Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It
Abstract
1. Introduction
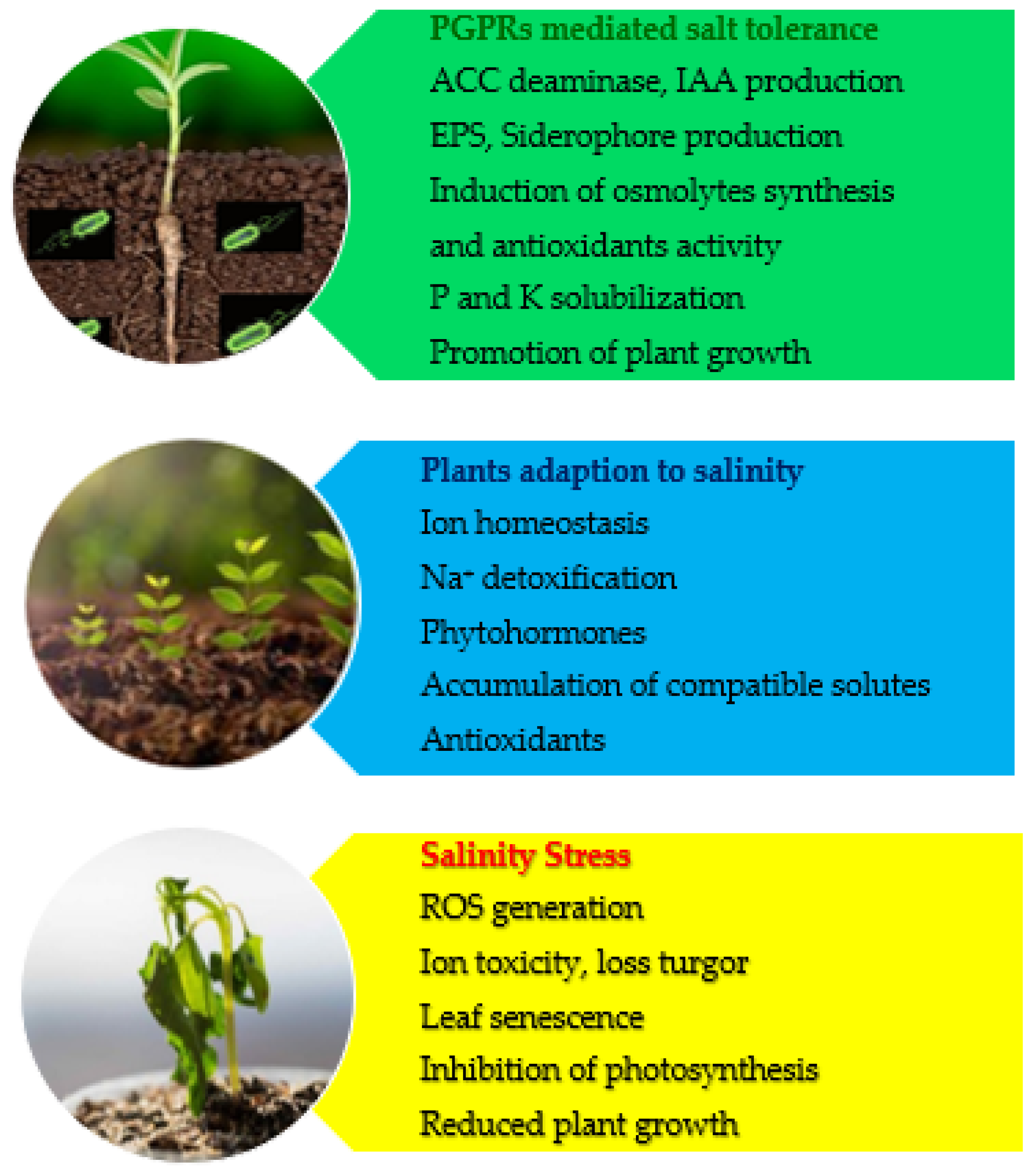
2. Salinity and Its Effects on Plants
3. Plant Strategies in Response to Salinity
3.1. Osmoprotectants
3.2. Antioxidants
3.3. Polyamines
3.4. Hormone Regulation
4. PGPR Enhance Salt Tolerance in Plants
4.1. Improvement of Nutrient Uptake and Solubilisation
4.2. Production of ACC Deaminase
4.3. Production of IAA
4.4. Production of EPS
4.5. Other Mechanisms
5. Formulation Technology
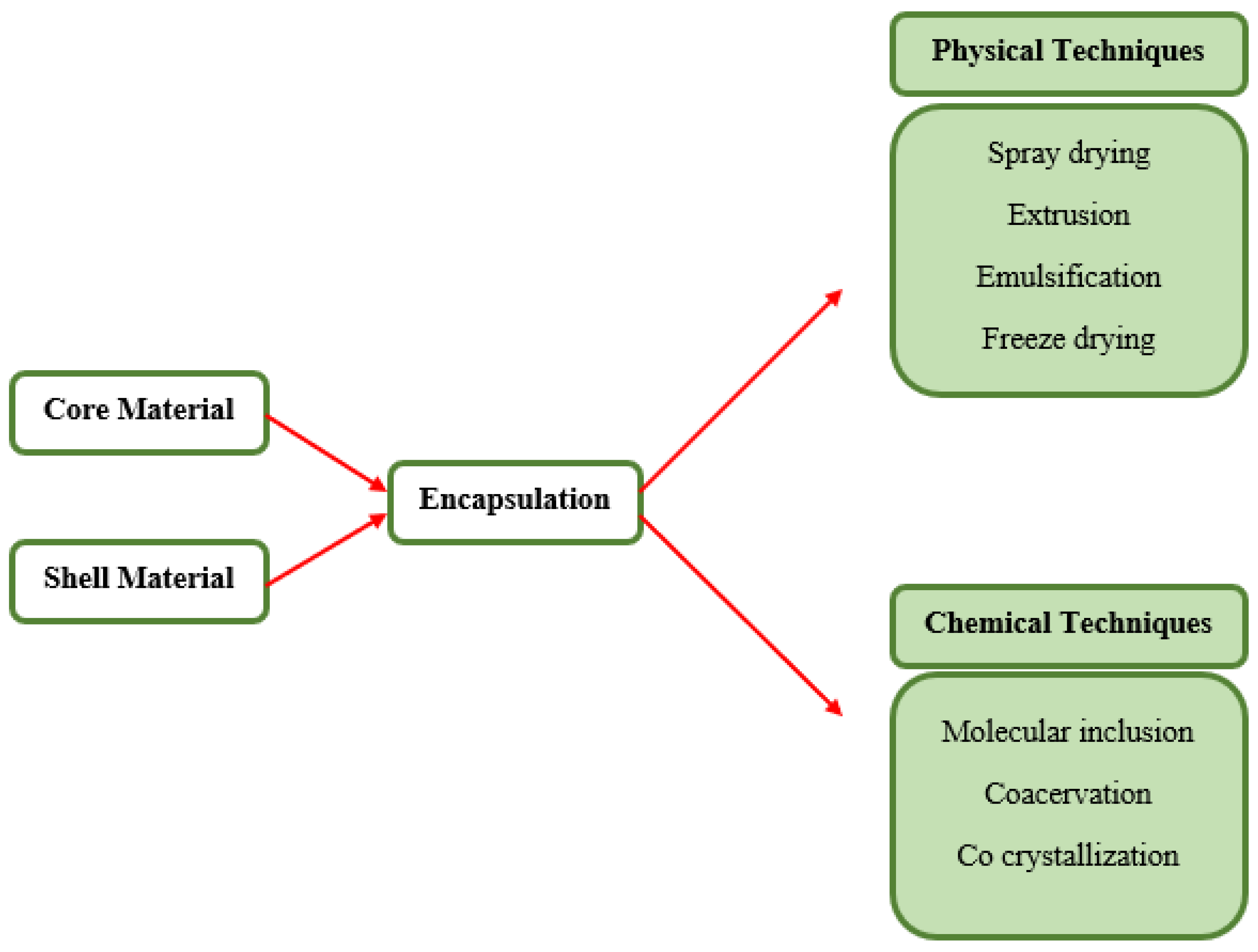
5.1. Encapsulation
5.2. Bacterial Encapsulation with Various Polymers against Salinity Stress
5.3. Examples of Using PGPR Encapsulation against Salinity Stress in Different Plants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterisation. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R.; Białkowska, A.; Canfora, L.; Pinzari, F. When salt meddles between plant, soil and microorganisms. Front. Plant Sci. 2020, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Moradi-Pour, M.; Saberi Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the formulation of alginate-gelatin encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M. A novel encapsulation of Streptomyces fulvissimus Uts22 by spray drying and its biocontrol efficiency against Gaeumannomyces graminis, the causal agent of take-all disease in wheat. Pest Manag. Sci. 2021, 2021. 77, 4357–4364. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M.; Mohammadinejad, R.; Thakur, V.K. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers 2021, 13, 1938. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Moradi Pour, M. The effect of Bacillus subtilis Vru1 encapsulated in alginate–bentonite coating enriched with titanium nanoparticles against Rhizoctonia solani on bean. Int. J. Biol. Macromol. 2020, 152, 1089–1097. [Google Scholar] [CrossRef]
- Datta, K.K.; Jong, C.D. Adverse effect of waterlogging and soil salinity on crop and land productivity in northwest region of Haryana, India. Agric. Water Manag. 2002, 57, 223–238. [Google Scholar] [CrossRef]
- Gkiougkis, I.; Kallioras, A.; Pliakas, F.-K.; Pechtelidis, A.; Diamantis, V.; Diamantis, J.; Ziogas, A.; Dafnis, I. Assessment of soil salinization at the eastern Nestos River Delta, N.E. Greece. Catena 2014, 128, 238–251. [Google Scholar] [CrossRef]
- Chang, T.T.; Zhang, Y.J.; Xu, H.L.; Shao, X.H.; Xu, Q.C.; Li, F.L. Osmotic adjustment and up-regulation expression of stress-responsive genes in tomato induced by soil salinity resulted from nitrate fertilisation. Int. J. Agric. Biol. Eng. 2018, 11, 126–136. [Google Scholar]
- De Souza, S.M.; Fay, F.E. Effect of salinity on soil microorganisms. In Soil Health and Land Use Management; Hernandez-Soriano, M.C., Ed.; IntechOpen: Rijeka, Croatia, 2014; pp. 177–198. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 29. [Google Scholar]
- Rogers, M.; Craig, A.D.; Munns, R.; Colmer, T.; Nichols, P.; Malcolm, C.; Barrett-Lennard, E.; Brown, A.; Semple, W.; Evans, P.; et al. The potential for developing fodder plants for the salt-affected areas of southern and eastern Australia: An overview. Aust. J. Exp. Agric. 2005, 45, 300–329. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Rahnama, A.; James, R.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinisation. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, S.; Quintero, F.; Cubero, B.; Ruiz, M.; Bressan, R.; Hasegawa, P.; Pardo, J. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002, 30, 529–539. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants—A review. Plant Soil Environ. 2008, 54, 89. [Google Scholar] [CrossRef]
- Yamada, T.; Takatsu, Y.; Manabe, T.; Kasumi, M.; Marubashi, W. Suppressive effect of trehalose on apoptotic cell death leading to petal senescence in ethylene-insensitive flowers of gladiolus. Plant Sci. 2003, 164, 213–221. [Google Scholar] [CrossRef]
- El-Shintinawy, F.; El-Shourbagy, M.N. Alleviation of changes in protein metabolism in NaCl-stressed wheat seedlings by thiamine. Biol. Plant. 2001, 44, 541–545. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Agastian, P.; Kingsley, S.J.; Vivekanandan, M. Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 2000, 38, 287–290. [Google Scholar] [CrossRef]
- Cha-Um, S.; Kirdmanee, C. Effect of glycinebetaine on proline, water use, and photosynthetic efficiencies, and growth of rice seedlings under salt stress. Turk. J. Agric. For. 2010, 34, 517–527. [Google Scholar]
- Rahman, S.; Miyake, H.; Takeoka, Y. Effects of exogenous glycinebetaine on growth and ultrastructure of salt-stressed rice seedlings (Oryza sativa L.). Plant Prod. Sci. 2002, 5, 33–44. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef]
- Meneguzzo, S.; Navam-Izzo, F.; Izzo, R. Antioxidative responses of shoots and roots of wheat to increasing NaCI concentrations. J. Plant Physiol. 1999, 155, 274–280. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, M.P.; Kerkeb, L.; Bueno, P.; Donaire, J.P. Changes induced by NaCl in lipid content and composition, lipoxygenase, plasma membrane H+-ATPase and antioxidant enzyme activities of tomato (Lycopersicon esculentum. Mill) calli. Plant Sci. 1999, 143, 143–150. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Shim, J.K.; Kim, D.H.; Lee, K.Y.; Lee, I.J. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J. Plant Growth Regul. 2014, 33, 137–149. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Sharkhuu, A.; Batelli, G.; Bressan, R.A.; Maggio, A. The Arabidopsis thaliana mutant air1 implicates SOS3 in the regulation of anthocyanins under salt stress. Plant Mol. Biol. 2013, 83, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Shaheen, R. Stimulation of antioxidant system and lipid peroxidation by abiotic stresses in leaves of Momordica charantia. Braz. J. Plant Physiol. 2007, 19, 149–161. [Google Scholar] [CrossRef]
- Munir, N.; Aftab, F. Enhancement of salt tolerance in sugarcane by ascorbic acid pretreatment. Afr. J. Biotechnol. 2011, 10, 18362–18370. [Google Scholar]
- Foyer, C.; López-Delgado, H.; Dat, J.; Scott, I. Hydrogen peroxide and glutathion-associated mechanisms of acclimatory stress tolerance and signaling. Physiol. Plant. 2006, 100, 241–254. [Google Scholar] [CrossRef]
- Kojo, S. Vitamin C: Basic metabolism and its function as an index of oxidative stress. Curr. Med. Chem. 2004, 11, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.M.; Römer, P.; Sumper, M. Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesise thermospermine rather than spermine. FEBS Lett. 2007, 581, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2009, 105, 1–6. [Google Scholar] [CrossRef]
- Roy, P.; Gupta, K.; SenGupta, D.N.; Ghosh, B. Spermidine treatment to rice seedlings recovers salinity stress induced damage of Plasma membrane and PM-bound H+- ATPase in salt-tolerant and salt sensitive Rice cultivars. Plant Sci. 2005, 168, 583–591. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef]
- Afzal, I.; Munir, F.; Ayub, C.; Basra, S.; Hameed, A.; Nawaz, A. Changes in antioxidant enzymes, germination capacity and vigour of tomato seeds in response of priming with polyamines. Seed Sci. Technol. 2009, 37, 765–770. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Miyazaki, A.; Takahashi, T.; Michael, A.; Kusano, T. The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 2006, 580, 6783–6788. [Google Scholar] [CrossRef]
- Shevyakova, N.; Musatenko, L.; Stetsenko, L.; Vedenicheva, N.; Voitenko, L.; Sytnik, K.; Kuznetsov, V. Effects of abscisic acid on the contents of polyamines and proline in common bean plants under salt stress. Russ. J. Plant Physiol. 2013, 60, 200–211. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Plant polyamine catabolism: The state of the art. Plant Signal. Behav. 2008, 3, 1061–1066. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole Iii, J.; Barceló, J.; Poschenrieder, C. Abscisic Acid Decreases Leaf Na+ Exclusion in Salt-Treated Phaseolus vulgaris L. J. Plant Growth Regul. 2009, 28, 187–192. [Google Scholar] [CrossRef]
- Fukuda, A.; Tanaka, Y. Effects of ABA, auxin, and gibberellin on the expression of genes for vacuolar H+-inorganic pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter in barley. Plant Physiol. Biochem. 2006, 44, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Shim, I.-S.; Usui, K. Induction of benzoic acid 2-hydroxylase and salicylic acid biosynthesis modulation by salt stress in rice seedlings. Plant Sci. 2006, 171, 263–270. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Arteca, R.N.; Foolad, M.R. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit. Rev. Plant Sci. 2010, 29, 162–190. [Google Scholar] [CrossRef]
- El-Tayeb, M.A. Response of barley grains to the interactive e.ect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- El-Mashad, A.A.; Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 2012, 249, 625–635. [Google Scholar] [CrossRef]
- Valencia-Cantero, E.; Hernández-Calderón, E.; Velázquez-Becerra, C.; López-Meza, J.E.; Alfaro-Cuevas, R.; López-Bucio, J. Role of dissimilatory fermentative iron-reducing bacteria in Fe uptake by common bean (Phaseolus vulgaris L.) plants grown in alkaline soil. Plant Soil 2007, 291, 263–273. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbial. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Akhtar, J.; Haq, M.A.U. Interactive effects of salinity and iron deficiency on different rice genotypes. J. Plant Nutr. Soil Sci. 2015, 178, 306–311. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Verma, J.P.; Prakash, S.; Meena, V.S.; Meena, R.S. Potassium as an important plant nutrient in sustainable agriculture: A state of the art. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 21–29. [Google Scholar]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Sobti, S.; Belhadj, H.A.; Djaghoubi, A. Isolation and characterisation of the native Rhizobia under hyper-salt edaphic conditions in Ouargla (southeast Algeria). Energy Procedia 2015, 74, 1434–1439. [Google Scholar] [CrossRef]
- Goswami, D.; Parmar, S.; Vaghela, H.; Dhandhukia, P.; Thakker, J.N. Describing Paenibacillus mucilaginosus strain N3 as an efficient plant growth promoting rhizobacteria (PGPR). Cogent Food Agric. 2015, 1, 1000714. [Google Scholar] [CrossRef]
- Choudhary, D.K. Microbial rescue to plant under habitat-imposed abiotic and biotic stresses. Appl. Microbiol. Biotechnol. 2012, 96, 1137–1155. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Sindhu, S.; Dua, S.; Verma, M.; Khandelwal, A. Growth Promotion of Legumes by Inoculation of Rhizosphere Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 195–235. [Google Scholar]
- Saghafi, D.; Delangiz, N.; Lajayer, B.A.; Ghorbanpour, M. An overview on improvement of crop productivity in saline soils by halotolerant and halophilic PGPRs. 3 Biotech 2019, 9, 261. [Google Scholar] [CrossRef]
- Etesami, H.; Mirseyed, H.; Alikhani, H. In planta selection of plant growth promoting endophytic bacteria for rice (Oryza sativa L.). J. Soil Sci. Plant Nutr. 2013, 14, 491–503. [Google Scholar] [CrossRef]
- Aznar, A.; Dellagi, A. New insights into the role of siderophores as triggers of plant immunity: What can we learn from animals? J. Exp. Bot. 2015, 66, 3001–3010. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Santos, S.N.; Silva, J.L.; Parma, M.M.; Avila, L.A.; Visconti, A.; Zucchi, T.D.; Taketani, R.G.; Andreote, F.D.; Melo, I.S. Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol. Res. 2013, 168, 183–191. [Google Scholar] [CrossRef]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2013, 2, 6. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Samiyappan, R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J. Appl. Microbial. 2007, 102, 1283–1292. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Damodharan, K.; Yang, S.H.; Suh, J.W. Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J. Appl. Microbiol. 2014, 117, 766–773. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp. SL-12 isolated from a salt lake. Symbiosis 2016, 69, 101–111. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhisation in Pisum sativum. J. Plant Physiol. 2014, 171, 884–894. [Google Scholar] [CrossRef]
- Kiani, M.; Ali, A.; Sultan, T.; Ahmad, R.; Hydar, S. Plant growth promoting rhizobacteria having 1-Aminocyclopropane-1-Carboxylic Acid Deaminase to induce salt tolerance in sunflower (Helianthus annus L.). Nat. Resour. 2015, 6, 391–397. [Google Scholar] [CrossRef]
- Din, B.U.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Hussain Munis, M.F.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC- deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar]
- Sarkar, A.; Pramanik, K.; Mitra, S.; Soren, T.; Maiti, T.K. Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 2018, 231, 434–442. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Bhise, K.K.; Bhagwat, P.K.; Dandge, P.B. Synergistic effect of Chryseobacterium gleum SUK with ACC deaminase activity in alleviation of salt stress and plant growth promotion in Triticum aestivum L. 3 Biotech 2017, 7, 105. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; He, Y.; Huang, Y.; Li, X.; Ye, B.C. Plant growth promotion and alleviation of salinity stress in Capsicum annuum L. by Bacillus isolated from saline soil in Xinjiang. Ecotoxicol. Environ. Saf. 2018, 164, 520–529. [Google Scholar] [CrossRef]
- Suarez, C.; Cardinale, M.; Ratering, S.; Steffens, D.; Jung, S.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Plant growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl. Soil Ecol. 2015, 95, 23–30. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbial. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Aeron, A.; Kumar, S.; Pandey, P.; Maheshwari, D.K. Emerging role of plant growth promoting rhizobacteria in agrobiology. In Bacteria in Agrobiology: Crop Ecosystems; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–36. [Google Scholar]
- Shrivastava, S.; D’Souza, S.; Desai, P. Production of indole-3-acetic acid by immobilised actinomycetes (Kitasatospora sp.) for soil application. Curr. Sci. 2008, 94, 1595–1604. [Google Scholar]
- El-Tarabily, K.A.; Sivasithamparam, K. Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol. Biochem. 2006, 38, 1505–1520. [Google Scholar] [CrossRef]
- Péret, B.; Svistoonoff, S.; Lahouze, B.; Auguy, F.; Santi, C.; Doumas, P.; Laplaze, L. A role for auxin during actinorhizal symbioses formation? Plant Signal. Behav. 2008, 3, 34–35. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Djebaili, R.; Pellegrini, M.; Smati, M.; Del Gallo, M.; Kitouni, M. Actinomycete strains isolated from saline soils: Plant growth promoting traits and inoculation effects on Solanum lycopersicum. Sustainability 2020, 12, 4617. [Google Scholar] [CrossRef]
- Saghafi, D.; Ghorbanpour, M.; Asgari Lajayer, B. Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress. J. Soil Sci. Plant Nutr. 2018, 18, 253–268. [Google Scholar] [CrossRef]
- Abeer, H.; Abdallah, E.F.; Alqarawi, A.A.; Al-Huqail, A.S.; Alshalawi, S.R.; Wirth, S.; Dilfuza, E. Impact of plant growth promoting Bacillus subtilis on growth and physiological parameters of Bassia indica (Indian bassia) grown udder salt stress. Pak. J. Bot. 2015, 47, 1735–1741. [Google Scholar]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, S.H.; Wang, S.M.; Luo, H.Y. Characterisation of extracellular polymeric substances from biofilm in the process of starting-up a partial nitrification process under salt stress. Appl. Microbiol. Biotechnol. 2011, 89, 1563–1571. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Touraine, B. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef] [PubMed]
- Barihi, R.; Panahpour, E.; Beni, M.H.M. Super absorbent polymer (hydrogel) and its application in agriculture. World Sci. J. 2013, 1, 223–228. [Google Scholar]
- Karimi, E.; Rohani, H.; Zafari, D.; Khodakaramian, G.; Taghinasab, M. Biological control of vascular wilt disease of carnation caused by Fusarium oxysporum f. sp. dianthi by Bacillus and Pseudomonas strains isolated from rhizosphere of carnation. J. Sci. Technol. Agric. Nat. Resour. 2007, 11, 309–320. [Google Scholar]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite Modified Sodium Alginate Hydrogel Composite for Efficient Removal of Malachite Green Dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Moradi-Pour, M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Nano-encapsulation of plant growth-promoting rhizobacteria and their metabolites using alginate-silica nanoparticles and carbon nanotube improves ucb1 pistachio micropropagation. J. Microbiol. Biotechnol. 2019, 29, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Li, C.; Wu, Z.S.; Xu, X.L.; Ren, L.; Zhao, H.S. Adsorption of protein from model wine solution by different bentonites. China J. Chem. Eng. 2007, 15, 632–638. [Google Scholar] [CrossRef]
- Wu, Z.S.; He, Y.H.; Chen, L.J.; Han, Y.J.; Li, C. Characterisation of R. planticola Rs-2 microcapsule prepared with a blend of alginate and starch and its release behavior. Carbohyd. Polym. 2014, 110, 259–267. [Google Scholar] [CrossRef]
- Wu, Z.S.; Zhao, Y.F.; Kaleem, I.; Li, C. Preparation of calcium alginate microcapsuled microbial fertiliser coating Klebsiella oxytoca Rs-5 and its performance under salinity stress. Eur. J. Soil Biol. 2011, 47, 152–159. [Google Scholar] [CrossRef]
- Afzal, M.; Shabir, G.; Tahseen, R.; Islam, E.; Iqbal, S.; Khan, Q.M.; Khalid, Z.M. Endophytic Burkholderia sp. strain PsJN improves plant growth and phytoremediation of soil irrigated with textile effluent. CLEAN–Soil Air Water 2014, 42, 1304–1310. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, S.; Iqbal, S.; Mirza, M.S.; Khan, Q.M. Inoculation method affects colonisation and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int. Biodeter. Biodegr. 2013, 85, 331–336. [Google Scholar] [CrossRef]
- Anandham, R.; Choi, K.H.; Gandhi, P.I.; Yim, W.J.; Park, S.J.; Kim, K.A.; Madhaiyan, M.; Sa, T.M. Evaluation of shelf life and rock phosphate solubilisation of Burkholderia sp. in nutrient-amended clay, rice bran and rock phosphate-based granular formulation. World J. Microb. Biot. 2007, 23, 1121–1129. [Google Scholar] [CrossRef]
- Minaxi, J.; Saxena, J. Efficacy of rhizobacterial strains encapsulated in nontoxic biodegradable gel matrices to promote growth and yield of wheat plants. Appl. Soil Ecol. 2011, 48, 301–308. [Google Scholar] [CrossRef]
- Semary, N.A.H.; Alouane, M.H.; Nasr, O.; Aldayel, M.F.; Alhaweti, F.H.; Ahmed, F. Salinity stress mitigation using encapsulated biofertilizers for sustainable agriculture. Sustainability 2020, 12, 9218. [Google Scholar] [CrossRef]
- Çavusoglu, K.; Kabar, K. Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses. Eurasian J. Biosci. 2010, 4, 70–79. [Google Scholar] [CrossRef]
- Padorf, M.; Pourzahedi, L.; Gilbertson, L.; Lowry, G.V.; Herckes, P.; Westerhoff, P. Graphite nanoparticle addition to fertilisers reduces nitrate leaching in growth of lettuce (Lactuca sativa). Environ. Sci. 2020, 7, 127–138. [Google Scholar]
- Semary, N.A.; Fouda, M. Anticancer activity of Cyanothece sp. strain extracts from Egypt: First record. Asian Pac. J. Trop. Biomed. 2015, 5, 992–995. [Google Scholar] [CrossRef]
- Trejo, A.; de-Bashan, L.E.; Hartmann, A.; Hernandez, J.P.; Rothballer, M.; Schmid, M.; Bashan, Y. Recycling waste debris of immobilised microalgae and plant growth-promoting bacteria from wastewater treatment as a resource to improve fertility of eroded desert soil. Environ. Exp. Bot. 2012, 75, 65–73. [Google Scholar] [CrossRef]
- Wu, B.Z.; Peng, Y.; Guo, L. Chun Li Root colonisation of encapsulated Klebsiella oxytoca Rs-5 on cotton plants and its promoting growth performance under salinity stress. Eur. J. Soil Biol. 2014, 60, 8187. [Google Scholar] [CrossRef]
- Komal, K.B.; Padma, B.D. Alleviation of salinity stress in rice plant by encapsulated salt tolerant plant growth promoting bacteria Pantoea agglomerans strain KL and its root colonisation ability. Arch. Acker. 2019, 65, 1955–1968. [Google Scholar]
- Augustine, T.Z.; Lima, E.; Suna, H.; Marelli, B. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity. Sci. J. Biol. Sci. 2019, 116, 25555–25561. [Google Scholar]
- Abo-Kora, H.A.; Maie, M.A. Reducing effect of soil salinity through using some strains of Nitrogen fixers bacteria and compost on sweet basil plant. Int. J. Pharmtech. Res. 2016, 19, 187–214. [Google Scholar]
- Ordookhani, K.; Khavazi, K.; Moezzi, A.; Rejali, F. Influence of PGPR and AMF on antioxidant activity, lycopene and potassium contents in tomato. Afr. J. Agric. Res. 2010, 5, 1108–1116. [Google Scholar]
- Fathy, N.O. Impact of compost on the availability and nutrients content of Vicia faba grown on saline waterirrigated soil. Minufiya J. Agr. Res. 2010, 35, 1573–1585. [Google Scholar]
- Baniaghil, N.; Arzanesh, M.H.; Ghorbanli, M.; Shahbazi, M. The effect of plant growth promoting rhizobacteria on growth parameters, antioxidant enzymes and microelements of Canola under salt stress. J. Appl. Environ. Biol. Sci. 2013, 3, 17–27. [Google Scholar]
- Maged, M.; Abo-Koura, H.A.; Bishara, M.M.; Gomaa, I.M. Microencapsulation: Toward the Reduction of the Salinity Stress Effect on Wheat Plants Using NPK Rhizobacteria. Biotechnol. J. Int. 2019, 23, 1–18. [Google Scholar]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Nozhkina, O.A.; Perfileva, A.I.; Graskova, I.A.; Dyakova, A.V.; Nurminsky, V.N.; Klimenkov, I.V.; Ganenko, T.V.; Borodina, T.N.; Aleksandrova, G.P.; Sukhov, B.G.; et al. The Biological Activity of a Selenium Nanocomposite Encapsulated in Carrageenan Macromolecules with Respect to Ring Rot Pathogenesis of Potato Plants. Nanotechnol. Russ. 2019, 14, 255–262. [Google Scholar] [CrossRef]
| Plant Species | Bacterial Strain | References |
|---|---|---|
| Wheat (Triticum aestivum L.) | Burkholderia sp. MTCC 12259 | [81] |
| Serratia sp. SL-12 | [77] | |
| Klebsiella sp. SBP-8 | [82] | |
| Bacillus pumilus FAB10 | [83] | |
| Chryseobacterium gleum sp. SUK | [84] | |
| Pea (Pisum sativum L.) | Arthrobacter protophormiae | [78] |
| Tomato (Solanum lycopersicum L.) | Streptomyces sp. | [76] |
| Peppers (Capsicum annuum L.) | Bacillus sp. | [85] |
| Arabidopsis thaliana Barley (Hordeum vulgare L.) | Hartmannibacter diazotrophicus E19 | [86] |
| Mung bean (Vigna radiata L.) | Pantoea sp., Enterococcus sp. | [87] |
| Pseudomonas syringae Pseudomonas fluorescens Rhizobium phaseoli | [88] |
| Bacteria | Coating | References |
|---|---|---|
| Parabukholderia phytofirmans | Alginate | [115] |
| Pseudomonas fluorescens | Alginate | [118] |
| Burkholderia cepacia | Alginate | [118] |
| Cyanobacteria cyanothece | Methyl salicylate | [119] |
| Rhizobacter enterobacter | Methyl salicylate | [119] |
| Azospirillum lipoferum | Alginate | [123] |
| Pantoea agglomerans | Alginate | [125] |
| Klebsiella oxytoca | Calcium-Alginate | [114] |
| Azosprillum lipoferum | Alginate | [127] |
| Paenibacillus polymyxa | Alginate | [127] |
| Paenibacillus polymyxa | Alginate | [131] |
| Bacillus nakamurai | Alginate | [131] |
| Bacillus pacificus | Alginate | [131] |
| Cyanothece sp. | Methyl salicylate | [119] |
| Enterobacter cloacae | Methyl salicylate | [119] |
| Clavibacter michiganensis | Selenium + Aggregates | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Tamanadar, E.; Moradi Pour, M.; Thakur, V.K. Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It. Sustainability 2021, 13, 12758. https://doi.org/10.3390/su132212758
Saberi Riseh R, Ebrahimi-Zarandi M, Tamanadar E, Moradi Pour M, Thakur VK. Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It. Sustainability. 2021; 13(22):12758. https://doi.org/10.3390/su132212758
Chicago/Turabian StyleSaberi Riseh, Roohallah, Marzieh Ebrahimi-Zarandi, Elahe Tamanadar, Mojde Moradi Pour, and Vijay Kumar Thakur. 2021. "Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It" Sustainability 13, no. 22: 12758. https://doi.org/10.3390/su132212758
APA StyleSaberi Riseh, R., Ebrahimi-Zarandi, M., Tamanadar, E., Moradi Pour, M., & Thakur, V. K. (2021). Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It. Sustainability, 13(22), 12758. https://doi.org/10.3390/su132212758







