Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
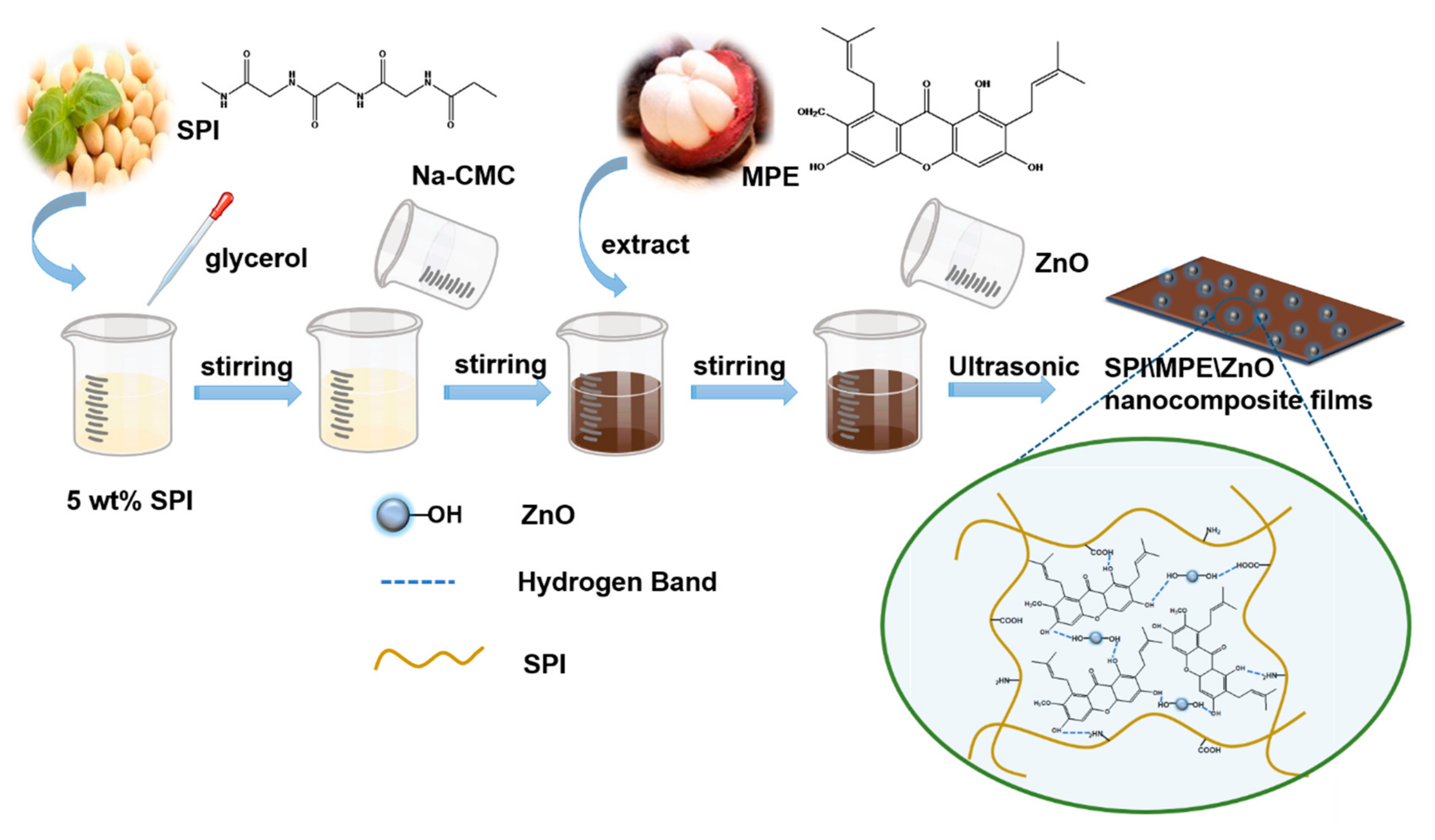
2.2. Preparation of Nanocomposite Films
2.3. Characterization of Films
2.3.1. Mechanical Properties
2.3.2. Water Solubility (WS)
2.3.3. Water Vapour Permeability (WVP)
2.3.4. Water Contact Angle (WCA)
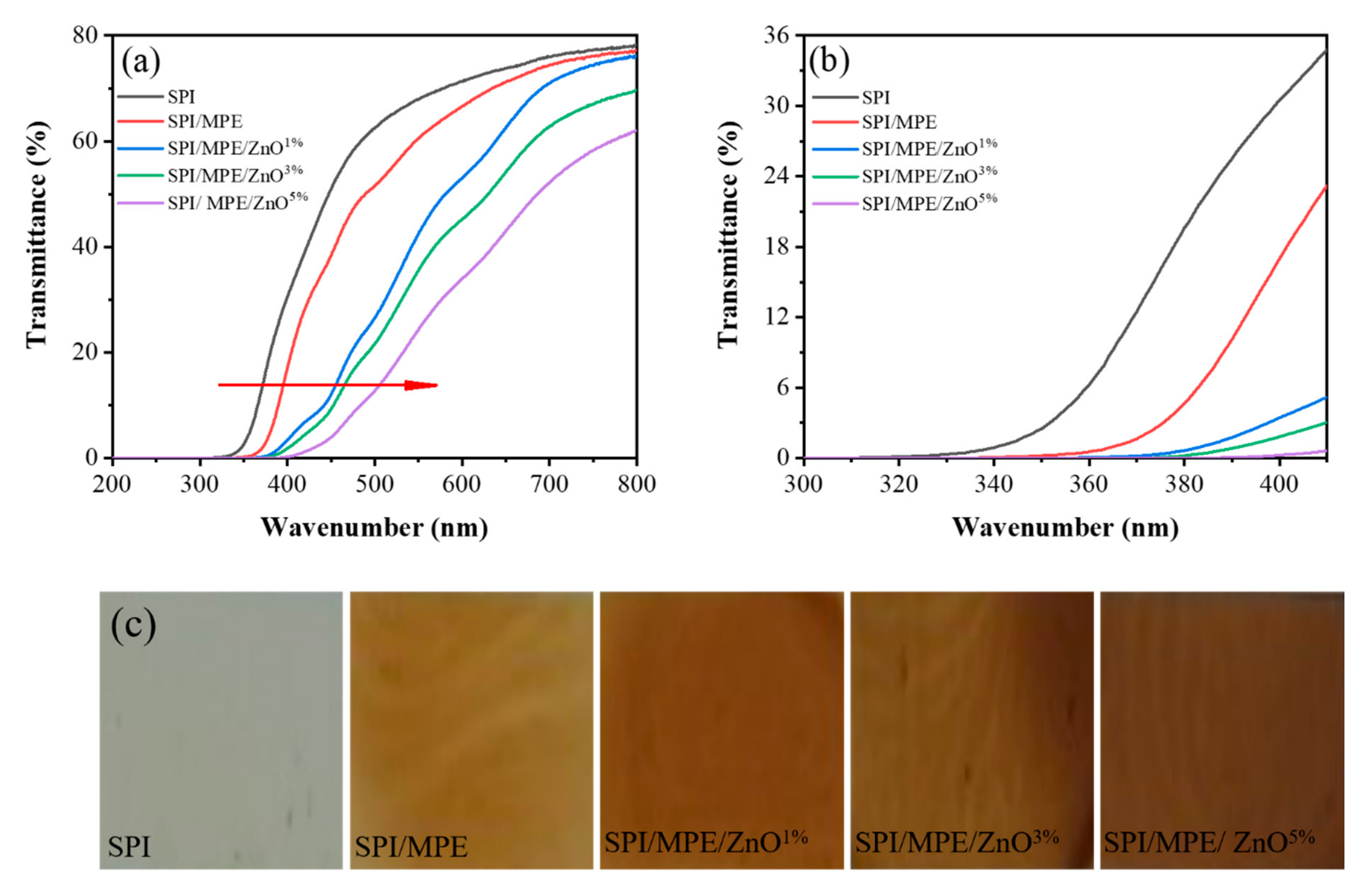
2.3.5. Films Transmittance and Opacity
2.3.6. Colour Measurement
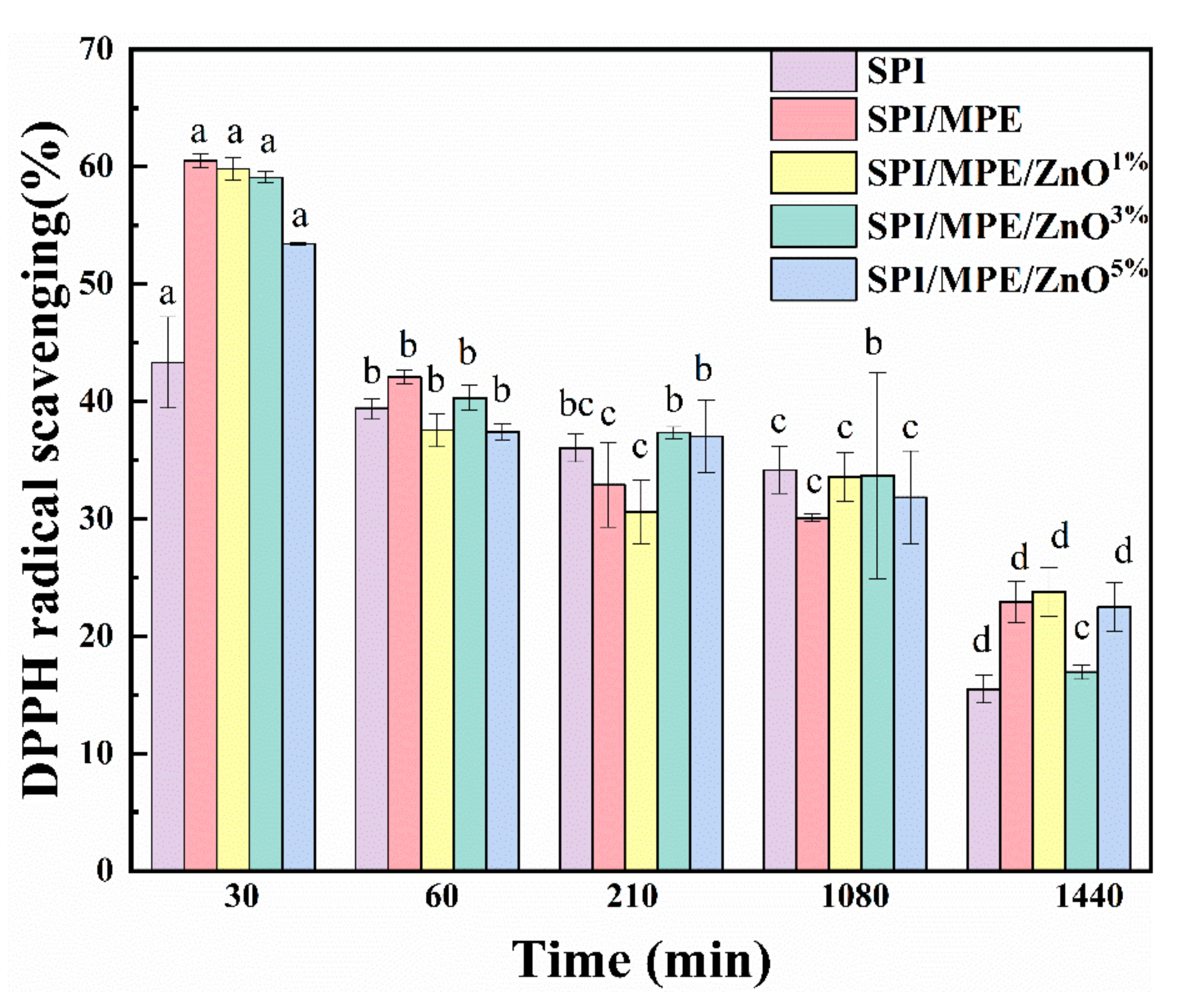
2.3.7. Antioxidant Activity
2.3.8. Antimicrobial Activity
2.3.9. Film Characterization
2.4. Statistical Analysis
3. Results
3.1. Mechanical Properties
3.2. WS, WVP, and WCA
3.3. UV-Vis Light Barrier Property, Colour, and Opacity
3.4. Antioxidant Activity and Antimicrobial Activity
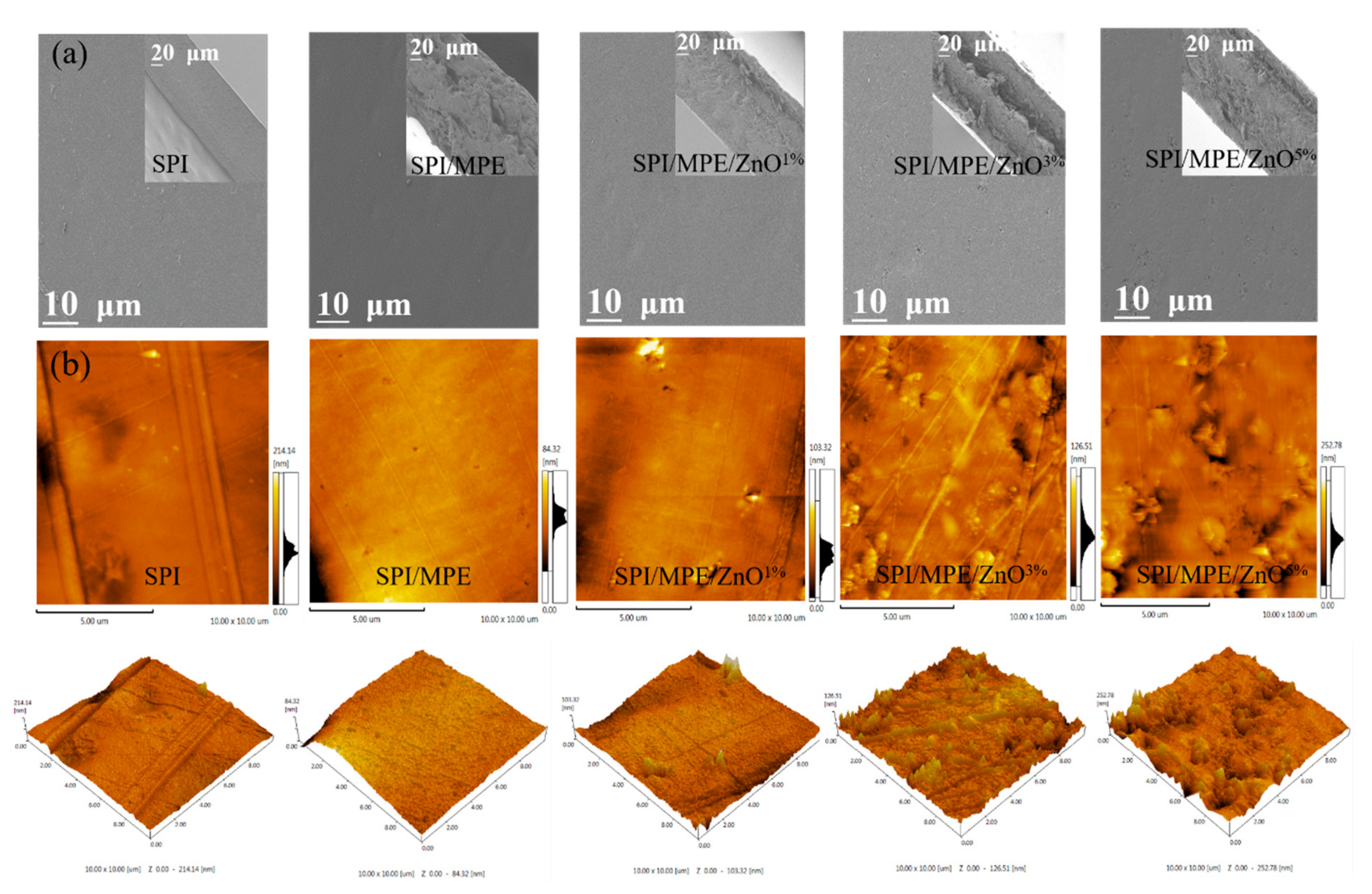
3.5. SEM and AFM
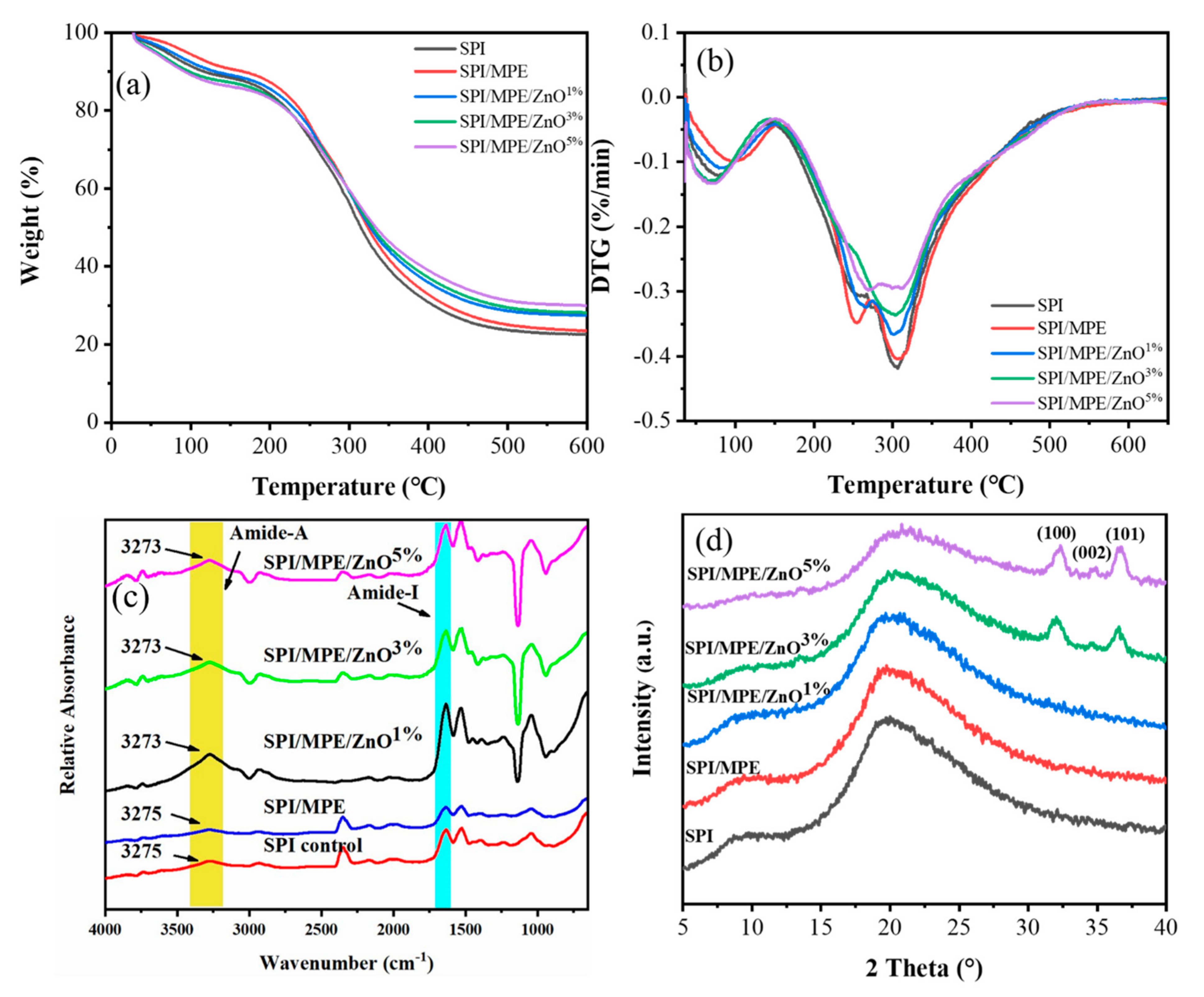
3.6. TGA
3.7. ATR-FTIR, XRD, and XPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The Potential of Proteins for Producing Food Packaging Materials: A Review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.W. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Environment friendly green composites based on soy protein isolate—A review. Food Hydrocoll. 2015, 50, 174–192. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Tao, Y.; Ling, Z.; Huang, C.; Lai, C.; Yong, Q. Fabrication of anti-bacterial, hydrophobic and UV resistant galactomannan-zinc oxide nanocomposite films. Polymer 2021, 215, 123412. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yong, H.; Qin, Y.; Liu, J.; Jin, C. Development of antioxidant and antimicrobial packaging films based on chitosan and mangosteen (Garcinia mangostana L.) rind powder. Int. J. Biol. Macromol. 2020, 145, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Chaivisuthangkura, A.; Malaikaew, Y.; Chaovanalikit, A.; Jaratrungtawee, A.; Panseeta, P.; Ratananukul, P.; Suksamrarn, S. Prenylated Xanthone Composition of Garcinia mangostana (Mangosteen) Fruit Hull. Chromatographia 2008, 69, 315–318. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Biological activity and stability of mangosteen as a potential natural color. Biosci. Biotechnol. Biochem. 2011, 75, 2257–2259. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Lin, Y.-M.; Wei, S.-D.; Tam, N.F.-Y. Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chem. 2011, 129, 1710–1720. [Google Scholar] [CrossRef]
- Hiranrangsee, L.; Kumaree, K.K.; Sadiq, M.B.; Anal, A.K. Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by Ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. J. Food Sci. Technol. 2016, 53, 3806–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widyarman, A.S.; Lay, S.H.; Wendhita, I.P.; Tjakra, E.E.; Murdono, F.I.; Binartha, C.T.O. Indonesian Mangosteen Fruit (Garcinia mangostana L.) Peel Extract Inhibits Streptococcus mutans and Porphyromonas gingivalis in Biofilms In vitro. Contemp. Clin. Dent. 2019, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2020, 13, 17–26. [Google Scholar] [CrossRef]
- Feng, Z.; Lu, X.; Gan, L.; Zhang, Q.; Lin, L. Xanthones, A Promising Anti-Inflammatory Scaffold: Structure, Activity, and Drug Likeness Analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef] [Green Version]
- Rukachaisirikul, V.; Phainuphong, P.; Sukpondma, Y.; Phongpaichit, S.; Taylor, W.C. Antibacterial caged-tetraprenylated xanthones from the stem bark of Garcinia scortechinii. Planta Med. 2005, 71, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Na, Y. Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 2009, 61, 707–712. [Google Scholar] [CrossRef]
- Ioset, J.R.; Marston, A.; Gupta, M.P.; Hostettmann, K. Antifungal Xanthones from Roots of Marila laxiflora. Pharm. Biol. 2004, 36, 103–106. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Dai, Q.; Huang, X.; Qin, Z. Preparation and characterizations of antibacterial–antioxidant film from soy protein isolate incorporated with mangosteen peel extract. e-Polymers 2021, 21, 575–589. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll. 2011, 25, 1085–1097. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Fabrication of natural-origin antibacterial nanocellulose films using bio-extracts for potential use in biomedical industry. Int. J. Biol. Macromol. 2020, 145, 914–925. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba Lophatheri extract on the physicochemical properties and biological activities of the chitosan film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crops Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT-Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Baek, S.-K.; Song, K.B. Development of Gracilaria vermiculophylla extract films containing zinc oxide nanoparticles and their application in smoked salmon packaging. LWT 2018, 89, 269–275. [Google Scholar] [CrossRef]
- Ramji, V.; Vishnuvarthanan, M. Influence of NiO Supported Silica Nanoparticles on Mechanical, Barrier, Optical and Antibacterial Properties of Polylactic Acid (PLA) Bio Nanocomposite Films for Food Packaging Applications. Silicon 2020. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles againstListeria monocytogeneson mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Anitha, S.; Brabu, B.; John Thiruvadigal, D.; Gopalakrishnan, C.; Natarajan, T.S. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2013, 97, 856–863. [Google Scholar] [CrossRef]
- Ahmadi, R.; Tanomand, A.; Kazeminava, F.; Kamounah, F.S.; Ayaseh, A.; Ganbarov, K.; Yousefi, M.; Katourani, A.; Yousefi, B.; Kafil, H.S. Fabrication and characterization of a titanium dioxide (TiO2) nanoparticles reinforced bio-nanocomposite containing Miswak (Salvadora persica L.) extract—The antimicrobial, thermo-physical and barrier properties. Int. J. Nanomed. 2019, 14, 3439–3454. [Google Scholar] [CrossRef] [Green Version]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; do Amaral Sobral, P.J.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Breda, C.A.; Morgado, D.L.; Assis, O.B.G.; Duarte, M.C.T. Processing and characterization of chitosan films with incorporation of ethanolic extract from “pequi” peels. Macromol. Res. 2017, 25, 1049–1056. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L. Developing a bio-based packaging film from soya by-products incorporated with valonea tannin. J. Clean. Prod. 2017, 143, 624–633. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.; Ma, Q.; Wang, L. Physical and antioxidant properties of flexible soy protein isolate films by incorporating chestnut (Castanea mollissima) bur extracts. LWT-Food Sci. Technol. 2016, 71, 33–39. [Google Scholar] [CrossRef]
- Yang, H.; Wen, X.L.; Guo, S.G.; Chen, M.T.; Jiang, A.M.; Lai, L.S. Physical, antioxidant and structural characterization of blend films based on hsian-tsao gum (HG) and casein (CAS). Carbohydr. Polym. 2015, 134, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Et Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, K.; Wang, Z.; Gindl-Altmutter, W.; Zhang, S.; Li, J. Fabrication of homogeneous and enhanced soybean protein isolate-based composite films via incorporating TEMPO oxidized nanofibrillated cellulose stablized nano-ZnO hybrid. Cellulose 2017, 24, 4807–4819. [Google Scholar] [CrossRef]
- Fang, Q.; Zhu, M.; Yu, S.; Sui, G.; Yang, X. Studies on soy protein isolate/polyvinyl alcohol hybrid nanofiber membranes as multi-functional eco-friendly filtration materials. Mater. Sci. Eng. B 2016, 214, 1–10. [Google Scholar] [CrossRef]
- Fu, F.; Li, L.; Liu, L.; Cai, J.; Zhang, Y.; Zhou, J.; Zhang, L. Construction of cellulose based ZnO nanocomposite films with antibacterial properties through one-step coagulation. ACS Appl. Mater. Interfaces 2015, 7, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Garrido, T.; Leceta, I.; de la Caba, K. Films based on proteins and polysaccharides: Preparation and physical–chemical characterization. Eur. Polym. J. 2013, 49, 3713–3721. [Google Scholar] [CrossRef]
- Liu, X.; Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Simultaneously Toughening and Strengthening Soy Protein Isolate-Based Composites via Carboxymethylated Chitosan and Halloysite Nanotube Hybridization. Materials 2017, 10, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Films | Thickness (mm) | TS (MPa) | EAB (%) | WS (%) | WVP × 10−3 (g mm/m2 h Pa) | WCA (°) |
|---|---|---|---|---|---|---|
| SPI | 0.10 ± 0.00 c | 5.02 ± 0.11 c | 82.87 ± 1.96 d | 41.86 ± 5.19 a | 19.53 ± 3.28 a | 57.80 ± 3.97 c |
| SPI/MPE | 0.14 ± 0.0 b | 6.57 ± 0.10 b | 59.95 ± 4.96 c | 33.92 ± 0.08 b | 19.23 ± 3.76 a | 71.30 ± 3.78 a |
| SPI/MPE/ZnO1% | 0.19 ± 0.04 a | 8.11 ± 0.26 a | 75.23 ± 27.98 b | 31.76 ± 0.30 bc | 19.67 ± 2.97 a | 75.08 ± 0.30 a |
| SPI/MPE/ZnO3% | 0.16 ± 0.0 b | 8.23 ± 0.31 a | 53.95 ± 6.68 b | 30.30 ± 1.69 bc | 13.67 ± 8.74 ab | 62.78 ± 0.28 b |
| SPI/MPE/ZnO5% | 0.12 ± 0.06 c | 8.84 ± 0.48 a | 47.88 ± 17.34 a | 28.71 ± 1.07 c | 9.23 ± 4.70 b | 58.75 ± 0.81 bc |
| Films | Bacteria | Viable Colony Numbers (CFU/mL) | Antibacterial Potency (%) |
|---|---|---|---|
| SPI | E. coli | 926 ± 4.24 a | 0 |
| S. aureus | 813 ± 32.53 a | 0 | |
| SPI/MPE | E. coli | 730 ± 8.49 b | 21.17 |
| S. aureus | 329 ± 6.36 b | 59.53 | |
| SPI/MPE/ZnO1% | E. coli | 512 ± 33.23 c | 44.71 |
| S. aureus | 57 ± 0.71 c | 92.99 | |
| SPI/MPE/ZnO3% | E. coli | 0 | 100 |
| S. aureus | 22 ± 1.41 c | 97.29 | |
| SPI/MPE/ZnO5% | E. coli | 0 | 100 |
| S. aureus | 21 ± 0.71 c | 97.42 |
| Films | % α-Helix | % β-Sheet | % β-Turn | Random Coil (%) |
|---|---|---|---|---|
| SPI | 13.02 | 51.81 | 18.34 | 13.64 |
| SPI/MPE | 12.90 | 52.52 | 21.22 | 13.36 |
| SPI/MPE/ZnO1% | 15.89 | 50.99 | 14.85 | 18.27 |
| SPI/MPE/ZnO3% | 15.13 | 50.59 | 16.99 | 17.29 |
| SPI/MPE/ZnO5% | 14.86 | 50.30 | 17.60 | 17.24 |
| Films | C (at. %) | O (at. %) | N (at. %) | Zn (at. %) |
|---|---|---|---|---|
| SPI | 69.12 | 18.94 | 8.04 | 0 |
| SPI/MPE | 65.62 | 22.41 | 8.15 | 0 |
| SPI/MPE/ZnO1% | 65.19 | 22.74 | 8.57 | 0.1 |
| SPI/MPE/ZnO3% | 65.51 | 22.25 | 7.84 | 0.35 |
| SPI/MPE/ZnO5% | 68.14 | 20.67 | 7.43 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhou, X.; Dai, Q.; Qin, Z. Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles. Nanomaterials 2021, 11, 3337. https://doi.org/10.3390/nano11123337
Huang X, Zhou X, Dai Q, Qin Z. Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles. Nanomaterials. 2021; 11(12):3337. https://doi.org/10.3390/nano11123337
Chicago/Turabian StyleHuang, Xi, Xin Zhou, Qingyin Dai, and Zhiyong Qin. 2021. "Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles" Nanomaterials 11, no. 12: 3337. https://doi.org/10.3390/nano11123337
APA StyleHuang, X., Zhou, X., Dai, Q., & Qin, Z. (2021). Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles. Nanomaterials, 11(12), 3337. https://doi.org/10.3390/nano11123337





