Method Categorization of Stem Cell Therapy for Degenerative Osteoarthritis of the Knee: A Review
Abstract
:1. Introduction
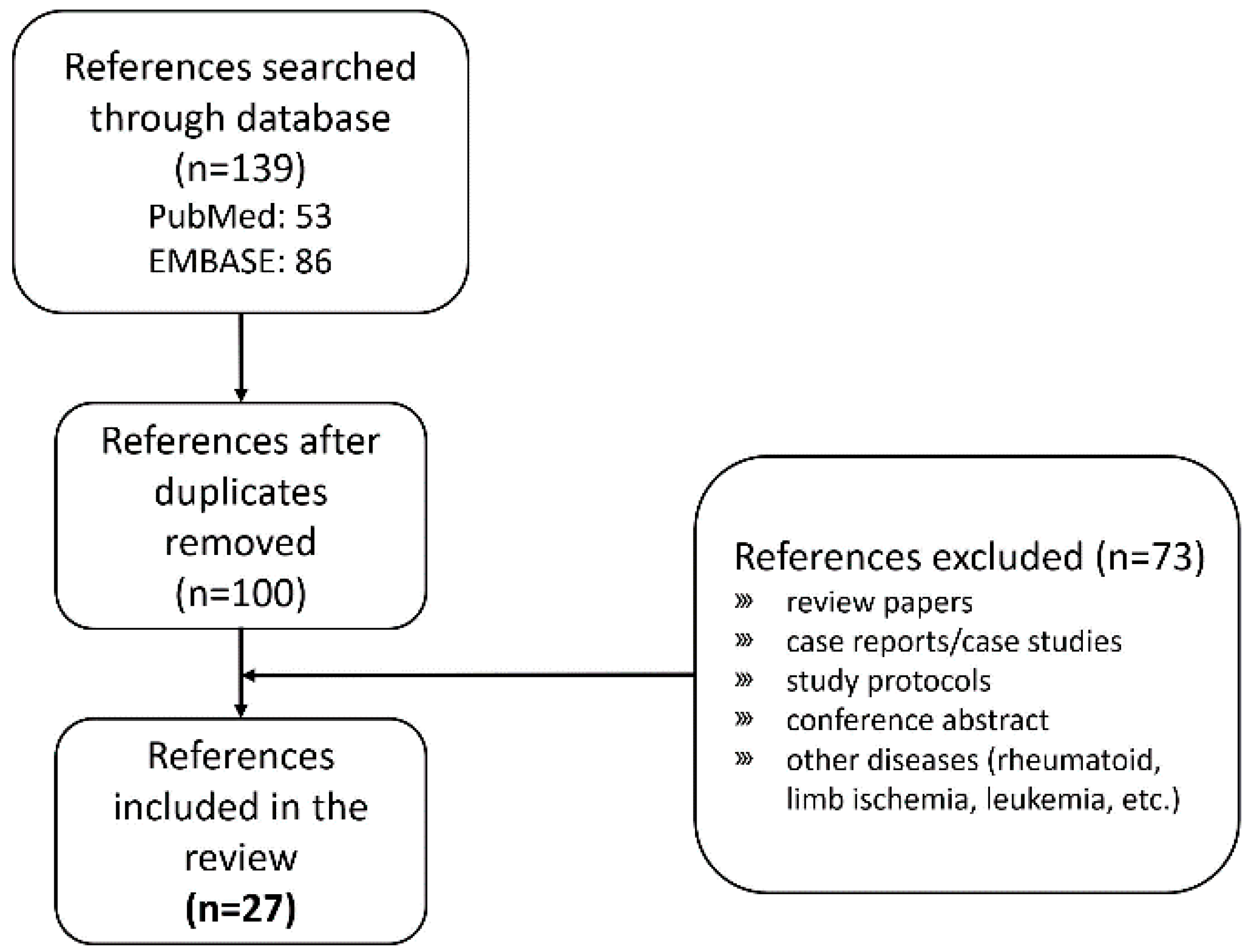
2. Search and Selection of Clinical Trials Applying Stem Cell Treatment
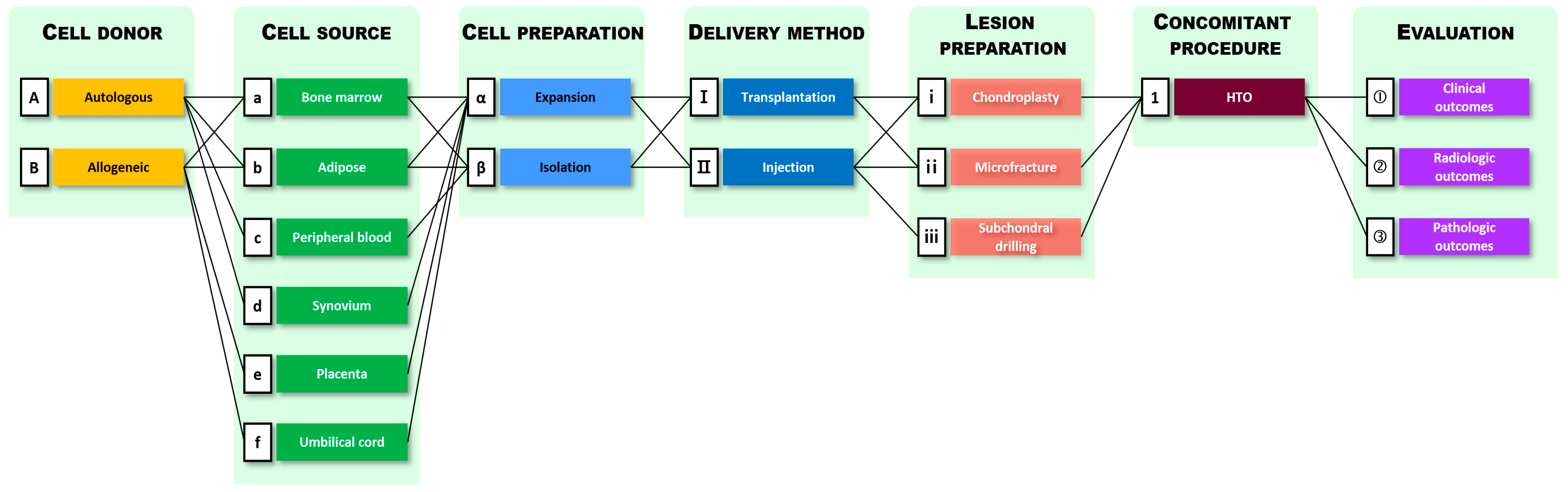
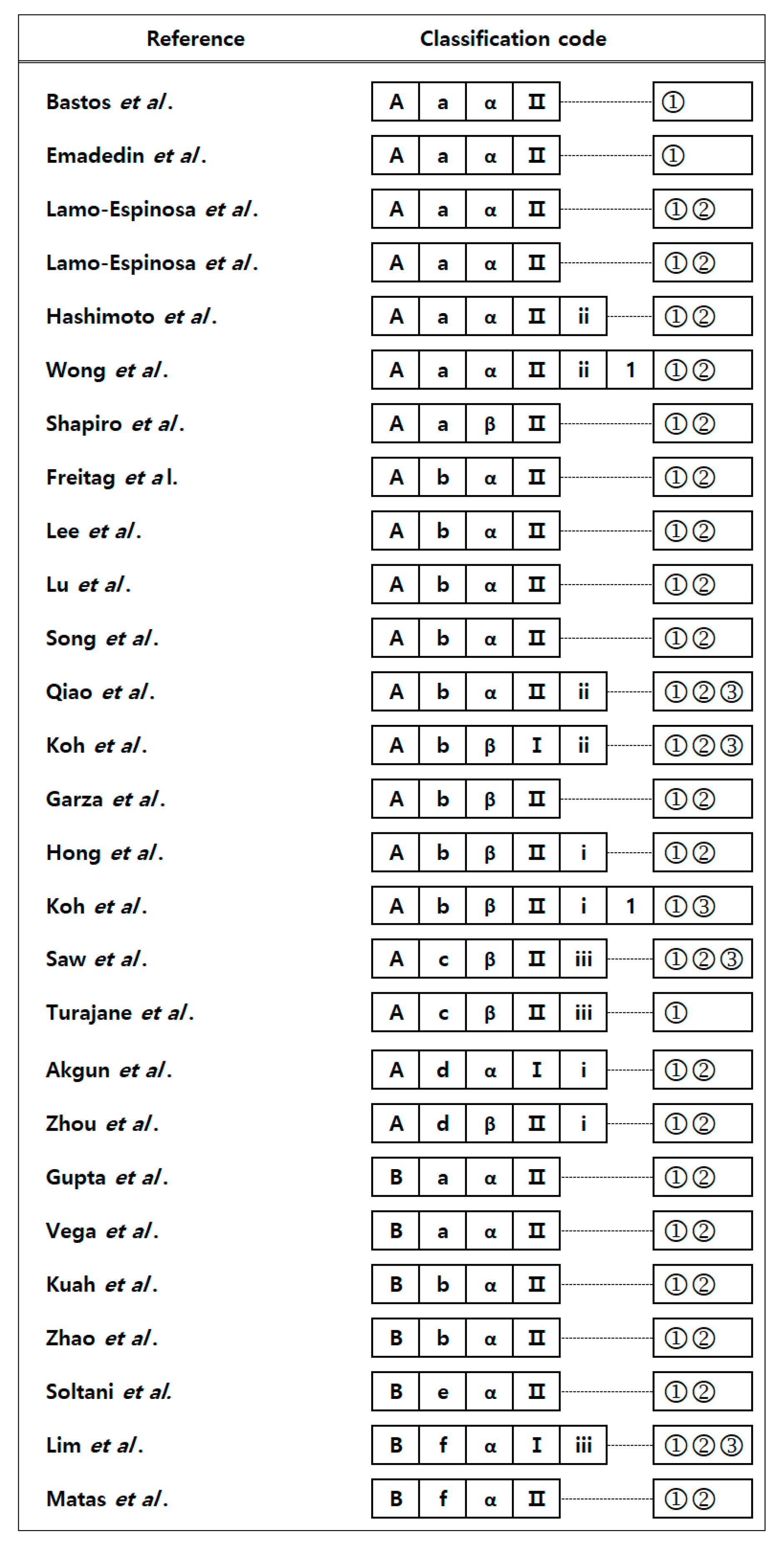
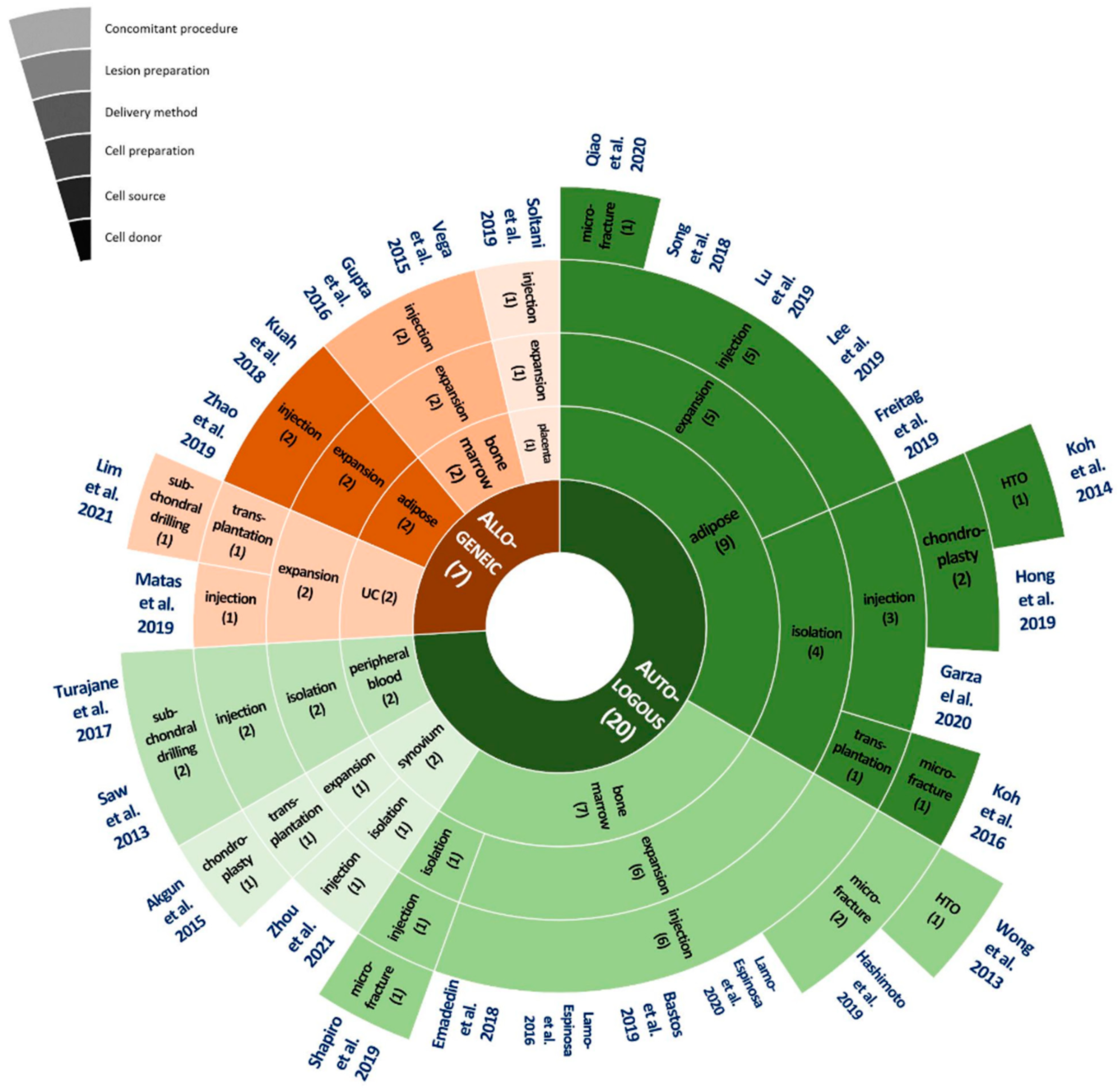
3. Method Categorization of Current Stem Cell Treatment Methods for Degenerative OA
4. Treatment Methods by Process
4.1. Patient Selection and Randomization
4.2. Cell Donor
4.2.1. Autologous MSCs
4.2.2. Allogeneic MSCs
4.3. MSC Sources and Preparation
4.3.1. Bone Marrow-Derived MSCs
4.3.2. Adipose Tissue Derived MSCs
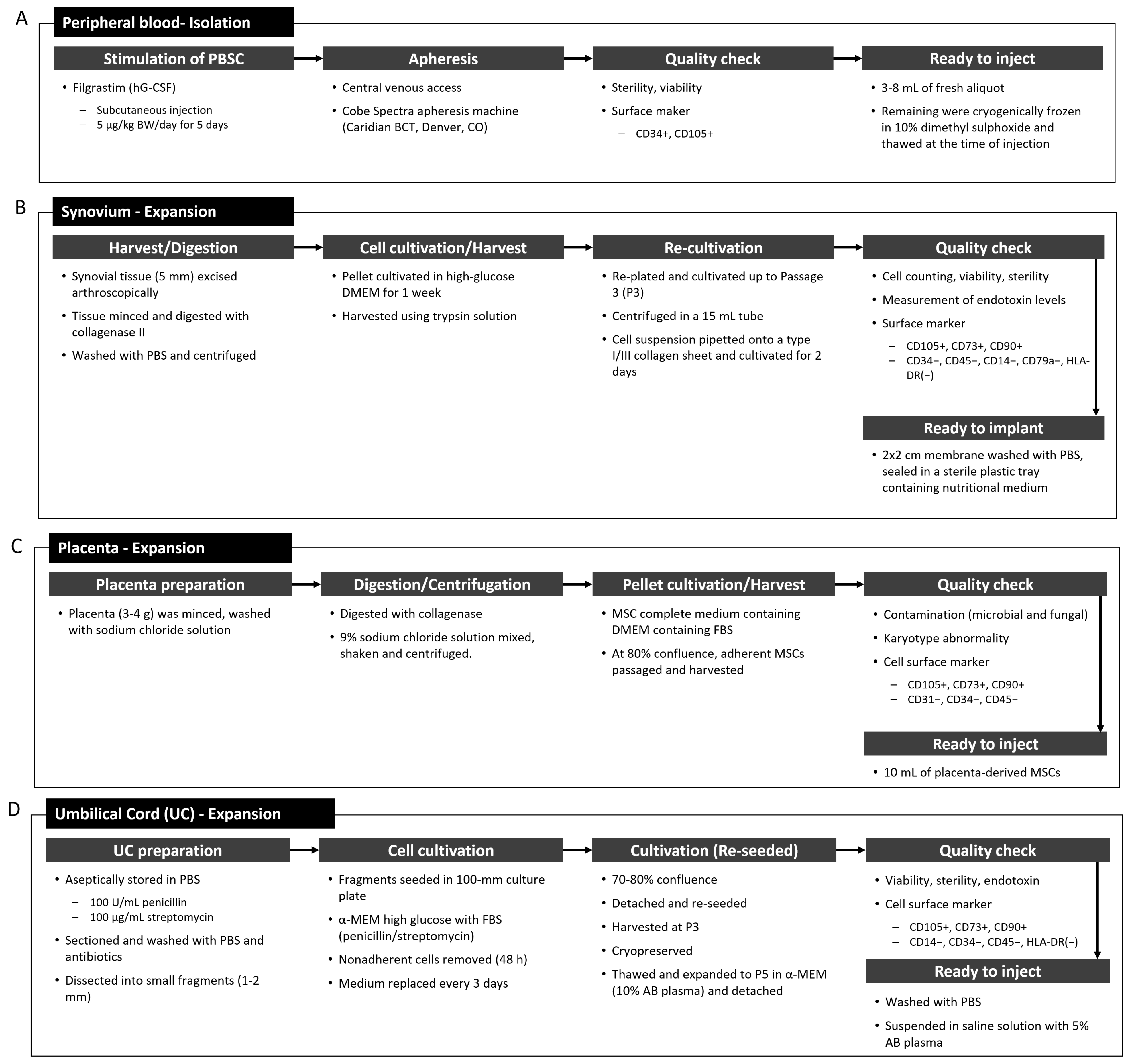
4.3.3. Peripheral Blood Stem Cells and Synovium-Derived MSCs
4.3.4. Placenta- and Umbilical Cord-Derived MSCs
4.4. Delivery Methods for MSCs
4.4.1. Transplantation
4.4.2. Injection
4.5. Lesion Preparation
4.6. Concomitant Procedure
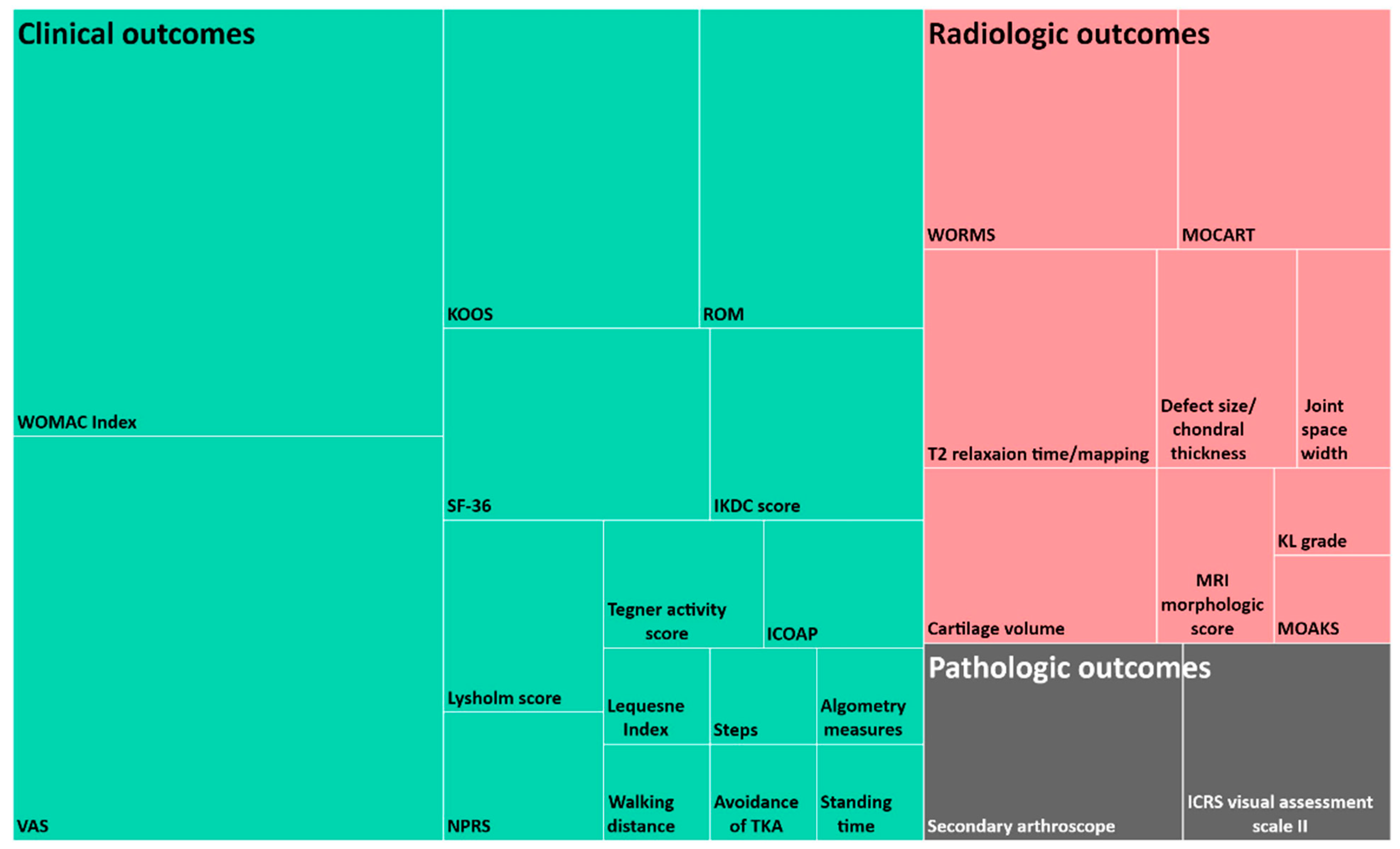
4.7. Evaluation Process
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Jung, H.H. Estimation of years lived with disability due to noncommunicable diseases and injuries using a population-representative survey. PLoS ONE 2017, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and Curr.ent treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, N.S.; Cruess, R.L. Classification of degenerative arthritis. Can. Med. Assoc. J. 1977, 117, 763–765. [Google Scholar] [PubMed]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeppel, J.P.; Crist, J.D.; Anderson, H.C.; Wang, J. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol. Histopathol. 2011, 26, 377–394. [Google Scholar] [CrossRef]
- Van der Kraan, P.M.; Van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Front. Bioeng. Biotechnol. 2019, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; van Dijk, N.; Della Villa, D. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. [Google Scholar] [CrossRef]
- Chappell, A.S.; Ossanna, M.J.; Liu-Seifert, H.; Iyengar, S.; Skljarevski, V.; Li, L.C.; Bennett, R.M.; Collins, H. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: A 13-week, randomized, placebo-controlled trial. Pain 2009, 146, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Lo, G.H. The Management of osteoarthritis: An overview and call to appropriate conservative treatment. Med. Clin. North Am. 2009, 93, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, W.; Lavelle, E.D.; Lavelle, L. Intra-Articular Injections. Anesthesiol. Clin. 2007, 25, 853–862. [Google Scholar] [CrossRef]
- Yen, Y.M.; Cascio, B.; O’Brien, L.; Stalzer, S.; Millett, P.J.; Steadman, J.R. Treatment of osteoarthritis of the knee with microfracture and rehabilitation. Med. Sci. Sports Exerc 2008, 40, 200–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lützner, J.; Kasten, P.; Günther, K.P.; Kirschner, S. Surgical options for patients with osteoarthritis of the knee. Nat. Rev. Rheumatol. 2009, 5, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Total joint replacement in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2006, 20, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Bertolusso, R.; Karastinos, A.; Conditt, M.; Noble, P.C.; Parsley, B.S. Implant durability and knee function after total knee arthroplasty in the morbidly obese patient. J. Arthroplast. 2009, 24, 89–94.e3. [Google Scholar] [CrossRef] [PubMed]
- Cherian, J.J.; Jauregui, J.J.; Banerjee, S.; Pierce, T.; Mont, M.A. What host factors affect aseptic loosening after THA and TKA? Clin. Orthop. Relat. Res. 2015, 473, 2700–2709. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-based therapy approaches: The hope for incurable diseases. Regen Med. 2014, 9, 649–672. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Rocke, J.P.J.; De Bari, C. Cell-based approaches to joint surface repair: A research perspective. Osteoarthr. Cartil. 2013, 21, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J.; Tak, D.H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.T.; Feng, Y.; Jia, H.H.; Zhao, M.; Yu, H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J. Stem Cells 2019, 11, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Ikpeama, U.; Rothenberg, J.B.; Malanga, G.A. Bone marrow–derived and adipose-derived mesenchymal stem cell therapy in primary knee osteoarthritis: A narrative review. PM R 2019, 11, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.S.; Lee, C.Y.; Lee, J.; Seo, H.H.; Choi, C.H.; Lee, S.; Hwang, K.C. Priming stem cells with protein kinase C activator enhances early stem cell-chondrocyte interaction by increasing adhesion molecules. Biol. Res. 2018, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Le, N.X.T.; Nguyen, T.P.T.; Chae, D.S.; Park, S.J.; Lee, N.Y. Nanohybrid biodegradable scaffolds for TGF-β3 release for the chondrogenic differentiation of human mesenchymal stem cells. Int. J. Pharm. 2020, 581, 1–10. [Google Scholar] [CrossRef]
- Yu, D.A.; Han, J.; Kim, B.S. Stimulation of chondrogenic differentiation of mesenchymal stem cells. Int. J. Stem Cells 2012, 5, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Wang, X.; Lin, Q.; Li, X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: A systematic review and meta-analysis. Int. Orthop. 2015, 39, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Yubo, M.; Yanyan, L.; Li, L.; Tao, S.; Bo, L.; Lin, C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Wang, Y.Y.; Li, C.J.; Shi, C.H.; Wang, W.S. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp. Ther. Med. 2016, 12, 3390–3400. [Google Scholar] [CrossRef] [Green Version]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emadedin, M.; Labibzadeh, N.; Liastani, M.G.; Karimi, A.; Jaroughi, N.; Bolurieh, T.; Hosseini, S.E.; Baharvand, H.; Aghdami, N. Intra-articular implantation of autologous bone marrow–derived mesenchymal stromal cells to treat knee osteoarthritis: A randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018, 20, 1238–1246. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondia, J.M.; Aquereta, J.D.; Andreu, J.A.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Z.; Tang, J.; Yue, B.; Wang, J.; Zhang, J.; Xuan, L.; Dai, C.; Li, S.; Li, M.; Xu, C.; et al. Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: A Phase IIa trial. Regen Med. 2020, 15, 1193–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy 2014, 30, 1453–1460. [Google Scholar] [CrossRef]
- March, L.; Cross, M.; Lo, C.; Arden, N.K.; Gates, L.; Leyland, K.M.; Hawker, G.; King, L.; Leyland, K. Osteoarthritis: A Serious Disease, Submitted to the U. S. Food and Drug Administration. Available online: https://www.oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf (accessed on 1 May 2021).
- Allen, K.; Golightlya, Y.M. Epidemiology of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 276–283. [Google Scholar] [CrossRef]
- Allen, K.D. Racial and ethnic disparities in osteoarthritis phenotypes. Curr. Opin. Rheumatol. 2010, 22, 528–532. [Google Scholar] [CrossRef]
- Cruz-Almeida, Y.; Sibille, K.T.; Goodin, B.R.; Petrov, M.E.; Bartley, E.J.; Riley, J.L., III; King, C.D.; Glover, T.L.; Sotolongo, A.; Herbert, M.S.; et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 2014, 66, 1800–1810. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: A phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [Green Version]
- FDA Consumer Updates Webpage. Available online: https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies (accessed on 1 August 2021).
- Coppens, D.G.; Hoekman, J.; De Bruin, M.L.; Slaper-Cortenbach, I.C.; Leufkens, H.G.; Meij, P.; Gardarsdottir, H. Advanced therapy medicinal product manufacturing under the hospital exemption and other exemption pathways in seven European Union countries. Cytotherapy 2020, 22, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kuah, D.; Sivell, S.; Longworth, T.; James, K.; Guermazi, A.; Cicuttini, F.; Wang, Y.; Craig, S.; Comin, G.; Robinson, D.; et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 2018, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Visawanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.A.; Park, M.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H. RNA sequencing reveals a transcriptomic portrait of human mesenchymal stem cells from bone marrow, adipose tissue, and palatine tonsils. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bastos, R.R.; Mathias, M.; Andrade, R.; Amaral, R.J.F.C.; Schott, V.; Balduino, A.; Bastos, R.; Oliveira, J.M.; Reis, R.L.; Rodeo, S.; et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2019, 28, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.L.; Lee, K.B.L.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H.P. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Nishida, Y.; Takahashi, S.; Nakamura, H.; Mera, H.; Kashiwa, K.; Yosiya, S.; Inagaki, Y.; Uematsu, K.; Tanaka, Y.; et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: A multicenter prospective randomized control clinical trial. Regen Ther. 2019, 11, 106–113. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Arthurs, J.R.; Heckman, M.G.; Bestic, J.M.; Kazmerchak, S.E.; Diehl, N.N.; Zubair, A.C.; O’Connor, M.I. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage 2019, 10, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Blanco, J.B.; Sanchez, M.; Moreno, V.; Granero-Molto, F.; Sanchez-Guijo, F.; Crespo-Cullel, I.; Mora, G.; Vicente, D.D.S.; Pompei-Fernández, O.; et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J. Transl. Med. 2020, 18, 356. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. A 2017, 105, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded mesenchymal stem cells for regenerative medicine: Yield in stromal vascular fraction from adipose tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef]
- Malanga, G.A.; Dona, S.; Borg-Stein, J.; Auriemma, M.; Singh, J.R. Refractory knee osteoarthritis: Adipose-derived stromal cells versus bone marrow aspiration concentrate. PM R 2018, 10, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Qing, B. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Liangjing, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Maria, D.S.; Tucker, B.S. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Zhao, X.; Ruan, J.; Tang, H.; Li, J.; Shi, Y.; Li, M.; Li, S.; Xu, C.; Lu, Q.; Dai, C. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Turajane, T.; Chaveewanakorn, U.; Fongsarun, W.; Aojanepong, J.; Papadopoulos, K.I. Avoidance of total knee arthroplasty in early osteoarthritis of the knee with intra-articular implantation of autologous activated peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial with differential effects of growth factor. Stem Cells Int. 2017, 2017, 8925132. [Google Scholar] [CrossRef] [Green Version]
- Saw, K.Y.; Anz, A.; Siew-Yoke, J.C.; Merican, S.; Ching-Soong, N.G.R.; Roohi, S.A.; Ragavanaidu, K. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial. Arthroscopy 2013, 29, 684–694. [Google Scholar] [CrossRef]
- Fan, J.; Varshney, R.R.; Ren, L.; Cai, D.; Wang, D.A. Synovium-derived mesenchymal stem cells: Anew cell source for musculoskeletal regeneration. Tissue Eng. Part B Rev. 2009, 15, 75–86. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheumatol. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xiang, D.; Shao, J.; Fu, Q.; Han, Y.; Zhu, J.; Chen, Y.; Qirong, Q. The clinical efficacy of arthroscopic therapy with knee infrapatellar fat pad cell concentrates in treating knee cartilage lesion: A prospective, randomized, and controlled study. J. Orthop. Surg. Res. 2021, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.K.; Forogh, B.; Ahmadbeigi, N.; Hadizadeh, K.H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-miranda, F.; Gonzalez, P.L.; Muse, E.; Khoury, M.; et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: Repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.C.; Park, Y.B.; Ha, C.W.; Cole, B.J.; Lee, B.L.; Jeong, H.J.; Kim, M.K.; Bin, S.I.; Choi, C.H.; Choi, C.H.; et al. Allogeneic umbilical cord blood–derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: A multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop. J. Sports Med. 2021, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Malinin, T.; Ouellette, E.A. Articular cartilage nutrition is mediated by subchondral bone: A long-term autograft study in baboons. Osteoarthr. Cartil. 2000, 8, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Goebel, L.K.H.; Orth, P.; Cucchiarini, M.; Madry, H. Subchondral drilling for articular cartilage repair: A systematic review of translational research. Dis. Model. Mech. 2018, 11, dmm034280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.; Zhao, Z.D.; Wang, Q.; Li, Z.L.; Huang, Y.; Zhao, S.; Hu, W.; Liang, J.W.; Li, P.L.; Wang, H.; et al. Biological potential alterations of migratory chondrogenic progenitor cells during knee osteoarthritic progression. Arthritis Res. Ther. 2020, 22, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Inclusion Criteria | |
| General | Males and females aged 18–75 years |
| Patients in stable health | |
| OA diagnosis | Symptomatic and radiographic OA |
| Kellgren–Lawrence grade 2 to 4 | |
| ICRS articular injury classification ≥ 3 | |
| Exclusion criteria | |
| General | BMI ≥ 40 kg/m2 |
| Pregnancy or lactation | |
| Mental disorder | |
| Those participating in another clinical trial | |
| Chronic treatment with immunosuppressive or anticoagulant drugs | |
| Alcoholism, drug abuse | |
| Unable to answer subjective questionnaires and inability to provide informed consent | |
| OA diagnosis | Secondary arthritis (related to rheumatoid arthritis, spondyloarthritis, or previous major knee traumas) |
| Mechanical pain caused by meniscal tears (including flap tears, bucket-handle tears, and complex tears) | |
| Bi-compartmental and tri-compartmental OA | |
| Malalignment/ deformities | Malalignment of the knee from femoral causes |
| Fixed flexion deformity of the knee | |
| Collateral ligament instability | |
| Joint line congruity angle of more than 2° | |
| Severe mechanistic extra-articular deformation (varus/valgus, >15°) | |
| Treatment related | Allergic reaction to components of study treatment and/or study implantation procedure |
| Unable to tolerate magnetic resonance imaging scans | |
| Previous treatment | Previous meniscectomy/significant partial meniscectomy |
| Prior stem cell treatment | |
| Intra-articular injection of hyaluronic acid or corticosteroid in the preceding 2 months | |
| Undergone previous cartilage procedures, such as microfracture or chondroplasty | |
| Arthroscopy or intraarticular infiltration in the last 6 months | |
| Corticosteroid treatment in the 3 last months | |
| Nonsteroidal anti-inflammatory drug therapy in the last 15 days | |
| Previous surgical treatment for anterior and/or posterior cruciate ligament reconstruction within 2 months | |
| Other diseases/ comorbidities | History of autoimmune disease |
| Malignancy, organ failure | |
| Cardiovascular disease, hypertension | |
| Positive viral markers (HIV, HBV, HCV, and HTLV-1/2), syphilis | |
| Bleeding disorder, i.e., hemophilia | |
| Poorly controlled diabetes mellitus | |
| Delivery Method | Substances | |
|---|---|---|
| Transplantation | Collagen matrix | Collagen type I/III membrane |
| Collagen sheet | ||
| Fibrin glue | Fibrin glue product (fibrinogen and thrombin) | |
| Injection | Basal medium | Minimum essential medium |
| Normal saline | ||
| Human serum | ||
| Albumin | ||
| Platelet poor plasma | ||
| Hyaluronic acid | Hyalone® (Hyaluronic acid sodium salt 4 mL/60 mg) | |
| Artz® (Hyaluronic acid sodium salt) | ||
| Platelet-rich plasma | Concentrated platelets from the autologous blood | |
| Growth factor | Human granulocyte colony-stimulating factor | |
| References | Patients | Follow Up | MSC Doner and Source | Number of MSCs | Delivery Method | Lesion Preparation/Concomitant Procedure | Clinical, Radiological, and Histological Outcomes | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Bastos et al. (2019) | n = 47 | 12 months | Autologous bone marrow | 4 × 106 | Injection | None |
| Treatments were effective in improving the function and decreasing symptoms. |
| Emadedin et al. (2018) | n = 43 | 6 months | Autologous bone marrow | 4 × 107 | Injection | None |
| Significant and clinically relevant pain relief was observed. |
| Lamo-Espinosa et al. (2016) | n = 30 | 12 months | Autologous bone marrow | 1 × 107 1 × 108 | Injection | None |
| Clinical and functional improvement of knee OA was observed. |
| Lamo-Espinosa et al. (2020) | n = 56 | 12 months | Autologous bone marrow | 100 × 106 | Injection | None |
| BM-MSC injection with PRP was a viable therapeutic option in the treatment of OA of the knee. |
| Hashimoto et al. (2019) | n = 11 | 48 weeks | Autologous bone marrow | 10 × 106 100 × 106 | Injection | Microfracture |
| A better quality of articular surface and improved symptomatic cartilage defect of the knee was observed. High dose (100 × 106) was more effective. |
| Wong et al. (2013) | n = 56 | 24 months | Autologous bone marrow | 1.46 × 107 | Injection | Microfracture/HTO |
| The treatment was effective in improving both short-term clinical and MOCART outcomes. |
| Shapiro et al. (2018) | n = 25 | 12 months | Autologous bone marrow | 1.7 × 105 (MSCs) 2.2 × 107 (HSCs) | Injection | None |
| BMAC is safe to perform but showed no superiority to saline injection. MRI cartilage sequences failed to show regenerative benefit. |
| Freitag et al. (2019) | n = 30 | 12 months | Autologous adipose | 1 ×108 1 × 108 × 2 | Injection | None |
| Clinically significant pain and function improvement was observed. MOAKS indicated that the disease progression was modified. |
| Lee et al. (2019) | n = 32 | 6 months | Autologous adipose | 1 × 108 | Injection | None |
| Satisfactory functional improvement and pain relief was observed. The treatment inhibited the progression of cartilage defects. |
| Lu et al. (2019) | n = 47 | 13 months | Autologous adipose | 5 × 107 × 2 | Injection | None |
| Treatments proved significant improvements in joint function, pain, quality of life, and cartilage regeneration. |
| Song et al. (2018) | n = 14 | 96 weeks | Autologous adipose | 1 × 107 × 3 2 × 107 × 3 5 × 107 × 3 | Injection | None |
| Treatments was effective in pain reduction, function improvements and the cartilage volume increase. The dosage of 5 × 107 showed the highest improvement. |
| Qiao et al. (2020) | n = 23 | 24 months | Autologous adipose | 5 × 107 × 2 | Injection | Microfracture |
| Function of the knee joint was clinically improved. The treatment promoted the decrease of cartilage defect and cartilage regeneration. |
| Koh et al. (2016) | n = 80 | 24 months | Autologous adipose | 4.97 × 106 | Transplantation | Microfracture |
| Pain and symptom improvements were observed. The appearance of cartilage lesions was improved. |
| Garza et al. (2020) | n = 39 | 12 months | Autologous adipose (SVF) | 1.5 × 107 SVF 3.0 × 107 SVF | Injection | None |
| The treatment significantly decreased knee OA pain and symptoms, and the high dose group showed better results. |
| Hong et al. (2018) | n = 16 | 12 months | Autologous adipose (SVF) | 2.98 × 107 | Injection | Chondroplasty |
| The treatment effectively relieved pain, improved function, and repaired cartilage defects. |
| Koh et al. (2014) | n = 44 | 24.4 months | Autologous adipose | 4.11 × 106 | Injection | Chondroplasty/HTO |
| Treatments was clinically effective and mildly improved cartilage healing. |
| Saw et al. (2013) | n = 49 | 18 months | Autologous peripheral blood | 2 × 107 × 8 (CD105+) 3 × 106 × 8 (CD34+) | Injection | Subchondral drilling |
| The quality of articular cartilage repair was improved. |
| Turajane et al. (2017) | n = 60 | 12 months | Autologous peripheral blood | 1.7 × 106 × 3 (CD104+) 1.2 × 106 × 3 (CD34+) | Injection | Subchondral drilling |
| Treatments showed promise in disease modification with potential inhibition of OA progression. |
| Akgun et al. (2014) | n = 14 | 24 months | Autologous synovium | 8 × 106 | Transplantation | Chondroplasty |
| Treatments effectively accelerate the recovery of chondral lesion of the knee. |
| Zhou et al. (2021) | n = 57 | 12 months | Autologous infrapatellar fat pad | 3.91 × 106 | Injection | Chondroplasty |
| The treatment provided an assistance in reducing pain and improving function of the knee. |
| Gupta et al. (2016) | n = 60 | 12 months | Allogeneic bone marrow | 25 × 106 50 × 106 75 × 106 150 × 106 | Injection | None |
| A trend toward pain reduction was observed at the lowest cell dose of 25 million. |
| Vega et al. (2015) | n = 30 | 12 months | Allogeneic bone marrow | 40 ×106 | Injection | None |
| Treatments provided clinically effective pain relief and improved the quality of cartilage. |
| Kuah et al. (2018) | n = 20 | 12 months | Allogeneic adipose | 3.9 × 106 6.7 × 106 | Injection | None |
| Pain reduced in intervention group, and lateral tibial cartilage loss was halted in the 3.9 M group, while the placebo group showed a significant cartilage loss. |
| Zhao et al. (2019) | n = 18 | 48 weeks | Allogeneic adipose | 1.0 × 107 × 2 2.0 × 107 × 2 5.0 × 107 × 2 | Injection | None |
| The treatment alleviated OA symptoms, and possible compositional changes of cartilage were suggested by quantitative MRI measurements. |
| Soltani et al. (2019) | n = 20 | 24 weeks | Allogeneic placenta | 0.5~0.6 × 108 | Injection | None |
| The treatments provided clinical improvements. |
| Lim et al. (2021) | n = 89 | 60 months | Allogeneic umbilical cord blood | 7.5 × 106 | Transplantation | Subchondral drilling |
| UCB-MSC can be a viable regenerative treatment option. |
| Matas et al. (2018) | n = 26 | 12 months | Allogeneic umbilical cord | 20 × 106 20 × 106 × 2 | Injection | None |
| It was observed that repeated MSC treatment was superior to active comparator in knee OA. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Shim, D.W.; Kang, K.-Y.; Chae, D.-S.; Lee, W.-S. Method Categorization of Stem Cell Therapy for Degenerative Osteoarthritis of the Knee: A Review. Int. J. Mol. Sci. 2021, 22, 13323. https://doi.org/10.3390/ijms222413323
Lee JS, Shim DW, Kang K-Y, Chae D-S, Lee W-S. Method Categorization of Stem Cell Therapy for Degenerative Osteoarthritis of the Knee: A Review. International Journal of Molecular Sciences. 2021; 22(24):13323. https://doi.org/10.3390/ijms222413323
Chicago/Turabian StyleLee, Jae Sun, Dong Woo Shim, Kyung-Yil Kang, Dong-Sik Chae, and Woo-Suk Lee. 2021. "Method Categorization of Stem Cell Therapy for Degenerative Osteoarthritis of the Knee: A Review" International Journal of Molecular Sciences 22, no. 24: 13323. https://doi.org/10.3390/ijms222413323






