 6.13 | 2.06 (m) | 0.09 (vl) | 538 (vh) | 26 (m) | ||
| B4P3 | 21 | 38 | 41 | 6.18 | 1.15 (l) | 0.09 (vl) | 334 (vh) | 30 (m) | ||
| B4P4 | 30 | 38 | 32 | 5.85 | 1.14 (l) | 0.08 (vl) | 474 (vh) | 29 (m) | ||
| B5P1 | 17 | 39 | 44 | 6.21 | 1.16 (l) | 0.09 (vl) | 206 (vh) | 58 (h) | (20) (vl) | 0–5 |
| B5P2 | 19 | 44 | 37 | 6.27 | 1.21 (l) | 0.1 (l) | 226 (vh) | 69 (vh) | ||
| B5P4 | 22 | 37 | 41 | 6.57 | 1.41 (l) | 0.09 (vl) | 409 (vh) | 54 (h) | ||
| Parameter | 6 MAP | 12 MAP | 18 MAP | 24 MAP | ||||
|---|---|---|---|---|---|---|---|---|
| F-Value | Sig p Value | F-Value | Sig p Value | F-Value | Sig p Value | F-Value | Sig p Value | |
| Survival | 7.200 | 0.005 * | 18.860 | 0.000 * | 18.860 | 0.000 * | 19.990 | 0.000 * |
| Height | 74.560 | 0.000 * | 33.880 | 0.000 * | 48.200 | 0.000 * | 30.990 | 0.000 * |
| Diameter | 74.040 | 0.000 * | 55.290 | 0.000 * | 166.390 | 0.000 * | 77.930 | 0.000 * |
| Crown area | 34.700 | 0.000 * | ||||||
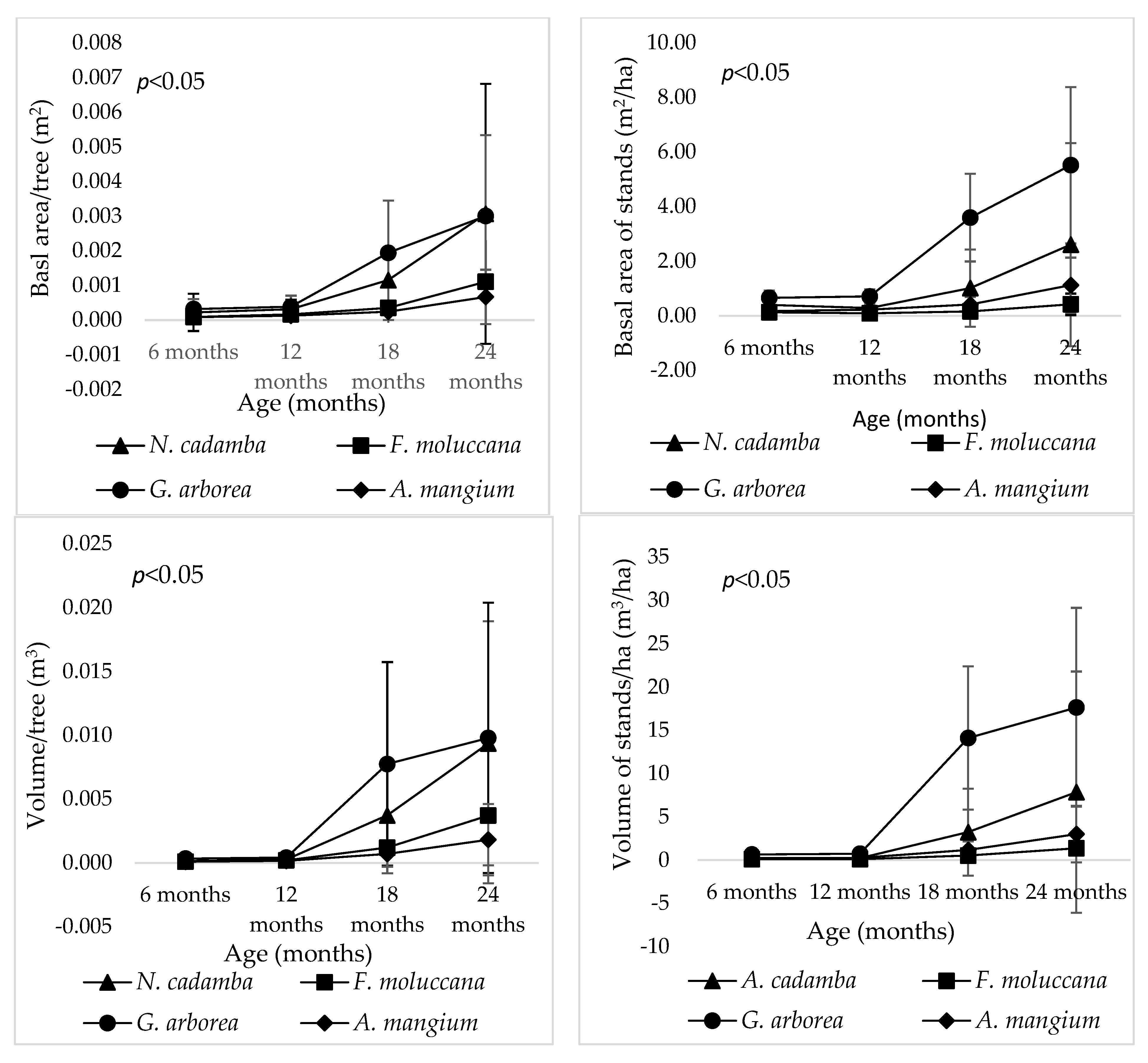
| BA/tree | 26.284 | 0.000 * | 43.064 | 0.000 * | 103.212 | 0.001 * | 47.360 | 0.000 * |
| BA/ha | 6.8820 | 0.006 * | 7.1890 | 0.005 * | 11.166 | 0.001 * | 5.459 | 0.013 * |
| Volume/tree | 32.727 | 0.000 * | 21.859 | 0.000 * | 65.305 | 0.000 * | 41.762 | 0.000 * |
| Volume/ha | 7.138 | 0.005 * | 7.159 | 0.005 * | 8.823 | 0.002 * | 3.838 | 0.039 * |
| Characteristics of Site | Type of Soil | Elevation (masl) | Slope (%) | Soil pH | Rainfall (mm/year) | Dry Months | Temperature |
|---|---|---|---|---|---|---|---|
| Site trial | Litosol [43] | 210 masl | 0–50 | 5–6.5 | 1.837 [63] | 6 [63] | 17.3–35.5 °C [63] |
| G. arborea | clay loam soils [4] and Dust clay [68] | 0–800 masl [68] | None | 4–7 [68] | 1.778–2.286 [68] | 2–4 [68] or 6–7 [67] | Optimum 21–28 °C, Min. 18–26 °C, max. 24–35 °C [68] |
| N. cadamba | Moist alluvial soil [30] Various soil types with sufficient aeration [78] | 300–800 masl [30] | None | 4.5–8.5 [30] | 1.500–5.000 [30] | 19 °C–33 °C [30] | |
| A. mangium | Various soil types [37] laterite soil [77,80] | 480–800 masl [37] | None | None | 1.446–2.970 [37] | 4 [37] | 12–34 °C [37] |
| F. moluccana | latosols, andosols, aluvial and red-yellow podzolic soils. ref. [35] Solum 30–90 cm [78] | 0 < 2000 and optimum 1600 [78] | 8–15 [35] | 4.5–7.5 [35] | 2.000–3.500 [35] | >4 months (15 days rain/dry months) [35] | 19–28 °C [1], 20–34 [10] and optimum 22 °C and 29 °C [78] |
| No | Species | Height Growth (m/year) | Diameter Growth (cm/year) | Height (m) | Diameter (cm) | Age (Months; Year) | Location and Reference |
|---|---|---|---|---|---|---|---|
| 1 | G. arborea | 2.05. | 2.88 | 4.11 | 5.76 | 2 years | Trial site (Gunungkidul) |
| 1.25 | 1.42 | 187.33 | 3.467 | 18 months | Dry land, Ciamis, West Java [14] | ||
| 2.02–2.18 | 1.9–2.04 | 101.12–109.35 | 0.95–1.02 | 6 months | Dry land Trenggalek, East Java [89] | ||
| 1.43 | 1.21 | 11.47 | 9.7 | 8 years | Dry land Banjar dan Tasikmalaya (West Java) [31] | ||
| 1.00 | 2.7 | 10 | 24 | 10 years | Dry land, Gorontalo, North Sulawesi [90] | ||
| 2 | N. cadamba | 1.86. | 2.74 | 3.72. | 5.48 | 2 years | Trial site (Gunungkidul) |
| 1.04 | 1.29 | 4.17 m | 5.15 | 4 year | Peat Soil at Riau [82] | ||
| 2.76–4.49 | 2.61–3.40 | 9.38–10.15 | 11.73–15.30 | 54 months | Dry land, Bogor, West Java [86] | ||
| 1.62 | 2.03 | 3.24 | 4.06 | 2 years | Dry land, Cianjur West Java [85] | ||
| 4.21 | 5.25 | 16.84 | 21.0 | 4 years | Dry land, Cianjur West Java [85] | ||
| 4.25 | 5.97 | 17 | 23.9 | 4 years | Dry land, South Kalimantan [35] | ||
| 2.09 | 3.86 | 22 | 40.5 | 10.5 | Dry land, West Java [78] | ||
| 1.70 | 1.72 | 2.59 | 3.74 | 2 years | Peat soil Pelalawan District, Riau [16] | ||
| 22.1 | 35.9 | 2.76 | 4.48 | 8 years | Dry land, Pakenjeng, Garut, West Java [29] | ||
| 3 | F. moluccana | 1.7. | 1.93 | 3.49. | 3.87 | 2 years | Trial site (Gunungkidul) |
| 3.31 | 2.77 | 496.8 | 4.16 | 18 months | Dry land, Ciamis, West Java [14] | ||
| 182 | 1.85 | 364 | 3.69 | 2 years | Dry land, Panjalu West Java [91] | ||
| 2.34–3.9 | 3.74–3.76 | 11.7–20.5 | 11.3–18.7 | 3–5 years | Dry land, Kediri, East Java [92] | ||
| 3.64 | 4.74 | 7.28 | 9.48 | 2 years | Sandy Soil, Dry land, Tasikmalaya West Java [47] | ||
| 1.485 | 1.53 | 1.98 | 2.04 | 9 months | Dry Land Tasikmlaya West Java [47] | ||
| 4 | A. mangium | 1.40. | 1.26 | 2.81 | 2.52 | 2 years | Trial Site (Gunungkidul) |
| 2.67 | 2.23 | 401.3 | 3.35 | 18 months | Dry land Ciamis, West Java [14] | ||
| 2.23 | 2.17 | 134 | 13 | 6 years | Dry land, Guangdong China [88] | ||
| 1.8–5.8 | 1.4–7.3 | 10–15 | 15 | 2–3 years | Dry land at Some sites [30] | ||
| 3.71 | 4.50 | 5.57 | 6.76 | 18 months | Dry land, Malaysia [73]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudomo, A.; Maharani, D.; Swestiani, D.; Sabastian, G.E.; Roshetko, J.M.; Perdana, A.; Prameswari, D.; Fambayun, R.A. Intercropping Short Rotation Timber Species with Teak: Enabling Smallholder Silviculture Practices. Forests 2021, 12, 1761. https://doi.org/10.3390/f12121761
Sudomo A, Maharani D, Swestiani D, Sabastian GE, Roshetko JM, Perdana A, Prameswari D, Fambayun RA. Intercropping Short Rotation Timber Species with Teak: Enabling Smallholder Silviculture Practices. Forests. 2021; 12(12):1761. https://doi.org/10.3390/f12121761
Chicago/Turabian StyleSudomo, Aris, Dewi Maharani, Dila Swestiani, Gerhard E. Sabastian, James M. Roshetko, Aulia Perdana, Diana Prameswari, and Rizki A. Fambayun. 2021. "Intercropping Short Rotation Timber Species with Teak: Enabling Smallholder Silviculture Practices" Forests 12, no. 12: 1761. https://doi.org/10.3390/f12121761
APA StyleSudomo, A., Maharani, D., Swestiani, D., Sabastian, G. E., Roshetko, J. M., Perdana, A., Prameswari, D., & Fambayun, R. A. (2021). Intercropping Short Rotation Timber Species with Teak: Enabling Smallholder Silviculture Practices. Forests, 12(12), 1761. https://doi.org/10.3390/f12121761








