Approaches to Inactivating Aflatoxins—A Review and Challenges
Abstract
:1. Introduction
2. Characteristics of Aflatoxins and Detoxification Methods
2.1. Toxicity and Properties
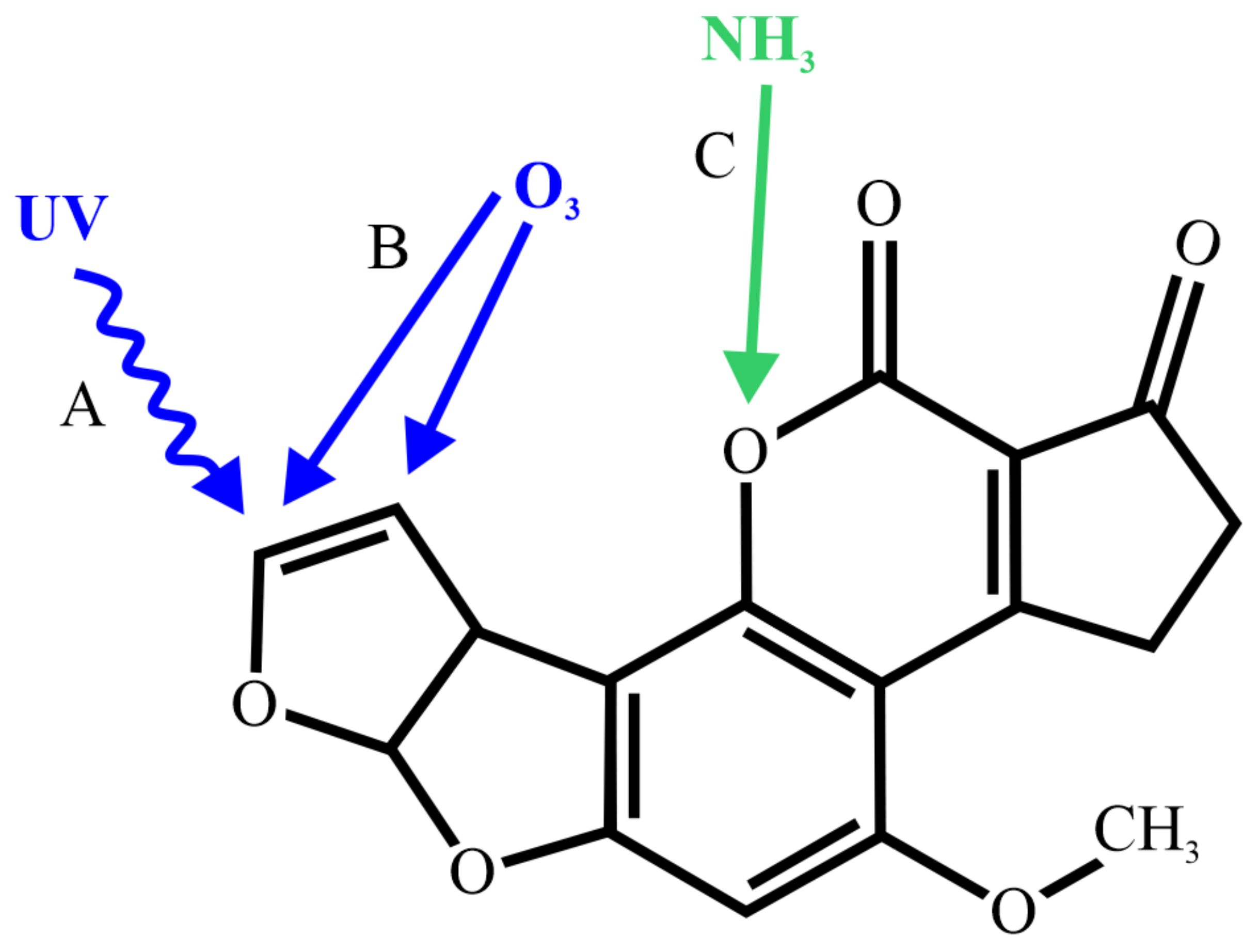
2.2. UV Radiation and Pulsed Light Treatment
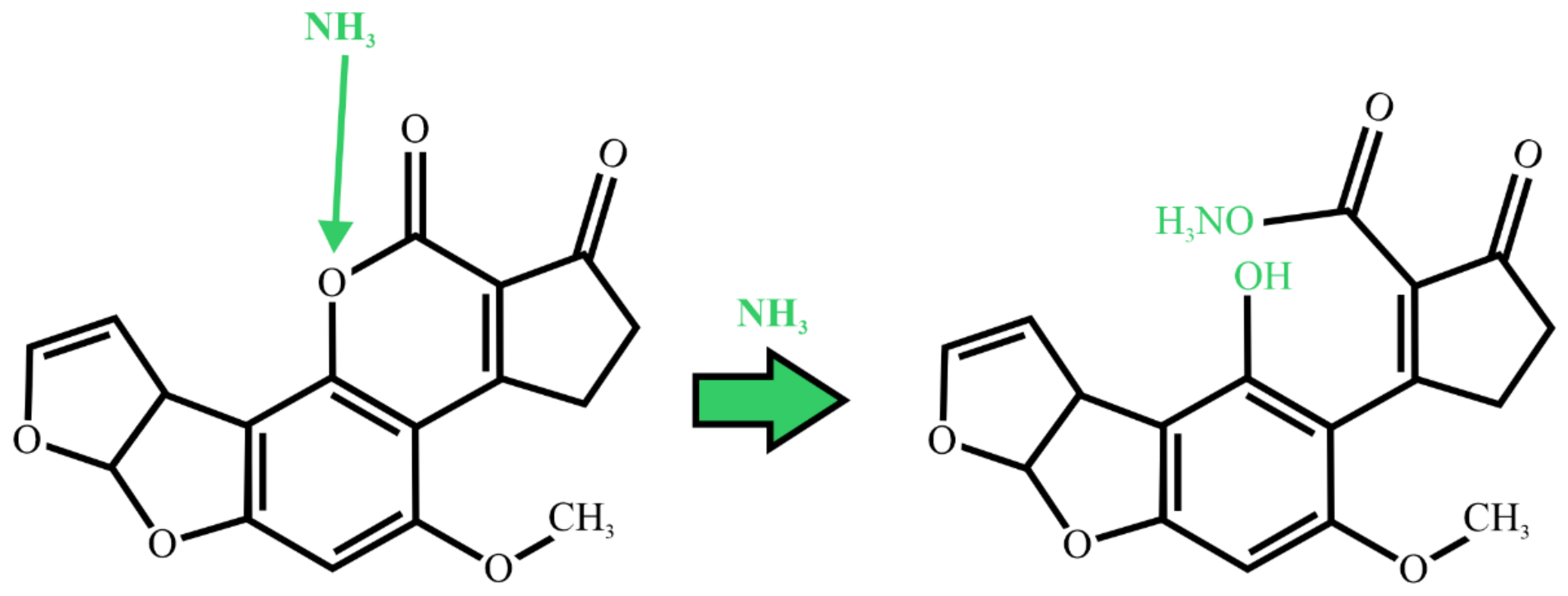
2.3. Ammoniation
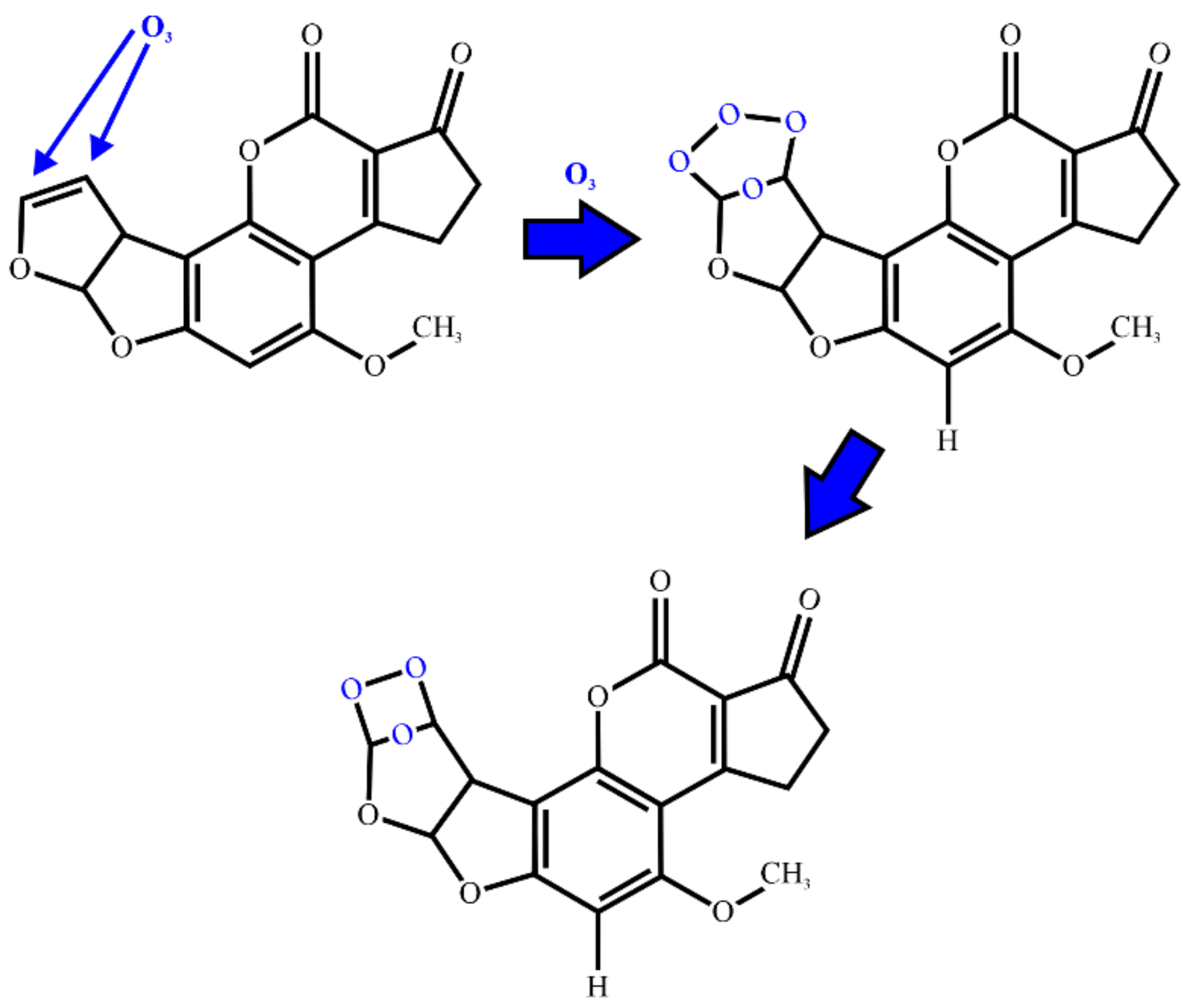
2.4. Ozonation
3. Characteristics of Aflatoxins and Detoxification Methods
3.1. Relevance of Non-Thermal Gas Discharge Plasmas for Agriculture
3.2. Degradation of Mycotoxins by Discharge Plasmas
3.3. Decontamination of Artificially Infected Seeds by Discharge Plasmas
4. Main Points and Future Perspectives
- The destruction of toxins remains both a scientific and technological challenge.
- The reported treatment times are prohibitively long for industrial application.
- The incomplete description of the particularities of the experimental setups and treatment conditions prevents valuable conclusions from being drawn.
- The plasma methods seem promising, but upscaling poses a significant challenge.
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Varga, J.; Tóth, B. Novel strategies to control mycotoxins in feeds: A review. Acta Vet. Hung. 2005, 53, 189–203. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranyi, N.; Kocsubé, S.; Vágvölgyi, C.; Varga, J. Current trends in aflatoxin research. Acta Biol. Szeged. 2013, 57, 95–107. [Google Scholar]
- Veldman, A.; Meijs, J.A.C.; Borggreve, G.J.; Heeres-van der Tol, J.J. Carry-over of aflatoxin from cows’ food to milk. Anim. Sci. 1992, 55, 163–168. [Google Scholar] [CrossRef]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel strategies for degradation of aflatoxins in food and feed: A review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Johnson, W.W.; Shimada, T.; Ueng, Y.-F.; Yamazaki, H.; Langouët, S. Activation and detoxication of aflatoxin B1. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 121–128. [Google Scholar] [CrossRef]
- Neal, G.E.; Eaton, D.L.; Judah, D.J.; Verma, A. Metabolism and toxicity of aflatoxins M1 and B1 in human-derived in vitro systems. Toxicol. Appl. Pharmacol. 1998, 151, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- MdQuadri, S.H.; Niranjan, M.S.; Chaluvaraju, K.C.; Shantaram, U.; Enamul, H.Y.S.M. An overview on chemistry, toxicity, analysis and control of aflatoxins. Int. J. Chem. Life Sci. 2013, 2, 1071–1078. [Google Scholar]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Samarajeewa, U.; Sen, A.C.; Cohen, M.D.; Wei, C.I. Detoxification of aflatoxins in foods and feeds by physical and chemical methods. J. Food Prot. 1990, 53, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.E.; Marth, E.H. Kinetics of interaction of aflatoxin M1 in aqueous solutions irradiated with ultraviolet energy. J. Agric. Food Chem. 1987, 35, 785–789. [Google Scholar] [CrossRef]
- Liu, R.; Jin, Q.; Tao, G.; Shan, L.; Huang, J.; Liu, Y.; Wang, X.; Mao, W.; Wang, S. Photodegradation kinetics and byproducts identification of the aflatoxin B1 in aqueous medium by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Mass Spectrom. 2010, 45, 553–559. [Google Scholar] [CrossRef]
- Patras, A.; Julakanti, S.; Yannam, S.; Bansode, R.R.; Burns, M.; Vergne, M.J. Effect of UV irradiation on aflatoxin reduction: A cytotoxicity evaluation study using human hepatoma cell line. Mycotoxin Res. 2017, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Atalla, M.M.; Hassanein, N.M.; El-Beih, A.A.; Youssef, Y.A. Effect of fluorescent and UV light on mycotoxin production under different relative humidities in wheat grains. Acta Pharm. Turc. 2004, 46, 205–222. [Google Scholar]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Reverberi, M.; Salustri, M.; Masi, P.; Cavella, S. Technological properties of durum wheat semolina treated by heating and UV irradiation for reduction of mycotoxin content. J. Food Process Eng. 2019, 42, e13006. [Google Scholar] [CrossRef]
- Basaran, P. Reduction of Aspergillus parasiticus on hazelnut surface by UV-C treatment. Int. J. Food Sci. Technol. 2009, 44, 1857–1863. [Google Scholar] [CrossRef]
- Jubeen, F.; Bhatti, I.A.; Khan, M.Z.; Zahoor-Ul, H.; Shahid, M. Effect of UVC irradiation on aflatoxins in ground nut (Arachis hypogea) and tree nuts (Juglans regia, prunus duclus and pistachio vera). J. Chem. Soc. Pakistan 2012, 34, 1366–1374. [Google Scholar]
- Moreau, M.; Lescure, G.; Agoulon, A.; Svinareff, P.; Orange, N.; Feuilloley, M. Application of the pulsed light technology to mycotoxin degradation and inactivation. J. Appl. Toxicol. 2013, 33, 357–363. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Park, D.L.; Price, W.D. Reduction of aflatoxin hazards using ammoniation. Rev. Environ. Contam. Toxicol. 2001, 171, 139–176. [Google Scholar]
- Schroeder, T.; Zweifel, U.; Sagelsdorff, P.; Friederich, U.; Luethy, J.; Schlatter, C. Ammoniation of aflatoxin-containing corn: Distribution, in vivo covalent deoxyribonucleic acid binding, and mutagenicity of reaction products. J. Agric. Food Chem. 1985, 33, 311–316. [Google Scholar] [CrossRef]
- Cucullu, A.F.; Lee, L.S.; Pons, W.A.; Stanley, J.B. Ammoniation of aflatoxin B1. Isolation and characterization of a product with molecular weight 206. J. Agric. Food Chem. 1976, 24, 408–410. [Google Scholar] [CrossRef]
- Lee, L.S.; Stanley, J.B.; Cucullu, A.F.; Pons, W.A.J.; Goldblatt, L.A. Ammoniation of aflatoxin B1: Isolation and identification of the major reaction product. J. Assoc. Off. Anal. Chem. 1974, 57, 626. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.J.; Weng, C.Y.; Park, D.L. Distribution of ammonia/aflatoxin reaction products in corn following exposure to ammonia decontamination procedure. Food Addit. Contam. 1994, 11, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.Y.; Martinez, A.J.; Park, D.L. Efficacy and permanency of ammonia treatment in reducing aflatoxin levels in corn. Food Addit. Contam. 1994, 11, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.N.E.; Ayesh, A.M.; Abdel Galil, M.M.; Naguib, K. Effect of high pressure ammoniation procedure on the detoxification of aflatoxins. Mycotoxin Res. 1997, 13, 23–34. [Google Scholar] [CrossRef]
- Norred, W.P. Ammonia treatment to destroy aflatoxins in corn. J. Food Prot. 1982, 45, 972–976. [Google Scholar] [CrossRef]
- McKenzie, K.S.; Kubena, L.F.; Denvir, A.J.; Rogers, T.D.; Hitchens, G.D.; Bailey, R.H.; Harvey, R.B.; Buckley, S.A.; Phillips, T.D. Aflatoxicosis in turkey poults is prevented by treatment of naturally contaminated corn with ozone generated by electrolysis. Poult. Sci. 1998, 77, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.S.; Sarr, A.B.; Mayura, K.; Bailey, R.H.; Miller, D.R.; Rogers, T.D.; Norred, W.P.; Voss, K.A.; Plattner, R.D.; Kubena, L.F.; et al. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food Chem. Toxicol. 1997, 35, 807–820. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Bian, Y.; Chen, Z. Effect of ozone treatment on aflatoxin B1 and safety evaluation of ozonized corn. Food Control 2014, 37, 171–176. [Google Scholar] [CrossRef]
- Prudente, A.D., Jr.; King, J.M. Efficacy and safety evaluation of ozonation to degrade aflatoxin in corn. J. Food Sci. 2002, 67, 2866–2872. [Google Scholar] [CrossRef]
- Proctor, A.D.; Ahmedna, M.; Kumar, J.V.; Goktepe, I. Degradation of aflatoxins in peanut kernels/flour by gaseous ozonation and mild heat treatment. Food Addit. Contam. 2004, 21, 786–793. [Google Scholar] [CrossRef]
- Ta, E.-D.; Ama, S.; El-Desouky, A.I.; Ha, E.-M. Effect of ozone gas on degradation of aflatoxin B1 and Aspergillus Flavus fungal. J. Environ. Anal. Toxicol. 2012, 2, 128. [Google Scholar] [CrossRef]
- Allen, B.; Wu, J.; Doan, H. Inactivation of fungi associated with barley grain by gaseous ozone. J. Environ. Sci. Health Part B 2003, 38, 617–630. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Bittencourt, K.O.; Scussel, V.M. Ozone treatment efficiency on Fusarium graminearum and deoxynivalenol degradation and its effects on whole wheat grains (Triticum aestivum L.) quality and germination. J. Stored Prod. Res. 2014, 59, 245–253. [Google Scholar] [CrossRef]
- Kells, S.A.; Mason, L.J.; Maier, D.E.; Woloshuk, C.P. Efficacy and fumigation characteristics of ozone in stored maize. J. Stored Prod. Res. 2001, 37, 371–382. [Google Scholar] [CrossRef]
- Liebermann, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1994; ISBN 0-471-00577-0. [Google Scholar]
- Eremin, D.; Bienholz, S.; Szeremley, D.; Trieschmann, J.; Ries, S.; Awakowicz, P.; Mussenbrock, T.; Brinkmann, R.P. On the physics of a large CCP discharge. Plasma Sources Sci. Technol. 2016, 25, 025020. [Google Scholar] [CrossRef]
- Mozetič, M.; Primc, G.; Vesel, A.; Zaplotnik, R.; Modic, M.; Junkar, I.; Recek, N.; Klanjšek-Gunde, M.; Guhy, L.; Sunkara, M.K.M.K.; et al. Application of extremely non-equilibrium plasmas in the processing of nano and biomedical materials. Plasma Sources Sci. Technol. 2015, 24, 015026. [Google Scholar] [CrossRef]
- Kutasi, K.; Saoudi, B.; Pintassilgo, C.D.; Loureiro, J.; Moisan, M. Modelling the Low-Pressure N2—O2 Plasma Afterglow to Determine the Kinetic Mechanisms Controlling the UV Emission Intensity and Its Spatial Distribution for Achieving an Efficient Sterilization Process. Plasma Process. Polym. 2008, 5, 840–852. [Google Scholar] [CrossRef]
- Moisan, M.; Boudam, K.; Carignan, D.; Kéroack, D.; Levif, P.; Barbeau, J.; Séguin, J.; Kutasi, K.; Elmoualij, B.; Thellin, O.; et al. Sterilization/disinfection of medical devices using plasma: The flowing afterglow of the reduced-pressure N2-O2 discharge as the inactivating medium. Eur. Phys. J. Appl. Phys. 2013, 63, 10001. [Google Scholar] [CrossRef] [Green Version]
- Nagatsu, M.; Terashita, F.; Koide, Y. Low-Temperature Sterilization with Surface-Wave-Excited Oxygen Plasma. Jpn. J. Appl. Phys. 2003, 42, L856–L859. [Google Scholar] [CrossRef]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-thermal atmospheric pressure discharges. J. Phys. D Appl. Phys. 2005, 38, R1–R24. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Šimor, M.; Ráhel’, J.; Vojtek, P.; Černák, M.; Brablec, A. Atmospheric-pressure diffuse coplanar surface discharge for surface treatments. Appl. Phys. Lett. 2002, 81, 2716–2718. [Google Scholar] [CrossRef]
- Černák, M.; Černáková, L.; Hudec, I.; Kováčik, D.; Zahoranová, A. Diffuse Coplanar Surface Barrier Discharge and its applications for in-line processing of low-added-value materials. Eur. Phys. J. Appl. Phys. 2009, 47, 22806. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.E.; Klämpfl, T.G.; Steffes, B.; Thomas, H.M.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Wemlinger, E.; Pedrow, P.; Barbosa-Cánovas, G.; Garcia-Perez, M. Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food Control 2013, 34, 149–157. [Google Scholar] [CrossRef]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of Seed Germination Performance through Cold Plasma Chemistry Technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.-D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Bethke, P.; Gubler, F.; Jacobsen, J.; Jones, R. Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 2004, 219, 847–855. [Google Scholar] [CrossRef]
- Ma, Z.; Marsolais, F.; Bykova, N.V.; Igamberdiev, A.U. Nitric Oxide and Reactive Oxygen Species Mediate Metabolic Changes in Barley Seed Embryo during Germination. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Brasoveanu, M.; Nemţanu, M.; Carmen, S.-B.; Karaca, G.; Erper, İ. Effect of Glow Discharge Plasma on Germination and Fungal Load of some Cereal Seeds. Rom. Rep. Phys. 2015, 67. [Google Scholar]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Sera, B.; Spatenka, P.; Sery, M.; Vrchotova, N.; Hruskova, I. Influence of Plasma Treatment on Wheat and Oat Germination and Early Growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Szőke, C.; Nagy, Z.; Gierczik, K.; Székely, A.; Spitkól, T.; Zsuboril, Z.T.; Galiba, G.; Marton, C.L.; Kutasi, K. Effect of the afterglows of low pressure Ar/N2-O2 surface-wave microwave discharges on barley and maize seeds. Plasma Process. Polym. 2018, 15, 1700138. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Rossi, F.; Kylián, O.; Rauscher, H.; Hasiwa, M.; Gilliland, D. Low pressure plasma discharges for the sterilization and decontamination of surfaces. New J. Phys. 2009, 11, 115017. [Google Scholar] [CrossRef]
- Raballand, V.; Benedikt, J.; Wunderlich, J.; von Keudell, A. Inactivation of Bacillus atrophaeus and of Aspergillus niger using beams of argon ions, of oxygen molecules and of oxygen atoms. J. Phys. D Appl. Phys. 2008, 41, 115207. [Google Scholar] [CrossRef]
- Philip, N.; Saoudi, B.; Crevier, M.-C.; Moisan, M.; Barbeau, J.; Pelletier, J. The respective roles of UV photons and oxygen atoms in plasma sterilization at reduced gas pressure: The case of N2-O2 mixtures. IEEE Trans. Plasma Sci. 2002, 30, 1429–1436. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.-H.; Lee, M.-H.; Han, D.-W.; Chung, K.-H.; Hyun, S.O.; Park, J.-C. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Huang, G.-Q.; Li, Y.-P.; Xiao, J.-X.; Zhang, Y.; Jiang, W.-L. Degradation of aflatoxin B1 by low-temperature radio frequency plasma and degradation product elucidation. Eur. Food Res. Technol. 2015, 241, 103–113. [Google Scholar] [CrossRef]
- Siciliano, I.; Spadaro, D.; Prelle, A.; Vallauri, D.; Cavallero, M.; Garibaldi, A.; Gullino, M. Use of cold atmospheric plasma to detoxify hazelnuts from aflatoxins. Toxins 2016, 8, 125. [Google Scholar] [CrossRef]
- Kutasi, K.; Popović, D.; Krstulović, N.; Milošević, S. Tuning the composition of plasma-activated water by a surface-wave microwave discharge and a kHz plasma jet. Plasma Sources Sci. Technol. 2019, 28, 095010. [Google Scholar] [CrossRef] [Green Version]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control 2016, 70, 1–8. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Shi, H.; Ileleji, K.; Stroshine, R.L.; Keener, K.; Jensen, J.L. Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food Bioprocess Technol. 2017, 10, 1042–1052. [Google Scholar] [CrossRef]
- Shi, H.; Cooper, B.; Stroshine, R.L.; Ileleji, K.E.; Keener, K.M. Structures of degradation products and degradation pathways of aflatoxin B1 by high-voltage atmospheric cold plasma (HVACP) treatment. J. Agric. Food Chem. 2017, 65, 6222–6230. [Google Scholar] [CrossRef] [PubMed]
- McDonough, M.X.; Campabadal, C.A.; Mason, L.J.; Maier, D.E.; Denvir, A.; Woloshuk, C. Ozone application in a modified screw conveyor to treat grain for insect pests, fungal contaminants, and mycotoxins. J. Stored Prod. Res. 2011, 47, 249–254. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Wang, Y.; Chen, Z. Structure elucidation and toxicity analyses of the degradation products of aflatoxin B1 by aqueous ozone. Food Control 2013, 31, 331–336. [Google Scholar] [CrossRef]
- Dolezal, A.L.; Shu, X.; OBrian, G.R.; Nielsen, D.M.; Woloshuk, C.P.; Boston, R.S.; Payne, G.A. Aspergillus flavus infection induces transcriptional and physical changes in developing maize kernels. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Brera, C.; Catano, C.; de Santis, B.; Debegnach, F.; de Giacomo, M.; Pannunzi, E.; Miraglia, M. Effect of industrial processing on the distribution of aflatoxins and zearalenone in corn-milling fractions. J. Agric. Food Chem. 2006, 54, 5014–5019. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetič, M. Applications of highly non-equilibrium low-pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control. Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]


| C19H18O8 | |||
| C17H17O9 | C17H12O7 | C17H14O7 | |
| C16H17O9 | C16H17O7 | C16H17O8 | C16H16O6 |
| C14H12O5 | C14H10O6 | ||
| C12H14O4 |
| System | Inoculated Seeds | Quantity | Treatment Time | Input Power | Initial AFB1 Concentration | Reduction |
|---|---|---|---|---|---|---|
| N2 DBD [71] | hazelnut | 40 g | 12 min | 1150 W | 20 ng/g | 70% |
| air ICP [75] | hazelnut | 8 g | 20 min | 300 W | 950 ng/g | 50% |
| 45%RH air CCP [76] | groundnuts | 10 g | 12 min | 60 W | 9.84 ng/g | 95% |
| 40%RH air DBD [77] | corn | 25 g | 1 min | 200 W | 420 ng/g | 62% |
| 10 min | 82% | |||||
| 30 min | 90% | |||||
| air RF jet [73] | maize | 40 g | 5 min | 655 W | 107 cfu/g | 5 log |
| air ICP [74] | maize | 8 g | 20 min | 300 W | 5 × 106 cfu/g | 1 log |
| 45%RH air CCP [76] | groundnuts | 10 g | 24 min | 60 W | 1.45 × 103 cfu/g | 2 log |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutasi, K.; Recek, N.; Zaplotnik, R.; Mozetič, M.; Krajnc, M.; Gselman, P.; Primc, G. Approaches to Inactivating Aflatoxins—A Review and Challenges. Int. J. Mol. Sci. 2021, 22, 13322. https://doi.org/10.3390/ijms222413322
Kutasi K, Recek N, Zaplotnik R, Mozetič M, Krajnc M, Gselman P, Primc G. Approaches to Inactivating Aflatoxins—A Review and Challenges. International Journal of Molecular Sciences. 2021; 22(24):13322. https://doi.org/10.3390/ijms222413322
Chicago/Turabian StyleKutasi, Kinga, Nina Recek, Rok Zaplotnik, Miran Mozetič, Mitja Krajnc, Peter Gselman, and Gregor Primc. 2021. "Approaches to Inactivating Aflatoxins—A Review and Challenges" International Journal of Molecular Sciences 22, no. 24: 13322. https://doi.org/10.3390/ijms222413322
APA StyleKutasi, K., Recek, N., Zaplotnik, R., Mozetič, M., Krajnc, M., Gselman, P., & Primc, G. (2021). Approaches to Inactivating Aflatoxins—A Review and Challenges. International Journal of Molecular Sciences, 22(24), 13322. https://doi.org/10.3390/ijms222413322








