Biodistribution of Mesoporous Carbon Nanoparticles via Technetium-99m Radiolabelling after Oral Administration to Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of Ordered Mesoporous CMK-1 Carbon Spheres
2.2. Characterisation
2.3. Radiolabeling of Carbon Nanoparticles
2.4. In Vitro Stability Studies
2.5. Biodistribution Studies
3. Results and Discussion
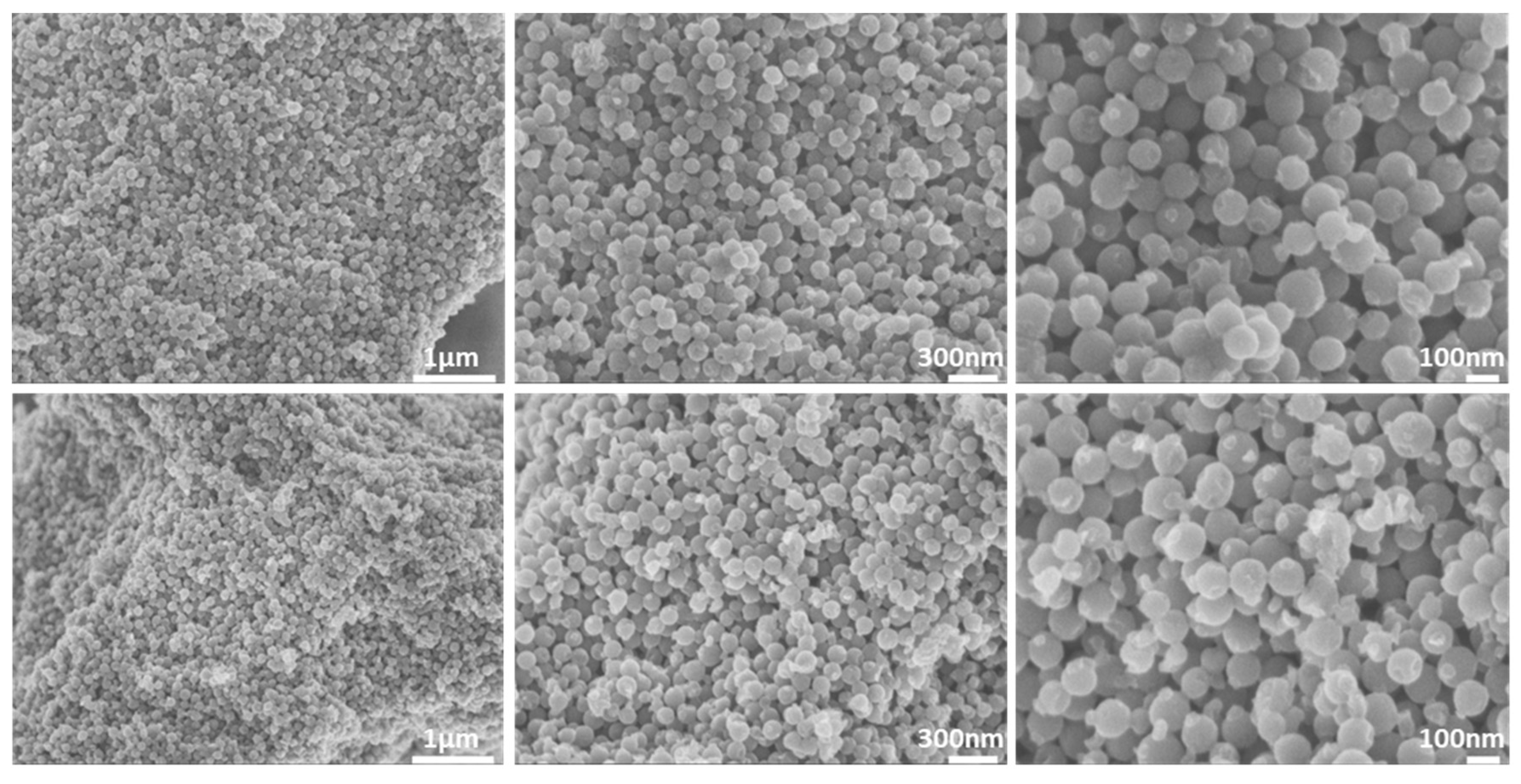
3.1. Morphological Properties
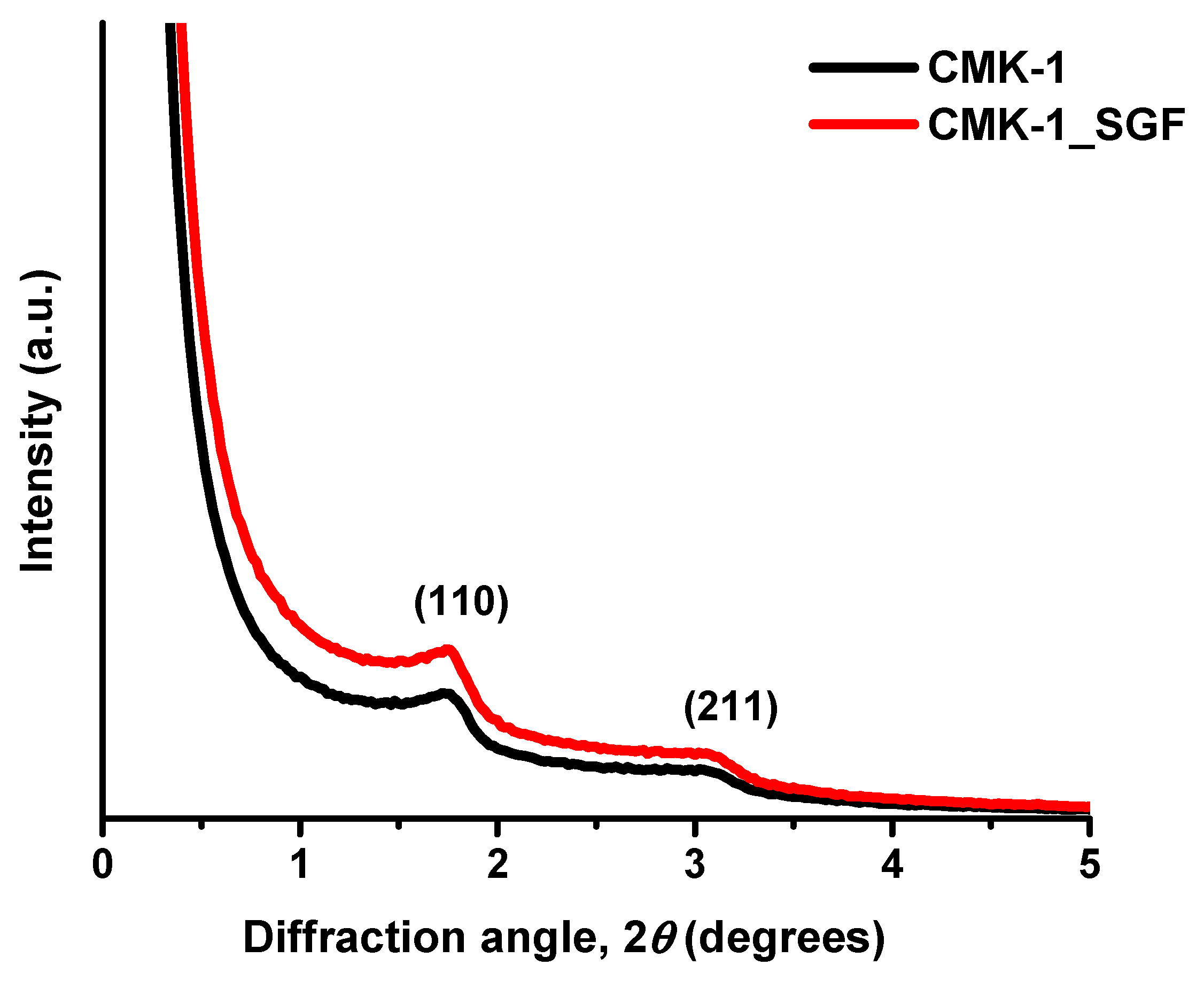
3.2. Structural Properties
3.3. Pore Properties
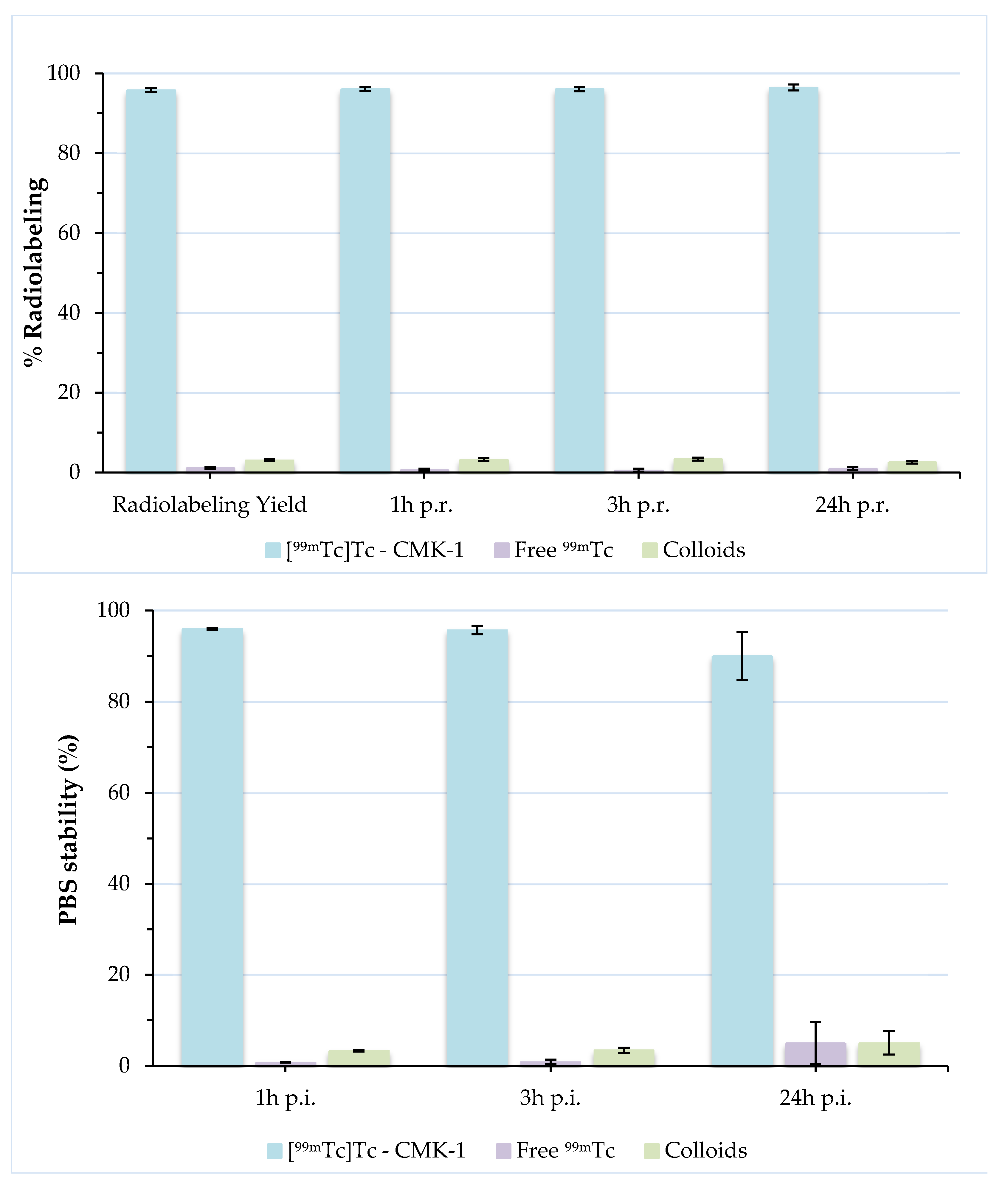
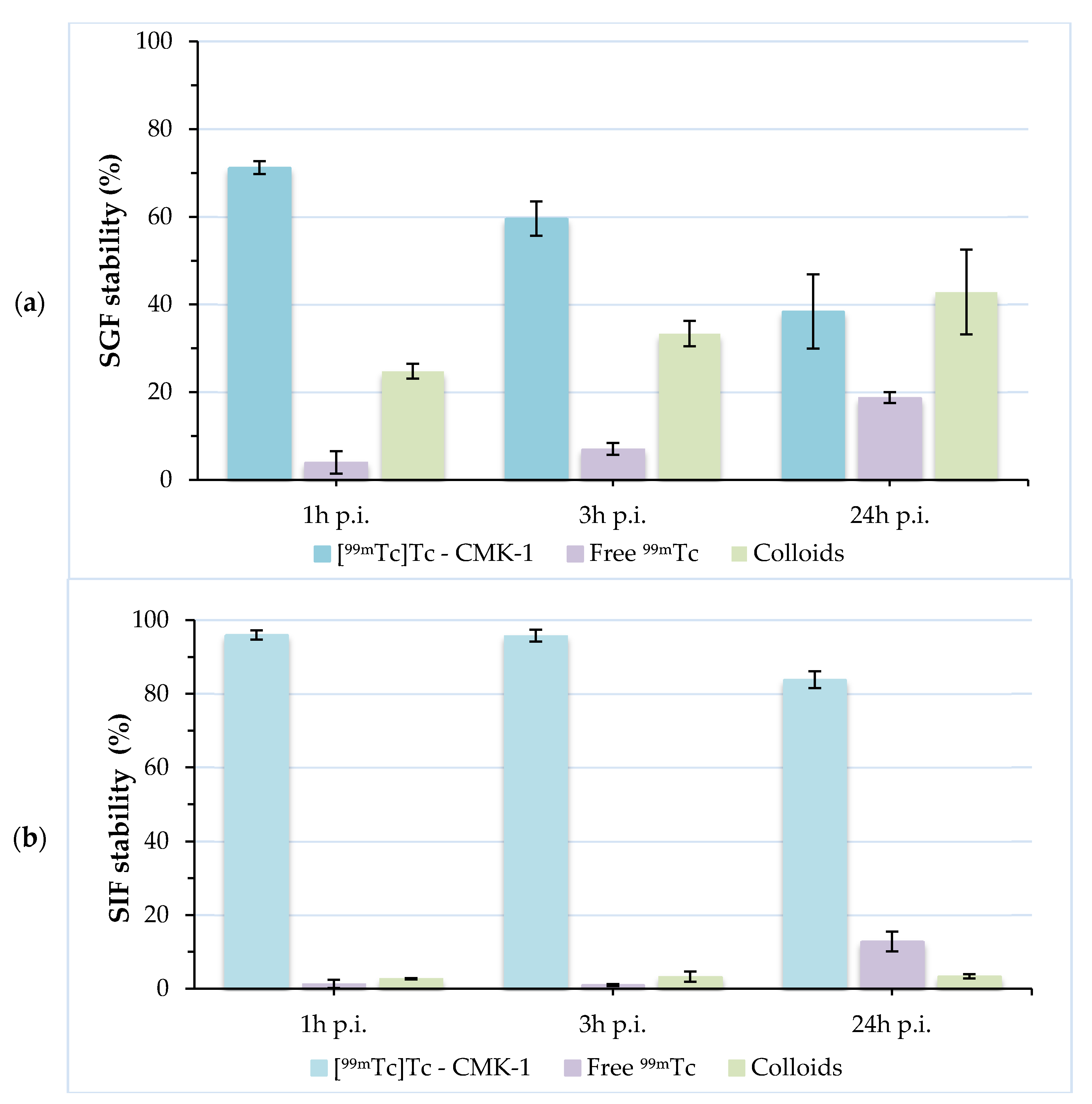
3.4. Radiolabeling of CMK-1 and In Vitro Stability Assessment
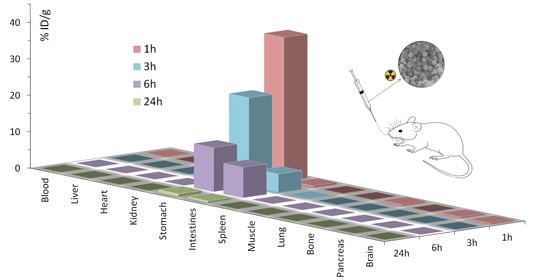
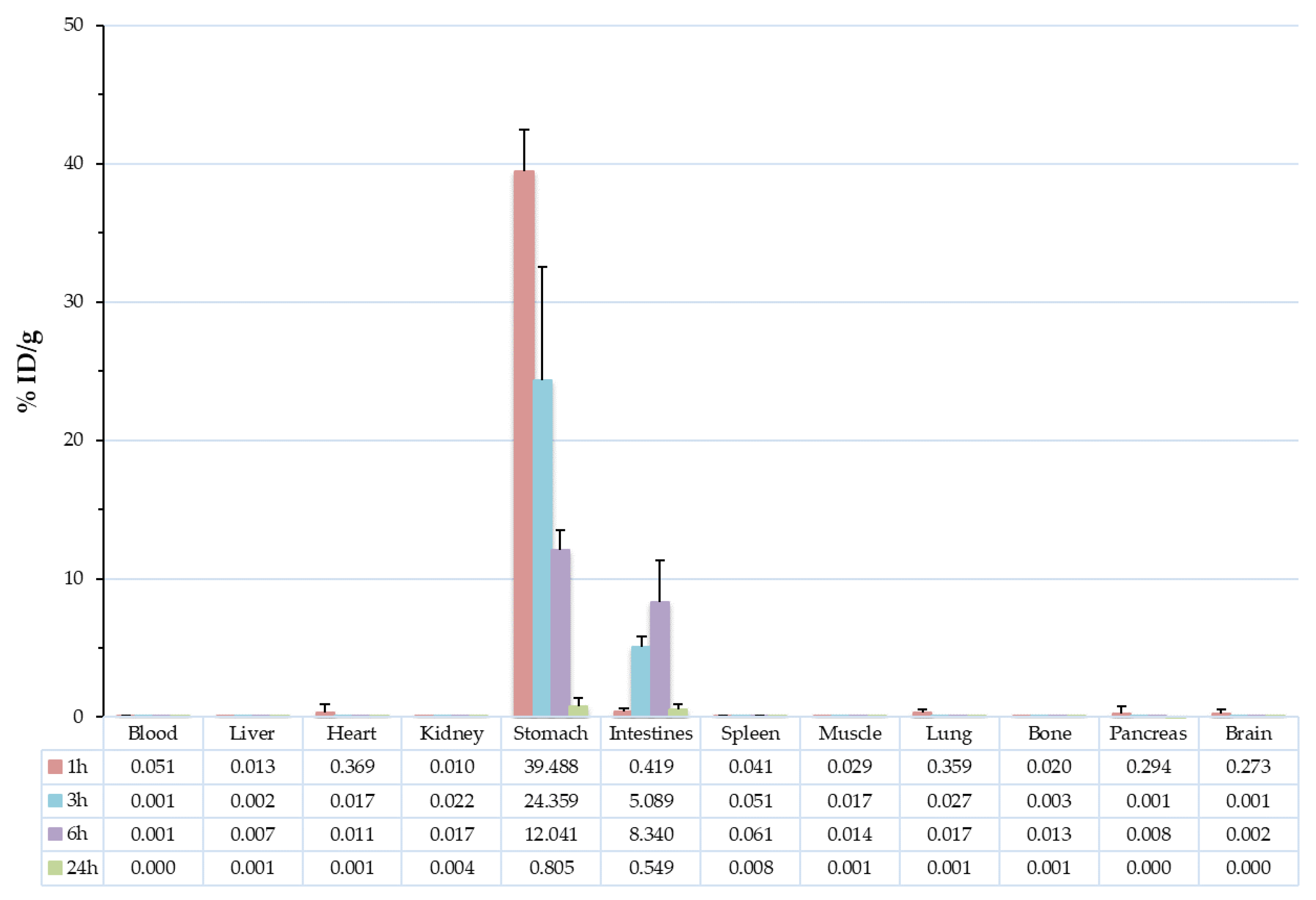
3.5. Biodistribution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery—A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Ting, J.; Tale, S.; Purchel, A.A.; Jones, S.D.; Widanapathirana, L.; Tolstyka, Z.P.; Guo, L.; Guillaudeu, S.; Bates, F.S.; Reineke, T.M. High-throughput excipient discovery enables oral delivery of poorly soluble pharmaceuticals. ACS Cent. Sci. 2016, 2, 748–755. [Google Scholar] [CrossRef]
- Goldberg, M.; Gomez-Orellana, I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2003, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.; Ko, B.; Park, S.; Lee, J. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, P.; Zhao, Q.; Wang, B.; Wang, S. The mechanism for increasing the oral bioavailability of poorly water-soluble drugs using uniform mesoporous carbon spheres as a carrier. Drug Deliv. 2014, 23, 420–428. [Google Scholar] [CrossRef]
- Boyd, B.J.; Bergström, C.A.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Multifunctional theranostic nanoparticles for biomedical cancer treatments—A comprehensive review. Mater. Sci. Eng. C 2021, 127, 112199. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Prakash, G.; Öztürk, A.B.; Saghazadeh, S.; Sohail, M.F.; Seo, J.; Dokmeci, M.R.; Zhang, Y.S.; Khademhosseini, A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, B.; Sun, J.; Hu, W.; Wang, H. Recent advances in porous nanostructures for cancer theranostics. Nano Today 2021, 38, 101146. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.I.; Alonso-Morales, N.; Baeza, J.A.; Rodríguez, J.J.; Gilarranz, M.A. High load drug release systems based on carbon porous nanocapsule carriers. Ibuprofen case study. J. Mater. Chem. B 2020, 8, 5293–5304. [Google Scholar] [CrossRef]
- Lu, H.; Yang, G.; Ran, F.; Gao, T.; Sun, C.; Zhao, Q.; Wang, S. Polymer-functionalized mesoporous carbon nanoparticles on overcoming multiple barriers and improving oral bioavailability of Probucol. Carbohydr. Polym. 2019, 229, 115508. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.A.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G.; et al. Engineered pH-responsive mesoporous carbon nanoparticles for drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 14946–14957. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Yue, Q.; Li, J.; Gao, M.; Yang, X.; Ren, Y.; Cheng, X.; Cui, P.; Deng, Y. Smart cargo delivery system based on mesoporous nanoparticles for bone disease diagnosis and treatment. Adv. Sci. 2021, 8, 2004586. [Google Scholar] [CrossRef]
- Christoforidou, T.; Giasafaki, D.; Andriotis, E.; Bouropoulos, N.; Theodoroula, N.; Vizirianakis, I.; Steriotis, T.; Charalambopoulou, G.; Fatouros, D. Oral drug delivery systems based on ordered mesoporous silica nanoparticles for modulating the release of aprepitant. Int. J. Mol. Sci. 2021, 22, 1896. [Google Scholar] [CrossRef]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.-P.N.; et al. Electrosprayed mesoporous particles for improved aqueous solubility of a poorly water soluble anticancer agent: In vitro and ex vivo evaluation. J. Control Release 2018, 278, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Galabova, B.B. Mesoporous silica nanoparticles: Synthesis, functionalization, drug loading and release—A review. Trop. J. Pharm. Res. 2021, 20, 1091–1100. [Google Scholar] [CrossRef]
- Björk, E.M.; Atakan, A.; Wu, P.-H.; Bari, A.; Pontremoli, C.; Zheng, K.; Giasafaki, D.; Iviglia, G.; Torre, E.; Cassinelli, C.; et al. A shelf-life study of silica- and carbon-based mesoporous materials. J. Ind. Eng. Chem. 2021, 101, 205–213. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Warren, K.E.; Naskar, A.K. Soft-templated mesoporous carbons as potential materials for oral drug delivery. Carbon 2014, 71, 47–57. [Google Scholar] [CrossRef]
- Xin, W.; Song, Y. Mesoporous carbons: Recent advances in synthesis and typical applications. RSC Adv. 2015, 5, 83239–83285. [Google Scholar] [CrossRef]
- Karavasili, C.; Amanatiadou, E.P.; Sygellou, L.; Giasafaki, D.K.; Steriotis, T.A.; Charalambopoulou, G.C.; Vizirianakis, I.S.; Fatouros, D.G. Development of new drug delivery system based on ordered mesoporous carbons: Characterisation and cytocompatibility studies. J. Mater. Chem. B 2013, 1, 3167–3174. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Sun, C.; Jiang, T.; Zhang, J.; Zhang, Q.; Sun, J.; Deng, Y.; Wang, S. Uniform mesoporous carbon as a carrier for poorly water soluble drug and its cytotoxicity study. Eur. J. Pharm. Biopharm. 2012, 80, 535–543. [Google Scholar] [CrossRef]
- Torre, E.; Giasafaki, D.; Steriotis, T.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Charalambopoulou, G.; Iviglia, G. Silver decorated mesoporous carbons for the treatment of acute and chronic wounds, in a tissue regeneration context. Int. J. Nanomed. 2019, 14, 10147–10164. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Ara, M.; Alim, M.; Uddin, S.; Najda, A.; Albadrani, G.; Sayed, A.; Mousa, S.; Abdel-Daim, M. Mesoporous carbon: A versatile material for scientific applications. Int. J. Mol. Sci. 2021, 22, 4498. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1915–1924. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Xu, Y.; Zeng, J.; Wang, J.; Chen, Q.; Su, L.; Wang, Z.; Deng, R.; Chu, F.; et al. Colon tissue-accumulating mesoporous carbon nanoparticles loaded with Musca domestica cecropin for ulcerative colitis therapy. Theranostics 2021, 11, 3417–3438. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Eleftheriadis, G.; Filippousi, M.; Tsachouridou, V.; Darda, M.-A.; Sygellou, L.; Kontopoulou, I.; Bouropoulos, N.; Steriotis, T.; Charalambopoulou, G.; Vizirianakis, I.; et al. Evaluation of mesoporous carbon aerogels as carriers of the non-steroidal anti-inflammatory drug ibuprofen. Int. J. Pharm. 2016, 515, 262–270. [Google Scholar] [CrossRef]
- Boffito, M.; Laurano, R.; Giasafaki, D.; Steriotis, T.; Papadopoulos, A.; Tonda-Turo, C.; Cassino, C.; Charalambopoulou, G.; Ciardelli, G. Embedding ordered mesoporous carbons into thermosensitive hydrogels: A cutting-edge strategy to vehiculate a cargo and control its release profile. Nanomaterials 2020, 10, 2165. [Google Scholar] [CrossRef]
- Niu, X.; Wan, L.; Hou, Z.; Wang, T.; Sun, C.; Sun, J.; Zhao, P.; Jiang, T.; Wang, S. Mesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailability. Int. J. Pharm. 2013, 452, 382–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Gao, C.; Li, X.; Li, L. Highly ordered mesoporous carbon nanomatrix as a new approach to improve the oral absorption of the water-insoluble drug, simvastatin. Eur. J. Pharm. Sci. 2013, 49, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhi, Z.; Li, X.; Gao, J.; Song, Y. Carboxylated mesoporous carbon microparticles as new approach to improve the oral bioavailability of poorly water-soluble carvedilol. Int. J. Pharm. 2013, 454, 403–411. [Google Scholar] [CrossRef]
- Han, C.; Huang, H.; Dong, Y.; Sui, X.; Jian, B.; Zhu, W. A comparative study of the use of mesoporous carbon and mesoporous silica as drug carriers for oral delivery of the water-insoluble drug carvedilol. Molecules 2019, 24, 1770. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhao, Y.; Zhao, C.; Hu, T.; Wang, J.; Liu, Y.; Tian, Y.; Wang, X. Controlled fabrication of orchid-like nitrogen-doped hierarchical porous carbon and hollow carbon nanospheres. J. Mater. Sci. 2020, 55, 16143–16157. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Zhang, J.; Zhu, X.; Liu, J.; Zhang, Y. Phase-change nanotherapeutic agents based on mesoporous carbon for multimodal imaging and tumor therapy. ACS Appl. Bio Mater. 2020, 3, 8705–8713. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Teng, Y.; Wang, S.; Guo, J.; Wang, C. NIR-controlled HSP90 inhibitor release from hollow mesoporous nanocarbon for synergistic tumor photothermal therapy guided by photoacoustic imaging. Nanoscale 2020, 12, 14775–14787. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Yang, M.; Li, X.; Mao, Y.; Guan, X.; Di, D.; Wang, S. Multi-stimuli responsive mesoporous carbon nano-platform gated by human serum albumin for cancer thermo-chemotherapy. Colloids Surf. B Biointerfaces 2019, 184, 110532. [Google Scholar] [CrossRef]
- Qiu, Y.; Ding, D.; Sun, W.; Feng, Y.; Huang, D.; Li, S.; Meng, S.; Zhao, Q.; Xue, L.-J.; Chen, H. Hollow mesoporous carbon nanospheres for imaging-guided light-activated synergistic thermo-chemotherapy. Nanoscale 2019, 11, 16351–16361. [Google Scholar] [CrossRef]
- Zhou, L.; Jing, Y.; Liu, Y.; Liu, Z.; Gao, D.; Chen, H.; Song, W.; Wang, T.; Fang, X.; Qin, W.; et al. Mesoporous carbon nanospheres as a multifunctional carrier for cancer theranostics. Theranostics 2018, 8, 663–675. [Google Scholar] [CrossRef]
- Li, C.; Qian, M.; Wang, S.; Jiang, H.; Du, Y.; Wang, J.; Lu, W.; Murthy, N.; Huang, R. Aptavalve-gated mesoporous carbon nanospheres image cellular mucin and provide on-demand targeted drug delivery. Theranostics 2017, 7, 3319–3325. [Google Scholar] [CrossRef]
- Li, C.; Meng, Y.; Wang, S.; Qian, M.; Wang, J.; Lu, W.; Huang, R. Mesoporous carbon nanospheres featured fluorescent aptasensor for multiple diagnosis of cancer in vitro and in vivo. ACS Nano 2015, 9, 12096–12103. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional carbon-based nanomaterials: Applications in biomolecular imaging and therapy. ACS Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Grosse, J.; Asad, A.B.M.A.; Radda, G.K.; Golay, X. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Ansar, F.H.Z.; Latifah, S.Y.; Kamal, W.H.B.W.; Khong, K.C.; Ng, Y.; Foong, J.N.; Gopalsamy, B.; Ng, W.K.; How, C.W.; Ong, Y.S.; et al. Pharmacokinetics and biodistribution of thymoquinone-loaded nanostructured lipid carrier after oral and intravenous administration into rats. Int. J. Nanomed. 2020, 15, 7703–7717. [Google Scholar] [CrossRef]
- Priyadarshani, A.; Chuttani, K.; Mittal, G.; Bhatnagar, A. Radiolabeling, biodistribution and gamma scintigraphy of noscapine hydrochloride in normal and polycystic ovary induced rats. J. Ovarian Res. 2010, 3, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Marouzi, S.; Darroudi, M.; Hekmat, A.; Sadri, K.; Oskuee, R.K. One-pot hydrothermal synthesis of carbon quantum dots from Salvia hispanica L. seeds and investigation of their biodistribution, and cytotoxicity effects. J. Environ. Chem. Eng. 2021, 9, 105461. [Google Scholar] [CrossRef]
- Bayoumi, N.A.; Emam, A.N. 99mTc radiolabeling of polyethylenimine capped carbon dots for tumor targeting: Synthesis, characterization and biodistribution. Int. J. Radiat. Biol. 2021, 97, 977–985. [Google Scholar] [CrossRef]
- Gharepapagh, E.; Fakhari, A.; Firuzyar, T.; Shomali, A.; Azimi, F. Preparation, biodistribution and dosimetry study of Tc-99m labeled N-doped graphene quantum dot nanoparticles as a multimodular radiolabeling agent. New J. Chem. 2021, 45, 3909–3919. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Yang, M.; Yudasaka, M.; Okazaki, T. Comparative assessments of the biodistribution and toxicity of oxidized single-walled carbon nanotubes dispersed with two different reagents after intravenous injection. Nanotoxicology 2021, 15, 798–811. [Google Scholar] [CrossRef]
- Genady, A.R.; Fong, D.; Slikboer, S.R.; El-Zaria, M.E.; Swann, R.; Janzen, N.; Faraday, A.; McNelles, S.A.; Rezvani, M.; Sadeghi, S.; et al. 99mTc-functionalized single-walled carbon nanotubes for bone targeting. ACS Appl. Nano Mater. 2020, 3, 11819–11824. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-dependent pulmonary impact of thin graphene oxide sheets in mice: Toward safe-by-design. Adv. Sci. 2020, 7, 1903200. [Google Scholar] [CrossRef]
- Psimadas, D.; Bouziotis, P.; Georgoulias, P.; Valotassiou, V.; Tsotakos, T.; Loudos, G. Radiolabeling approaches of nanoparticles with 99mTc. Contrast Media Mol. Imaging 2013, 8, 333–339. [Google Scholar] [CrossRef]
- Fragogeorgi, E.A.; Savina, I.; Tsotakos, T.; Efthimiadou, E.; Xanthopoulos, S.; Palamaris, L.; Psimadas, D.; Bouziotis, P.; Kordas, G.; Mikhalovsky, S.; et al. Comparative in vitro stability and scintigraphic imaging for trafficking and tumor targeting of a directly and a novel 99mTc(I)(CO)3 labeled liposome. Int. J. Pharm. 2014, 465, 333–346. [Google Scholar] [CrossRef]
- Tsoukalas, C.; Psimadas, D.; Kastis, G.A.; Koutoulidis, V.; Harris, A.L.; Paravatou-Petsotas, M.; Karageorgou, M.; Furenlid, L.R.; Moulopoulos, L.; Stamopoulos, D.; et al. A novel metal-based imaging probe for targeted dual-modality SPECT/MR imaging of angiogenesis. Front. Chem. 2018, 6, 224. [Google Scholar] [CrossRef] [Green Version]
- Psimadas, D.; Baldi, G.; Ravagli, C.; Bouziotis, P.; Xanthopoulos, S.; Franchini, M.C.; Georgoulias, P.; Loudos, G. Preliminary evaluation of a 99mTc labeled hybrid nanoparticle bearing a cobalt ferrite core: In vivo biodistribution. J. Biomed. Nanotechnol. 2012, 8, 575–585. [Google Scholar] [CrossRef]
- Orocio-Rodríguez, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Ramírez, F.D.M.; Ocampo-García, B.E.; Azorin-Vega, E.P.; Sánchez-García, F.M. Two novel nanosized radiolabeled analogues of somatostatin for neuroendocrine tumor imaging. J. Nanosci. Nanotechnol. 2015, 15, 4159–4169. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Khalaj, A.; Sabzevari, O.; Badrzadeh, L.; Mohammadzadeh, P.; Motlagh, S.S.M.; Bitarafan-Rajabi, A.; Ardestani, M.S. Technetium-99m chelator-free radiolabeling of specific glutamine tumor imaging nanoprobe: In vitro and in vivo evaluations. Int. J. Nanomed. 2018, 13, 4671–4683. [Google Scholar] [CrossRef] [Green Version]
- Ardestani, M.S.; Bitarafan-Rajabi, A.; Mohammadzadeh, P.; Mortazavi, S.; Sabzevari, O.; Azar, A.D.; Kazemi, S.; Hosseini, S.R.; Ghoreishi, S.M. Synthesis and characterization of novel 99mTc-DGC nano-complexes for improvement of heart diagnostic. Bioorg. Chem. 2020, 96, 103572. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Q.; Zeng, J.; Gu, Z.; Gao, M. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials 2019, 228, 119553. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kim, T.-W.; Chung, P.-W.; Slowing, I.; Tsunoda, M.; Yeung, E.S.; Lin, V.S.-Y. Structurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cells. Nano Lett. 2008, 8, 3724–3727. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.J.; Park, J.Y.; Kim, Y.-I.; Lee, Y.-S.; Jeong, J.M.; Kim, J.; Kim, E.E.; Kang, K.W.; Lee, D.S.; Jeong, S.; et al. Image-based analysis of tumor localization after intra-arterial delivery of technetium-99m-labeled SPIO using SPECT/CT and MRI. Mol. Imaging 2017, 16, 1536012116689001. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-W.; Solovyov, L.A. Synthesis and characterization of large-pore ordered mesoporous carbons using gyroidal silica template. J. Mater. Chem. 2006, 16, 1445–1455. [Google Scholar] [CrossRef]
- Kaneda, M.; Tsubakiyama, T.; Carlsson, A.; Sakamoto, Y.; Ohsuna, A.T.; Terasaki, O.; Joo, S.H.; Ryoo, R. Structural study of mesoporous MCM-48 and carbon networks synthesized in the spaces of MCM-48 by electron crystallography. J. Phys. Chem. B 2002, 106, 1256–1266. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Das, M.; Datir, S.R.; Singh, R.P.; Jain, S. Augmented anticancer activity of a targeted, intracellularly activatable, theranostic nanomedicine based on fluorescent and radiolabeled, methotrexate-folic acid-multiwalled carbon nanotube conjugate. Mol. Pharm. 2013, 10, 2543–2557. [Google Scholar] [CrossRef]
- Reddy, L.H.; Sharma, R.K.; Chuttani, K.; Mishra, A.K.; Murthy, R.R. Etoposide-incorporated tripalmitin nanoparticles with different surface charge: Formulation, characterization, radiolabeling, and biodistribution studies. AAPS J. 2004, 6, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.H.; Sharma, R.K.; Murthy, R.S.R. Enhanced tumour uptake of doxorubicin loaded poly(butyl cyanoacrylate) nanoparticles in mice bearing Dalton’s lymphoma tumour. J. Drug Target. 2004, 12, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G.; Davison, A. The chemistry of technetium I, II, III and IV. Int. J. Appl. Radiat. Isot. 1982, 33, 867–874. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Cui, Y.; Yue, Y.; Gao, Y.; Zhao, Q.; Liu, J.; Wang, S. A Eu3+/Gd3+-EDTA-doped structurally controllable hollow mesoporous carbon for improving the oral bioavailability of insoluble drugs and in vivo tracing. Nanotechnology 2016, 27, 315101. [Google Scholar] [CrossRef]
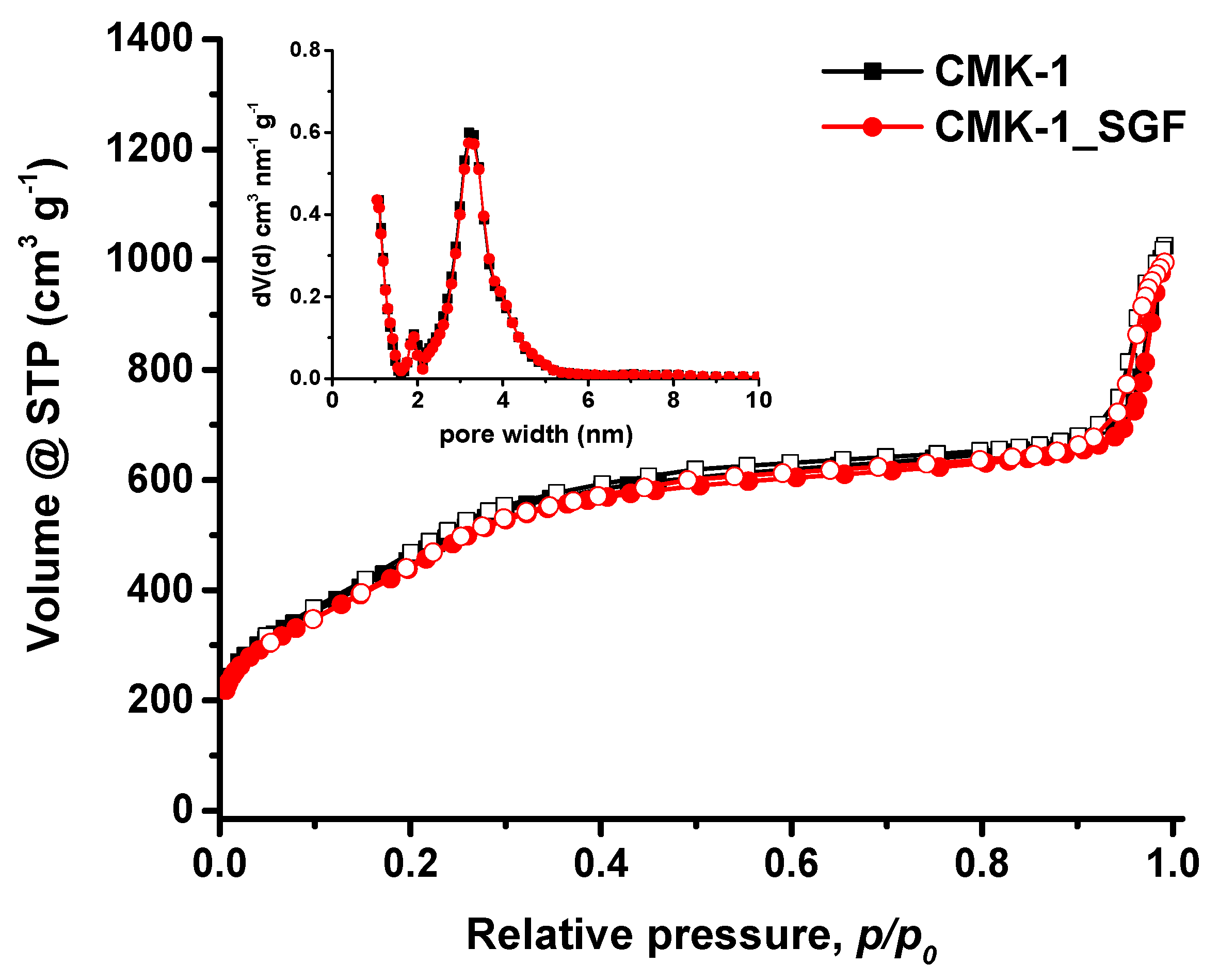
| Sample | SBET (m2/g) | TPV (cm3/g) at p/p0 = 0.90 | Vmicro (cm3/g) | Vmeso (cm3/g) | Pore Width (nm) |
|---|---|---|---|---|---|
| CMK-1 | 1513 | 1.03 | 0.22 | 0.81 | 3.20 |
| CMK-1_SGF | 1506 | 1.01 | 0.21 | 0.80 | 3.20 |
| 60 min | 3 h | 6 h | 24 h | |||||
|---|---|---|---|---|---|---|---|---|
| %ID/g | STDV | %ID/g | STDV | %ID/g | STDV | %ID/g | STDV | |
| Blood | 0.05 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Liver | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 |
| Heart | 0.37 | 0.58 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 |
| Kidney | 0.01 | 0.00 | 0.02 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 |
| Stomach | 39.49 | 2.97 | 24.36 | 8.17 | 12.04 | 1.49 | 0.81 | 0.60 |
| Intestines | 0.42 | 0.24 | 5.09 | 0.74 | 8.34 | 3.01 | 0.55 | 0.42 |
| Spleen | 0.04 | 0.06 | 0.05 | 0.01 | 0.06 | 0.01 | 0.01 | 0.01 |
| Muscle | 0.03 | 0.02 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Lung | 0.36 | 0.20 | 0.03 | 0.04 | 0.02 | 0.02 | 0.00 | 0.00 |
| Bone | 0.02 | 0.02 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 |
| Pancreas | 0.29 | 0.47 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Brain | 0.27 | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamai, M.; Giasafaki, D.; Salvanou, E.-A.; Charalambopoulou, G.; Steriotis, T.; Bouziotis, P. Biodistribution of Mesoporous Carbon Nanoparticles via Technetium-99m Radiolabelling after Oral Administration to Mice. Nanomaterials 2021, 11, 3260. https://doi.org/10.3390/nano11123260
Mamai M, Giasafaki D, Salvanou E-A, Charalambopoulou G, Steriotis T, Bouziotis P. Biodistribution of Mesoporous Carbon Nanoparticles via Technetium-99m Radiolabelling after Oral Administration to Mice. Nanomaterials. 2021; 11(12):3260. https://doi.org/10.3390/nano11123260
Chicago/Turabian StyleMamai, Maria, Dimitra Giasafaki, Evangelia-Alexandra Salvanou, Georgia Charalambopoulou, Theodore Steriotis, and Penelope Bouziotis. 2021. "Biodistribution of Mesoporous Carbon Nanoparticles via Technetium-99m Radiolabelling after Oral Administration to Mice" Nanomaterials 11, no. 12: 3260. https://doi.org/10.3390/nano11123260
APA StyleMamai, M., Giasafaki, D., Salvanou, E.-A., Charalambopoulou, G., Steriotis, T., & Bouziotis, P. (2021). Biodistribution of Mesoporous Carbon Nanoparticles via Technetium-99m Radiolabelling after Oral Administration to Mice. Nanomaterials, 11(12), 3260. https://doi.org/10.3390/nano11123260