1. Introduction
Leucine-rich repeat kinase 2 (LRRK2) is a multidomain protein pertaining to the Roco protein family [
1] that has become the topic of an increasing number of studies since it was first associated with Parkinson’s disease (PD) [
2,
3,
4]. Since then, numerous mutations of the LRRK2 gene have been identified, making LRRK2 a major genetic risk factor for inherited PD. Subsequent evidence indicates that LRRK2 mutations are also present in a small percentage of sporadic PD cases [
1,
5,
6].
In the mammalian brain, LRRK2 is highly expressed in brain areas recipient of dopaminergic projections, such as the cerebral cortex, striatum, and the olfactory bulb (OB), both in neurons and in microglial cells [
7]. At the cellular level, LRRK2 is enriched at the presynaptic terminal, where, interacting with several proteins such as Rabs [
8,
9], EndoA [
10,
11], N-ethylmaleimide sensitive factor (NSF) [
5,
12], orchestrates vesicle trafficking and neurotransmitter release [
5,
10,
13,
14,
15]. The fact that LRRK2 operates at the synapse, as do most of the proteins encoded by genes associated with PD [
6,
16], prompted the hypothesis that the synapse is the first target of PD pathology. Indeed, the emerging vision is that defects in the synaptic functionality precede degeneration and neuronal loss. However, despite accumulating evidence on the action of LRRK2 at the cellular and molecular level in the presynaptic compartment, the impact of LRRK2 at systems level remains unknown. Addressing this question is critical to gain insight into the role of LRRK2 in physiological conditions.
The synapse is the elementary unit that allows the structural and functional communication among neurons, resulting in the formation of neuronal circuits. However, the impact of the synapse functionality on the information processing capability of neuronal circuits and, ultimately on the behavioral outcome, can be understood only by analyzing the synaptic activity at system level. Several theoretical and experimental evidence indicates that neuronal circuits are built to generate emergent functional states [
17,
18]. These states consist of recurrent patterns of activity among ensembles of neurons that serve the dynamic coordination of the neuronal responses within local and wider networks. These coactive groups of neurons (ensembles) are considered the functional units that process and encode sensory, cognitive, and motor functions [
19,
20].
At a different scale, oscillations, quasi-periodic and large modulations of neuronal activity present both at rest and during task execution, represent a form of highly coherent activity established to integrate neuronal responses locally and among different brain regions [
21,
22]. One of the brain areas where oscillations have been first identified and characterized is the OB [
23]. The OB exhibits a wide range of oscillations that differ in the frequency and in the neuronal circuits that generate them. A growing body of evidence indicates that these oscillations are associated with distinct behaviors, being engaged in specific sensory and cognitive processes [
24,
25]. Interestingly, the OB is also one of the first brain areas involved in PD pathology [
26]. Indeed, olfactory deficits precede motor impairments by several years [
27]. The OB is, therefore, an interesting brain area to investigate whether and how the action of LRRK2 affects neuronal network dynamics and oscillations. To address this issue, we took advantage of a transgenic line of mice carrying a null mutation in LRRK2 (LRRK2 KO mouse). We found that, at the behavioral level, LRRK2 KO mice exhibit impairments in the olfactory discrimination test. At the circuit level, the lack of LRRK2 leads to a decrease in the amplitude of the neuronal oscillations in the 40–100 Hz band, mostly during active sniffing. Functional recording from population of neurons in the OB revealed that, upon odorant stimulation, mutant neurons have smaller and less reliable responses and exhibit a spatiotemporal profile with reduced correlation.
2. Materials and Methods
2.1. Animals
Animals were housed in filtered cages in a temperature-controlled room with a 12/12-h dark/light cycle and had access to water and food ad libitum. All procedures were conformed to the EU Directive 2010/63/EU for animal experiments and to the ARRIVE guidelines. Experimental protocols were approved by the Italian Ministry of Health.
Experiments were performed on female and male leucine-rich repeat kinase 2 knockout (LRRK2 KO) mice (C57BL/6-Lrrk2tm1.1Mjff/J, The Jackson Laboratory, Stock No: 016121) and their wild-type (WT) littermates (C57BL/6NJ background, The Jackson Laboratory, Stock No: 005304), used as internal controls, at the age of about 8 weeks. All analyses were performed blind to the genotype.
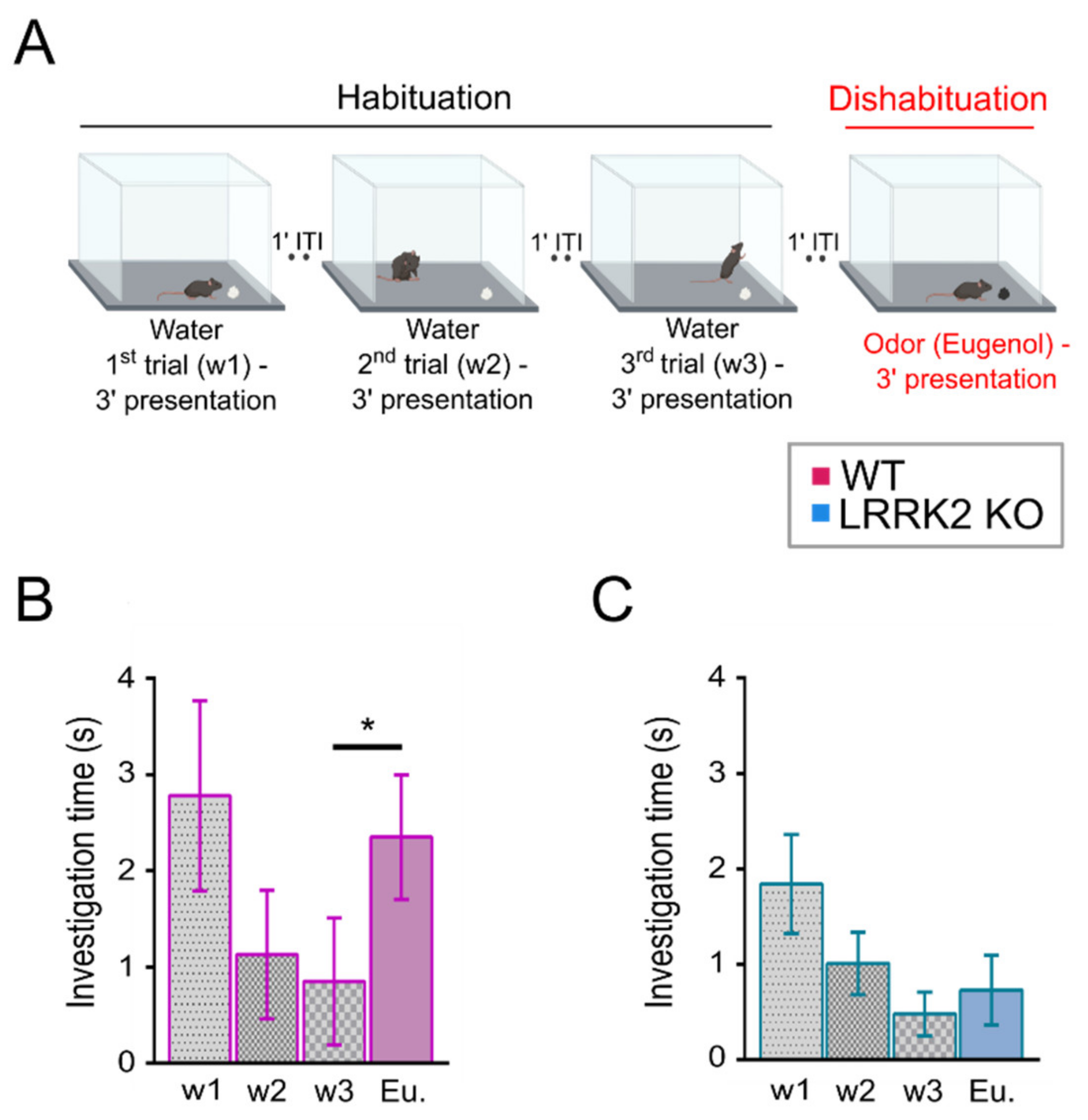
2.2. Habituation Dishabituation Test
The test was performed as described in [
28] with minor modifications on LRRK2 KO and WT littermate control mice. During the two days preceding the experimental day, mice were put in a polycarbonate cage (20 cm × 15 cm × 13 cm) identical to the test cage for 30 min, once per day, to allow habituation to the setup.
The day of the experiment, mice were placed in the test cage for about 30 min to familiarize with the environment. After this habituation period, a piece of filtered paper (2 cm × 2 cm) with double-distilled water was presented always in the same position in the cage for 3 min. This was repeated three times with a 1-min interval (these first three trials represent the habituation trials). On the fourth trial (the dishabituation trial), filtered paper scented with eugenol (1% v/v in mineral oil) was presented into the cage. Investigation time, defined as the period of active sniffing with nasal contact with the filtered paper, was measured for each trial. To avoid odor contamination, clean cages were used for each trial. All behavioral tests were conducted during the same daytime period and were video-recorded with a CMOS camera (30 frames per sec, 2592 × 1944 pixels) for extracting the locomotion and behavioral parameters.
2.3. Surgical Procedure for LFP Electrode Implantation
Animals were anesthetized with a mixture of Zoletil100 (a combination of Zolazepam and Tiletamin, 1:1, 10 mg/kg; Virbac, Carros, Cedex, France) and Rompun (Xylazine 2%, 0.06 mL/kg; Bio98, Milan, Italy) and placed into a custom stereotaxic frame. A small hole was made in the skull above the OB to allow the insertion of a custom-made bipolar stainless-steel electrode into the OB at the following stereotaxic coordinates: anterior = 800 μm, lateral = 500 μm, depth = 180 μm, with respect to the intersection of the midline and the inferior cerebral vein. A small screw, used as a reference electrode, was fixed on the skull above the cerebellum. All the components were soldered to a small fingerprint connector and secured to the skull with dental cement (Paladur, AgnTho’s, Lidingö, Sweden). Mice were immediately treated with post-operative analgesia (Tramadol 10 mg/kg, Formevet, Milan, Italy). Upon waking up from the anesthesia, animals were placed in individual cages for 3 days to allow complete recovery before initiation of LFP recordings.
2.4. Local Field Potentials (LFP) Recordings in Freely Behaving Mice
LFP signal was amplified by a mini head-stage preamplifier (NPI electronic GmbH, Tamm, ermany) and by a 2-channels extracellular amplifier (EXT-02F NPI electronic Germany). Amplified signals were band-pass filtered (0.3–300 Hz) (EXT-02F NPI electronic, Germany) and digitized at 10 kHz (National Instruments, Austin, TX, USA).
LFPs were recorded in LRRK2 KO and WT littermate control mice. Mice were free to move in a plexiglass arena (35 cm × 35 cm × 40 cm) placed within a Faraday cage. Before beginning the recording session, mice were habituated to the apparatus for about 30 min.
Each mouse was recorded in two distinct sessions, in two consecutive days. Each session lasted for 2 h. During the recordings in freely exploring conditions, mice were video recorded with a CMOS camera (30 frames per sec, 2592 × 1944 pixels)
Recorded videos were manually scored (MPC HC software, 1.9.5,) to identify two main behaviors: resting and exploring-sniffing. Resting indicates the behavior of an animal that is still for at least one minute. Exploring-sniffing denotes a mouse that moves around and sniffs the environment without being engaged in other tasks such as grooming. Aggregated recording datasets of 15 min were then processed for the LFP analysis.
2.5. Local Field Potentials Data Analysis
The raw signal acquired at 10 kHz was filtered with a 5th order band-pass Butterworth filter in 1–150 Hz range and then down-sampled at 250 Hz.
Traces for different spectral bands were obtained using filters with different band-pass ranges (4–12 Hz, 15–30 Hz, 40–70 Hz, 70–100 Hz).
Power spectral density (PSD) of LFP was computed over each behavior time interval with Welch’s method, with a Hann window with a length of 0.5 s and with 0.25 s overlap between windows, using the SciPy package for Python (
https://www.scipy.org/, accessed on 1 August 2020). Spectrograms were calculated with the same parameters.
Mean power for Figure 2G was averaged over the band ranges for each mouse.
All data are expressed as mean ± SD. Mann–Whitney test or Wilcoxon test were performed in a Python environment with the SciPy package to assess the statistical significance of the data.
2.6. Surgical Procedures for Functional In Vivo Imaging
2.6.1. Virus Injections
Animals were anesthetized with a mixture of Zoletil 100 (a combination of Zolazepam and Tiletamin, 1:1, 10 mg/kg;) and Rompun (Xylazine 2%, 0.06 mL/kg;) and placed into a stereotaxic frame. Adeno-associated viruses (AAVs) expressing the calcium indicator GCaMP6s (pAAV9.Syn.GCaMP6s.WPRE.SV40, Addgene (Watertown, MA, USA), catalog number: 100843-AAV9) were injected in each OB (500 nl of AAV vector, 70 nl/min) at the following stereotaxic coordinates: anterior = 1 mm; lateral = 500 μm; depth = 400 μm, relative to the intersection of the midline and the inferior cerebral vein. Mice were immediately treated with post-operative analgesia (Tramadol 10 mg/kg,).
2.6.2. In Vivo Imaging Window Implantation
The day of the functional imaging experiment, 15–18 days after viral injection, mice were anesthetized with isoflurane (4% for induction and 1% for maintenance) and placed into a custom stereotaxic frame. Body temperature was continuously monitored and maintained at 37 °C by a thermostatic electric blanket during the entire experiment. A craniotomy (1–2 mm diameter) was made over each olfactory bulb, leaving the dura intact. The craniotomy was filled with agarose (2%) and closed with a glass window (a cover-glass 5 mm diameter, 150 μm thick; Warner Instruments, Hamden, CT, USA). The window was secured to the skull with dental cement (Paladur, AgnTho’s, Lidingö, Sweden).
2.7. Two-Photon Functional Imaging
In the OB, neuronal activity recordings were acquired using a two-photon microscope (Bergamo I series, Thorlabs, Newton, NJ, US). The light source, a Ti:Sapphire laser (Chameleon Ultra II, Coherent), was tuned at 920 nm to excite the genetically encoded calcium indicator GCaMP6s, and its intensity modulated using a Pockell cell (Conopitcs, Danbury, CT, USA). A main dichroic mirror (690 nm LP, Semrock, West Henrietta, NY, USA) in combination with a 525/60 nm short pass emission filter (Semrock, West Henrietta, NY, USA) directed the fluorescence photons toward the photomultiplier tube (Hamamatsu, Tokyo, Japan, H7422PA-40 model). Images were acquired at 30 frames per second, 512 × 512 pixel resolution, with a water-dipping 16x objective (Nikon, Tokyo, Japan, LWD DIC N2, N.A. 0.8) at a depth of 200–220 μm below the OB surface. Mitral cell spontaneous activity was recorded in different OB regions, imaging the same field of view (FOV) for 5 min in LRRK2 KO and WT littermate control mice. For the characterization of the sensory-induced activity, odorant stimulation was delivered by a custom olfactometer. A simple code controlling an Arduino Uno board (Arduino, Boston, Massachusset, USA) synchronized the image acquisition with the aperture of the valves of the olfactometer. For each FOV, odorant stimuli were presented in pseudo-random order (7 with the odorant mixture and 3 with mineral oil), interleaved with clean airflow. Odorant stimuli consisted of an odor mixture composed of: propionaldehyde, butyraldehyde, valeraldehyde, (+)-Carvon, citral, (−)-limonene, ethyl tiglate, eugenol (all from Merck, Danvers, Massachusset, USA). Isofluorane anesthesia at 1% was used in all the imaging experiments. All odorants were diluted 1% v/v in mineral oil.
2.8. Functional Imaging Analysis
Time series with functional activity were processed by means of Suite2p (in Python environment) for motion correction and semi-automatic segmentation of the cells. For each cell, the extracted fluorescence trace was filtered to reduce detection noise and slow drifts in the overall fluorescence using a 5th order digital Butterworth filter with a bandpass range from 0.005 to 0.8 Hz. The Z-score was calculated for all the traces. To quantify activation and duration of events during spontaneous activity, data were binarized based on a threshold defined as 2.5 × (σFOV + MedianCELL), where σFOV is the standard deviation of the entire field of view and MedianCELL corresponds to the median value of the intensity distribution of each cell. For each cell the amplitude, the rate, and the duration of the activity events were calculated and compared between the two groups. Correlation between amplitude and duration was assessed fitting the data to a sigmoid curve to estimate the plateau value for each strain, defined as , where D represents the maximal duration at the higher amplitudes a, k the slew rate and a0 the amplitude value corresponding to half of the duration maximum. The goodness of fit was assessed by calculating the coefficient of determination (R2).
Kernel density estimations (KDEs) of these data were calculated with a top-hat kernel, with a bandwidth of 0.75 using the scikit-learn package (
https://scikit-learn.org/stable/index.html, accessed on 15 June 2021. The spatial structure of the activity was estimated by calculating the correlation between all the possible pairs of neurons as a function of their relative distance within the same FOV. For this purpose, Pearson’s correlation coefficient between the corresponding time series was used. For the quantification of the odor-evoked activity, a regression-based analysis was implemented, where the regression functions corresponded to the expected response of a cell to the presentation of one specific odorant. The regressors that are always zero except during the period of the stimulation, are in a number corresponding to the number of stimulation events and were convolved with an exponential decay kernel accounting for the impulsive response function of the fluorescent reporter GCaMP6s and the cell response delay (τ
ON, GCaMP6s = 0.11 s and τ
OFF,GCaMP6s = 0.52 s and response delay = 5.6 s). Using a linear regression routine available in the Scipy’s Python package (
https://www.scipy.org/, accessed on 10 October 2021, the cell responses were fit to obtain a series of coefficients, each representing the amplitude of the response for the particular stimulation. Along with the amplitude of the responses, the effective duration was compared between the two groups along with the correlation between these parameters following the same procedure described above for the spontaneous activity. As for the spontaneous activity, also for the stimulated activity Pearson’s correlation between all the possible pairs of neurons was used to assess the spatial structure of the activation.
For each field with stimulation, a vector with the average response rate to each stimulus was computed, and for spontaneous activity, a similar average activity vector was calculated over 33.3 s periods.
The vectors were clustered with hierarchical clustering using the ward method, and then the relative presence of wild-type vs. mutant field of views was computed in the unbiased clusters.
2.9. Statistical Analysis
Statistical significance was assessed mostly using Mann–Whitney’s test and Kruskall–Wallis test where indicated.
4. Discussion
In this paper, we analyzed the impact of LRRK2 on the neuronal activity at system level, combining behavioral tests, electrophysiological and optical recordings of a large population of neurons in the OB of LRRK2 KO mice. The emerging picture is that the lack of LRRK2 expression significantly impairs odorant-evoked responses and the spatial correlation in the activity among the stimulus-responsive neurons, with limited effects on spontaneous neuronal activity.
At the behavioral level, LRRK2 KO mice were tested with a habituation–dishabituation test. This behavioral paradigm, in its simplicity, allows to investigate several aspects of olfactory functions. We found that LRRK2 KO mice can detect the same cue presented in subsequent trials, showing habituation. However, the absence in KO mice of a significant rise in the exploration time at the presentation of a novel odorant after the habituation, is indicative of the inability to discriminate a new scent.
This deficit in olfactory response was confirmed by the electrophysiological recordings in freely behaving mice, where we measured the OB neuronal correlates underlying the act of freely sniffing. To this end, the analysis of the LFPs recordings focused on comparing the resting and active sniffing behavior. A striking difference in neuronal network dynamics emerged during the sniffing phase in KO mice. In physiological conditions, theta oscillations largely reflect the discharge of sensory afferents from the nasal epithelium to the OB, along with low-frequency burst firing of specific OB neuronal populations, namely external tufted cells (ETCs). These oscillations track the respiratory rhythm, which is in the range 1–4 Hz during quiet-waking state and increases to 4–12 Hz during novel odor exploration and discrimination [
24]. Focusing our analysis on the active exploration and sniffing behavior, we found that control animals show an increase in the power of theta in the range (4–12Hz), as expected from a higher correlated activity among the odorant-activated sensory inputs and the ETCs.
In contrast, in LRRK2 KO mice, the increase of the theta power was smaller. This difference, although not significant, points to an altered coordination in the olfactory behavior. Discriminating new odors elicits beta and gamma oscillations, whose appearance reflects the specific behavioral tasks. As in our experimental conditions, animals typically experience different environmental circumstances and behavioral states; controls show a power increase in both frequency bands. Again, LRRK2 KO mice present a more erratic trend, which results in a lower beta and gamma power increase. Interestingly, in KO mice, we observed this erratic pattern for all the frequency bands, as if sensory inputs were unable to elicit a consistent and reliable integration of neuronal responses in the OB of KO mice. With regards to gamma oscillations, it has been shown that this oscillatory activity reflects the level of synchrony of MCs firing [
35,
36]. Therefore, reduced gamma power indicates that MCs fire with a larger jitter relative to the gamma oscillation phase, presenting less correlated activity overall. Although we do not have a clear explanation of the strongest impairment of gamma oscillations in LRRK2 KO mice, we could speculate that this defect is due to the fact that gamma are the fastest oscillations in the OB, and this offers a workload that the LRRK2 KO networks cannot cope with. When the neuronal circuits are at rest or engaged in tasks that do not require orchestrating synaptic activity at high frequencies coherently, the network dynamics can keep up with the task, even though less reliably. However, when a more demanding performance is required, the missing role of LRRK2 in regulating the synaptic machinery significantly impairs the dynamics of neuronal networks, i.e., reduced gamma-band power.
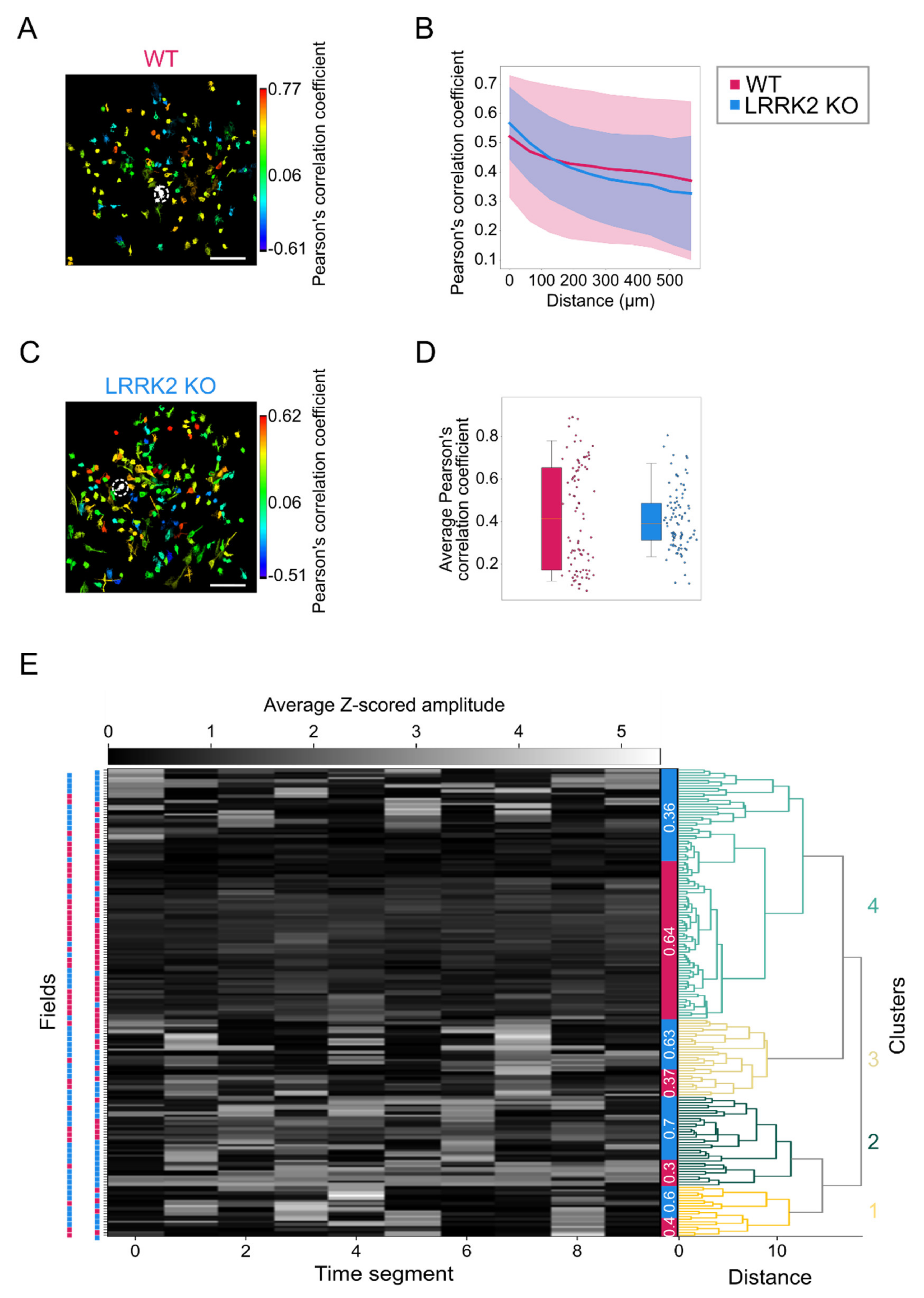
Complementary to observations in the LFP recordings, spontaneous events recorded by means of functional imaging in single cells exhibit a similar spatio-temporal profile in the two groups, although M/TCs tend to present larger and longer responses in LRRK2 KO than in WT mice. The limited impairments of the neuronal activity at rest were further corroborated by a similar spatial profile of the correlation in neuronal activity in WT and LRRK2 KO mice. In addition, the average profile of spontaneous activity was comparable, as demonstrated also by the hierarchical clustering of all the FOVs recorded.
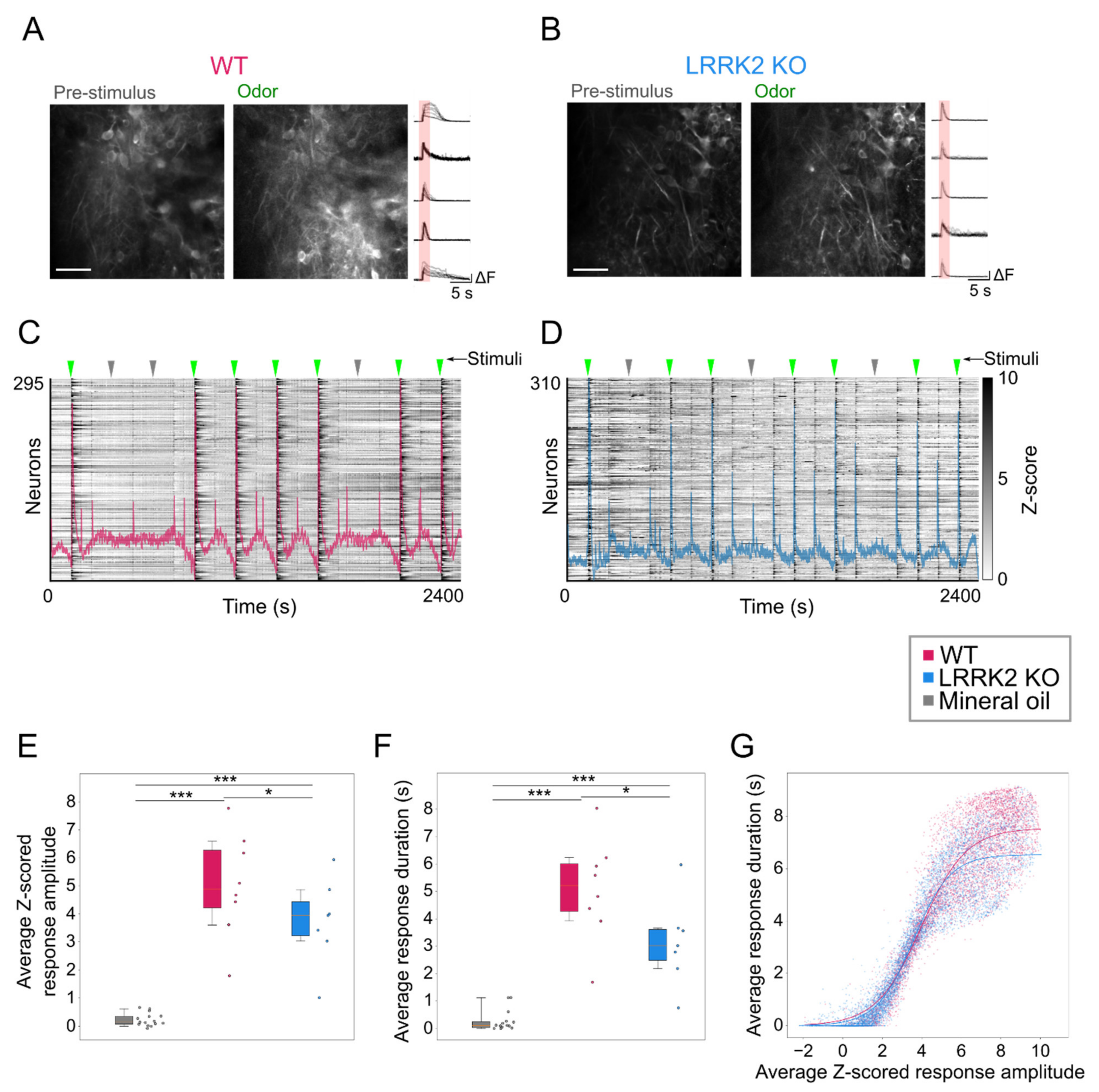
In contrast, odorant-evoked responses showed significant differences in the two groups of mice. Even during the online optical recordings, the FOVs in response to odorant stimulation appeared less bright in LRRK2 KO mice than in controls (
Figure 5A,B). As the functional imaging relies on increases in fluorescence to track neuronal responses (i.e., putative action potentials burst) [
37], the reduced change in brightness suggests a reduced response to the odorants in LRRK2 KO mice, as subsequently confirmed by the quantitative analysis. The alterations in odorant responses emerged in two different aspects. First, in KO mice, M/TCs exhibit significantly shorter and smaller odorant responses (
Figure 5E,F). These results were confirmed by the distinct probability distribution maps of the response amplitude as a function of the response duration for the two genotypes. Further evidence of this trend came from the fitting of the data, characterized by a significant shift of the maximal duration of the responses towards lower values for LRRK2 KO neurons with respect to controls. Second, we found that M/TCs in the two groups show different reliability in the response to the series of successive odorant presentations. An increased number of mutant neurons responded to none or only a few scent trials, resulting in a limited number of cells that responded to the entire cycle of sensory stimulation (i.e., 7 odorant presentations). In line with these results, the recorded FOVs with lower evoked-activity originate mainly from KO mice and form a homogeneous cluster, clearly separated from the cluster with higher activity in which most FOVs are from WT mice.
This altered reliability profile was associated with a generally reduced correlated activity among all M/TCs and its spatial gradient. This lower correlation level may be linked to the reduced gamma power observed in the LFP recordings. Even though the temporal dynamics of odorant responses are complex, and it is odorant-, concentration-, and task- specific in most species, from flies to mammals, odorant presentation elicits dynamically correlated activity among the ensembles of responsive neurons [
24,
38,
39,
40,
41,
42,
43]. This signature appears compromised in KO mice, as already observed in the LFPs recordings. As the coherence disruption in odor-encoding assemblies was reported to impair olfactory discrimination [
38], we speculate that reduced correlated activity among MT/Cs may contribute to the observed impairment in discrimination for LRRK2 KO mice.
Altogether our data indicate that, at rest, the absence of LRRK2 expression does not impair the neuronal network dynamics in a significant manner. However, odor response is clearly compromised in LRRK2 KO mice. These results are compatible with functional and/or structural alterations at the level of the synapse. As evidence indicates that LRRK2 orchestrates vesicles trafficking and endocytosis in vitro [
5,
11,
12,
44,
45], we favor the hypothesis that in the absence of LRRK2, these processes are slowed down. These kinds of synaptic deficits may cause mild alterations of the synaptic functionality at rest, as we observed in the LFP and functional recordings of spontaneous activity. However, they might significantly impair neurotransmission and neuronal communication during intense synaptic activity (as during high-frequency oscillations and evoked-activity), when vesicle recycling is critical to maintain proper neurotransmitter release. The lack of coordination in the molecular machinery at LRRK2 KO presynaptic terminals may therefore lead to the abnormal response profile of the single neurons and impair the activity correlation among the ensembles of the responsive cells. Along with its presynaptic role, LRRK2 was reported to be involved on the postsynaptic side in actin cytoskeleton dynamics [
46] that regulates dendrite outgrowth and dendritic spine formation and consolidation. These neuronal processes exert a critical role in establishing structural connectivity among neurons. It is therefore not surprising that LRRK2 expression during development coincides with the phase of spine formation [
47]. In line with the role of LRRK2 in regulating neuronal morphology via actin remodeling, it was reported that lack of LRRK2 is associated with altered spinogenesis [
46,
48]. These structural defects could further contribute to altered communication among neurons and altered neuronal plasticity, mostly during intense neuronal network activity. In conclusion, our work provides evidence of the physiological role of LRRK2 at system level, in coordinating the spatio-temporal pattern of neuronal activity among ensembles of neurons that are critical to process neuronal information.