Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Viscosity and Water Content of DES
2.3. Electrochemical Measurements
2.4. Carbon Material Modification
2.5. Carbon Material Characterization
3. Results and Discussion
3.1. Viscosity and Water Content of Ethaline 200
3.2. Characterization of the Carbon Materials
3.2.1. Surface Area by Brunauer–Emmet–Teller Method
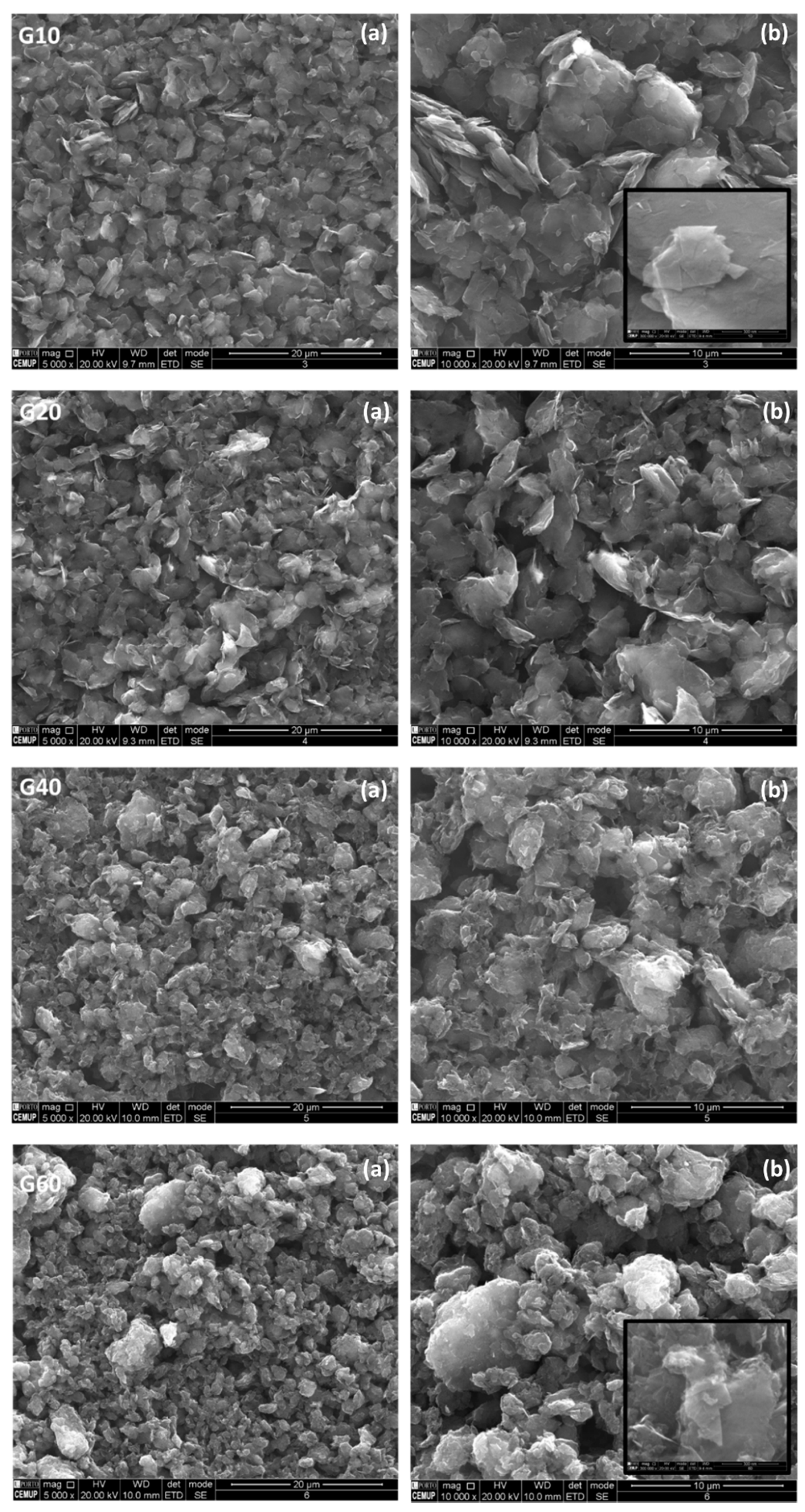
3.2.2. Scanning Electron Microscopy Characterization
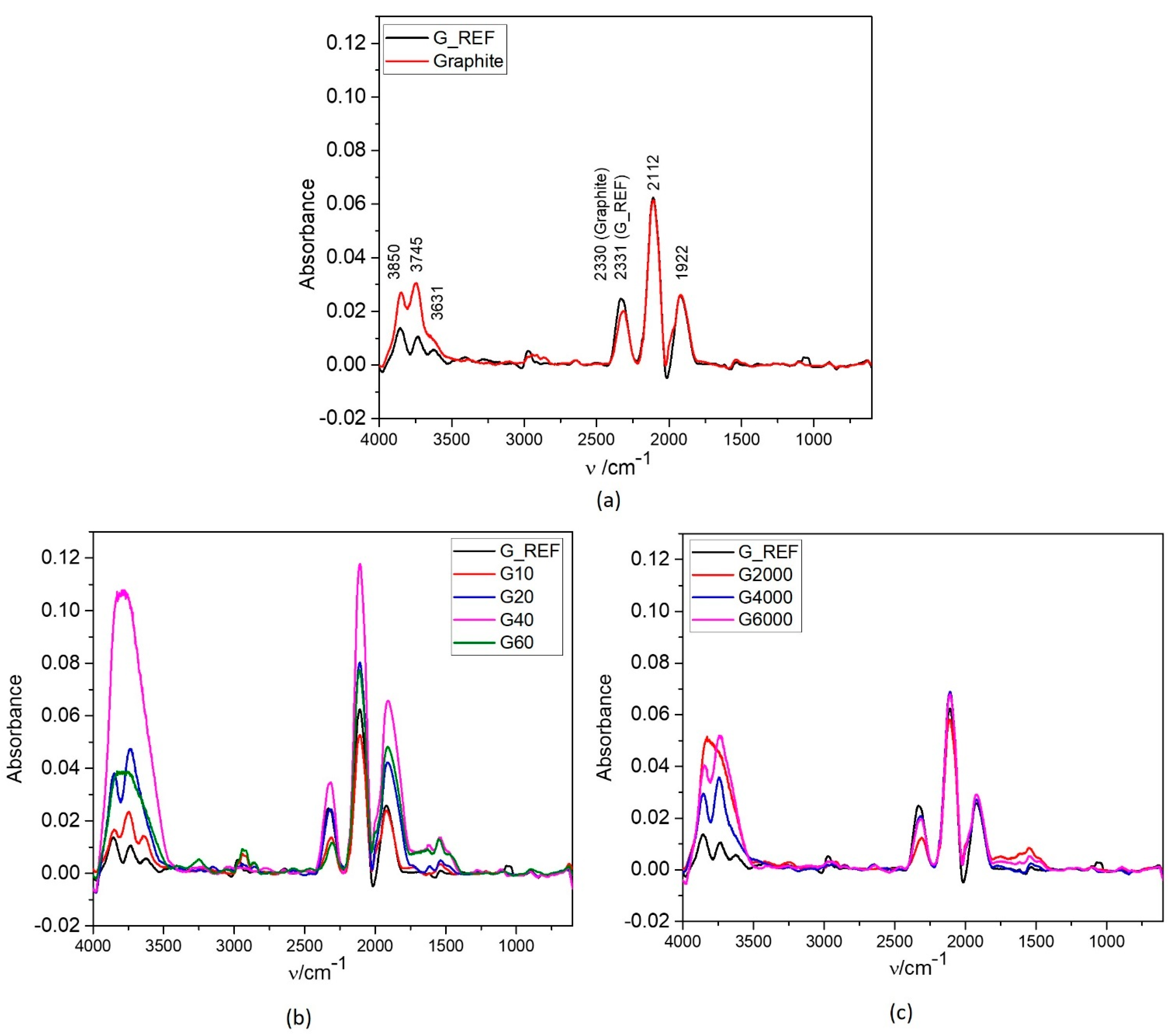
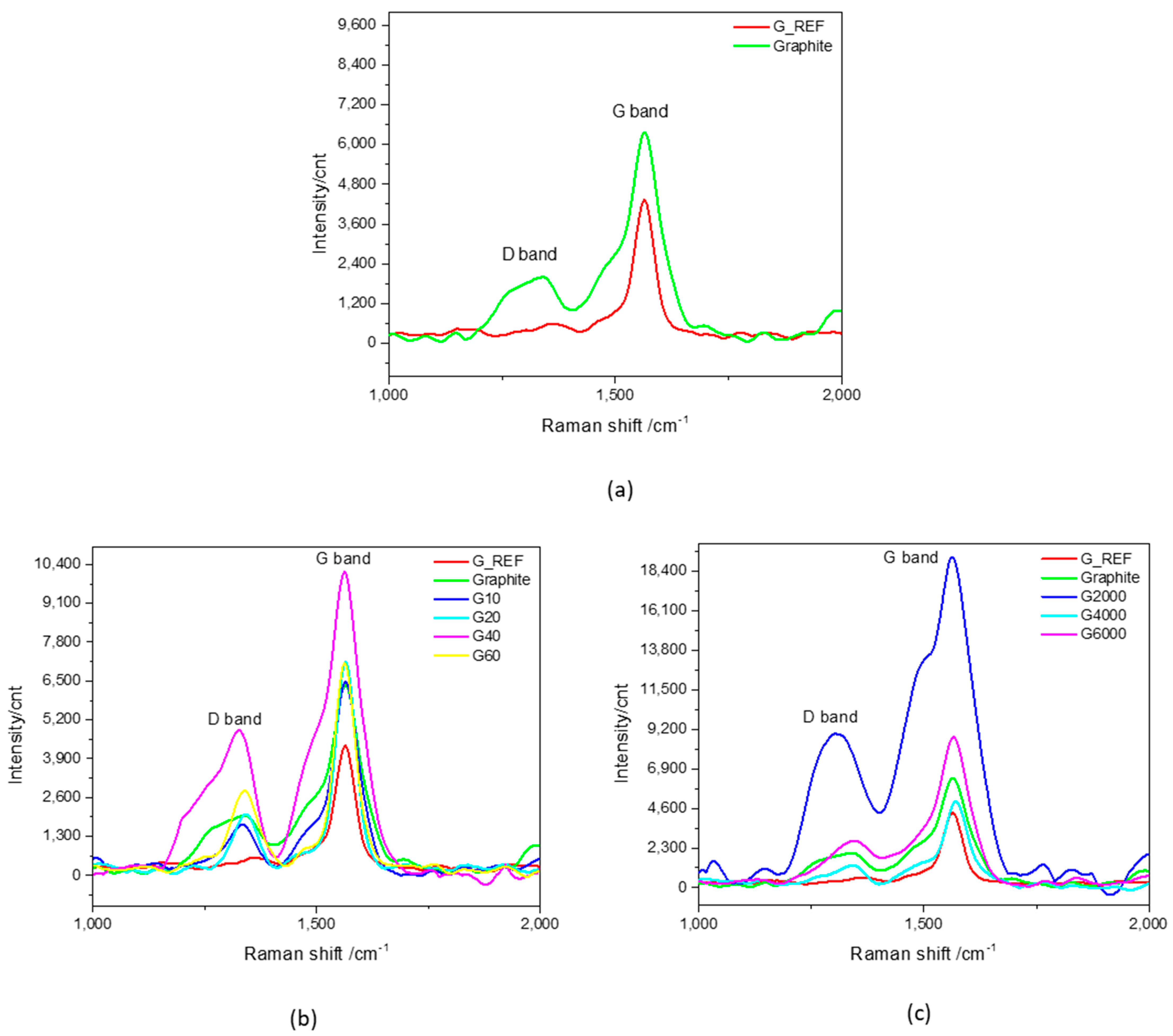
3.2.3. ATR−IR and Raman Spectroscopy Characterization
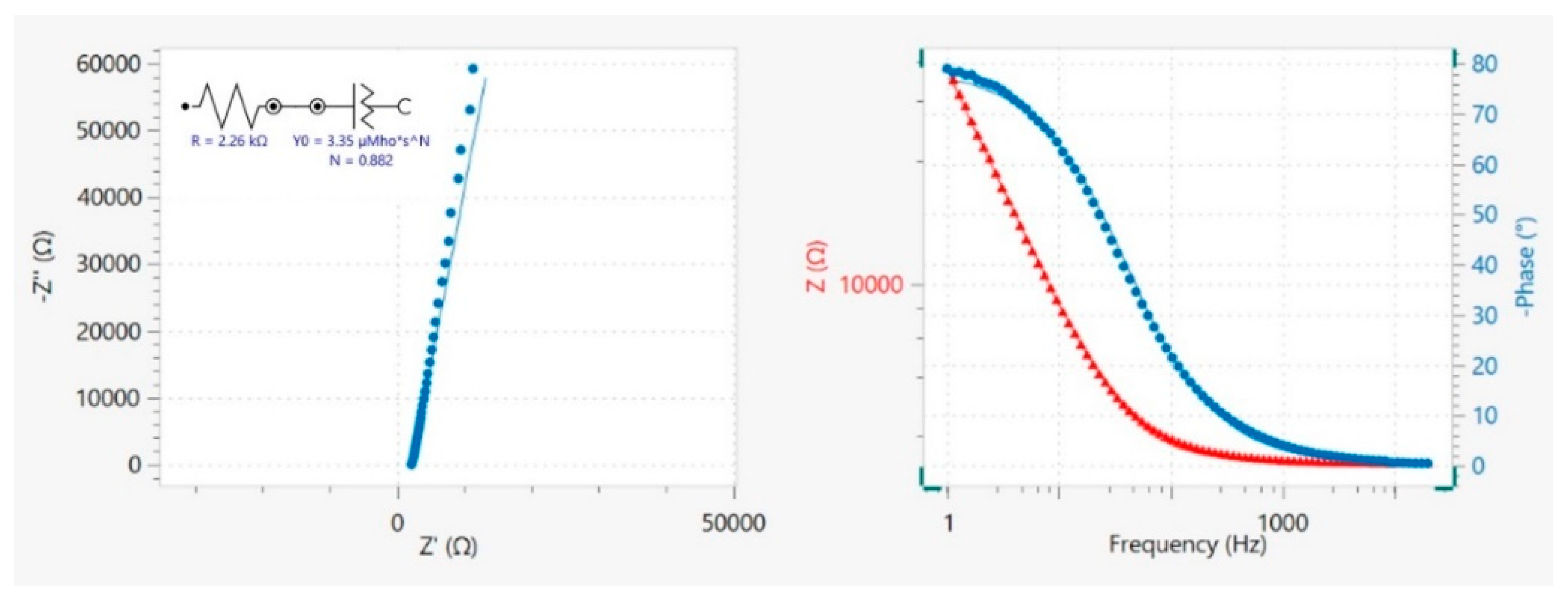
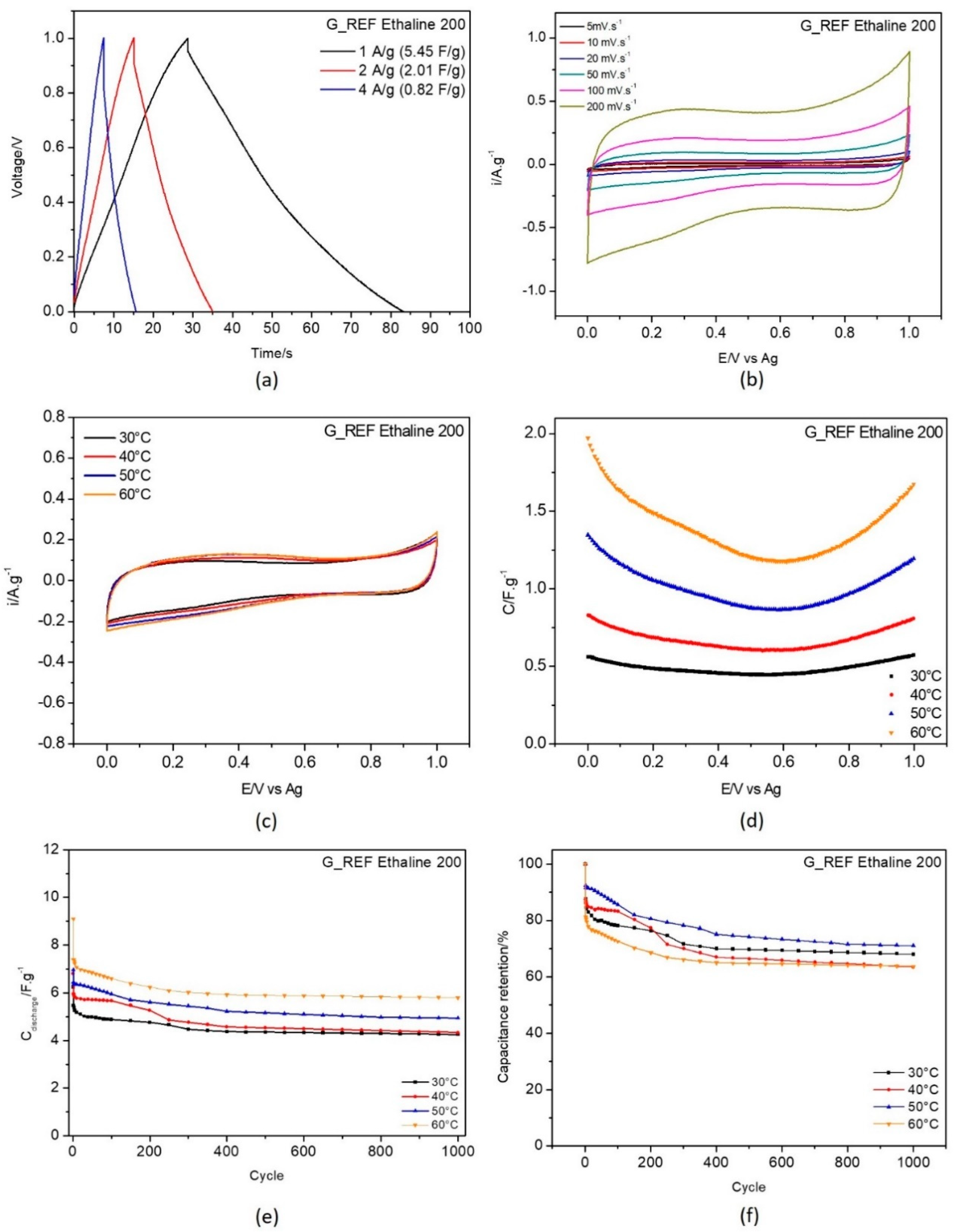
3.3. Electrochemical Behavior of G_REF and Commercial Graphite Modified Electrodes
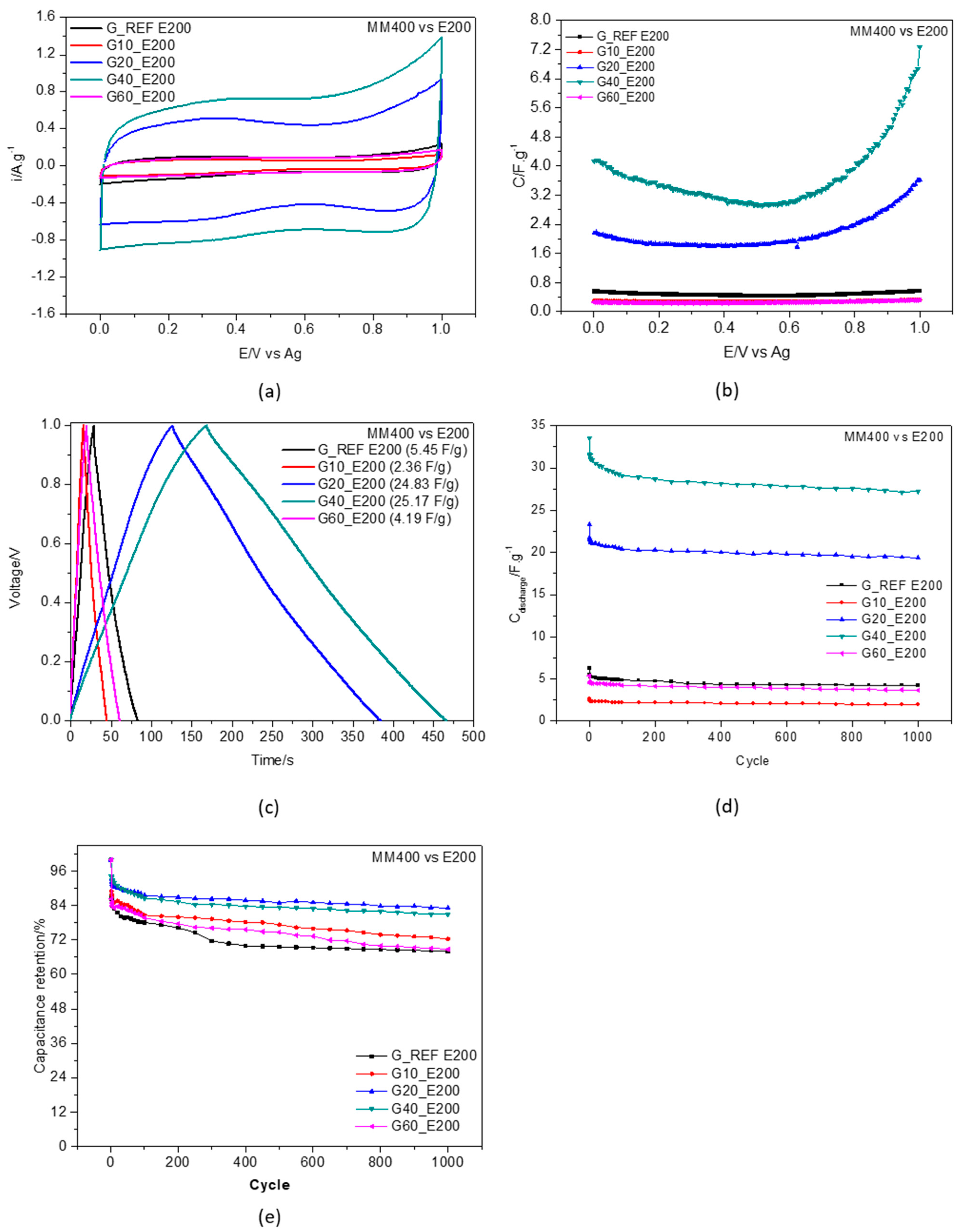
3.4. Electrochemical Behavior of Ball−Milled Graphite Modified Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratajczak, P.; Suss, M.E.; Kaasik, F.; Béguin, F. Carbon electrodes for capacitive technologies. Energy Storage Mater. 2019, 16, 126–145. [Google Scholar] [CrossRef]
- Pareek, A.; Shanthi Sravan, J.; Venkata Mohan, S. Exploring chemically reduced graphene oxide electrode for power generation in microbial fuel cell. Mater. Sci. Energy Technol. 2019, 2, 600–606. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.; Chang, N. Fuel economy analysis of fuel cell and supercapacitor hybrid systems. Int. J. Hydrog. Energy 2016, 41, 1381–1390. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Siddique, S.; Khan, A.; Haider, S.S.; Khalid, M. Recent developments in graphene based novel structures for efficient and durable fuel cells. Mater. Res. Bull. 2019, 122, 110674. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Eftekhari, A. On the mechanism of microporous carbon supercapacitors. Mater. Today Chem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon N. Y. 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced Design and Synthesis of Composite Photocatalysts for the Remediation of Wastewater: A Review. Catal. 2019, 9, 122. [Google Scholar] [CrossRef]
- Laskar, M.A.; Siddiqui, S. Chapter 12-Nanomaterials—based on graphene oxide and its derivatives—for separation and preconcentration of metal ions. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–219. ISBN 978-0-12-815811-1. [Google Scholar]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Frackowiak, E.F.B.; Frackowiak, E. Carbon Materials for the Electrochemical Storage of Energy in capacitors. J. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Frackowiak, E. Supercapacitors based on carbon materials and ionic liquids. J. Braz. Chem. Soc. 2006, 17, 1074–1082. [Google Scholar] [CrossRef]
- Frackowiak, E.; Abbas, Q.; Béguin, F. Carbon/carbon supercapacitors. J. Energy Chem. 2013, 22, 226–240. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Gorska, B.; Frackowiak, E.; Beguin, F. Redox active electrolytes in carbon/carbon electrochemical capacitors. Curr. Opin. Electrochem. 2018, 9, 95–105. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Rout, C.S.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, C.N.R. Graphene-based electrochemical supercapacitors. J. Chem. Sci. 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Sato, K.; Saito, R.; Oyama, Y.; Jiang, J.; Cançado, L.G.; Pimenta, M.A.; Jorio, A.; Samsonidze, G.G.; Dresselhaus, G.; Dresselhaus, M.S. D-band Raman intensity of graphitic materials as a function of laser energy and crystallite size. Chem. Phys. Lett. 2006, 427, 117–121. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Yan, S.; Chen, T.; Fang, H.; Yuan, X. Wide-Field Super-Resolved Raman Imaging of Carbon Materials. ACS Photonics 2021, 8, 1801–1809. [Google Scholar] [CrossRef]
- Malka, D.; Berke, B.A.; Tischler, Y. Improving Raman spectra of pure silicon using super-resolved method. J. Opt. 2019, 21, 075801. [Google Scholar] [CrossRef]
- Malka, D.; Berkovic, G.; Hammer, Y.; Zalevsky, Z. Super-resolved Raman spectroscopy. Spectrosc. Lett. 2013, 46, 307–313. [Google Scholar] [CrossRef]
- Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Hashim, U. Synthesis and characterization of graphene and carbon nanotubes: A review on the past and recent developments. J. Ind. Eng. Chem. 2014, 20, 1171–1185. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Fan, X.; Chang, D.W.; Chen, X.; Baek, J.-B.; Dai, L. Functionalized graphene nanoplatelets from ball milling for energy applications. Curr. Opin. Chem. Eng. 2016, 11, 52–58. [Google Scholar] [CrossRef]
- Moreno-Fernández, G.; Boulanger, N.; Nordenström, A.; Iakunkov, A.; Talyzin, A.; Carriazo, D.; Mysyk, R. Ball-milling-enhanced capacitive charge storage of activated graphene in aqueous, organic and ionic liquid electrolytes. Electrochim. Acta 2021, 370. [Google Scholar] [CrossRef]
- Godse, L.S.; Karandikar, P.B.; Khaladkar, M.Y. Study of carbon materials and effect of its ball milling, on capacitance of supercapacitor. Energy Procedia 2014, 54, 302–309. [Google Scholar] [CrossRef]
- Nandhini, R.; Mini, P.A.; Avinash, B.; Nair, S.V.; Subramanian, K.R. V Supercapacitor electrodes using nanoscale activated carbon from graphite by ball milling. Mater. Lett. 2012, 87, 165–168. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Du, X.; Hong, S.; Zhao, S.; Chen, Y.; Chen, X.; Song, H. Boosting the Electrical Double-Layer Capacitance of Graphene by Self-Doped Defects through Ball-Milling. Adv. Funct. Mater. 2019, 29, 1901127. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, J.; Huang, L.; He, C.; Zhang, J.; Wang, X.; Yang, Y. One-pot mechanochemical exfoliation of graphite and in situ polymerization of aniline for the production of graphene/polyaniline composites for high-performance supercapacitors. RSC Adv. 2020, 10, 44688–44698. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, J.; Zhou, Y.; Hao, L.; Xue, R.; Jiang, L.; Yi, B. Activated carbon/graphene composites with high-rate performance as electrode materials for electrochemical capacitors. J. Solid State Electrochem. 2013, 17, 2949–2958. [Google Scholar] [CrossRef]
- Chen, Z.; Ludwig, M.; Warr, G.G.; Atkin, R. Effect of cation alkyl chain length on surface forces and physical properties in deep eutectic solvents. J. Colloid Interface Sci. 2017, 494, 373–379. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Solid-state gel polymer electrolytes based on ionic liquids containing imidazolium cations and tetrafluoroborate anions for electrochemical double layer capacitors: Influence of cations size and viscosity of ionic liquids. J. Power Sources 2018, 406, 128–140. [Google Scholar] [CrossRef]
- Zarrougui, R.; Hachicha, R.; Rjab, R.; Ghodbane, O. 1-Allyl-3-methylimidazolium-based ionic liquids employed as suitable electrolytes for high energy density supercapacitors based on graphene nanosheets electrodes. J. Mol. Liq. 2018, 249, 795–804. [Google Scholar] [CrossRef]
- Jänes, A.; Permann, L.; Arulepp, M.; Lust, E. Electrochemical characteristics of nanoporous carbide-derived carbon materials in non-aqueous electrolyte solutions. Electrochem. Commun. 2004, 6, 313–318. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, C.; Woodward, C.E.; Wang, X. Bridging the gap between macroscopic electrochemical measurements and microscopic molecular dynamic simulations: Porous carbon supercapacitor with ionic liquids. Electrochim. Acta 2018, 289, 29–38. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Martins, V.L.; Rennie, A.J.R.; Sanchez-Ramirez, N.; Torresi, R.M.; Hall, P.J. Improved Performance of Ionic Liquid Supercapacitors by using Tetracyanoborate Anions. ChemElectroChem 2018, 5, 598–604. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A. Studies on electrical double layer capacitor with a low-viscosity ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate as electrolyte. Bull. Mater. Sci. 2013, 36, 729–733. [Google Scholar] [CrossRef]
- Laheäär, A.; Arenillas, A.; Béguin, F. Change of self-discharge mechanism as a fast tool for estimating long-term stability of ionic liquid based supercapacitors. J. Power Sources 2018, 396, 220–229. [Google Scholar] [CrossRef]
- Timperman, L.; Vigeant, A.; Anouti, M. Eutectic mixture of Protic Ionic Liquids as an Electrolyte for Activated Carbon-Based Supercapacitors. Electrochim. Acta 2015, 155, 164–173. [Google Scholar] [CrossRef]
- Tuhania, P.; Singh, P.K.; Bhattacharya, B.; Dhapola, P.S. PVDF-HFP and 1-ethyl-3-methylimidazolium thiocyanate—Doped polymer electrolyte for efficient supercapacitors. High Perform. Polym. 2018, 30, 911–917. [Google Scholar] [CrossRef]
- David, M. Applications of Ionic Liquids in Polymer Science and Technology, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 23–45. ISBN 9783662449035. [Google Scholar]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A. Supercapacitors utilising ionic liquids. Energy Storage Mater. 2017, 9, 47–69. [Google Scholar] [CrossRef]
- Lee, Y.R.; Row, K.H. Comparison of ionic liquids and deep eutectic solvents as additives for the ultrasonic extraction of astaxanthin from marine plants. J. Ind. Eng. Chem. 2016, 39, 87–92. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S.; Forrest, G. A Comparative Study of Nickel Electrodeposition Using Deep Eutectic Solvents and Aqueous Solutions. Electrochim. Acta 2015, 176, 718–726. [Google Scholar] [CrossRef]
- Abbott, A.P.; Al-Barzinjy, A.A.; Abbott, P.D.; Frisch, G.; Harris, R.C.; Hartley, J.; Ryder, K.S. Speciation, physical and electrolytic properties of eutectic mixtures based on CrCl3·6H2O and urea. Phys. Chem. Chem. Phys. 2014, 16, 9047–9055. [Google Scholar] [CrossRef]
- Abbott, A.; Aldous, L.; Borisenko, N.; Coles, S.; Fontaine, O.; Gamarra Garcia, J.D.; Gardas, R.; Hammond, O.; Hardwick, L.J.; Haumesser, P.H.; et al. Electrochemistry: General discussion. Faraday Discuss. 2018, 206, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Salomé, S.; Pereira, N.M.; Ferreira, E.S.; Pereira, C.M.; Silva, A.F. Tin electrodeposition from choline chloride based solvent: Influence of the hydrogen bond donors. J. Electroanal. Chem. 2013, 703, 80–87. [Google Scholar] [CrossRef]
- Costa, R.; Pereira, C.M.; Silva, A.F. Insight on the effect of surface modification by carbon materials on the Ionic Liquid Electric Double Layer Charge Storage properties. Electrochim. Acta 2015, 176, 880–886. [Google Scholar] [CrossRef]
- Sopcic, S.; Kraljic Rokovic, M.; Mandic, Z. Preparation and characterization of RuO2/polyaniline/polymer binder composite electrodes for supercapacitor application. J. Electrochem. Sci. Eng. 2012, 2, 41–52. [Google Scholar] [CrossRef]
- López-Chavéz, R.; Cuentas-Gallegos, A.K. The Effect of Binder in Electrode Materials for Capacitance Improvement and The Effect of Binder in Electrode Materials for Capacitance Improvement and EDLC Binder-free Cell Design. J. New Mater. Electrochem. Syst. 2013, 16, 139–251. [Google Scholar]
- Silva, F.; Gomes, C.; Figueiredo, M.; Costa, R.; Martins, A.; Pereira, C.M. The electrical double layer at the [BMIM][PF6] ionic liquid/electrode interface—Effect of temperature on the differential capacitance. J. Electroanal. Chem. 2008, 622, 153–160. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- De Oliveira, R.R.L.; Albuquerque, D.A.C.; Cruz, T.G.S. Measurement of the Nanoscale Roughness by Atomic Force Microscopy: Basic Principles and Applications. Available online: https://www.intechopen.com/chapters/33450 (accessed on 10 September 2021).
- Ghosh, A.; Subrahmanyam, K.S.; Krishna, K.S.; Datta, S.; Govindaraj, A.; Pati, S.K.; Rao, C.N.R. Uptake of H2 and CO2 by Graphene. J. Phys. Chem. C 2008, 112, 15704–15707. [Google Scholar] [CrossRef]
- Wasalathilake, K.C.; Ayoko, G.; Yan, C. Porous Graphene Materials for Energy Storage and Conversion Applications. Recent Adv. Graphene Res. 2016, 2, 196. [Google Scholar]
- Bourlinos, A.B.; Steriotis, T.A.; Karakassides, M.; Sanakis, Y.; Tzitzios, V.; Trapalis, C.; Kouvelos, E.; Stubos, A. Synthesis, characterization and gas sorption properties of a molecularly-derived graphite oxide-like foam. Carbon N. Y. 2007, 45, 852–857. [Google Scholar] [CrossRef]
- Disma, F. Effect of Mechanical Grinding on the Lithium Intercalation Process in Graphites and Soft Carbons. J. Electrochem. Soc. 1996, 143, 3959. [Google Scholar] [CrossRef]
- Welham, N.J.; Williams, J.S. Extended milling of graphite and activated carbon. Carbon N. Y. 1998, 36, 1309–1315. [Google Scholar] [CrossRef]
- Chen, Y.; Fitz Gerald, J.; Chadderton, L.T.; Chaffron, L. Nanoporous carbon produced by ball milling. Appl. Phys. Lett. 1999, 74, 2782–2784. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Zhan, L.; Qiao, W.; Liang, X.; Ling, L. Effect of ball-milling technology on pore structure and electrochemical properties of activated carbon. J. Shanghai Univ. Engl. Ed. 2008, 12, 372–376. [Google Scholar] [CrossRef]
- Mhadhbi, M. Modelling of the High-Energy Ball Milling Process. Adv. Mater. Phys. Chem. 2021, 11, 31–44. [Google Scholar] [CrossRef]
- Xing, T.; Li, L.H.; Hou, L.; Hu, X.; Zhou, S.; Peter, R.; Petravic, M.; Chen, Y. Disorder in ball-milled graphite revealed by Raman spectroscopy. Carbon N. Y. 2013, 57, 515–519. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Wang, G.-C.; Lu, T.-M.; Palasantzas, G.; De Hosson, J.T.M. Surface-roughness effect on capacitance and leakage current of an insulating film. Phys. Rev. B 1999, 60, 9157–9164. [Google Scholar] [CrossRef]
- Palasantzas, G.; De Hosson, J.T.M. The effect of mound roughness on the electrical capacitance of a thin insulating film. Solid State Commun. 2001, 118, 203–206. [Google Scholar] [CrossRef][Green Version]
- Patrikar, R.M.; Dong, C.Y.; Zhuang, W. Modelling interconnects with surface roughness. Microelectron. J. 2002, 33, 929–934. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Breeding, C.; Shigley, J. The “type” classification system of diamonds and its importance in gemology. Gems Gemol. 2009, 45, 96–111. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Chegel, V.I.; Gridina, N.Y.; Repnytska, O.P.; Shirshov, Y.M.; Tryndiak, V.P.; Todor, I.M.; Solyanik, G.I. Surface enhanced IR absorption of nucleic acids from tumor cells: FTIR reflectance study. Biopolym. Biospectroscopy Sect. 2002, 67, 470–486. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Nicolaisen, F.M. IR absorption spectrum (4200–3100 cm−1) of H2O and (H2O)2 in CCl4. Estimates of the equilibrium constant and evidence that the atmospheric water absorption continuum is due to the water dimer. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 2060–2076. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Q.; Li, Y.; Zhang, M.; Wei, X. Preparation and characterisation of graphene. Mater. Res. Innov. 2015, 19, S9344–S9350. [Google Scholar] [CrossRef]
- Çiplak, Z.; Yildiz, N.; Cąlimli, A. Investigation of graphene/Ag nanocomposites synthesis parameters for two different synthesis methods. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 361–370. [Google Scholar] [CrossRef]
- Coates, J. Encyclopedia of Analytical Chemistry-IInterpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2004, 1, 23. [Google Scholar]
- Wang, X.; Christopher, J.W.; Swan, A.K. 2D Raman band splitting in graphene: Charge screening and lifting of the K-point Kohn anomaly. Sci. Rep. 2017, 7, 13539. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, L. Structural Quantification for Graphene and Related Two-Dimensional Materials by Raman Spectroscopy. Anal. Chem. 2019, 91, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Pardanaud, C.; Cartry, G.; Lajaunie, L.; Arenal, R. Investigating the Possible Origin of Raman Bands in Defective sp2/sp3 Carbons below 900 cm−1: Phonon Density of States or Double Resonance Mechanism at Play? C 2019, 5, 79. [Google Scholar] [CrossRef]
- Bratek, W.; Świątkowski, A.; Pakuła, M.; Biniak, S.; Bystrzejewski, M.; Szmigielski, R. Characteristics of activated carbon prepared from waste PET by carbon dioxide activation. J. Anal. Appl. Pyrolysis 2013, 100, 192–198. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Sharma, R.; Meduri, P.; Arey, B.W.; Schaef, H.T.; Lutkenhaus, J.L.; Lemmon, J.P.; Thallapally, P.K.; Nandasiri, M.I.; McGrail, B.P.; et al. In Situ One-Step Synthesis of Hierarchical Nitrogen-Doped Porous Carbon for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7214–7222. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.S.; Otubo, L.; Ferreira, O.P.; de Gimenez, I.F.; Mazali, I.O.; Barreto, L.S. Biomorphic activated porous carbons with complex microstructures from lignocellulosic residues. Microporous Mesoporous Mater. 2008, 107, 276–285. [Google Scholar] [CrossRef]
- Bonso, J.S.; Kalaw, G.D.; Ferraris, J.P. High surface area carbon nanofibers derived from electrospun PIM-1 for energy storage applications. J. Mater. Chem. A 2014, 2, 418–424. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Puech, P.; Kandara, M.; Paredes, G.; Moulin, L.; Weiss-Hortala, E.; Kundu, A.; Ratel-Ramond, N.; Plewa, J.M.; Pellenq, R.; Monthioux, M. Monthioux Analyzing the Raman Spectra of Graphenic Carbon Materials from Kerogens to Nanotubes: What Type of Information Can Be Extracted from Defect Bands? C 2019, 5, 69. [Google Scholar]
- Labunov, V.A.; Tabulina, L.V.; Komissarov, I.V.; Mikhnavets, L.A.; Tkach, A.N. Reduction of graphene from graphene oxide in different media. Mater. Phys. Mech. 2019, 41, 1–7. [Google Scholar]
- Dubale, A.A.; Su, W.N.; Tamirat, A.G.; Pan, C.J.; Aragaw, B.A.; Chen, H.M.; Chen, C.H.; Hwang, B.J. The synergetic effect of graphene on Cu2O nanowire arrays as a highly efficient hydrogen evolution photocathode in water splitting. J. Mater. Chem. A 2014, 2, 18383–18397. [Google Scholar] [CrossRef]
- Costa, R.; Figueiredo, M.; Pereira, C.M.; Silva, F. Electrochemical double layer at the interfaces of Hg/choline chloride based solvents. Electrochim. Acta 2010, 55, 8916–8920. [Google Scholar] [CrossRef]
- Costa, R.; Pereira, C.; Silva, A. Ionic liquids at electrified interfaces for advanced energy/charge storage applications. In Ionic Liquids; Fehrmann, R., Ed.; De Gruyter: Villeurbanne, France, 2019; pp. 101–128. [Google Scholar]
- Heimböckel, R.; Hoffmann, F.; Fröba, M. Insights into the influence of the pore size and surface area of activated carbons on the energy storage of electric double layer capacitors with a new potentially universally applicable capacitor model. Phys. Chem. Chem. Phys. 2019, 21, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Taer, E.; Agustino, A.; Farma, R.; Taslim, R.; Paiszal, M.; Ira, A.; Yardi, S.D.; Sari, Y.P.; Yusra, H.; Nurjanah, S.; et al. The relationship of surface area to cell capacitance for monolith carbon electrode from biomass materials for supercapacitor aplication. J. Phys. Conf. Ser. 2018, 1116, 032040. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, L.; Abbas, M.A.; Bang, J.H. The influence of surface area, porous structure, and surface state on the supercapacitor performance of titanium oxynitride: Implications for a nanostructuring strategy. Phys. Chem. Chem. Phys. 2017, 19, 21140–21151. [Google Scholar] [CrossRef]
- Eguchi, T.; Kanamoto, Y.; Tomioka, M.; Tashima, D.; Kumagai, S. Effect of ball milling on the electrochemical performance of activated carbon with a very high specific surface area. Batteries 2020, 6, 22. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Chen, R.; Cao, G.; Chen, S.; Wang, G.; Yang, Y. Room temperature molten salt as electrolyte for carbon nanotube-based electric double layer capacitors. J. Power Sources 2006, 158, 773–778. [Google Scholar] [CrossRef]
- Schutjajew, K.; Yan, R.; Antonietti, M.; Roth, C.; Oschatz, M. Effects of carbon pore size on the contribution of ionic liquid electrolyte phase transitions to energy storage in supercapacitors. Front. Mater. 2019, 6, 65. [Google Scholar] [CrossRef]
- Zdolšek, N.; Rocha, R.P.; Krstić, J.; Trtić-petrović, T.; Šljukić, B.; Figueiredo, J.L.; Vujković, M.J. Electrochemical investigation of ionic liquid-derived porous carbon materials for supercapacitors: Pseudocapacitance versus electrical double layer. Electrochim. Acta 2019, 298, 541–551. [Google Scholar] [CrossRef]
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayeva, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; Talyzin, A.V. Activated graphene as a material for supercapacitor electrodes: Effects of surface area, pore size distribution and hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912. [Google Scholar] [CrossRef] [PubMed]


| DES | Composition | Molar Ratio | Choline Chloride | HBD | Water Content (wt.%) ** | Viscosity (Cp) *** |
|---|---|---|---|---|---|---|
| ethaline 200 * (E200) | Choline chloride (ChCl) + ethylene glycol (EG) | 1 (ChCl):2 EG | 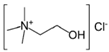 |  | 8.8 ± 0.5 | 30 °C: 64.3 ± 1.3 40 °C: 48.7 ± 1.8 50 °C: 35.1 ± 0.9 60 °C: 25.3 ± 0.3 |
| Carbon Material | Terminology | Code | Milling | Treatment |
|---|---|---|---|---|
| Graphene * | Commercial graphene (11–15 nm, 99.5%, Iolitec nanomaterials) | G_REF | − | As received |
| Graphite | Commercial graphite (<20 µm, synthetic, Sigma Aldrich) | Graphite | − | As received |
| 2000 rpm | G@2000 | ULTRA−TURRAX® | Ball milling for 1 h changing the rotation speed | |
| 4000 rpm | G@4000 | |||
| 6000 rpm | G@6000 | |||
| 10 min. MM400 | G10 | Retsch MM400 | Ball milling at 25 Hz changing the milling time | |
| 20 min. MM400 | G20 | |||
| 40 min. MM400 | G40 | |||
| 60 min. MM400 | G60 |
| Samples | SBET (m2·g−1) | Pore Volume (cm3·g−1) | Specific Capacitance (F·g−1) |
|---|---|---|---|
| G_REF | 45.14 | 0.00461 | 5.45 ± 0.96 |
| Graphite | 10.74 | 0.00028 | 4.27 ± 0.85 |
| G10 | 18.15 | 0.00050 | 2.36 ± 0.21 |
| G20 | 235.80 | 0.02227 | 24.83 ± 2.33 |
| G40 | 308.58 | 0.03163 | 25.10 ± 2.22 |
| G60 | 41.84 | 0.00419 | 4.19 ± 1.01 |
| G@2000 | 9.81 | 0.00103 | 1.95 ± 0.35 |
| G@4000 | 10.80 | 0.00081 | 2.57 ± 0.44 |
| G@6000 | 11.33 | 0.00088 | 2.59 ±0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11, 3258. https://doi.org/10.3390/nano11123258
Brandão ATSC, Costa R, Silva AF, Pereira CM. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials. 2021; 11(12):3258. https://doi.org/10.3390/nano11123258
Chicago/Turabian StyleBrandão, Ana T. S. C., Renata Costa, A. Fernando Silva, and Carlos M. Pereira. 2021. "Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte" Nanomaterials 11, no. 12: 3258. https://doi.org/10.3390/nano11123258
APA StyleBrandão, A. T. S. C., Costa, R., Silva, A. F., & Pereira, C. M. (2021). Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials, 11(12), 3258. https://doi.org/10.3390/nano11123258