Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Histopathological Analysis
2.4. Measurement of AFB1-DNA Adduct in Liver Genomic DNA
2.5. Measurement of Total P450 Content in Liver
2.6. Measurement of Antioxidant Indices of Liver
2.7. Plasma Biochemical Analysis
2.8. RNA Isolation and Quantitative Real-Time PCR Analysis (qPCR)
2.9. Preparation of Protein Extracts and Western Blot Analysis
2.10. Statistical Analysis
3. Results
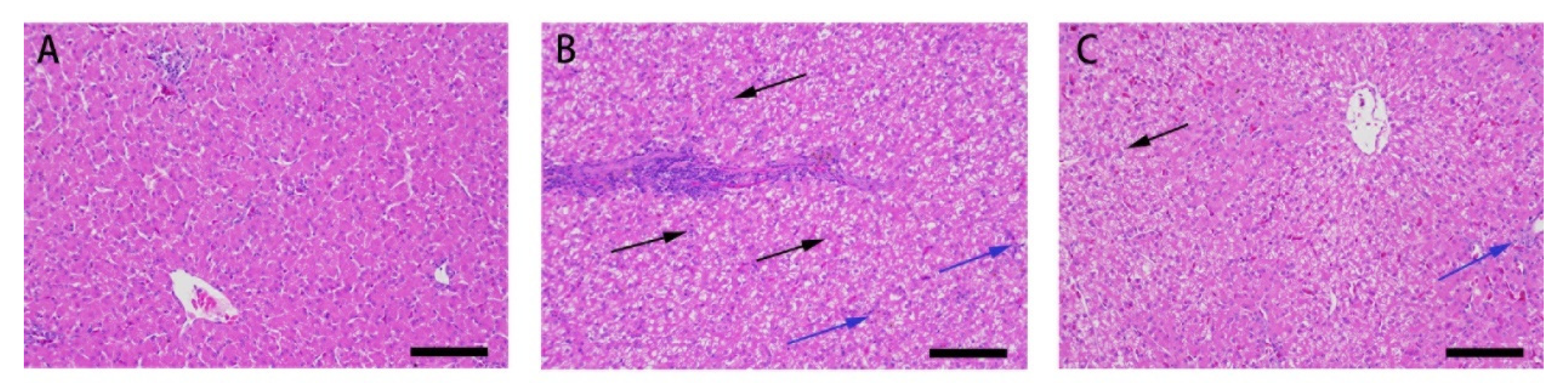
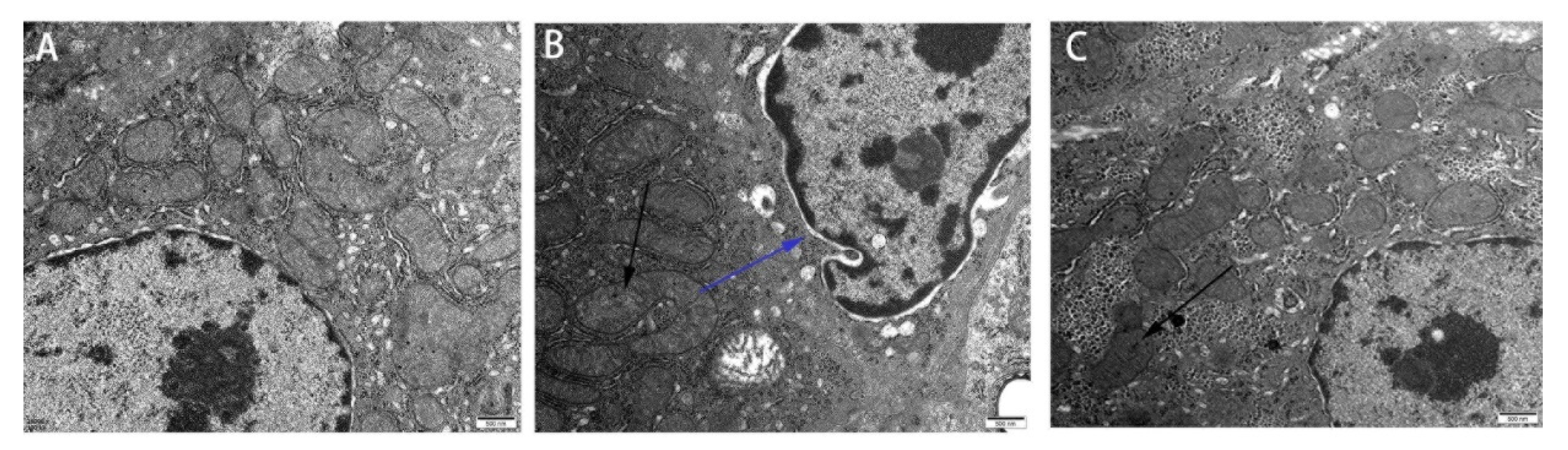
3.1. Effect of Res on the Liver Microstructure and Ultrastructure of Duck Exposed in AFB1
3.2. Effect of Res on Liver Function Impaired by AFB1
3.3. Effect of Res on the Liver Antioxidation Status of Ducks Exposed to AFB1
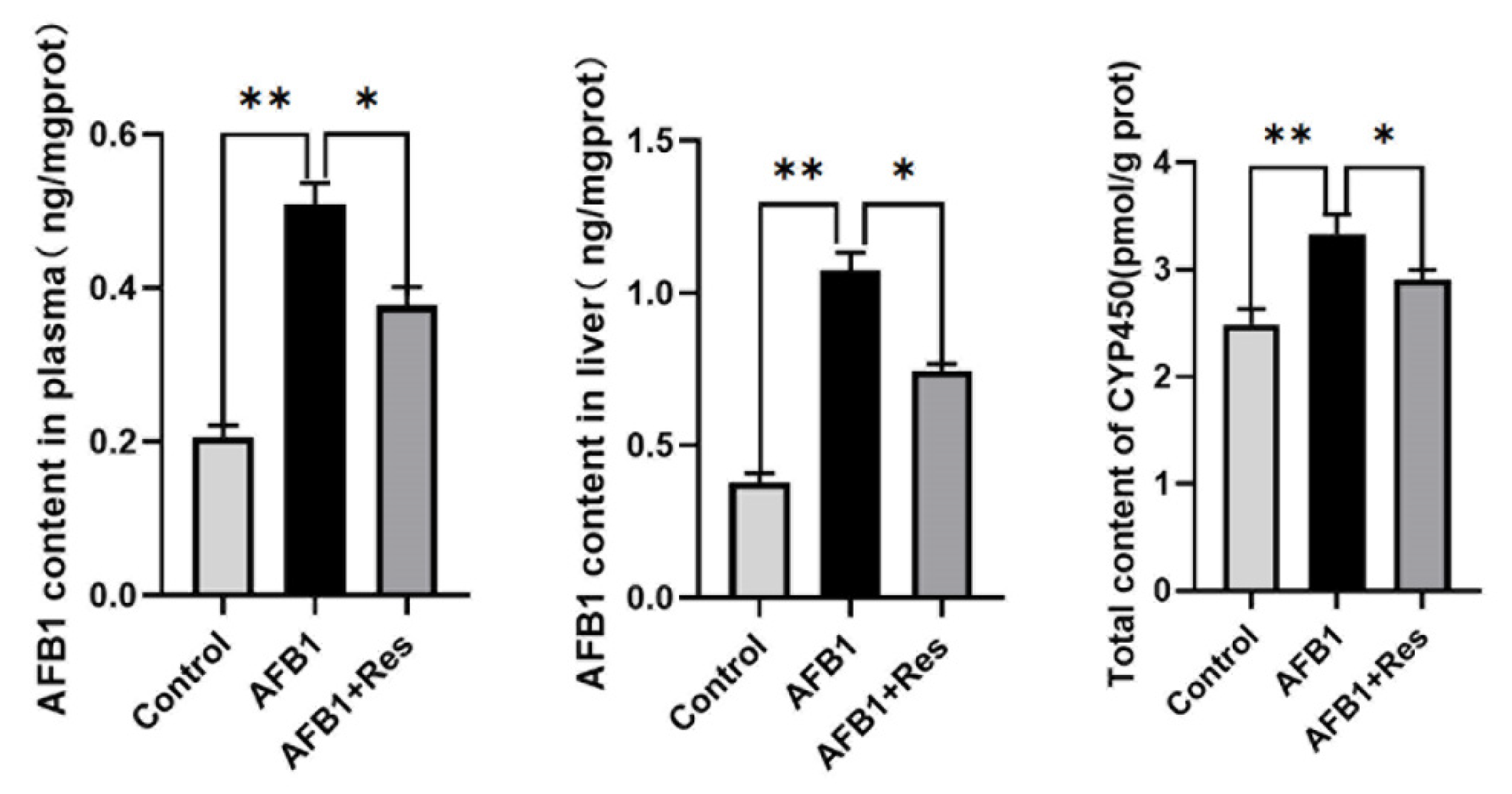
3.4. Effect of Res on the Content of AFB1-DNA Adduct and CYP450 Content in the Ducks’ Livers and Plasma Exposed to AFB1
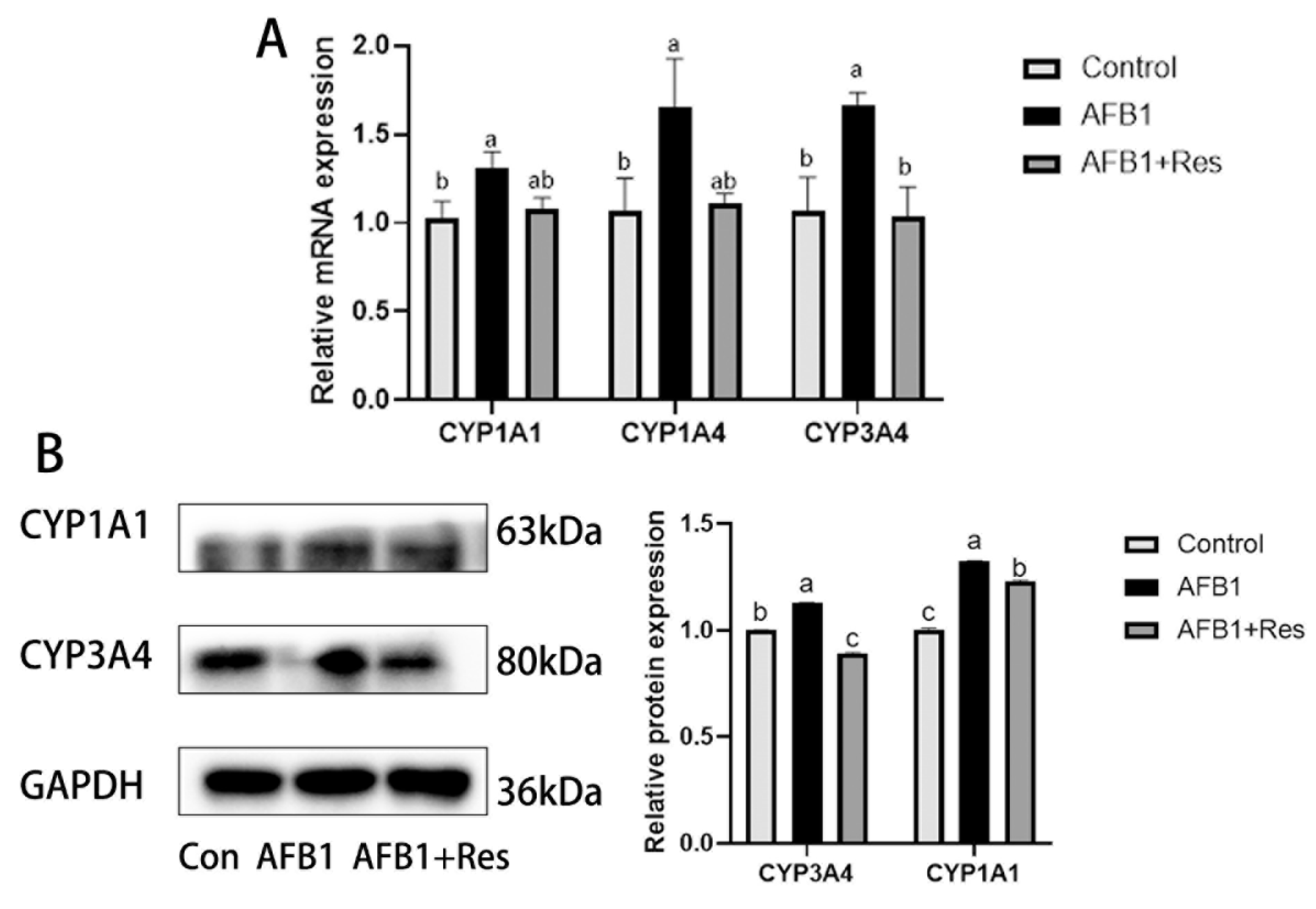
3.5. Effect of Res on the Expression of Phase-I Metabolic Enzyme CYP450 in AFB1
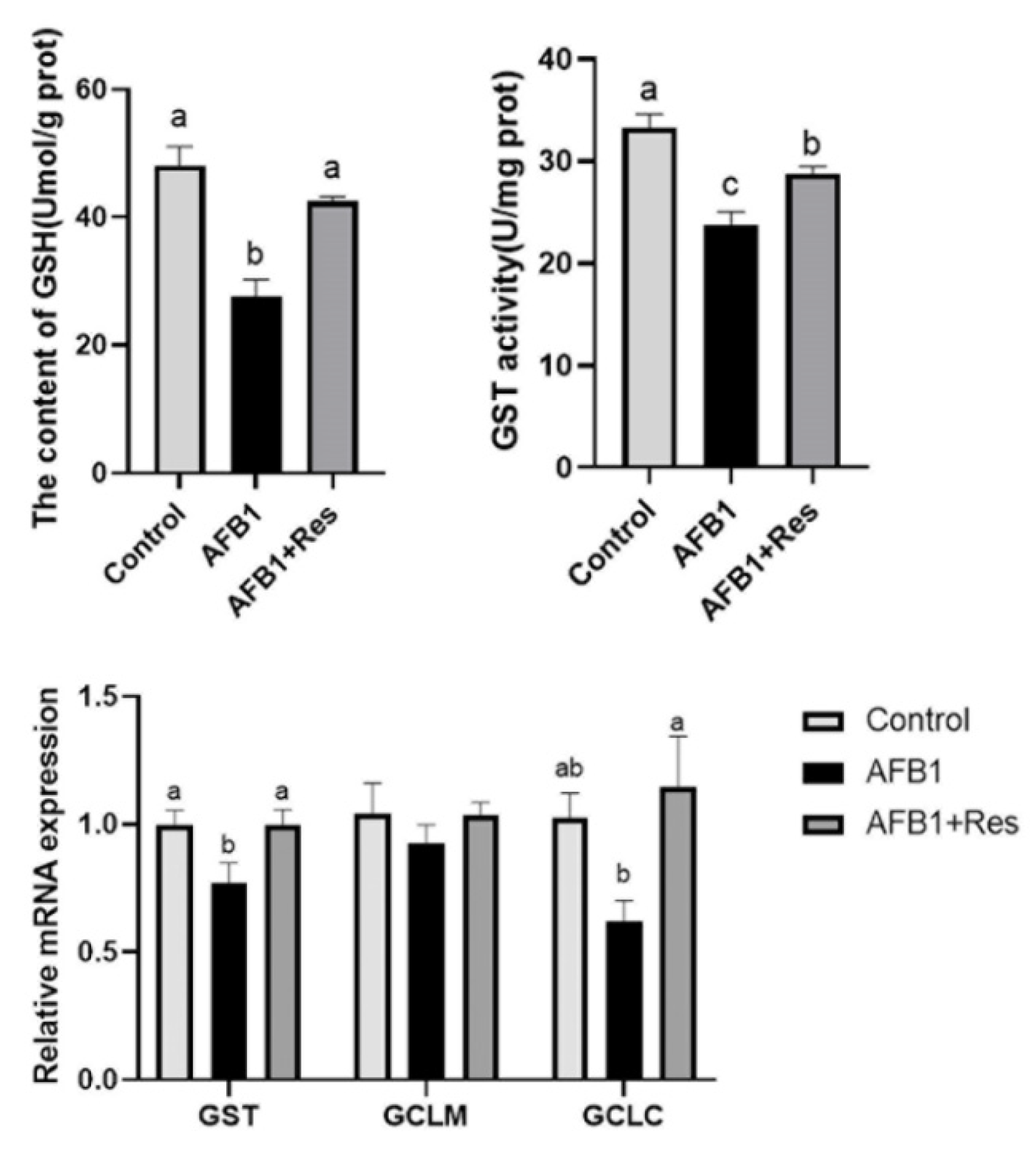
3.6. Effect of Res on GSH Content and mRNA Expression of Related Regulatory Genes in Liver of AFB1-Exposed Duck
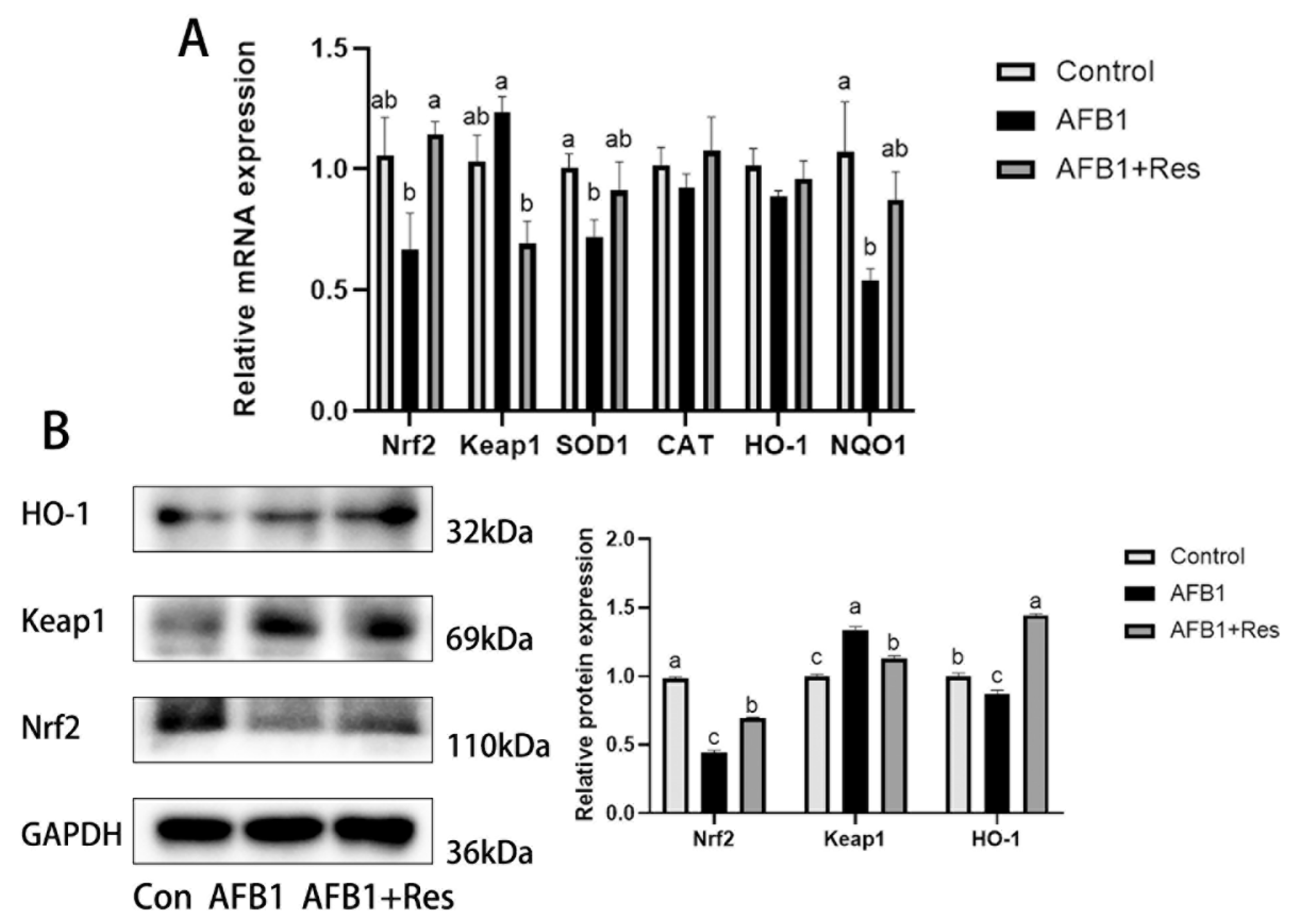
3.7. Effect of Res and AFB1 on Nrf2 Signaling Pathway in Liver
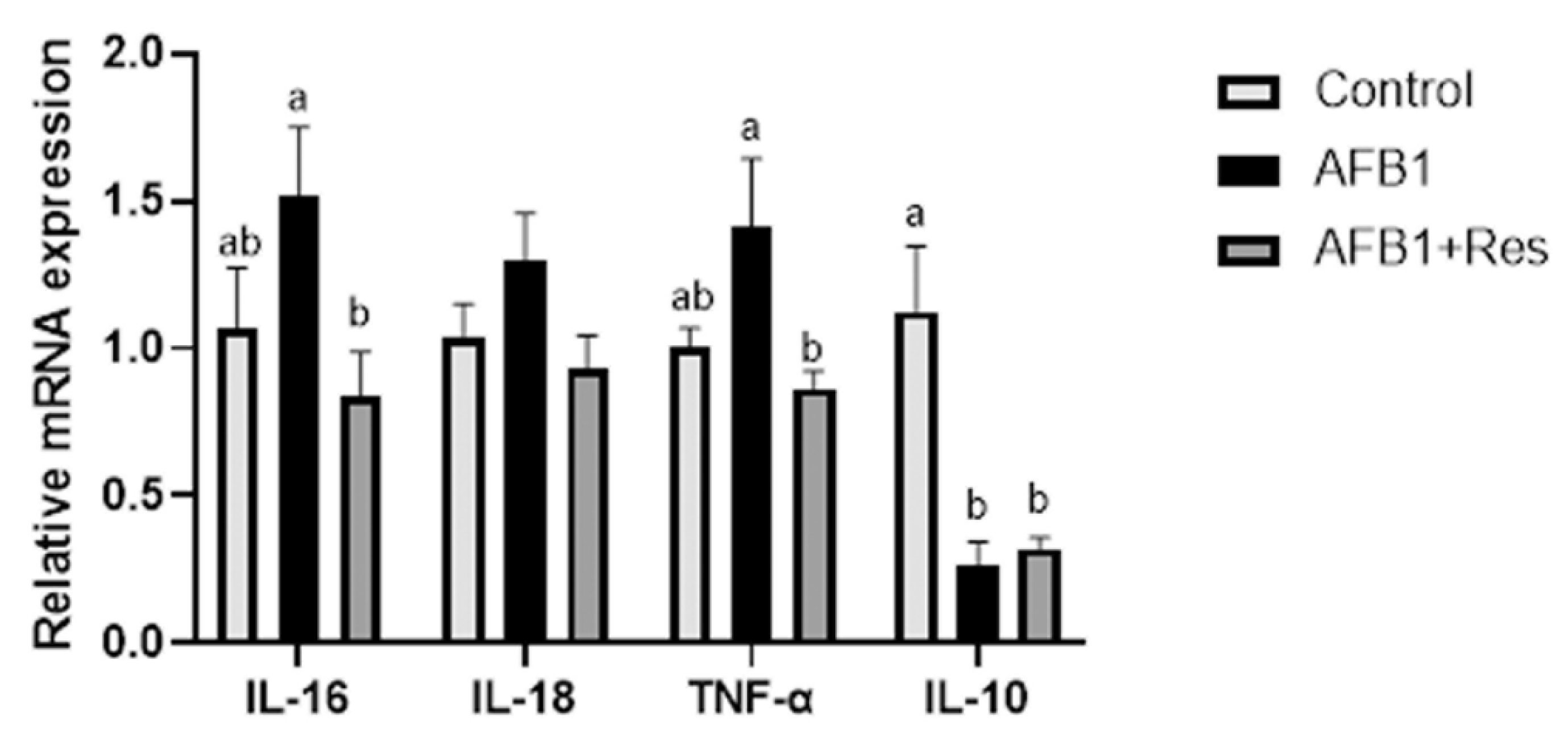
3.8. Effect of Res on Inflammatory Response of Duck Induced by AFB1
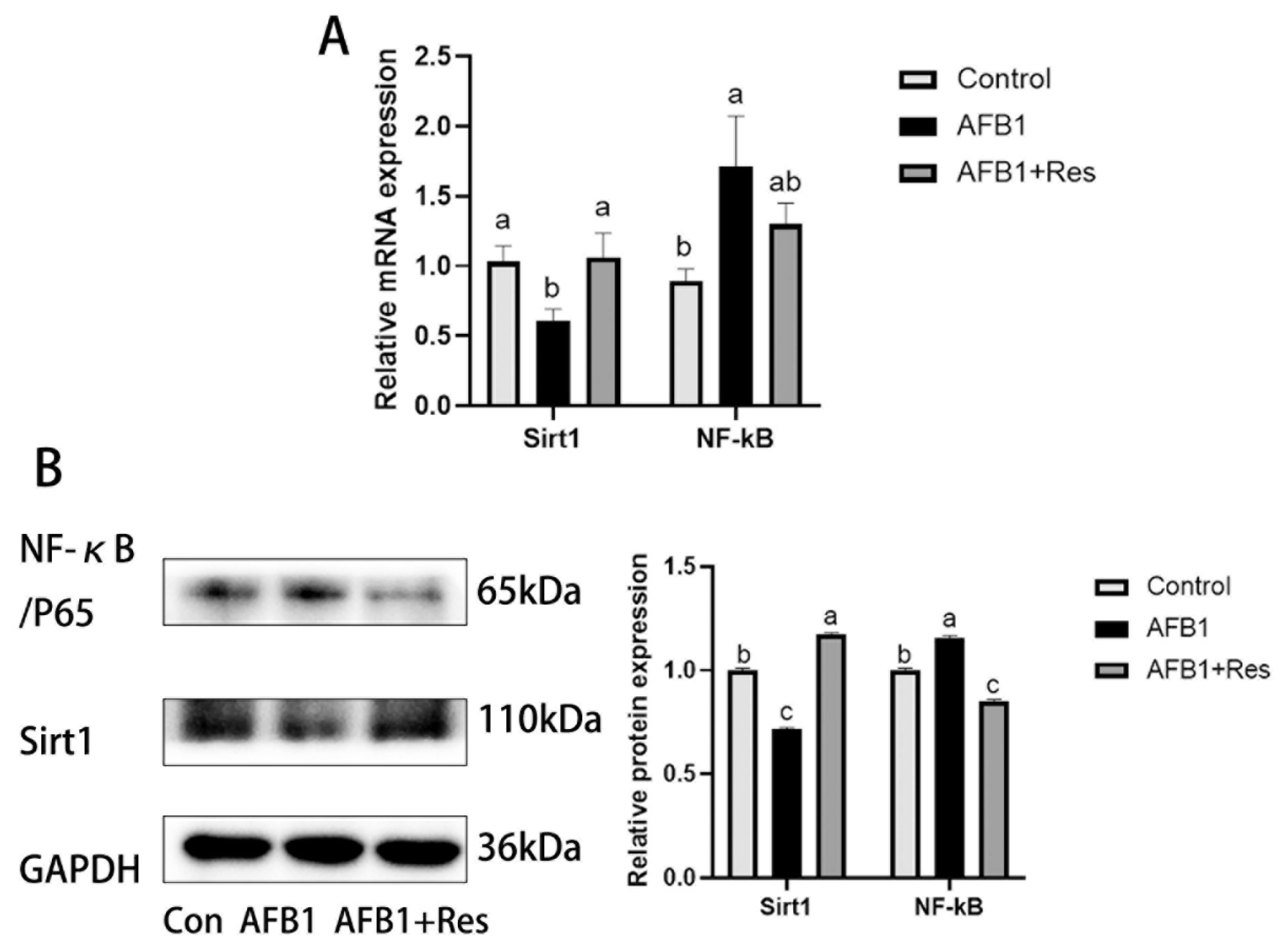
3.9. Effect of AFB1 and Res on the Expression of Sirt1 and NF-κB in Liver
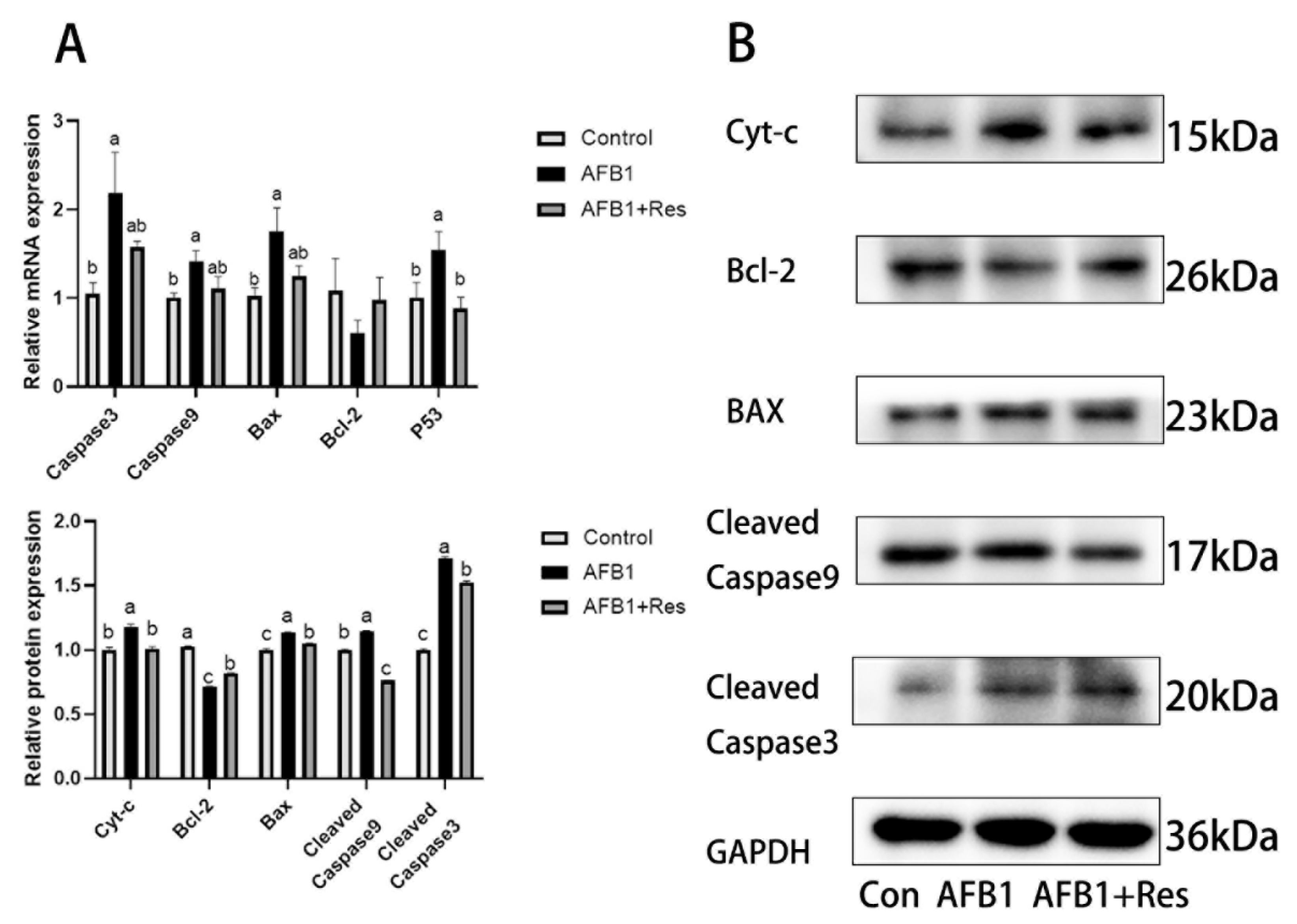
3.10. Effect of Res on Mitochondrial Apoptosis Pathway Induced by AFB1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.C.; Chou, C.C.; Liu, J.R.; Cheng, K.C. Protective and Detoxifying Effects Conferred by Dietary Selenium and Curcumin against AFB1-Mediated Toxicity in Livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Kumar Mishra, S.; Wang, T.; Xu, Z.; Zhao, X.; Wang, Y.; Yin, H.; Fan, X.; Zeng, B.; Yang, M.; et al. AFB1 Induced Transcriptional Regulation Related to Apoptosis and Lipid Metabolism in Liver of Chicken. Toxins 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Zaghini, A.; Martelli, G.; Roncada, P.; Simioli, M.; Rizzi, L. Mannanoligosaccharides and aflatoxin B1 in feed for laying hens: Effects on egg quality, aflatoxins B1 and M1 residues in eggs, and aflatoxin B1 levels in liver. Poult. Sci. 2005, 84, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Ali Rajput, S.; Sun, L.; Zhang, N.; Mohamed Khalil, M.; Gao, X.; Ling, Z.; Zhu, L.; Khan, F.A.; Zhang, J.; Qi, D. Ameliorative Effects of Grape Seed Proanthocyanidin Extract on Growth Performance, Immune Function, Antioxidant Capacity, Biochemical Constituents, Liver Histopathology and Aflatoxin Residues in Broilers Exposed to Aflatoxin B1. Toxins 2017, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Han, X.-Y.; Huang, Q.-C.; Li, W.-F.; Jiang, J.-F.; Xu, Z.-R. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livest. Sci. 2008, 119, 216–220. [Google Scholar] [CrossRef]
- Li, L.; Wang, H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016, 379, 191–197. [Google Scholar] [CrossRef]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB 1 -induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Zhang, N.Y.; Qi, M.; Zhao, L.; Zhu, M.K.; Guo, J.; Liu, J.; Gu, C.Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.S.; et al. Curcumin Prevents Aflatoxin B(1) Hepatoxicity by Inhibition of Cytochrome P450 Isozymes in Chick Liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Bunderson, B.R.; Croasdell, A.; Reed, K.M.; Coulombe, R.A., Jr. Alpha-class glutathione S-transferases in wild turkeys (Meleagris gallopavo): Characterization and role in resistance to the carcinogenic mycotoxin aflatoxin B1. PLoS ONE 2013, 8, e60662. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Rawal, S.; Kim, J.E.; Coulombe, R., Jr. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef]
- Sun, L.H.; Zhang, N.Y.; Zhu, M.K.; Zhao, L.; Zhou, J.C.; Qi, D.S. Prevention of Aflatoxin B1 Hepatoxicity by Dietary Selenium Is Associated with Inhibition of Cytochrome P450 Isozymes and Up-Regulation of 6 Selenoprotein Genes in Chick Liver. J. Nutr. 2015, 146, 655–661. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Johnson, W.W.; Shimada, T.; Ueng, Y.F.; Yamazaki, H.; Langouët, S. Activation and detoxication of aflatoxin B1. Mutat. Res. 1998, 402, 121–128. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, L.; Makarova, A.V.; Burgers, P.M.; Stone, M.P.; Lloyd, R.S. Error-prone replication bypass of the primary aflatoxin B1 DNA adduct, AFB1-N7-Gua. J. Biol. Chem. 2014, 289, 18497–18506. [Google Scholar] [CrossRef] [Green Version]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Shi, X.; Tang, X.; Wang, Y.; Shen, F.; Zhang, Q.; Wang, C.; Jiang, M.; Liu, M.; Yu, L. Aflatoxin B1 Induces Reactive Oxygen Species-Mediated Autophagy and Extracellular Trap Formation in Macrophages. Front. Cell Infect. Microbiol. 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary Curcumin Alleviated Acute Ileum Damage of Ducks (Anas platyrhynchos) Induced by AFB1 through Regulating Nrf2-ARE and NF-κB Signaling Pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, J.; Cao, Z.; Ji, Q.; Han, Y.; Song, M.; Shao, B.; Li, Y. Lycopene attenuates AFB1-induced renal injury with the activation of the Nrf2 antioxidant signaling pathway in mice. Food Funct. 2018, 9, 6427–6434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Shan, A.; Feng, X. Avian host defense cathelicidins: Structure, expression, biological functions, and potential therapeutic applications. Poult. Sci. 2020, 99, 6434–6445. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Pang, Q.; Yang, H.; Diao, X.; Shan, A.; Feng, X. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (Anas platyrhynchos). Food Chem. 2021, 363, 130263. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.; Suganthi, R.U.; Thammiaha, V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1094–1104. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Gan, Z.; Zhuo, R.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates Aflatoxin B(1)-Induced ROS Formation and Increase of m(6)A RNA Methylation. Animals 2020, 10, 677. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, Y.; Jin, S.; Pang, Q.; Shan, A.; Feng, X. Dietary resveratrol alleviated lipopolysaccharide-induced ileitis through Nrf2 and NF-κB signalling pathways in ducks (Anas platyrhynchos). J. Anim. Physiol. Anim. Nutr. 2021. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Yuan, S.; Peng, X.; Fang, J.; Wang, F.; Cui, H.; Chen, Z.; Yuan, J.; Geng, Y. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicol. Ind. Health 2016, 32, 278–284. [Google Scholar] [CrossRef]
- Rotimi, O.A.; Rotimi, S.O.; Goodrich, J.M.; Adelani, I.B.; Agbonihale, E.; Talabi, G. Time-Course Effects of Acute Aflatoxin B1 Exposure on Hepatic Mitochondrial Lipids and Oxidative Stress in Rats. Front. Pharmacol. 2019, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Gunduz, A.; Yalcin, E.; Cavusoglu, K. Combined toxic effects of aflatoxin B2 and the protective role of resveratrol in Swiss albino mice. Sci. Rep. 2021, 11, 18081. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010, 89, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual Role of Dietary Curcumin Through Attenuating AFB(1)-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB(1) Bioactivation and Detoxification. Front. Pharmacol. 2018, 9, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Nrf2: A main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021, 95, 1521–1533. [Google Scholar] [CrossRef]
- Ji, Y.; Nyamagoud, S.B.; SreeHarsha, N.; Mishra, A.; Gubbiyappa, S.K.; Singh, Y. Sitagliptin protects liver against aflatoxin B1-induced hepatotoxicity through upregulating Nrf2/ARE/HO-1 pathway. Biofactors 2020, 46, 76–82. [Google Scholar] [CrossRef]
- Li, S.; Muhammad, I.; Yu, H.; Sun, X.; Zhang, X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol. Environ. Saf. 2019, 176, 137–145. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, Y.S.; Chen, J.S.; Huang, C.F.; Su, S.L.; Lu, K.C.; Lin, Y.F.; Chu, P.; Lin, S.H.; Sytwu, H.K. Resveratrol ameliorates renal damage, increases expression of heme oxygenase-1, and has anti-complement, anti-oxidative, and anti-apoptotic effects in a murine model of membranous nephropathy. PLoS ONE 2015, 10, e0125726. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, G.; Chen, L.; Ding, Y.; Lian, J.; Hong, G.; Lu, Z. Resveratrol protects mice from paraquat-induced lung injury: The important role of SIRT1 and NRF2 antioxidant pathways. Mol. Med. Rep. 2016, 13, 1833–1838. [Google Scholar] [CrossRef]
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 2012, 84, 1332–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ai, Y.; Niu, X.; Shang, F.; Li, Z.; Liu, H.; Li, W.; Ma, W.; Chen, R.; Wei, T.; et al. Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem.-Biol. Interact. 2020, 317, 108972. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Knutson, M.D.; Leeuwenburgh, C. Resveratrol and novel potent activators of SIRT1: Effects on aging and age-related diseases. Nutr. Rev. 2008, 66, 591–596. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 2010, 332, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Rajendrasozhan, S.; Yang, S.R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, M.H.; Jeong, J.K.; Lee, Y.J.; Seol, J.W.; Jackson, C.J.; Park, S.Y. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthr. Cartil. 2013, 21, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar]
- Wang, W.J.; Xu, Z.L.; Yu, C.; Xu, X.H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017, 88, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, Z.; Duan, C.; Wang, C.; Zhao, Y.; Yang, G.; Gao, L.; Niu, C.; Xu, J.; Li, S. Lactobacillus plantarum C88 protects against aflatoxin B(1)-induced liver injury in mice via inhibition of NF-κB-mediated inflammatory responses and excessive apoptosis. BMC Microbiol. 2019, 19, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, S.A.; Zhang, C.; Feng, Y.; Wei, X.T.; Khalil, M.M.; Rajput, I.R.; Baloch, D.M.; Shaukat, A.; Rajput, N.; Qamar, H.; et al. Proanthocyanidins Alleviates AflatoxinB1-Induced Oxidative Stress and Apoptosis through Mitochondrial Pathway in the Bursa of Fabricius of Broilers. Toxins 2019, 11, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Muhammad, I.; Sun, X.; Han, M.; Hamid, S.; Zhang, X. Protective role of curcumin in ameliorating AFB(1)-induced apoptosis via mitochondrial pathway in liver cells. Mol. Biol. Rep. 2018, 45, 881–891. [Google Scholar] [CrossRef] [PubMed]

| Item | 1–4 Weeks | 5–8 Weeks | 9–10 Weeks |
|---|---|---|---|
| Ingredient | |||
| Corn (7.9) | 61.70 | 68.94 | 75.80 |
| Soybean meal (45) | 26.09 | 26.80 | 20.10 |
| Corn protein flour (55) | 7.90 | — | — |
| Dicalcium phosphate | 1.40 | 1.40 | 1.40 |
| Limestone | 1.08 | 1.06 | 1.06 |
| Salt | 0.38 | 0.38 | 0.38 |
| DL-Methionine | 0.15 | 0.22 | 0.16 |
| L-Lysine | 0.20 | 0.10 | 0.00 |
| choline chloride (50%) | 0.10 | 0.10 | 0.10 |
| Premix | 1.00 1 | 1.00 2 | 1.00 3 |
| Total | 100 | 100 | 100 |
| Nutritional level | |||
| Calculated nutrient 4 | |||
| Metabolizable energy (MJ/kg) | 12.14 | 11.98 | 12.21 |
| CP (%) | 20.67 | 17.51 | 15.03 |
| Calcium (%) | 0.9 | 0.9 | 0.88 |
| Total phosphorus (%) | 0.68 | 0.67 | 0.65 |
| Non-phytate phosphorus (%) | 0.44 | 0.44 | 0.44 |
| Lysine (%) | 1.07 | 0.95 | 0.71 |
| Methionine (%) | 0.48 | 0.48 | 0.39 |
| Methionine +cystine (%) | 0.81 | 0.75 | 0.63 |
| Threonine (%) | 0.75 | 0.66 | 0.56 |
| Tryptophane (%) | 0.21 | 0.19 | 0.16 |
| Transcripts | Sequence (5′–3′) | Product Size (bp) | |
|---|---|---|---|
| Oxidative | Nrf2 | F: GGCCGCCTTGAAGCTCATCTC | 177 |
| stress | R: CTTGCCTCTCCTGCGTATATCTCG | ||
| Keap1 | F: TTTTCAAGACCTCACCCTCCATAAACC | 113 | |
| R: AGTAGCCCAAGGACTGCCGATAG | |||
| HO-1 | F: AGAGCCAGGAGAACGGTCACC | 114 | |
| R: TGCTTGTCCTCTCGCTTTCTTTCC | |||
| CAT | F: ATGGACCAATGTGCGTGACTGAC | 104 | |
| R: CATGCGGCTCTCCTTCACAACAG | |||
| SOD1 | F:TCTCTCTGACTGGACCACACTGC | 100 | |
| R: GTTAGCGTGCTCTCGTTGTCTCC | |||
| NQO1 | F: GCTCTCACGGCACGACATCAC | 84 | |
| R: TCCTTCCACGCTTCTCCCATCTC | |||
| GST | F: GACTTTCTTGTGGGGAACCGACTC | 102 | |
| R: AGGAAACCCAGAAAGCACAGCAG | |||
| GCLM | F: TGTTGTGTGATGCCACCTGATCTC | 150 | |
| R: CCATTCGTGTGCTTTGACGTTCTG | |||
| GCLC | F: TTCAGGTGACATTCCAGGCTTGC | 108 | |
| R: AGAACGGAGATGCAGCACTCAATG | |||
| Detoxification | CYP1A1 | F: AGGACGGAGGCTGACAAGGTG | 104 |
| R: AGGATGGTGGTGAGGAAGAGGAAG | |||
| CYP1A4 | F: AATGCTCGTTTCAGTGCCTTC | 127 | |
| R: CCTCCCCTGTCCTTTTCTCC | |||
| CYP3A4 | F: GACGTGCAGAAGCGACTCCAG | 161 | |
| R: TTCTTGCAGATCCGCTCAATCCG | |||
| Inflammation | IL-10 | F: CAACCTGCTGCTGAGCCTGAAG | 133 |
| R: CGCCTTGTAGATGCCGTTCTCG | |||
| IL-16 | F: TCAACGGACTCGCAGTAACTCAAC | 95 | |
| R: GATCTCTGAAGGGCACGGCTTTC | |||
| IL-18 | F: ACCCTCCATCGCTTCCTTCGG | 113 | |
| R: CCGCTGCCAGATTTCATCTCCTG | |||
| TNF-α | F: CCGTGGTCAGTTTCCATCAGG | 117 | |
| R: ACTTTGCAGTTAGGTGACGCT | |||
| NF-κB | F: GGAGCAGTGGCGGTGTCAAC | 126 | |
| R: AGTGCAGTTCATGTCATCGGTCTC | |||
| Sirt1 | F: TGACAGAGCCTCACATGCAAGTTC | 144 | |
| R: GGTGGAGGGATTGTTTCTGGTAGC | |||
| Apoptosis | Caspase3 | F: TGAGGCAGACAGTGGACCAGATG | 110 |
| R: CTGCATTCCGCCAGGAGTAATAGC | |||
| Caspase9 | F: TGGATTGCGATTCACCCGAAGATG | 83 | |
| R: ATTACCCGAGGGAGCCTGGAAAG | |||
| Bax | F: GCGGACGGAGCCTTCAACTG | 84 | |
| R: CTGCGAGAACAGAGCCTTGATGG | |||
| Bcl-2 | F: GAGTTCGGCGGCGTCATGTG | 155 | |
| R: CCATACAACTCCACGAAGGCATCC | |||
| P53 | F: ACTGCTACGTCGCGGCTCTC | 199 | |
| R: CGCTGGCAAGGCTGGTGAAC | |||
| Internal | β-actin | F: ATGTCGCCCTGGATTTCG | 62 |
| reference | R: ATGTCGCCCTGGATTTCG |
| Item | Control | AFB1 | AFB1 + Res |
|---|---|---|---|
| TP, g/L | 35.83 ± 1.62 a | 31.17 ± 1.14 b | 30.17 ± 0.95 b |
| AST, IU/L | 42.17 ± 9.72 | 45.20 ± 5.72 | 42.60 ± 5.45 |
| ALT, IU/L | 21.20 ± 0.80 b | 34.67 ± 3.04 a | 31.25 ± 1.49 a |
| ALP, IU/L | 285.75 ± 11.46 | 312.00 ± 18.80 | 304.25 ± 39.19 |
| TBIL, μmol/L | 1.43 ± 0.12 | 1.37 ± 0.049 | 1.32 ± 0.07 |
| ALB, g/L | 17.27 ± 0.60 a | 15.83 ± 0.55 a,b | 15.43 ± 0.44 b |
| GLO, g/L | 18.57 ± 1.1 a | 15.33 ± 0.65 b | 14.70 ± 0.64 b |
| LDH, U/L | 1042.24 ± 6.75 b | 1219.82 ± 62.32 a | 1126.60 ± 34.06 a,b |
| Item | Control | AFB1 | AFB1 + Res |
|---|---|---|---|
| SOD, U/mg | 572.25 ± 16.70 a | 382.44 ± 8.52 b | 538.71 ± 3.98 a |
| T-AOC, U/mg | 3.82 ± 0.09 a | 1.69 ± 0.08 c | 2.77 ± 0.13 b |
| H2O2, mmol/g | 7.50 ± 0.26 b | 8.30 ± 0.56 a | 7.19 ± 0.2 a,b |
| CAT, U/mg | 31.83 ± 0.49 a | 18.35 ± 1.51 c | 26.01 ± 0.52 b |
| MDA, U/mg | 1.17 ± 0.12 | 1.27 ± 0.10 | 1.29 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, Y.; Zhou, X.; Liu, M.; Jin, S.; Shan, A.; Feng, X. Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals 2021, 11, 3516. https://doi.org/10.3390/ani11123516
Liu F, Wang Y, Zhou X, Liu M, Jin S, Shan A, Feng X. Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals. 2021; 11(12):3516. https://doi.org/10.3390/ani11123516
Chicago/Turabian StyleLiu, Fangju, Yingjie Wang, Xin Zhou, Mengru Liu, Sanjun Jin, Anshan Shan, and Xingjun Feng. 2021. "Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways" Animals 11, no. 12: 3516. https://doi.org/10.3390/ani11123516
APA StyleLiu, F., Wang, Y., Zhou, X., Liu, M., Jin, S., Shan, A., & Feng, X. (2021). Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals, 11(12), 3516. https://doi.org/10.3390/ani11123516





