Nutritive Value Variation and In Vitro Digestibility of Hempseed Meal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Variability in Nutritive Value
2.3. In Vitro Dry Matter Digestibility Experiments
2.4. Statistical Analysis
3. Results
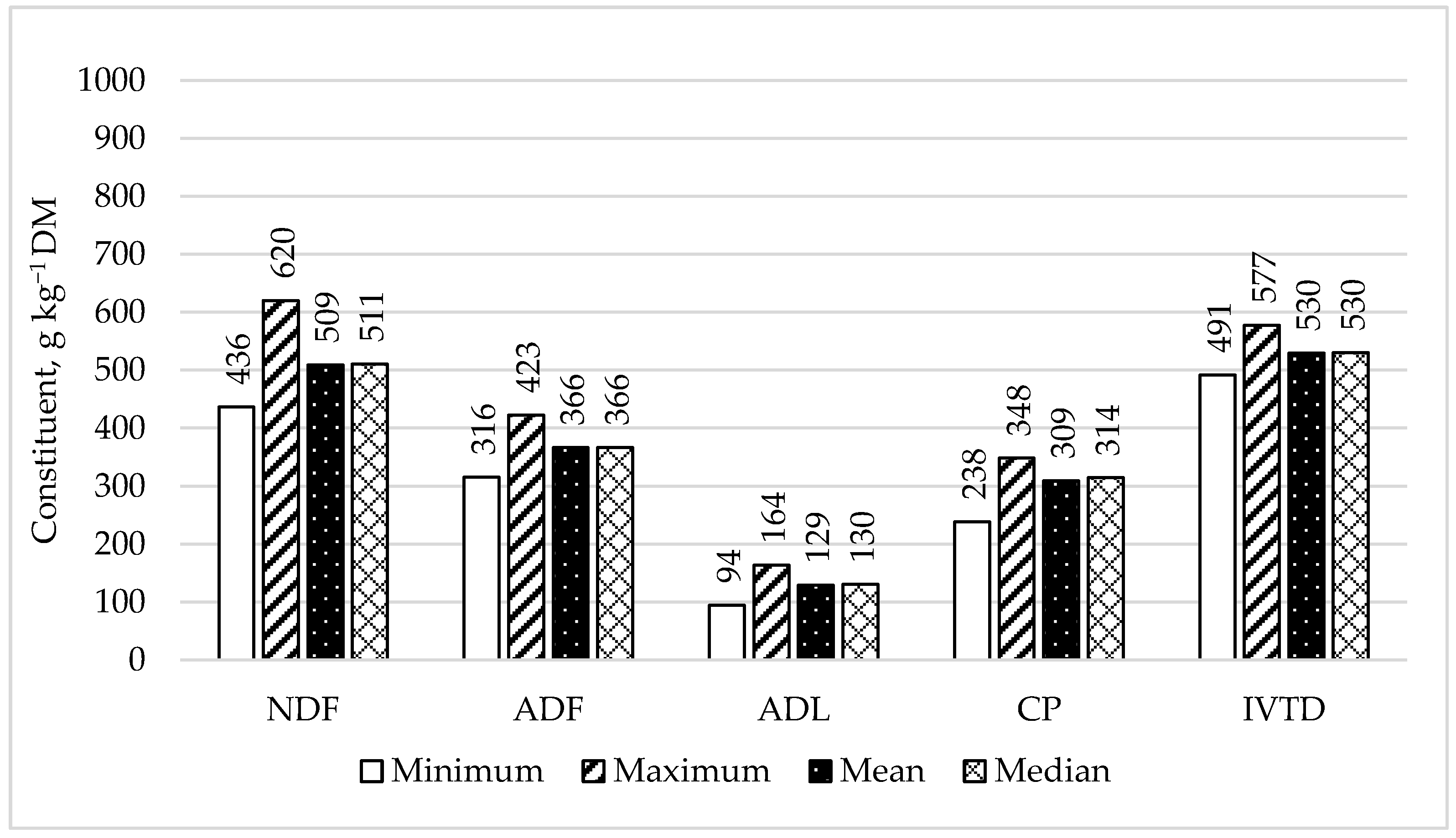
3.1. Variability in Nutritive Value
3.2. In Vitro Dry Matter Digestibility
4. Discussion
4.1. Variability in Nutritive Value
Sources of Nutritive Value Variation
4.2. In Vitro Dry Matter Digestibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Congress. 2018 United States Farm Bill. H.R. 2, 115th Congress, Washington, DC, USA. 2018. Available online: https://www.ams.usda.gov/sites/default/files/media/2018FarmBill.pdf (accessed on 23 August 2021).
- Sawyer, J.; Stout, J.M.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The genetic structure of marijuana and hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef] [Green Version]
- Pojić, M.; Mišan, A.; Sakač, M.; Hadnađev, T.D.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of byproducts originating from hemp oil processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Ruiz-Morena, M.; Stern, M.D.; Martinsson, K. Effects of temperature during moist heat treatment on ruminal degradability and intestinal digestibility of protein and amino acids in hempseed cake. Asian-Australas. J. Anim. Sci. 2012, 25, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, A.F.; McKinnon, J.J.; Christensen, D.A. The nutritive value of hemp meal for ruminants. Can. J. Anim. Sci. 1998, 79, 91–95. [Google Scholar] [CrossRef]
- Silversides, F.G.; LefranÇois, M.R. The effect of feeding hemp seed meal to laying hens. Br. Poult. Sci. 2005, 46, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Finell, M.; Martinsson, K. Effects of increasing amounts of hempseed cake in the diet of dairy cows on the production and composition of milk. Animal 2010, 4, 1854–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in seed traits in a collection of Cannabis sativa L. genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984. [Google Scholar]
- Vogel, K.P.; Pedersen, J.F.; Masterson, S.D.; Toy, J.J. Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop Sci. 1999, 39, 276–279. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B. Systems of analysis for evaluating fibrous feeds. In Standardization of Analytical Methodology of Feeds, Proceedings of the International Workshop, Ottawa, ON, Canada, 12–14 March 1980; Pigden, W.J., Ed.; Unipub: New York, NY, USA, 1980; pp. 49–60. [Google Scholar]
- Lowrey, R.S. The nylon bag technique for the estimation of forage quality. In Proceedings of the National Conference on Forage Quality, Evaluation, and Utilization, Lincoln, NE, USA, 14 September 1969; University of Georgia: Athens, GA, USA, 1969; pp. 1–12. [Google Scholar]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- ANKOM Technology. Method 3: In Vitro True Digestibility Using the DaisyII Incubator. 2017. Available online: https://www.ankom.com/sites/default/files/document-files/D200_D2001_Manual.pdf (accessed on 23 August 2021).
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Royston, P. Approximating the Shapiro-Wilk W-Test for non-normality. Stat. Comput. 1992, 2, 117–119. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemp Feed Coalition; Loveland, CO, USA. Personal communication, 2021.
- Hessel, A.; Eriksson, M.; Nadeau, E.; Turner, T.; Johansson, B. Cold-pressed hempseed cake as a protein feed for growing cattle. Acta Agric. Scand. Sect. A 2008, 58, 136–145. [Google Scholar] [CrossRef]
- Suriyong, S.; Vearasilp, S.; Krittigamas, N.; Rinmanee, S.; Punyalue, A. Effect of seed maturity on seed physiological quality, oil content and fatty acid composition of hemp seed. Chian Mai Univ. J. Nat. Sci. 2012, 11, 351–358. [Google Scholar]
- Sainio, P.P.; Jauhiainen, L.; Hakala, K.; Ojanen, H. Climate changes and prolongation of growing season: Changes in regional potential for field crop production in Finland. Agric. Food Sci. 2009, 18, 171–190. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Evaluation of protein concentration, amino acid profile, and antinutritional compounds in hempseed meal from dioecious and monoecious varieties. Am. J. Plant Sci. 2015, 6, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Grieshop, C.M.; Kadzere, C.T.; Clapper, G.M.; Flickinger, E.A.; Bauer, L.L.; Frazier, R.L.; Fahey, G.C. Chemical and nutritional characteristics of United States soybeans and soybean meals. J. Agric. Food Chem. 2003, 51, 7684–7691. [Google Scholar] [CrossRef] [PubMed]
| Experiment 1: Hempseed Meal Replacement of Concentrate, g kg−1 | Experiment 2: Hempseed Meal Replacement of Dietary Crude Protein, g kg−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feedstuff | 0 | 250 | 500 | 750 | 1000 | 0 | 250 | 500 | 750 |
| Steam-flaked corn | 600 | 450 | 300 | 150 | - | 310 | 310 | 310 | 315 |
| Bermudagrass hay | 400 | 400 | 400 | 400 | 400 | - | - | 120 | 250 |
| Alfalfa meal pellets | - | - | - | - | - | 620 | 530 | 280 | - |
| Soybean meal | - | - | - | - | - | 70 | 15 | - | - |
| Hempseed meal | - | 150 | 300 | 450 | 600 | - | 145 | 290 | 435 |
| Nutritive value | |||||||||
| NDF | - | - | - | - | - | 295 | 284 | 326 | 340 |
| ADF | - | - | - | - | - | 213 | 210 | 220 | 207 |
| ADL | - | - | - | - | - | 50 | 59 | 59 | 53 |
| CP | - | - | - | - | - | 120 | 140 | 150 | 190 |
| Effect | Estimate | SE 1 | Z-Value | p-Value | Contribution to Variance 2 |
|---|---|---|---|---|---|
| NDF | |||||
| Rep (source by batch) | 0 | - | - | - | - |
| Batch (source) | 0.000969 | 0.000434 | 2.23 | 0.01 | 65.08 |
| Source | 0 | - | - | - | - |
| Residual | 0.000520 | 0.000134 | 3.87 | < 0.01 | 34.92 |
| ADF | |||||
| Rep (source by batch) | 0 | - | - | - | - |
| Batch (source) | 0.000381 | 0.000172 | 2.21 | 0.01 | 44.82 |
| Source | 0.000375 | 0.000441 | 0.85 | 0.20 | 44.12 |
| Residual | 0.000094 | 0.000024 | 3.87 | <0.01 | 11.06 |
| ADL | |||||
| Rep (source by batch) | 0 | - | - | - | - |
| Batch (source) | 0.000287 | 0.000121 | 2.38 | <0.01 | 68.50 |
| Source | 0.000096 | 0.000143 | 0.67 | 0.25 | 22.91 |
| Residual | 0.000036 | 0.000009 | 3.87 | <0.01 | 8.59 |
| CP | |||||
| Rep (source by batch) | 0 | - | - | - | - |
| Batch (source) | 0.000964 | 0.000371 | 2.60 | <0.01 | 96.50 |
| Source | 0 | - | - | - | - |
| Residual | 0.000035 | 0.000013 | 2.74 | <0.01 | 3.50 |
| IVTD | |||||
| Rep (source by batch) | 0 | - | - | - | - |
| Batch (source) | 0.000050 | 0000068 | 0.73 | 0.23 | 9.58 |
| Source | 0 | - | - | - | - |
| Residual | 0.000472 | 0.000100 | 4.74 | <0.01 | 90.42 |
| Hempseed Meal Replacement of Concentrate, g kg−1 | Contrasts | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 250 | 500 | 750 | 1000 | Linear | Quadratic | |
| IVDMD, g kg−1 DM | 406 a | 379 ab | 332 ab | 285 ab | 225 b | <0.01 | 0.56 |
| Hempseed Meal Replacement of Crude Protein, g kg−1 | Contrasts | |||||
|---|---|---|---|---|---|---|
| 0 | 250 | 500 | 750 | Linear | Quadratic | |
| IVDMD, g kg−1 DM | 771 a | 716 b | 693 b | 643 c | <0.01 | 0.86 |
| Cultivar | Extraction Method | CP 1 | NDF 2 | ADF 3 | ADL 4 | Reference |
|---|---|---|---|---|---|---|
| CAN19 | Chemical | 345 | - | - | - | [8] |
| CAN20 | Chemical | 356 | - | - | - | [8] |
| CAN24 | Chemical | 331 | - | - | - | [8] |
| CAN 26 | Chemical | 345 | - | - | - | [8] |
| CAN39 | Chemical | 320 | - | - | - | [8] |
| CAN40 | Chemical | 354 | - | - | - | [8] |
| CAN48 | Chemical | 339 | - | - | - | [8] |
| CAN51 | Chemical | 336 | - | - | - | [8] |
| CAN58 | Chemical | 346 | - | - | - | [8] |
| Finola | Chemical | 317 | - | - | - | [8] |
| Carmagnola | Chemical | 334 | - | - | - | [8] |
| CS | Chemical | 316 | - | - | - | [8] |
| Fibranova | Chemical | 325 | - | - | - | [8] |
| Fedora | Chemical | 339 | - | - | - | [8] |
| Futura 75 | Chemical | 337 | - | - | - | [8] |
| Felina 32 | Chemical | 343 | - | - | - | [8] |
| Ferimon | Chemical | 344 | - | - | - | [8] |
| Codimono | Chemical | 336 | - | - | - | [8] |
| Carmaleonte | Chemical | 345 | - | - | - | [8] |
| Kc Dora | Chemical | 332 | - | - | - | [8] |
| Various | Cold-pressed | 335 | 436 | 346 | - | [19] |
| Finola | Cold-pressed | 385 | 449 | - | - | [20] |
| Finola | Cold-pressed | 369 | 434 | - | - | [20] |
| - | - | 344 | 393 | 103 | - | [7] |
| Finola | Cold-pressed | 336 | 382 | 336 | - | [4] |
| - | - | 320.8 | 507.9 | 390.4 | 131.9 | [5] |
| Carmagnola | Chemical | 337 | - | - | - | [21] |
| CS | Chemical | 348 | - | - | - | [21] |
| Fibranova | Chemical | 351 | - | - | - | [21] |
| Futura 75 | Chemical | 342 | - | - | - | [21] |
| Felina 32 | Chemical | 351 | - | - | - | [21] |
| Ferimon | Chemical | 331 | - | - | - | [21] |
| Unika-b | Cold-pressed | 249 | 372 | - | - | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobson, K.J.; Kinman, L.A.; Owsley, W.F.; Muir, J.P.; Smith, W.B. Nutritive Value Variation and In Vitro Digestibility of Hempseed Meal. Animals 2021, 11, 3481. https://doi.org/10.3390/ani11123481
Jacobson KJ, Kinman LA, Owsley WF, Muir JP, Smith WB. Nutritive Value Variation and In Vitro Digestibility of Hempseed Meal. Animals. 2021; 11(12):3481. https://doi.org/10.3390/ani11123481
Chicago/Turabian StyleJacobson, Kristen June, Lea Ann Kinman, Walter Franklin Owsley, James Pierre Muir, and William Brandon Smith. 2021. "Nutritive Value Variation and In Vitro Digestibility of Hempseed Meal" Animals 11, no. 12: 3481. https://doi.org/10.3390/ani11123481
APA StyleJacobson, K. J., Kinman, L. A., Owsley, W. F., Muir, J. P., & Smith, W. B. (2021). Nutritive Value Variation and In Vitro Digestibility of Hempseed Meal. Animals, 11(12), 3481. https://doi.org/10.3390/ani11123481






