Abstract
The multidisciplinary ‘Phyto-threats’ project was initiated in 2016 to address the increasing risks to UK forest and woodland ecosystems from trade-disseminated Phytophthora. A major component of this project was to examine the risk of Phytophthora spread through nursery and trade practices. Close to 4000 water and root samples were collected from plant nurseries located across the UK over a three-year period. Approximately half of the samples tested positive for Phytophthora DNA using a metabarcoding approach with 63 Phytophthora species identified across nurseries, including quarantine-regulated pathogens and species not previously reported in the UK. Phytophthora diversity within nurseries was linked to high-risk management practices such as use of open rather than closed water sources. Analyses of global Phytophthora risks identified biological traits and trade pathways that explained global spread and host range, and which may be of value for horizon-scanning. Phytophthoras having a higher oospore wall index and faster growth rates had wider host ranges, whereas cold-tolerant species had broader geographic and latitudinal ranges. Annual workshops revealed how stakeholder and sector ‘appetite’ for nursery accreditation increased over three years, although an exploratory cost-benefit analysis indicated that the predicted benefits of introducing best practice expected by nurseries outweigh their costs only when a wider range of pests and diseases (for example, Xylella) is considered. However, scenario analyses demonstrated the significant potential carbon costs to society from the introduction and spread of a new tree-infecting Phytophthora: Thus, the overall net benefit to society from nurseries adopting best practice could be substantial.
1. Introduction
The UK has a woodland resource of around 3.2 million hectares, which equates to 13% of the total land area of the country. Although this figure is low compared with the European country average of 37% woodland cover, the UK’s woodlands are nonetheless highly valued for a range of services including timber production, associated biodiversity, water and air quality improvement and numerous health and aesthetic benefits for society [1]. Taking into consideration the usages to which a monetary value can be ascribed, the asset value of UK woodlands was estimated to be worth £130 billion in 2017 [2]. Trees are also increasingly viewed as key in combating climate change threats through provision of carbon sequestration, flood mitigation and urban cooling. Such factors are driving policy change towards increasing woodland cover. In 2019–2020, 13,700 Ha of new woodland was created in the UK [3] and there are plans for future, large-scale woodland planting schemes aimed at flood mitigation and offsetting CO2 emissions, for example the new ‘northern forest’ scheme led by the UK’s Woodland Trust which will involve the planting of 50 million native trees over 25 years.
Despite these recognised values and ambitions to increase and enhance the UK’s woodland resource, at the same time the country’s trees have never before been under such pressure from the threat of new pests and diseases introduced through the ever-burgeoning plant trade and other human-mediated pathways [4]. For example, the value of ornamental plant imports to the UK increased by 71% in real terms between 1994 (£458 million) [5] and 2018 (£1.3 billion) [6]. The costs of trade-associated disease outbreaks are substantial. The first major epidemic of an imported tree disease in the UK with a huge ecological, landscape and societal impact was Dutch elm disease, which was brought in on Canadian elm logs and killed around 28 million elm trees between 1970 and 1990 [4]. More recently, the ash dieback epidemic, caused by the invasive fungal pathogen Hymenoscyphus fraxineus, is estimated to cost the UK around £15 billion, taking into account the loss of numerous ecosystem services associated with loss of the ash tree [7]. The authors also estimated that the total cost of ash dieback would be fifty times greater than the annual value of trade in live plants to and from Britain.
In the intervening period between Dutch elm disease and ash dieback, an accelerating series of pathogen outbreaks and disease epidemics affecting the nation’s trees have come from Phytophthora; a diverse genus of filamentous oomycete plant pathogens which infect a very broad range of herbaceous and woody hosts and are the causal agents of some of the most serious tree disease epidemics worldwide, including Phytophthora cinnamomi across several continents [8], P. ramorum [9] in the USA and UK, and P. agathidicida in New Zealand [10]. In the UK, P. alni has killed large numbers of alder since the 1990s [11] and, since the early 2000s, P. ramorum [12], P. kernoviae [13], P. lateralis [14], P. austrocedri [15] and P. pseudosyringae [16] have been causing serious damage to trees across a range of different environments and resulting in significant economic and ecological losses. In all the above cases, imported planting material has been either confirmed or strongly implicated as the most likely route of Phytophthora introduction. The effects are powerful. The loss of Japanese larch due to P. ramorum [9] leaves UK forestry in a vulnerable position with Sitka spruce now the sole commercially viable timber species suited to the western UK’s acid, upland forestry sites. The destruction of juniper by P. austrocedri leaves one of the UK’s three native conifer species and pioneer woodland colonisers at great risk of irreversible decline with serious ecological consequences [15].
Phytophthora is well suited to thriving in plant nursery environments as its free-swimming zoospores spread readily in water, and it can survive, often unseen, for long periods in soil and host material as thick-walled resting spores [17]. There is indeed strong evidence for the co-existence of multiple Phytophthora species in plant nurseries [18], which not only facilitates transmission across continents via traded plants but may also accelerate speciation and adaptation onto new hosts, for example as a result of hybridization, which is thought to be a common event in Phytophthora evolution [19]. Certainly, the hybrid species P. alni [20] has had devastating consequences for riparian alder across Europe.
One of the major tasks for Plant Health practitioners is to curb the spread of Phytophthora pathogens through trade. However, there are many challenges; Plant Health regulations are based on known species, but the genus Phytophthora is large and complex. Currently, there are approximately 180 species of Phytophthora provisionally named worldwide with new, cryptic species being described at an increasing rate as a result of global surveys for Phytophthora in many environments. It is thought that the genus may contain up to 500 species [4] and so the greatest future threat may come from the introduction of novel species with unknown host associations, which are ecologically adapted to establishing and spreading under UK environmental conditions. Indeed, the Plant Health protocols and phytosanitary regulations of The International Plant Protection Convention, EU and UK national authorities, and the World Trade Organisation are proving fallible when confronted with the huge volumes of containerised plants and cut flowers being brought into the UK from the EU and third countries, and the popularity of instant landscape schemes driving the importation of large, semi-mature or mature trees. The risky practices in the plant trade, and the flaws and loopholes in international and national Plant Health regulatory frameworks, are comprehensively outlined by Brasier [4] who called for the development of a national best practice certification scheme to combat the spread of plant pests and pathogens. The Nursery Industry Accreditation Scheme Australia (NIASA) is an example of such a scheme that sets guidelines for best practice for plant production nurseries and growing media manufacturers. The case for development of a similar scheme in the UK has continued to gather strength since the review of Brasier [4], not only to mitigate risk from Phytophthora but also in the face of a new threat posed by the broad host range bacterial plant pathogen Xylella fastidiosa now present on mainland Europe and causing multiple disease outbreaks on various horticultural and amenity plant species.
To address the increasing risks to UK forest and woodland ecosystems from trade-disseminated Phytophthora as implicated in the recent upsurge of Phytophthora diseases in the UK, and in light of the proposed massive increase in woodland planting across the country, the three-year multidisciplinary ‘Phyto-threats’ project was initiated in 2016. The project focused on understanding the drivers of emergence of Phytophthora species and opportunities for mitigation through three scientific objectives in which we sought to: (i) examine the distribution and diversity of Phytophthoras in different plant nursery management systems, (ii) assess the feasibility of accreditation by identifying social and economic opportunities and barriers to implementation of an accreditation scheme, and (iii) identify global Phytophthora risks to the UK by modelling the introduction, establishment and spread of species in relation to biological characteristics, environmental factors and trade flows.
In this paper, we present a broad overview of the project’s methods and main outcomes in relation to the key Phytophthora risks identified, the changes to management practice needed to mitigate these risks and the feasibility of achieving better biosecurity. We also appraise the multidisciplinary framework in which we sought to operate, how stakeholder engagement shaped our progress, and future opportunities and challenges to reducing Phytophthora spread in trade.
2. Overview of Methods
2.1. Phytophthora Distribution, Diversity and Management in UK Nursery Systems
The incidence and diversity of Phytophthora species in water and plant samples collected from nurseries located in England, Wales and Scotland was examined over a three-year period. The nursery survey was conducted on two scales; a fine-scale survey which involved the detailed sampling by the project team of fifteen partner plant nurseries operating a range of management practices including the production of bare root and containerised forest trees, wholesale horticultural plants, specialist native trees, stock for botanic garden collections and retail garden centres, and a broad-scale survey involving 118 nurseries and garden centres sampled systematically during annual statutory plant health inspections.
A key challenge was to secure partner nurseries willing to be subject to intensive sampling and scrutiny of results. Around twenty nurseries seeking to actively manage their Phytophthora risk were identified through the Plant Health authorities in England/Wales and Scotland. A letter and flier were sent to each nursery explaining the project’s aim and objectives, and importantly, what the project could offer to that nursery in terms of advice on Phytophthora management. It was emphasised at this point that all results would be anonymised, and that no nursery would be identified in any reports or publications. A follow-up phone call was held with each nursery to explain what would be required and any potential statutory implications should a quarantine-regulated species be found during the sampling. This approach succeeded in securing fifteen partner nurseries for the project’s fine-scale survey.
Since roots and water are the major transmission pathways of Phytophthora, both substrates were targeted for the fine-scale survey. The sampling method was not random but biased to finding Phytophthora. Roots were sampled from symptomatic plants as well as a range of asymptomatic plants that are known to be Phytophthora hosts. Irrigation water, run-off from potted plants, puddles and collection ponds were also sampled on-site by filtration methods that capture Phytophthora propagules [21] (Figure 1). For the broad-scale survey, root samples were collected.

Figure 1.
Fine-scale nursery sampling. Top, L-R: sampling roots of Chamaecyparis lawsoniana, irrigation reservoir, puddle. Bottom, L-R: water flow through sampling of Pinus sylvestris, water storage tank, filter after water sampling.
For each nursery sample, metadata were collected on associated host species, symptoms present at time of sampling, sample origin and location on nursery. This was supplemented with wider data on propagation and trading practices, irrigation sources and disease management practice for downstream analysis to inform best practice.
To try to capture the highest diversity of Phytophthora species possible in each sample DNA metabarcoding was used. This is a method which uses high-throughput sequencing technology to detect all species of a target group (in this case the Phytophthora genus) present within an environmental sample, including as-yet undescribed taxa [21,22]. Total eDNA (environmental DNA) is extracted from the sample and a target region of the genome unique to individual species, known as a ‘barcode’, is amplified and sequenced using high-throughput DNA sequencing. Thousands of DNA sequences can be generated from an individual sample and, following a number of quality control and data cleaning steps, each remaining sequence is compared to a database of reference sequences [23] to identify the species present. In this study the ITS1 genomic region, expected to identify uniquely Phytophthora species, was sequenced using the Illumina platform as described in [24] except that synthetic control sequences were used instead of positive control samples to estimate error, and Phytophthora species were identified using a Phytophthora classification tool developed as part of this project [25]. The development of this tool focused on maximising the accuracy of reporting while minimising the risk of reporting false positives. Much of the development work focused on identifying and accounting for technical variation in metabarcoding, determined through the use of synthetic DNA control sequences alongside real samples when sequencing.
2.2. Is Nursery Accreditation in the UK Feasible and If So, What Should It Look Like?
Mitigating the spread of plant diseases through nursery accreditation will only be successful if there is consumer and industry buy-in. To ensure effective liaison of the multidisciplinary science team with stakeholders, and to facilitate impact, the project operated within a collaborative learning platform which contained at its core a project Board composed of lead members of the science team plus a key industry stakeholder, and an Expert Advisory Panel (EAP) composed of external scientists from the UK and overseas, UK policymakers, Plant Health practitioners and industry representatives. The role of this panel was to help steer the project’s science outcomes to match stakeholder needs. A wide range of stakeholder and consumer attitudes to implementation of a UK-wide nursery ‘best practice’ accreditation scheme was identified through surveys and interviews [26,27]. These analyses involved growers, retailers, local authorities, landscape architects and the general public. An enhanced view of stakeholder perspectives was also obtained through three annual stakeholder workshops (described below) and attendance at key sector events, such as the National Plant Show, Britain’s largest plant trade show. To investigate the economics of introducing best practices from a nursery perspective, managers at 75 plant nurseries were interviewed to gather information on costs and benefits of introducing best practices [28]. A societal perspective was also explored, which estimated the timber production and carbon benefits associated with avoiding new tree disease epidemics as a result of introducing best practice [28].
2.3. Global Phytophthora Risks to the UK
Establishment and spread of forest pathogens following human introduction has been linked with biological traits [29] as well as characteristics of the invaded region [30] including its climatic similarity to the native range. In our study, Phytophthora risks to the UK were identified and ranked by modelling the introduction, establishment and spread of different Phytophthora species in relation to their biological characteristics (traits) as well as environmental and social factors; namely trade flows and climatic conditions. To facilitate these analyses, global databases of traits and national-scale [31] and local-scale occurrence of different species were collated from a wide range of sources including plant health diagnostic labs, culture collections, literature records, citizen science initiatives, government organisations and global distributional databases such as GBIF. Records were obtained from the forestry, agricultural and nursery sectors, and from natural/semi-natural forests, urban spaces, parks, as well as private and public gardens (Figure 2). Species names were matched to those in the Phyto-threats trait database using the Index Fungorum taxonomic backbone [32]. Country-level occurrence data was collated from CAB Abstracts, CABI Invasive Species Compendium datasheets, EPPO Global Database, GBIF, the DAISIE European Invasive Alien Species Gateway, NCBI Biosample, Index Fungorum and the Fungal Collection of the CBS strain database (Table 1).
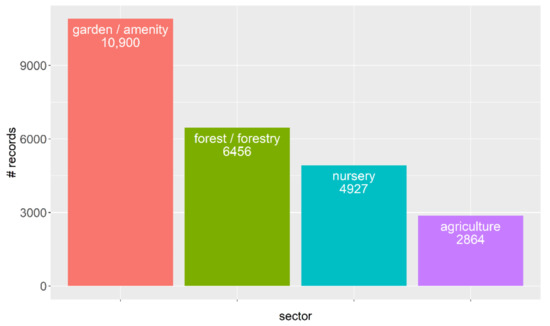
Figure 2.
Number of global Phytophthora records arising from the different sectors.

Table 1.
Sources used to extract country-level distribution data for Phytophthora ramorum species. Index Fungorum was used as the taxonomic backbone to match species names from multiple sources to those in the trait database.
Site-level (georeferenced) records were obtained from a combination of published records and unpublished data contributed by an international network of 107 plant pathologists. A literature search, with ongoing alerts, was performed in Google Scholar using the search terms ‘in title: “Phytophthora” AND “sp. nov”’ and ‘Phytophthora in title: “distribution” OR “occurrence” OR “diversity”’. The references (including supporting information) were screened for either georeferenced Phytophthora species records or records associated with site names and these data were extracted. Where available, data were also collected on the habitat, host, substrate, and disease symptom for both published and unpublished records, thus indicating potential behaviour of individual species across a range of environments and hosts. Additionally, where possible, survey metadata on the year, identification method, the sampling procedure and the spatial precision of records were recorded to support decisions about spatial recording intensity and the reliability and/or accuracy of individual records when analysing the data. For unpublished data, the owner of the data and permissions for use and presentation were also recorded.
Close to 40,000 Phytophthora records were collated from 179 countries (Figure 3), including approximately 28,000 site-level records. This equated to 1585 species x country combinations and included the year of the earliest species record in each country. Much of the recording effort for Phytophthora pathogens has been concentrated in the US, Australia, New Zealand and Europe. Despite the effort to accumulate these data, the number of site level data points yielded for individual Phytophthora species was low, exceeding 50 records for only 13/197 species. Data were therefore insufficient for most species to model the environmental niches or landscape-scale drivers of spread. This highlights the disproportionately low global recording effort for pathogen taxa (versus plant host taxa or taxa of conservation concern) and the importance of initiatives to develop centralised, cross-sectoral databases for plant pathogens to enable a comprehensive understanding of their behaviour and potential for spread between hosts and habitats [42]. The biological traits database [31] was compiled through literature review and expertise of project pathologists together with pathologists in Australia (T. Burgess and G. Hardy, Murdoch University) and New Zealand (P. Scott and N. Williams, Scion). It included all 179 Phytophthora species or sub-species known from forestry, agricultural and horticultural settings as of January 2018. Of these species, 166 are formally described, 13 are provisionally named and 8 are known hybrids. Ten traits were used to predict global impacts of Phytophthora (number of countries reached, latitudinal limits and number of host plant families). These traits are related to growth (i.e., minimum and optimum temperatures for growth, growth rate at optimum temperature), survival, persistence and reproduction (presence of hyphal swellings, chlamydospores, oospores and oospore wall index), dispersal, (i.e., caducous sporangia, proliferating sporangia), and disease symptoms (ability to cause root and foliar disease) [42]. Most of these traits have historically been collected for use in morphological identification of species; however, their functional significance is less well understood.
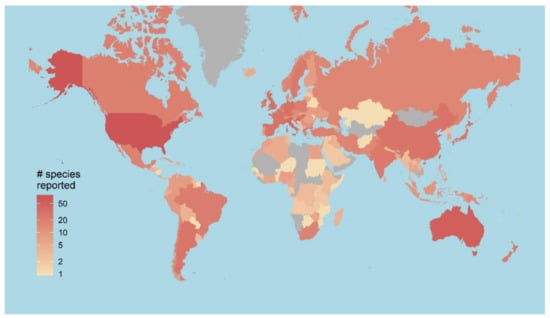
Figure 3.
The number of different Phytophthora species reported in each country, based on records in the global Phyto-threats database.
Global patterns of new Phytophthora arrivals at the country-level were analysed in relation to trade connectivity between source regions and importing countries (as a proxy for propagule pressure) [43]. The country-level reports of Phytophthora species were used to define a potential source range for each species (detections prior to 2000). Countries with new detections after 2000 were defined as new arrivals while countries without new detections were assigned as non-arrivals. Trade connectivity was measured as the sum of imports of live plants from all source countries where the Phytophthora species was present prior to 2000. The relationship between trade connectivity (fixed effect) and new Phytophthora arrivals was estimated using phylogenetic generalised linear mixed models fitted in R package ‘brms’ [44]. Phytophthora arrivals (1) and non-arrivals (0) were modelled as a Bernoulli response. Initially, random intercepts were estimated to quantify the magnitude of unexplained variance at the species and country level (for example due to species level traits and country level biosecurity). Phylogenetic non-independence among the species-level observations was accounted for using a unique species-level intercept with mean of zero and variance among species derived from their phylogenetic position. Species phylogenetic relationships were inferred from an ITS phylogeny (T. Burgess, unpublished data) including all 179 species in our trait database. The fitted models were used to predict import risks (probability of a new Phytophthora arrival) to the UK from specific source countries using data on live plant imports to the UK from each country. As the models are trained using global patterns of arrival and trade connectivity, they can equally be used to estimate import risk to any country, based on its connectivity to potential countries of Phytophthora that have yet to arrive.
3. Overview of Results and Discussion
3.1. A High Diversity of Phytophthora ramorum Species Was Found in UK Nurseries with Strong Evidence Linking Infestation Levels to Management Practices That Should Be Targeted in an Accreditation Scheme
Overall, the project collected 3624 samples from 163 host genera; the top five most frequently sampled species being Juniperus, Taxus, Viburnum, Pinus and Chamaecyparis (Figure 4).
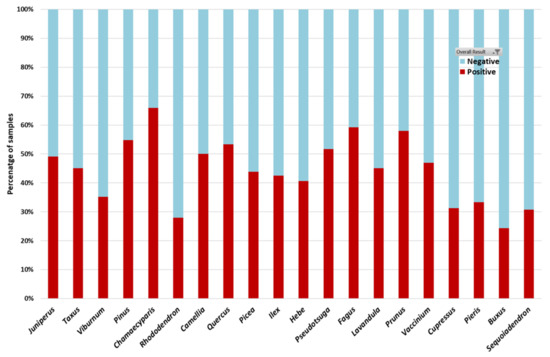
Figure 4.
The twenty host genera most frequently sampled in the fine-scale nursery survey. Data shown are the percentage of samples testing either positive (red shading) or negative (blue shading) for Phytophthora for each host.
The sample analysis was a two-stage process, with the first stage being a PCR test to determine whether Phytophthora was present in the sample or not. All Phytophthora-positive samples were then progressed to the second stage of Illumina sequencing to determine which Phytophthora ramorum species were present. Overall, ~50% of all nursery samples collected were positive for Phytophthora ramorum or closely related oomycetes. This varied according to host genus, with Chamaecyparis, Fagus, Prunus, Pseudotsuga and Pinus yielding the highest proportions of positive samples (Figure 4). In terms of Phytophthora ramorum test results in relation to nursery practices, the percentage of positive samples ranged from 20%–70% across the fifteen partner nurseries, and this reflected the variation in plant health status and nursery management practices across the nurseries. Sequences matching a total of 63 known Phytophthora ramorum species were detected in the nursery samples, the most common being P. gonapodyides, P. cinnamomi, P. cryptogea/pseudocryptogea, P. syringae, and P. lacustris. The species assemblages and their abundance also varied across the fifteen partner nurseries sampled for the fine-scale survey (Figure 5), and this could be related to observed management practices at sampling.
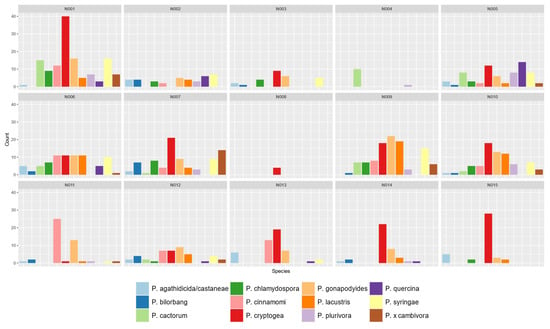
Figure 5.
Abundance (by number of samples) of the 12 Phytophthora ramorum species most frequently detected in the fifteen partner nurseries (N001-N015) in the fine-scale nursery survey. Note P. cryptogea values include those of P. pseudocryptogea.
The clade 6 taxa (such as P. gonapodyides and P. lacustris) are generally considered native and less pathogenic, and are abundant in rivers in Europe. However, the other abundant species (which, in addition to the above species, also included P. cactorum, P. cambivora, P. plurivora and P. nicotianae) are common pathogens on many hosts in the nursery industry. The important quarantine-regulated species P. ramorum was found in twelve samples overall and P. kernoviae not found at all, whereas other regulated pathogens P. lateralis and P. austrocedri were each found in 10 samples. The relatively low abundance of P. ramorum and absence of P. kernoviae, both of which are heavily targeted in Plant Health surveillance, suggests that existing phytosanitary measures may be working to some extent.
Other species of concern included P. cinnamomi which is adapted to warmer environmental conditions, has an exceptionally wide host range and was found to be widespread on a range of hosts particularly in nurseries in southern England. Phytophthora quercina was found in the majority of Quercus plants sampled in the survey. This species is thought to be native to Europe and implicated in root damage and progressive decline of oaks [45]. A DNA sequence matching (tropical) clade 5 species P. agathidicida/castanae/cocois was found in many water samples, particularly in southern UK nurseries. There is an open question as to whether components of peat-free potting media, namely coconut fibre (coir), imported directly from tropical countries such as Sri Lanka, could be the source of inoculum. There were also several new potential Phytophthora ramorum species records for the UK (i.e., P. castanetorum, P. palmivora, P. pseudotsugae, P. tentaculata, P. terminalis, P. uliginosa) and evidence for Phytophthora ramorum root infections in newly arrived plants imported from the European Union.
Management practices can affect pathogen arrival (source and health of plant material coming in, growing media, water source, mud on vehicles/boots), pathogen spread on site (including water management and hygiene) and pathogen dispersal off site (quality control at sale, water run-off, plant disposal). Some of the most diverse Phytophthora ramorum assemblages, including highly damaging species, were found in river water used to irrigate plants (up to eight known species in a single river sample) and in open reservoir irrigation sources, as well as in puddles (up to twelve known species in a single sample) which formed around plant stock, confirming that effective water treatment and good drainage are essential components of Phytophthora ramorum management. Indeed, preliminary analysis of Phytophthora ramorum communities in relation to management practices showed that the commonest Phytophthora ramorum species were more likely to occur in nurseries using open rather than closed water sources (Figure 6).
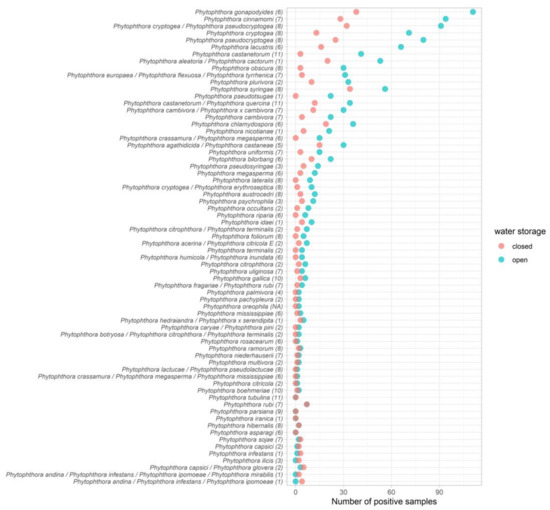
Figure 6.
Number of samples testing positive for each Phytophthora ramorum species at nurseries using open versus closed water sources. Taxonomic clade is presented in brackets after each species name. Data represent samples collected from the fifteen nurseries sampled in the fine-scale survey.
Plant disposal was clearly an issue across nurseries, with most disposal areas or ‘dumps’ containing Phytophthora-infected material which could be a source of inoculum. Phytophthoras were also detected in diseased shelterbelt or landscape trees present in and around some nurseries, acting as additional inoculum reservoirs. One outcome of this project was the recommendation that trees growing in the immediate environment of each nursery should be included in routine Statutory Plant Health inspections. Another issue highlighted by the survey was that, in some cases, native plant stock being raised from seed collected from ecologically sensitive sites and destined for planting back out at these sites were found to be harbouring damaging Phytophthora ramorum species. Also found was the quarantine regulated species P. lateralis infecting an ‘off-colour’ Chamaecyparis lawsoniana offered at a discounted price in the retail area of one nursery. Thus, this study identified a number of key practices that should be targeted in an accreditation scheme or in guidance documentation for woodland restoration.
Direct engagement with a range of nurseries operating different practices enabled the science team to understand the challenges faced by the industry and some of the key factors leading to good and poor biosecurity practice. Following the reporting of Phytophthora ramorum findings to each nursery, feedback and advice on improving practice was given using a ‘non-blaming’ approach to facilitate dialogue. A genuine lack of awareness of the risks and routes of Phytophthora ramorum contamination and spread was apparent in some cases, for example through asymptomatic imported plants, unclean water sources and inadequate drainage. There was also a frequently held assumption that symptoms of poor health in plants were caused by other factors such as frost or drying out when the subsequent sampling results confirmed infection by Phytophthora.
As the project progressed there was evidence of partner nurseries improving practice based on advice delivered by the science team as a result of Phytophthora ramorum findings, for example raising plants off the ground, improving drainage and taking decisions not to trade in high-risk hosts. Nurseries also started asking questions of growing media suppliers–what are the processes of sterilisation and can they guarantee pathogen-free components? and seed suppliers–has this seed come from disease-free orchards? In relation to raising from locally collected seed any native plant stock destined for restoration planting at ecologically vulnerable sites, one nursery manager is considering offering dedicated growing of stock to high biosecurity specification close to each restoration site and well away from the main commercial premises. Another partner nursery has employed a full-time plant health specialist to train staff on plant health issues, audit existing plant health processes within the nursery, and to update plant health standards.
3.2. Informing Accreditation and Broader Plant Health Surveillance
Parallel to the Phyto-threats project, a joint accreditation initiative led by the Horticultural Trades Association and industry was being developed. This is the Plant Healthy Certification Scheme (PHCS) (https://planthealthy.org.uk/) (accessed on 17 November 2021) which is based on the Plant Health Management Standard (PHMS) currently consisting of a check-list of 23 requirements that demonstrate that a business is operating responsibly. The PHMS is not prescriptive at this early stage in the development of the scheme but rather highlights problem areas on a nursery and offers advice on how they can be resolved. It is expected that as more information on biosecurity risk becomes available, more prescriptive measures will be incorporated. The Phyto-threats project science team are liaising with those leading on PHCS to provide the scientific basis for more prescriptive audited measures around water source and usage, plant disposal, growing media and raising plants off the ground to be referenced in the scheme guidance, since the project’s main outcomes strongly suggest that a set of priority prescriptive measures will be necessary for certification to be effective. Additionally, the project’s findings of widespread Phytophthora ramorum contamination have led to the recommendation that the PHCS audit process includes a component of targeted testing for pests and pathogens. To this end the various strands of engagement with the Statutory Plant Health teams in England/Wales and Scotland have facilitated a greater awareness of what the metabarcoding technology can offer and generated discussion of its potential for routine nursery testing, as part of regular statutory surveillance or for incorporation into an accreditation scheme.
3.3. Stakeholder Workshops Enabled Tracking of Wider Attitudes towards Accreditation
It is clear that nurseries can collectively play a key role in mitigating pests and diseases through attention to their daily practices (e.g., procuring plants, storage and management). More widespread adoption of biosecurity best practice in the sector could be promoted through the introduction of a UK-wide accreditation or certification scheme. However, the success of such a scheme is likely to depend not only on its uptake by nurseries but also the plant-purchasing behaviours of other key sectors in the plant supply chain. Research carried out by social scientists in the Phyto-threats project explored attitudes towards a hypothetical accreditation scheme. A survey of 100 UK garden centres and nurseries [26] identified strong agreement within these sectors that an accreditation scheme would provide greater biosecurity protection and ensure that consumers purchased better quality plants. However, there were concerns over costs and a degree of scepticism over the likely interest of consumers in accredited products. The appetite for accreditation was further explored amongst UK nurseries as well as public sector (e.g., local authorities) and commercial (e.g., large retailers) consumers through a series of qualitative semi-structured interviews [27]. Findings suggest that nurseries would be supportive of accreditation if costs were not prohibitive, actions required were not deemed unreasonable, the scheme provided a safety net and it had measures to deal with non-compliance with the required standards. Importantly, they were keen to see evidence of consumer demand for accredited goods. Large-scale consumers are undoubtedly key players in whether membership of an accreditation scheme is seen as viable. There was agreement amongst local authorities and retailers that accreditation would be valuable if it helped to define quality, drove up standards and provided accurate records of traceability. However, there were a number of questions that mirrored the concerns of nurseries e.g., Who/what would be accredited? What would the demand be? How would an accreditation scheme be policed? What resources would be needed to do it properly? How would it be financed? [27].
Three annual multi-stakeholder workshops were also held over the course of the project, each with ca. 45 attenders representing nursery managers, landscape architects, garden designers, Plant Health inspectors, foresters, policy makers, academics and others. The first workshop on ‘Improving nursery resilience against threats from Phytophthora’ was held in 2016. The objectives of this workshop were to introduce the scientific aims of the Phyto-threats project, develop collaborative networks across individuals and groups with an interest in working towards collective best practice in nurseries, and to share lessons and experiences around the challenges and opportunities of managing disease threats. The workshop included perspectives on plant health from a Defra policymaker, two forest nursery managers and a trader, followed by a panel discussion to elaborate on some of the challenges faced by different sectors. The use of correct tools for disease detection was said to be important, as was the closure of high-risk pathways in terms of transmission of pests and diseases. The value that plants add to the environment was believed to be greatly under-valued by society, which facilitates the desire for cheaper products and imports. Reducing bureaucracy was considered key to making changes in the sector as well as seeking opportunities to increase the quality and quantity of UK plant production. Feedback from an afternoon workshop session suggested that accreditation might need to be tailored for different stakeholders but possibly under a single umbrella. A scheme could include different levels of standards to encourage businesses to improve their practices, but it should have minimal bureaucracy and there would need to be consumer support for any scheme to provide an incentive for nurseries to be involved. Decisions over what the scheme should include would best be made by representatives from a mix of sectors. EU exit might provide an opportunity for the UK to promote its own best management practices and to have more control over quality of imports. There are practices (e.g., mail orders, TV garden shows, illegal trade in plants) that could undermine an accreditation scheme, so the scheme would require consumers to be informed and supportive.
The second workshop in 2017, ‘Reducing Phytophthora ramorum in trade and designing effective accreditation’, aimed to share science findings to date from the project which might help underpin accreditation, understand existing UK assurance schemes and how they might be supported, and generate ideas for how an accreditation scheme should work in order to be effective. Although attendance was similar to the previous year’s workshop, it was notable how the overall appetite for accreditation had increased across the range of stakeholders. The key outcomes of the workshop are summarised as follows:
- A single, all-encompassing UK accreditation scheme is preferred.
- Accreditation needs to cover the entire supply chain if it is to have impact.
- Demand for accredited products should be ensured to incentivise uptake in the scheme.
- Accreditation could also be incentivised by allowing access to grant funding, and through financial reimbursement when contracts to supply accredited plants are cancelled/altered.
- It is necessary to raise public awareness about the need and benefits of accreditation through highly visible campaigns and signage, including a recognisable logo.
- Training for growers and others in the sector should be integral to accreditation so that practices continue to improve.
- For a scheme to be respected and to generate improvement it must be effectively policed.
In 2019 a final stakeholder workshop was held to share the latest science findings from the project, to provide an interactive demonstration of science outcomes and tools and to explore how the project’s science outcomes can best be used to support the continued development of accreditation and plant health policy. An update was provided on the Plant Healthy certification scheme. It was clear that the project’s work on analysing Phytophthora ramorum diversity in nurseries had identified hosts and practices of high biosecurity risk that should be targeted in the Plant Health Management Standard. However, the stakeholder surveys undertaken as part of this project highlighted the challenges of securing uptake and consumer support for accreditation i.e., [26,27], one of the key challenges being the need for much better engagement on plant health risks to secure collaboration from all sectors.
3.4. Economics of Introducing Best Practice
It was apparent from interviews that few nurseries are willing or able to incur substantial costs to become accredited [27] and indeed doubted whether introducing best practice measures would be economically viable, currently. An exploratory economic analysis from a nursery perspective highlighted uncertainty and the difficulty in obtaining robust estimates of the costs and benefits of introducing the best practices [28]. A reduction in the frequency of disease outbreaks is the primary benefit to a nursery of implementing best practices, but the extent to which the risk of outbreaks falls is difficult to quantify in advance. Only a minority of the nursery managers were able to provide a quantitative estimate of, or range for, the cost that they anticipated, were a future Phytophthora ramorum outbreak to occur. In addition, for each best practice, only a minority of nursery managers provided a quantitative estimate of the implementation cost. Twelve best practice measures were initially considered [28]. Five of the measures (water storage in fully enclosed tanks, clean/covered storage of growing media, installation of drains or free-draining gravel beds, raised benches and tool disinfestation stations) were reported to have been implemented already by a majority of nurseries surveyed. The analysis focused on the remaining seven practices not currently commonly implemented (water testing for pathogens, water treatment facility, quarantine holding area for imported plants, composting/incineration facility for sick/unwanted plants, boot washing station, vehicle washing station, buy only from trusted or accredited UK suppliers).
Based on the limited available data, the analysis suggested that, over a 10-year nursery investment time horizon, the introduction of the five best practices is unlikely to be justified on the basis of avoided Phytophthora ramorum outbreaks alone, since the average benefit of avoiding a Phytophthora ramorum outbreak (mean £47,000) was far lower than the average cost (mean £386,000) for set-up and maintenance of the best practices over a 10-year investment horizon [28]. Roughly one Phytophthora ramorum outbreak per annum would need to be avoided for the mean benefits of introducing the best practices to exceed the mean costs, but such outbreaks appear far less common at present [28]. However, the introduction of the best practices could be expected to reduce the risks of other pest and disease outbreaks, such as Xylella fastidiosa. Only when reducing these additional outbreaks is considered too are the benefits likely to start to outweigh the costs—although the benefit of avoiding a Xylella outbreak to the nursery is also difficult to quantify [28].
From society’s viewpoint, the value of implementing phytosanitary measures in nurseries is the expected reduction in the losses that would result from reduced risks that infections spread into commercial forests and native woodlands. Using a representative social value of £100/tonne CO2, the cost to society of a new disease destroying 90% of the Sitka spruce resource (the key commercial conifer plantation species in the UK) in a similar manner to recent impacts of Phytophthora ramorum on larch is £11,400 million and for the loss of 40% of oak (a key component of native broadleaved woodland), £500 million [28]. If implementation of nursery best practice measures were to reduce the risk of an outbreak on Sitka spruce and oak by 1%, this would result in a combined, largely carbon, benefit to society of £120 million [28]. Thus, whereas there may not be a net benefit for individual nurseries of introducing best practice unless a high frequency of disease outbreaks is expected to be avoided, the overall benefit to society of an accreditation scheme that resulted in best practice introduction could be substantial.
3.5. Functional Significance of Phytophthora ramorum Traits: Do Biological Traits Explain Differences in Global Extent and Host Range between Phytophthora ramorum Species?
The global impact (extent and known host range) of Phytophthora ramorum species was modelled against ecological traits (those commonly collected when species are described) and time known to science, accounting for phylogenetic relatedness between species. The ability to cause disease symptoms (in roots or foliage), primary ecological traits and phylogenetic position could partially predict geographical spread and host ranges [42]. For example, having a higher oospore wall index and faster growth rates increased host ranges whereas cold-tolerance increased the number of countries and latitudinal limits reached [42]. These results reflected the different mechanisms underpinning Phytophthora ramorum impacts. Higher oospore wall index could allow greater persistence in the soil which might increase encounter rates with novel hosts. In a separate analysis, comparing the latitudinal extent reached by individual species in the wider environment in the UK, it was shown that species with lower minimum temperatures for growth established at higher altitudes. Our findings were consistent with prior studies showing that establishment of Phytophthora ramorum species outside of nurseries in Sweden was also positively associated with low temperature tolerance and the production of asexual survival structures [46].
These relationships may help to rank the risks from emerging pathogens, as they are discovered, on the basis of their trait and phylogenetic similarity to species that have already had high impact. A large amount of species-level variation in global impacts was not explained by the traits included in models, highlighting knowledge gaps surrounding the functional traits of plant pathogens which have adaptive value during invasions. Phylogenetic position, i.e., relatedness between species, explained 53% of species-level variation in the number of countries reached. Quantifying sporangial production, oospore production and chlamydospore production at different temperatures may be key to understanding the role of propagule pressure and cold tolerance in invasion success. Incorporating genomic traits associated with virulence and host range may also be of value for understanding the evolutionary potential of pathogens during invasions. Addressing these knowledge gaps may improve the predictive power of such horizon scanning frameworks [42].
3.6. What Is the Role of Trade Pathways, Climate Matching and Biosecurity Capacity in Driving Arrival of Phytophthora ramorum Species in New Countries?
At the country level, a greater number of Phytophthoras arrive in those countries that are more connected to source regions through a higher frequency of international trade. Amongst commodities, imports of live plants were best able to discriminate between arrivals and non-arrivals (Figure 7), significantly increasing the probability of detecting new Phytophthora ramorum species in a country (Figure 8; R2 = 0.33). This re-confirms that importation of plant material is the most likely pathway for Phytophthora ramorum introduction into new regions [4]. Major source countries posing a risk of contributing to UK Phytophthora ramorum arrivals, due to their reported Phytophthora ramorum richness and trade connectedness, included The Netherlands, Denmark, Belgium, Germany, and Ireland. (Figure 9).
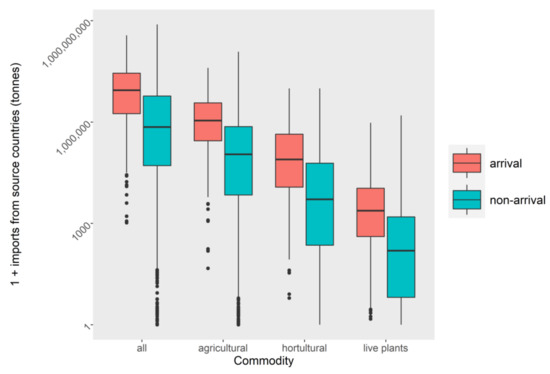
Figure 7.
Comparing rates of import of different commodities (from Phytophthora ramorum source countries) between countries in which Phytophthoras have arrived since 2000 (pink) versus those in which they did not arrive (blue).
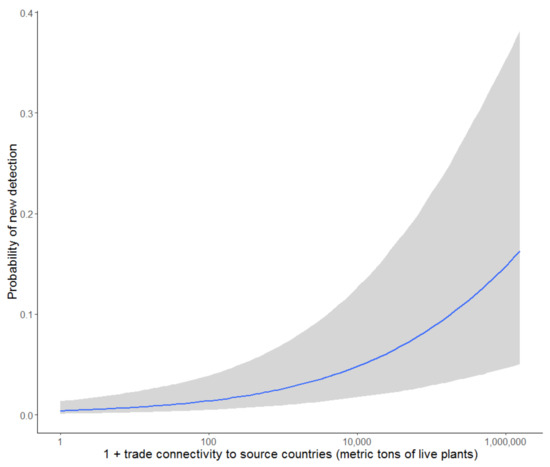
Figure 8.
Estimated relationship between Phytophthora ramorum arrival risk and imports of live plants from potential source regions, conditional on climate matching, national pest and pathogen reporting and biosecurity capacity. Grey shading represents 95% confidence intervals based on the fixed effects, excluding variance among species due to random effects.
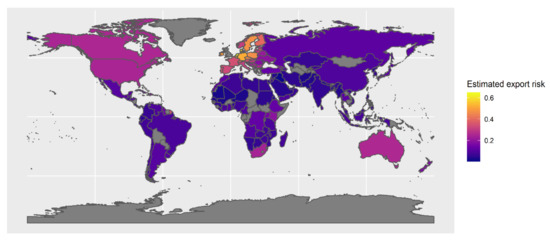
Figure 9.
Estimated risk of export of a new Phytophthora ramorum to the UK by exporting country, based on trade flows, climate-similarity, reported Phytophthora ramorum richness, plant health reporting activity and national biosecurity capacity.
These models have been extended (reported in a separate paper in preparation) with the addition of climate matching, biological traits, plant health reporting activity and biosecurity effort. These developments were intended to account for unexplained variance in trade models at the species-level and country level. The refined models were designed to understand which species with which traits are more likely to be introduced through trade, and whether source countries with more similar climate to sink countries and lower biosecurity effort pose a greater risk of introduction, whilst accounting for plant health reporting effort.
3.7. For Phytophthora ramorum Species with Sufficient Data, Global Niche Models Can Be Used to Understand Which Areas of the UK Are at Risk from Establishment
Models and maps of species’ environmental niches, indicating their potential distribution, can be developed by matching patterns in species occurrence (as in Figure 10) with patterns in key environmental drivers [47]. All eight Phytophthora ramorum species (starred in Figure 10) for which there were sufficient global records to make a niche model are already present in the UK. The UK data were reserved from the global niche models for each species and the wider environment data points were used as an independent validation dataset to determine whether environmental niche models can predict the occurrence of species in invaded areas accurately. Models additionally accounted for unsuitable habitat, dispersal distances and spatial biases in recording effort and used expert-elicitation to identify key environmental drivers as described in [48] and included metrics of seasonal climate as well as relevant land uses (forest, urban and agricultural).
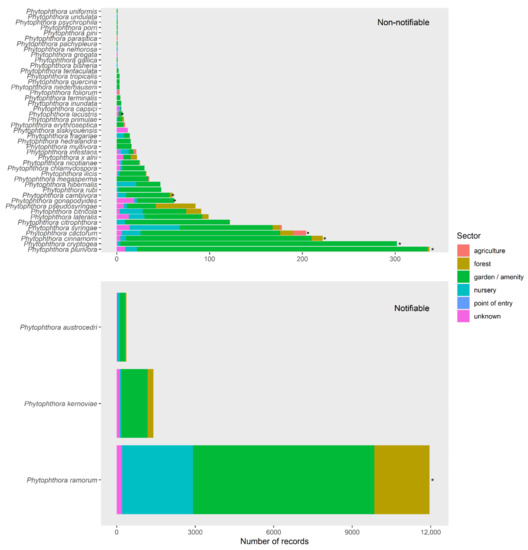
Figure 10.
Number of times each Phytophthora ramorum species has been recorded in the UK, in points of entry, the nursery, garden, forest or agricultural sectors for notifiable (lower graph) and non-notifiable (upper graph) species.
The fitted models predicted both the global and UK Phytophthora ramorum distributions with reasonable accuracy (global Area Under the Curve (AUC) values exceeding 0.972 for all species, UK AUC values exceeding 0.65 except for P. gonopodyides). Figure 11 illustrates how the UK distribution of P. ramorum in the wider environment was predicted accurately from the global niche model (UK AUC of 0.74). However, this assessment was hampered by low numbers of UK records for most species. It is concluded that global environmental niche models could provide useful risk maps for Phytophthora ramorum outbreaks in outdoor environments in the UK, but that centralised databases that draw together pathogen data across sectors will be required to compensate for the incomplete and extremely biased recording of Phytophthora ramorum species globally that poses major challenges for the modelling.
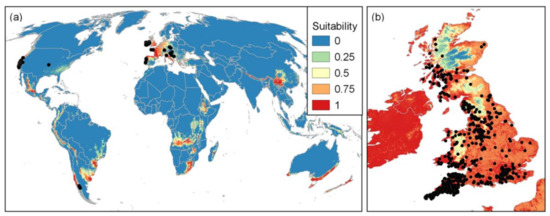
Figure 11.
Global habitat suitability maps for Phytophthora ramorum where values are the predicted probability of occurrence of this species overlaid with (a) the global training dataset occurrences (black dots) and (b) UK validation occurrences from natural and outdoor managed environments (black dots) (AUC = 0.747, vs. all UK background).
3.8. Tools for a Range of Stakeholders
The global Phytophthora ramorum databases developed as part of this study have been made available to researchers and other end-users and will be updated as new species are described and information becomes available. A number of model outputs were integrated into prototype tools to help influence policy and practice (https://loubar.github.io/PHC-plant-health-biosecurity-risks-Scotland/) (accessed on 17 November 2021) as part of a project for Scotland’s Plant Health Centre (PHC2019/05 and PHC2019/06). These included a Phytophthora ramorum importation tool which focusses on the role of international trade in the movement of Phytophthoras and allows users to visualise the Phytophthora ramorum diversity in exporting countries and the volume of imports from those locations. An interactive database of Phytophthora ramorum pathogens and associated hosts was also developed, which is searchable by host and by pathogen and links to risk maps of UK suitability for the pathogens. The hosts include UK woodland and commercial forestry species, which could be used to inform species choices for commercial planting and afforestation. A third tool allows users to interact with maps of Phytophthora ramorum disease records in the UK. The maps show which species are predominantly nursery-associated and which are common in the wider environment. The co-development of such tools with stakeholders across sectors is ongoing.
4. Concluding Remarks
The Phyto-threats project involved a consortium of scientists in disciplines ranging from plant pathology, ecological modelling, evolutionary genetics, economics, and social science as well as a range of consumer, practitioner, and policy stakeholders. Operating within a collaborative learning platform designed to promote interdisciplinary working and facilitate stakeholder participation and knowledge exchange, the consortium aimed to deliver the science base to support a UK-wide plant nursery accreditation scheme based on the rationale that the ‘consumer’ has considerable power to mitigate Phytophthora risks by driving a change in the culture of nursery practices. This evidence base was informed by a greater understanding of Phytophthora diversity in different UK nursery management systems, the feasibility of accreditation from social and economic perspectives, and the emerging Phytophthora threats from both global and evolutionary perspectives. At the end of the project a collaborative framework was established to ensure that results from the Phyto-threats project continue to feed into the development of a UK-wide certification scheme (the PHCS), to help ensure successful uptake and recognition of accreditation, and to establish protocols aimed at reducing the risks of introducing Phytophthora to the wider UK environment through new planting programmes.
Many of the approaches developed in this project are also applicable to the wider field of plant health, including the threat posed by X. fastidiosa. For example, the metabarcoding methodology can be applied to the broader detection of pathogens including bacterial pathogens in nurseries, fungal pathogens in spore traps and invertebrate pests in insect traps. The ecological model frameworks developed are widely transferable to other tree pests or diseases since they account for sparse, clustered recording effort and encompass a wide range of potential pathways and environmental factors that may influence arrival and spread of such species. The project also contributed to the body of knowledge of traits that underpin invasiveness for plant pathogens. Improving sanitation practices in plant nurseries and communication and engagement across a wide spectrum of stakeholders focused on the importance of high-health planting material has helped to raise the profile of plant health and associated risks across the plant supply chain. In 2020 a two-year EUPHRESCO project (Early detection of Phytophthora in nurseries and traded plants of EU and third countries) commenced involving the rolling out of the nursery sampling and metabarcoding methodology developed as part of the Phyto-threats project across twelve partner countries. This project takes core biological and social science elements developed as part of Phyto-threats out into the international sphere to develop a coordinated strategy for the early detection of Phytophthora pathogens in plant nurseries and traded plants for planting across EU and third countries. This new project with its much wider geographical focus will inform international best practice, complement phytosanitary regulation, and enhance global engagement on Plant Health.
Author Contributions
Conceptualization, methodology, sample collection, data analysis, validation, writing; S.G., D.E.L.C., B.P., M.M., L.B., D.S.C., L.P., P.J.A.C., P.T., M.D., G.V., C.P.; data curation and advice, A.P.-S., J.B.; sample collection and processing, D.F.-M., T.P., E.R., B.K., A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant funded jointly by the Biotechnology and Biological Sciences Research Council, the Department for Environment, Food and Rural affairs, the Economic and Social Research Council, the Forestry Commission, the Natural Environment Research Council and the Scottish Government, under the Tree Health and Plant Biosecurity Initiative, grant number BB/N023463/1.
Institutional Review Board Statement
The study was conducted according to the FOREST RESEARCH SERG ethical statement and monitored by an Ethics Committee comprising FOREST RESEARCH and FORESTRY COMMISSION experts on social, economic and natural sciences and policy.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The Phytophthora traits database is available at https://doi.org/10.5281/zenodo.4081474 (accessed on 17 November 2021).
Acknowledgments
The authors would like to thank Beatrice Hénricot, Mhairi Clark and Anna Harris for assistance with sample collection and data collation, and the members of our Expert Advisory Panel; Susan Frankel, Giles Hardy, Kelvin Hughes, Jon Knight, Richard McIntosh, John Morgan, David Slawson and John Spiers for advice and direction during the course of the project. We also wish to thank all the partner nursery managers for allowing us to sample their premises, enabling this project to go ahead, and the Plant Health inspectors for assistance with sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woodland Trust. The Economic Benefits of Woodland. A Report for the Woodland Trust Prepared by Europe Economics; The Woodland Trust: Grantham, UK, 2008; 24p, Available online: https://www.woodlandtrust.org.uk/publications/2017/01/economic-benefits-of-woodland/ (accessed on 17 November 2021).
- Office for National Statistics. Woodland Natural Capital Accounts, UK. 2020; 47p. Available online: https://www.ons.gov.uk/economy/environmentalaccounts/bulletins/woodlandnaturalcapitalaccountsuk/2020 (accessed on 17 November 2021).
- Forestry Commission. Forestry Statistics; Forestry Commission: Edinburgh, UK, 2020; 235p. Available online: https://www.forestresearch.gov.uk/tools-and-resources/statistics/forestry-statistics/ (accessed on 17 November 2021).
- Brasier, C.M. The Biosecurity Threat to the UK and Global Environment from International Trade in Plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Anon (2006) Future of UK Horticulture A case study analysis and overview of the UK horticultural production industry and its future over the next 10–20 years. National Horticultural Forum and Promar International. Available online: https://www.hortforum.net/uploads/7/2/9/5/7295387/nhf_future_of_hort_pdf3.pdf (accessed on 11 June 2012).
- Defra. Horticulture Statistics; Defra: London, UK, 2020; 10p. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/901689/hort-report-17jul20.pdf (accessed on 17 November 2021).
- Hill, L.; Jones, G.; Atkinson, N.; Hector, A.; Hemery, G.; Brown, N. The £15 Billion Cost of Ash Dieback in Britain. Curr. Biol. 2019, 29, R315–R316. [Google Scholar] [CrossRef]
- Dobrowolski, M.P.; Tommerup, I.C.; Shearer, B.L.; O’Brien, P.A. Three Clonal Lineages of Phytophthora Cinnamomi in Australia Revealed by Microsatellites. Phytopathology 2003, 93, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Goss, E.M.; Carbone, I.; Grünwald, N.J. Ancient Isolation and Independent Evolution of the Three Clonal Lineages of the Exotic Sudden Oak Death Pathogen Phytophthora Ramorum. Mol. Ecol. 2009, 18, 1161–1174. [Google Scholar] [CrossRef]
- Bradshaw, R.E.; Bellgard, S.E.; Black, A.; Burns, B.R.; Gerth, M.L.; McDougal, R.L.; Scott, P.M.; Waipara, N.W.; Weir, B.S.; Williams, N.M.; et al. Phytophthora Agathidicida: Research Progress, Cultural Perspectives and Knowledge Gaps in the Control and Management of Kauri Dieback in New Zealand. Plant Pathol. 2020, 69, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M.; Kirk, S.A.; Delcan, J.; Cooke, D.E.L.; Jung, J.; Man In’t Veld, W. Phytophthora Alni Sp. Nov. and Its Variants: Designation of Emerging Heteroploid Hybrid Pathogens Spreading on Alnus Trees. Mycol. Res. 2004, 108, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Webber, J.F. Sudden Larch Death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef]
- Brasier, C.M.; Beales, P.A.; Kirk, S.A.; Denman, S.; Rose, J. Phytophthora Kernoviae Sp. Nov., An Invasive Pathogen Causing Bleeding Stem Lesions on Forest Trees and Foliar Necrosis of Ornamentals in the UK. Mycol. Res. 2005, 109, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Green, S.; Brasier, C.M.; Schlenzig, A.; McCracken, A.; MacAskill, G.A.; Wilson, M.; Webber, J.F. The Destructive Invasive Pathogen Phytophthora Lateralis Found on Chamaecyparis Lawsoniana across the UK. For. Pathol. 2013, 43, 19–28. [Google Scholar]
- Green, S.; Elliot, M.; Armstrong, A.; Hendry, S.J. Phytophthora Austrocedrae Emerges as a Serious Threat to Juniper (Juniperus Communis) in Britain. Plant Pathol. 2015, 64, 456–466. [Google Scholar] [CrossRef]
- Scanu, B.; Webber, J.F. Dieback and Mortality of Nothofagus in Britain: Ecology, Pathogenicity and Sporulation Potential of the Causal Agent Phytophthora Pseudosyringae. Plant Pathol. 2016, 65, 26–36. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996; 562p. [Google Scholar]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, J.; Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; et al. Widespread Phytophthora Infestations in European Nurseries put Forest, Semi-Natural and Horticultural Ecosystems at High Risk of Phytophthora Diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Bertier, L.; Leus, L.; D’hondt, L.; de Cock, A.W.A.M.; Höfte, M. Host Adaptation and Speciation through Hybridization and Polyploidy in Phytophthora. PLoS ONE 2013, 8, e85385. [Google Scholar] [CrossRef] [Green Version]
- Ioos, R.; Andrieux, A.; Marçais, B.; Frey, P. Genetic Characterisation of the Natural Hybrid Species Phytophthora alni as Inferred from Nuclear and Mitocondrial DNA Analyses. Fung. Gen. Biol. 2006, 43, 511–529. [Google Scholar] [CrossRef] [Green Version]
- Scibetta, S.; Schena, L.; Chimento, A.; Cacciola, S.O.; Cooke, D.E.L. A Molecular Method to Assess Phytophthora Diversity in Environmental Samples. J. Microbiol. Meth. 2012, 88, 356–368. [Google Scholar] [CrossRef]
- Mendoza, M.L.Z.; Sicheritz-Pontén, T.; Gilbert, M.T.P. Environmental Genes and Genomes: Understanding the Differences and Challenges in the Approaches and Software for Their Analyses. Brief. Bioinform. 2015, 16, 745–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsoum, N.; A’Hara, S.; Cottrell, J.; Green, S. Using Developments in Molecular Techniques to Improve Forest Biodiversity Monitoring. In Forestry Commission Research Note 2018; Forestry Commission: Edinburgh, UK, 2018; Volume 32, 14p. Available online: https://www.forestresearch.gov.uk/research/using-dna-barcoding-and-metabarcoding-to-detect-species-and-improve-forest-biodiversity-monitoring/ (accessed on 17 November 2021).
- Riddell, C.E.; Frederickson-Matika, D.; Armstrong, A.C.; Elliot, M.; Forster, J.; Hedley, P.E.; Morris, J.; Thorpe, P.; Cooke, D.E.L.; Pritchard, P.; et al. Metabarcoding Reveals a High Diversity of Woody Host-Associated Phytophthora spp. in Soils at Public Gardens and Amenity Woodlands in Britain. PeerJ 2019, 7, e6931. [Google Scholar] [CrossRef] [Green Version]
- Cock, P.; Pritchard, L.; Thorpe, P.; Cooke, D.E.L. peterjc/thapbi-pict: THAPBI PICT. Zenodo 2021. [CrossRef]
- Dunn, M.; Marzano, M.; Forster, J. Buying Better Biosecurity: Plant Buying Behaviour and the Implications for an Accreditation Scheme in the Horticultural Sector. Plants. People Planet 2020, 2, 259–268. [Google Scholar] [CrossRef]
- Marzano, M.; Dunn, M.; Pettitt, T.; Green, S. Perceptions of Biosecurity-Based Accreditation in the Plant trade: A UK Example. Forests 2021. (accepted subject to final approval). [Google Scholar]
- Valatin, G.; Price, C.; Green, S. Reducing Disease Risks to British Forests: An Exploration of Costs and Benefits of Nursery Best Practices. Forests 2021. (accepted subject to final approval). [Google Scholar]
- Philibert, A.; Desprez-Loustau, M.L.; Fabre, B.; Frey, P.; Halkett, F.; Husson, C.; Lung-Escarmant, B.; Benoit, M.; Robin, C.; Vacher, C.; et al. Predicting Invasion Success of Forest Pathogenic Fungi from Species Traits. J. Appl. Ecol. 2011, 48, 1381–1390. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.-L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical Patterns and Determinants of Invasion by Forest Pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barwell, L.J.; Perez-Sierra, A.; Henricot, B.; Harris, A.; Burgess, T.; Hardy, G.; Scott, P.; Williams, N.; Cooke, D.E.L.; Green, S.; et al. Phytophthora Global Impacts (Version v1.0.0). Zenodo 2020. [Google Scholar] [CrossRef]
- Index Fungorum Partnership. 2018. Available online: http://www.indexfungorum.org/ (accessed on 6 August 2019).
- CABI. CAB Direct; CAB International: Wallingford, UK, 2019; Available online: https://www.cabi.org/publishing-products/cab-direct/ (accessed on 30 July 2019).
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2019; Available online: https://www.cabi.org/ISC (accessed on 6 August 2019).
- EPPO. EPPO Global Database. 2019. Available online: https://gd.eppo.int/ (accessed on 6 August 2019).
- Chamberlain, S.A.; Boettiger, C. R Python, and Ruby Clients for GBIF Species Occurrence Data. PeerJ PrePrints 2017. [CrossRef]
- GBIF.org. GBIF Occurrence Download. 2018. Available online: https://doi.org/10.15468/dl.dqsr1u (accessed on 4 April 2018).
- Roy, D.; Alderman, D.; Anastasiu, P.; Arianoutsou, M.; Augustin, S.; Bacher, S.; Başnou, C.; Beisel, J.; Bertolino, S.; Bonesi, L.; et al. DAISIE—Inventory of Alien Invasive Species in Europe. v1.0. Research Institute for Nature and Forest (INBO). Dataset/Checklist. 2019. Available online: https://ipt.inbo.be/resource?r=daisie-checklist&v=1.0 (accessed on 6 August 2019).
- Winter, D.J. Rentrez: An R Package for the NCBI eUtils API. R J. 2017, 9, 520–526. Available online: https://journal.r-project.org/archive/2017/RJ-2017-058/index.html (accessed on 6 August 2019). [CrossRef]
- NCBI. National Center for Biotechnology Information Biosample Records. 2018. Available online: https://www.ncbi.nlm.nih.gov/biosample (accessed on 6 August 2019).
- Chamberlain, S.; Szocs, E. Taxize—Taxonomic Search and Retrieval in R. F1000Research 2013, 2, 191. [Google Scholar] [CrossRef] [Green Version]
- Barwell, L.J.; Perez-Sierra, A.; Henricot, B.; Harris, A.; Burgess, T.; Hardy, G.; Scott, P.; Williams, N.; Cooke, D.E.L.; Green, S.; et al. Evolutionary Trait-Based Approaches for Predicting Future Global Impacts of Plant Pathogens in the Genus Phytophthora. J. Appl. Ecol. 2021, 58, 718–730. [Google Scholar] [CrossRef]
- Chapman, D.; Purse, B.; Roy, H.; Bullock, J. Trade Networks Explain the Distribution of Invasive Species. Glob. Ecol. Biogeog. 2017, 26, 907–917. [Google Scholar] [CrossRef]
- Bürkner, P.C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Cooke, D.E.L.; Jung, T.; Williams, N.A.; Schubert, R.; Oβwald, W.; Duncan, J.M. Genetic Diversity of European Populations of the Oak Fine-Root Pathogen Phytophthora quercina. For. Pathol. 2005, 35, 57–70. [Google Scholar] [CrossRef]
- Redondo, M.A.; Boberg, J.; Stenlid, J.; Oliva, J. Functional Traits Associated with the Establishment of Introduced Phytophthora Spp. in Swedish forests. J. Appl. Ecol. 2018, 55, 1538–1552. [Google Scholar] [CrossRef]
- Purse, P.V.; Golding, N. Tracking the Distribution and Impacts of Diseases with Biological Records and Distribution Modelling. Biol. J. Linn. Soc. 2015, 115, 664–677. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.; Pescott, O.L.; Roy, H.E.; Tanner, R. Improving Species Distribution Models for Invasive Non-Native Species with Biologically Informed Pseudo-Absence Selection. J. Biogeogr. 2019, 46, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).