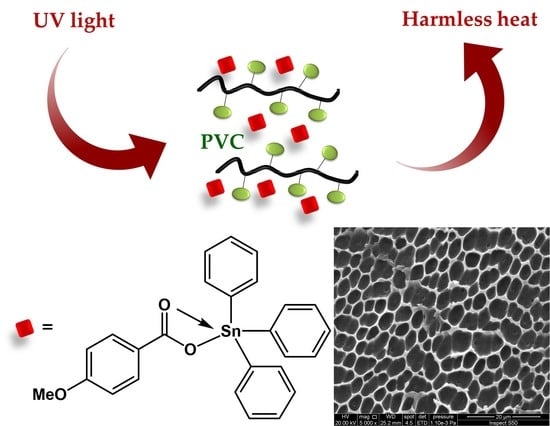
Substituted Organotin Complexes of 4-Methoxybenzoic Acid for Reduction of Poly(vinyl Chloride) Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
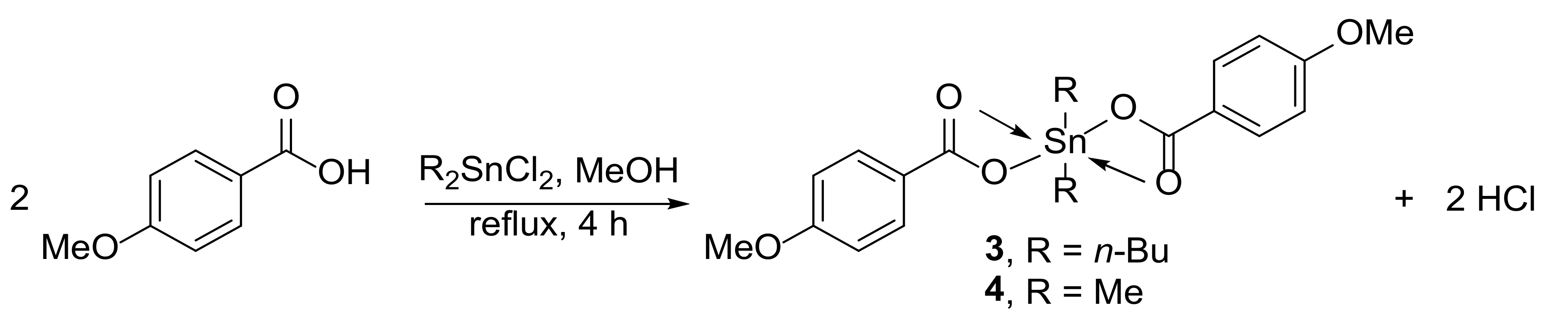
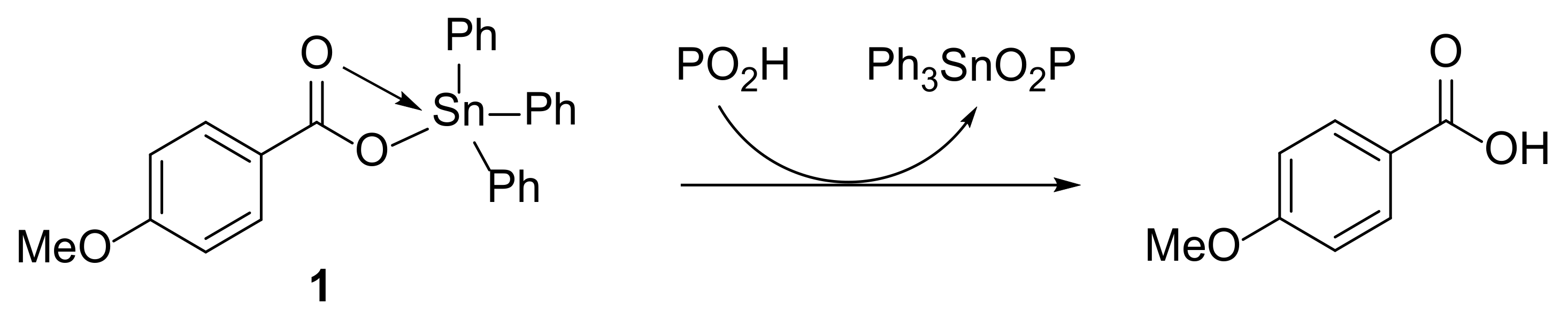
2.2. Synthesis of Complexes 1 and 2
2.3. Synthesis of Complexes 3 and 4
2.4. Preparation of PVC Thin Films
2.5. PVC Exposure to UV Light
3. Results and Discussion
3.1. Synthesis of Complexes 1–4
3.2. FTIR Spectroscopy of Complexes 1–4
3.3. NMR Spectroscopy of Complexes 1–4
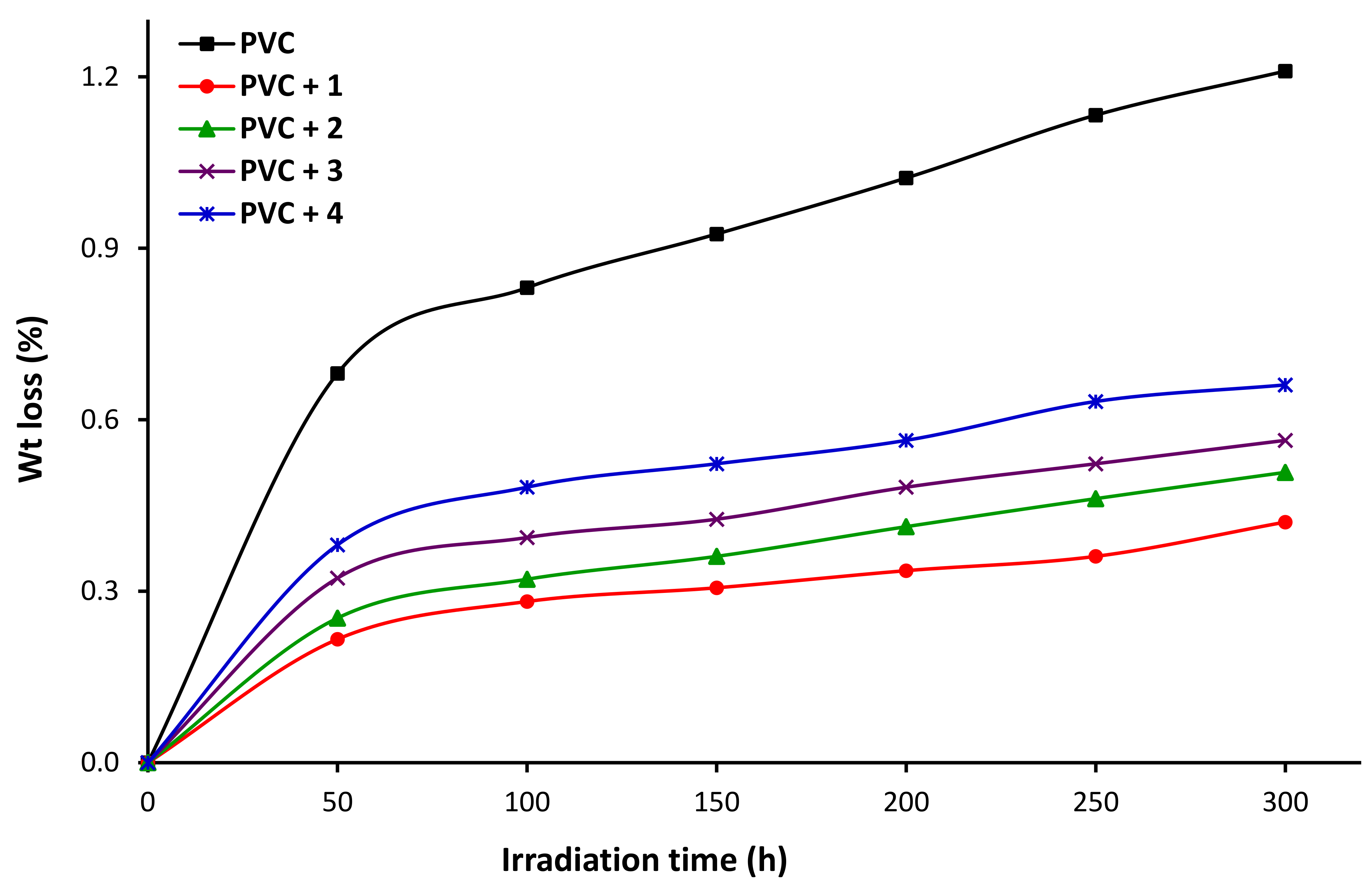
3.4. Impact of Irradiation on Weight of PVC
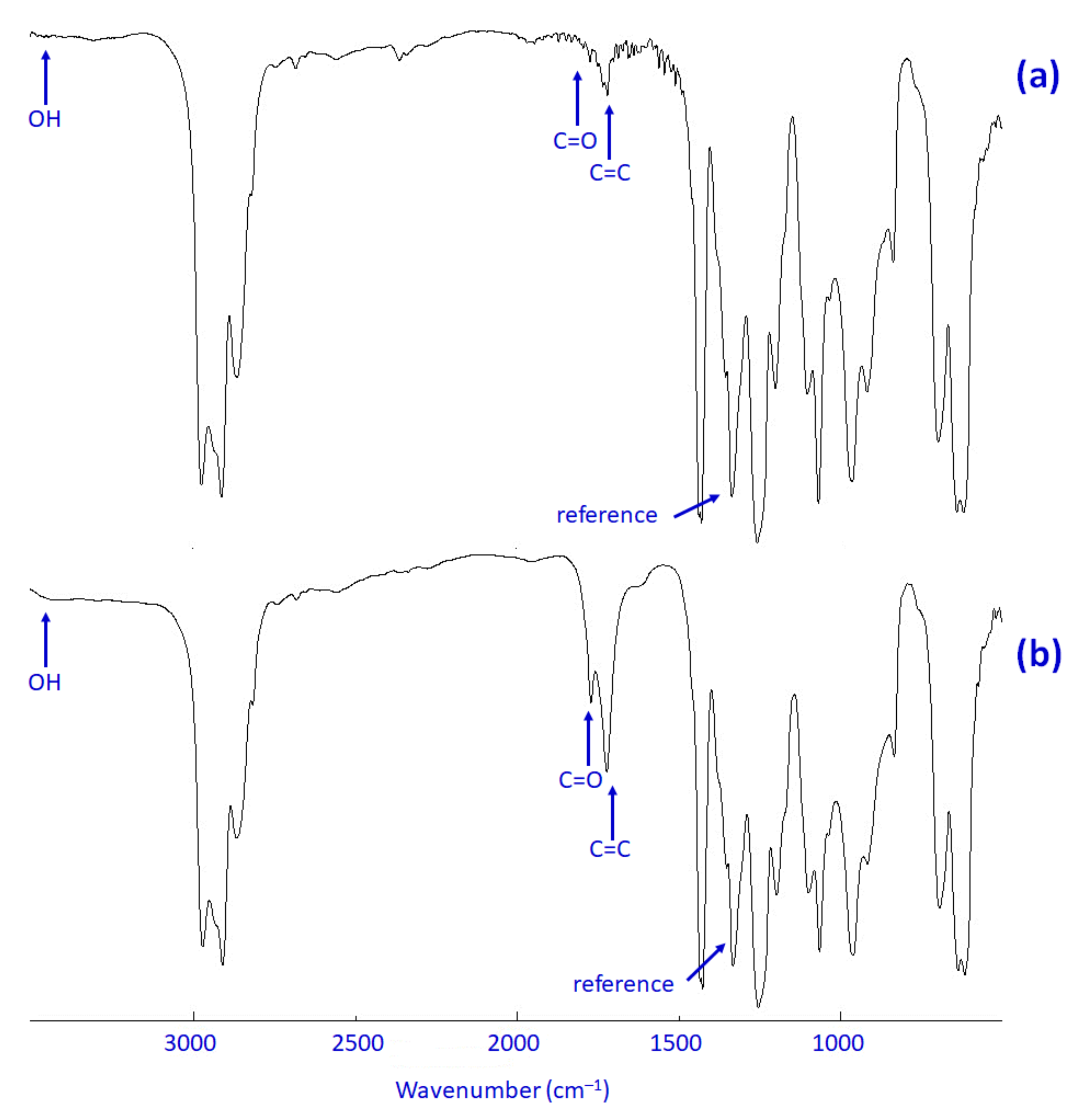
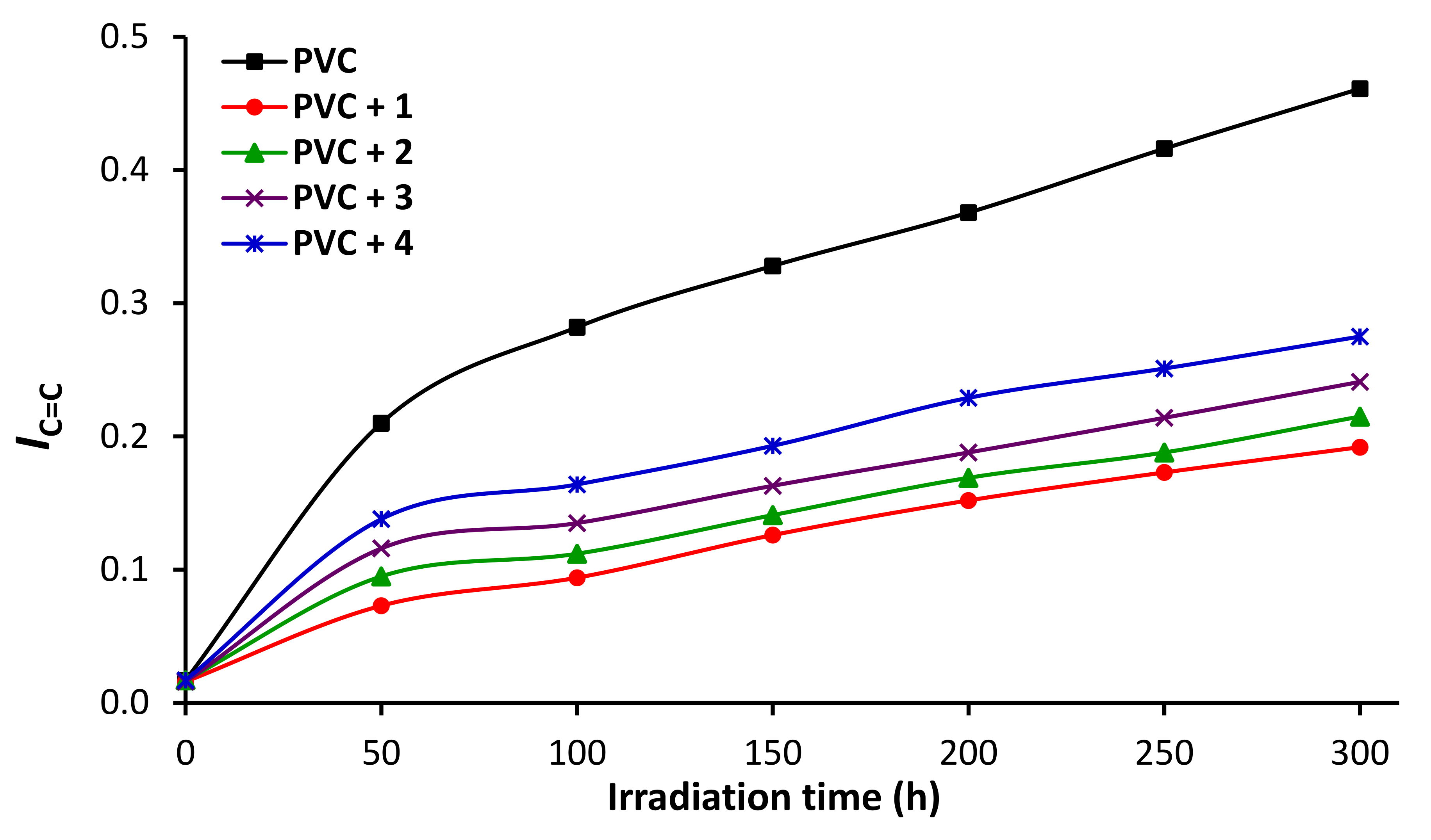
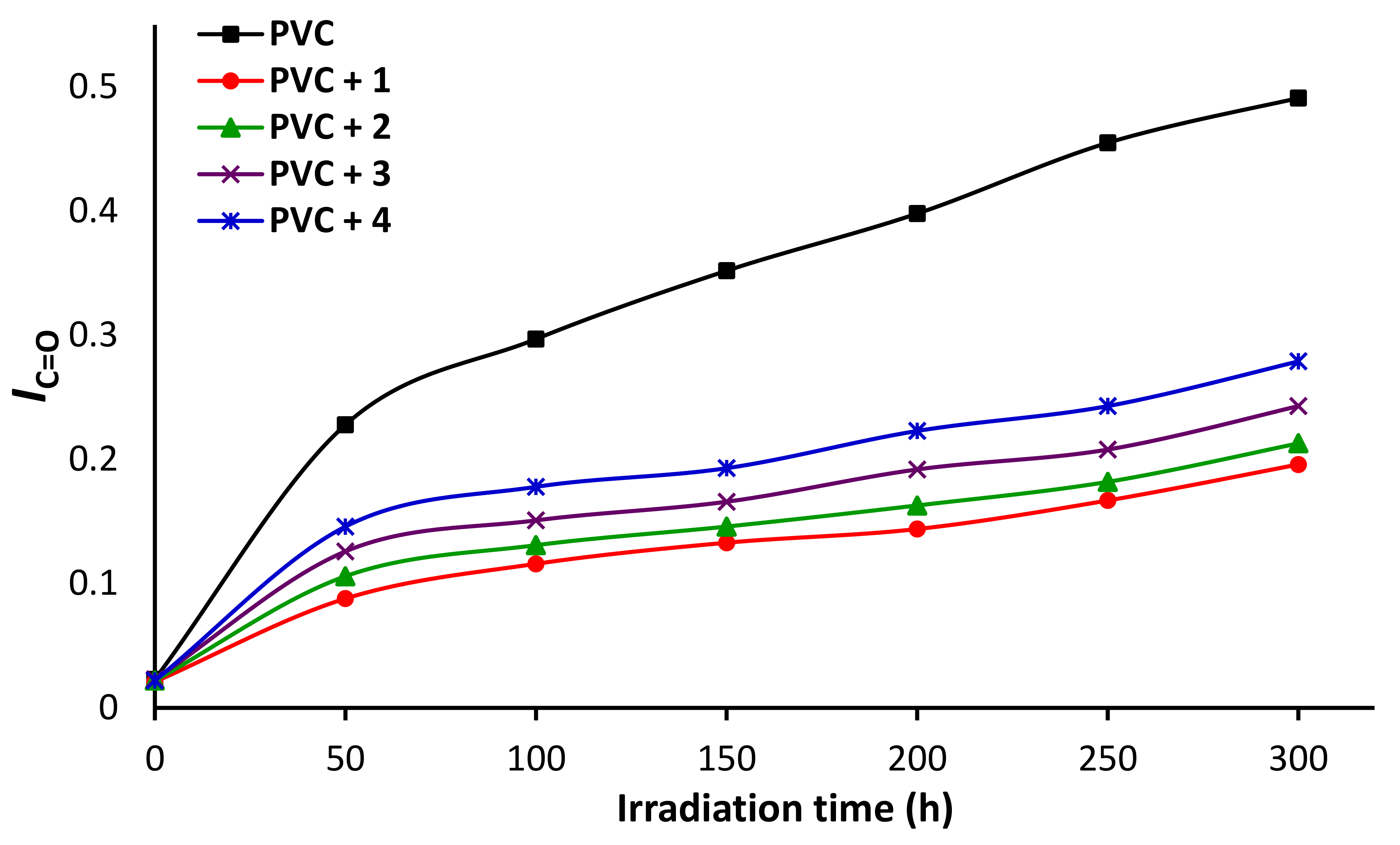
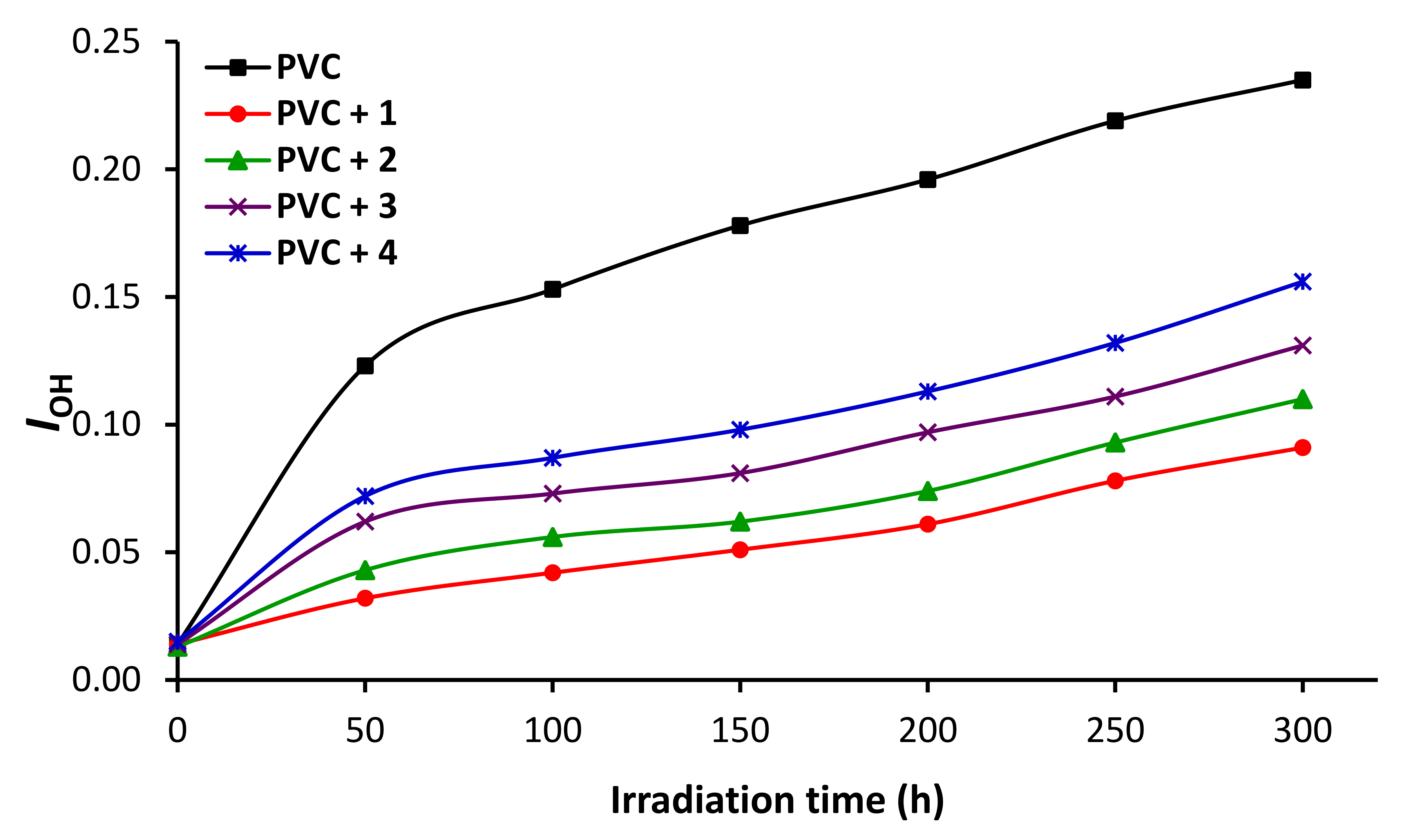
3.5. Impact of Irradiation on FTIR Spectroscopy of PVC
3.6. Impact of Irradiation on Molecular Weight of PVC
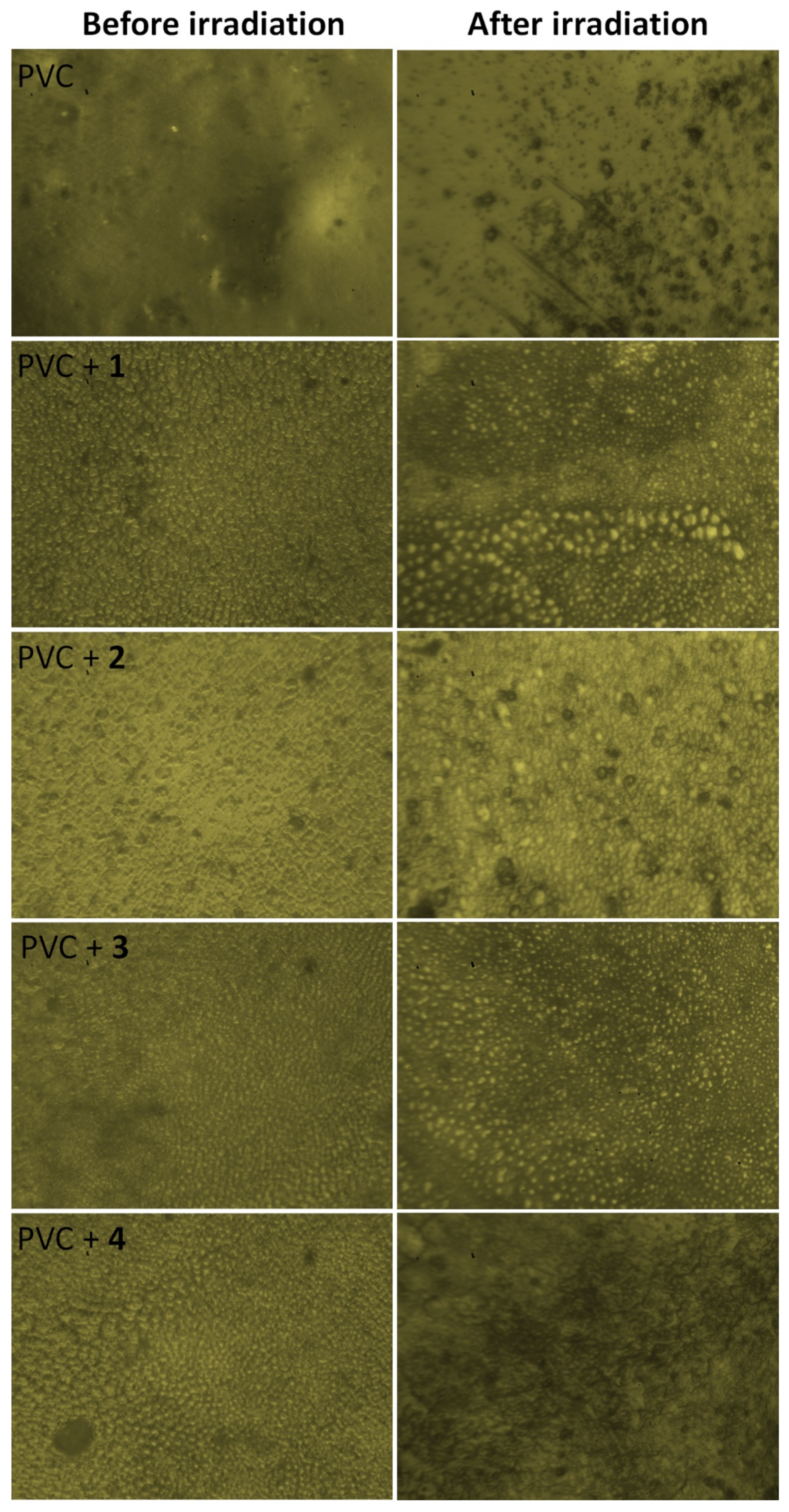
3.7. Impact of Irradiation on Surface Morphology of PVC
3.8. Impact of Sn Complexes on Photostabilization of PVC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Coltro, L.; Pitta, J.B.; Madaleno, E. Performance evaluation of new plasticizers for stretch PVC films. Polym. Test. 2013, 32, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Keane, M.A. Catalytic conversion of waste plastics: Focus on waste PVC. J. Chem. Technol. Biotechnol. 2007, 82, 787–795. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Liao, S.-L.; Li, Q.-G.; Guan, Q.; Jia, P.-Y.; Zhou, Y.-H. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration—Still on the run. React. Funct. Polym. 2019, 147, 104458. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.B.; Kumosa, M. Particle removal mechanisms in synergistic aging of polymers and glass reinforced polymer composites under combined UV and water. Compos. Sci. Technol. 2017, 153, 273–281. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Luo, Z.; Wang, H.; Wei, Z. Molecular chain model construction, thermo-stability, and thermo-oxidative degradation mechanism of poly(vinyl chloride). RSC Adv. 2016, 6, 31898–31905. [Google Scholar] [CrossRef]
- Torikai, A.; Hasegawa, H. Accelerated photodegradation of poly(vinyl chloride). Polym. Degrad. Stabil. 1999, 63, 441–445. [Google Scholar] [CrossRef]
- Salovey, R.; Gebauer, R.C. Chain scission during the irradiation of poly(vinyl chloride). J. Polym. Sci. A Polym. Chem. 1972, 10, 1533–1537. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stabil. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Malshe, V.C.; Waghoo, G. Weathering study of epoxy paints. Prog. Org. Coating 2004, 51, 267–272. [Google Scholar] [CrossRef]
- Yousif, Y.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [Green Version]
- Yousif, E.; Hasan, A. Photostabilization of poly(vinyl chloride) – Still on the run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef] [Green Version]
- Babinsky, R. PVC additives: A global review. Plast. Addit. Compd. 2006, 8, 38–40. [Google Scholar] [CrossRef]
- Das, P.; Roy, A.; Chakrabarti, S. Photocatalytic degradation of the nanocomposite film comprising polyvinyl chloride (PVC) and sonochemically synthesized iron-doped zinc oxide: A comparative study of performances between sunlight and UV radiation. J. Polym. Environ. 2017, 25, 1231–1241. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. 6—The interactions of microplastics and chemical pollutants. In Microplastic Pollutants; Elsevier Inc.: Oxford, UK, 2017; pp. 131–157. [Google Scholar]
- Chen, C.; Chen, L.; Yao, Y.; Artigas, F.; Huang, Q.; Zhang, W. Organotin release from polyvinyl chloride microplastics and concurrent photodegradation in water: Impacts from salinity, dissolved organic matter, and light exposure. Environ. Sci. Technol. 2019, 53, 10741–10752. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Khanal, S.; Zhang, C.; Xu, S. Photodegradation of polybenzimidazole/polyvinyl chloride composites and polybenzimidazole: Density functional theory and experimental study. J. Appl. Polym. Sci. 2021, 138, 49693. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Star, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly(vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef] [Green Version]
- Schiller, M. PVC Additives: Performance, Chemistry, Developments, and Sustainability; Carl Hanser Verlag: Munich, Germany, 2015. [Google Scholar]
- Chakrabarti, S.; Chaudhuri, B.; Bhattacharjee, S.; Das, P.; Dutta, B.K. Degradation mechanism and kinetic model for photocatalytic oxidation of PVC-ZnO composite film in presence of a sensitizing dye and UV radiation. J. Hazard. Mater. 2008, 154, 230–236. [Google Scholar] [CrossRef]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide particles on the surface morphology and the mechanical properties of PVC composites during QUV accelerated weathering. Polym. Compos. 2016, 37, 3391–3397. [Google Scholar] [CrossRef]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering. Polym. Degrad. Stab. 2014, 104, 33–39. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Yousif, E. SEM morphological analysis of irradiated polystyrene film doped by a Schiff base containing a 1,2,4-triazole ring system. Appl. Petrochem. Res. 2019, 9, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [Green Version]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Ahmed, A.; El-Hiti, G.A.; Hadi, A.G.; Ahmed, D.S.; Baashen, M.A.; Hashim, H.; Yousif, E. Photostabilization of poly(vinyl chloride) films blended with organotin complexes of mefenamic acid for outdoor applications. Appl. Sci. 2021, 11, 2853. [Google Scholar] [CrossRef]
- Arkış, E.; Balköse, D. Thermal stabilisation of poly(vinyl chloride) by organotin compounds. Polym. Degrad. Stab. 2005, 88, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, Z.N.; Yousif, E.; Alias, M.; El-Hiti, G.A.; Ahmed, D.S. Synthesis, characterization, properties, and use of new fusidate organotin complexes as additives to inhibit poly(vinyl chloride) photodegradation. J. Polym. Res. 2020, 27, 267. [Google Scholar] [CrossRef]
- Jasem, H.; Hadi, A.G.; El-Hiti, G.A.; Baashen, M.A.; Hashim, H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Tin-naphthalene sulfonic acid complexes as photostabilizers for poly(vinyl chloride). Molecules 2021, 26, 3629. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin(IV) dithiocarbamate complexes: Chemistry and biological activity. Molecules 2018, 23, 2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Deyab, S.S.; El-Newehy, M.H. Synthesis and characterization of novel organotin-phosphorous compounds II. Molecules 2010, 15, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.A.; Ashfaq, M.; Waseem, A.; Ahmed, M.M.; Najam, T.; Shaheen, S.; Rivera, G. Synthesis and biological activities of organotin(IV) complexes as antitumoral and antimicrobial agents. A review. Mini Rev. Med. Chem. 2015, 15, 406–426. [Google Scholar] [CrossRef]
- Siemann, U. Solvent Cast Technology—A Versatile Tool for Thin Film Production. In Scattering Methods and the Properties of Polymer Materials. Progress in Colloid and Polymer Science; Stribeck, N., Smarsly, B., Eds.; Springer: Berlin, Germany, 2005; Volume 130, pp. 1–14. [Google Scholar]
- Alcock, N.W.; Culver, J.; Roe, S.M. Secondary bonding. Part 15. Influence of lone pairs on coordination: Comparison of diphenyl-tin(IV) and –tellurium(IV) carboxylates and dithiocarbamates. J. Chem. Soc. Dalton Trans. 1992, 1477–1484. [Google Scholar] [CrossRef]
- Singh, H.L. Synthesis, spectroscopic, and theoretical studies of tin(II) complexes with biologically active Schiff bases derived from amino acids. Main Group Met. Chem. 2016, 39, 67–76. [Google Scholar] [CrossRef]
- Singh, H.L.; Singh, J. Synthesis, spectroscopic, molecular structure, and antibacterial studies of dibutyltin(IV) Schiff base complexes derived from phenylalanine, isoleucine, and glycine. Bioinorg. Chem. Appl. 2014, 2014, 716578. [Google Scholar] [CrossRef]
- Rehman, W.; Baloch, M.K.; Badshah, A.; Ali, S. Synthesis and characterization of biologically potent di-organotin(IV) complexes of mono-methyl glutarate. J. Chin. Chem. Soc. 2005, 52, 231–236. [Google Scholar] [CrossRef]
- Masood, H.; Ali, S.; Mazhar, M.; Shahzadi, S.; Shahid, K. 1H, 13C, 119Sn NMR, Mass, Mössbauer and biological studies of tri-, di- and chlorodiorganotin(IV) carboxylates. Turk. J. Chem. 2004, 28, 75–86. [Google Scholar]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chloride. J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Chaochanchaikul, K.; Rosarpitak, V.; Sombatsompop, N. Photodegradation profiles of PVC compound and wood/PVC composites under UV weathering. Express Polym. Lett. 2013, 7, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Nief, O.A. Photostabilization of polyvinyl chloride by some new thiadiazole derivatives. Eur. J. Chem. 2015, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Pi, H.; Xiong, Y.; Guo, S. The kinetic studies of elimination of HCl during thermal decomposition of PVC in the presence of transition metal oxides. Polym. Plast. Technol. Eng. 2005, 44, 275–288. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Padil, V.V.T.; Nagalakshmaiah, M.; Waclawek, S.; Černík, M.; Varma, R.S. Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers 2020, 12, 512. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, L.C.; Grubb, D.T.; Meyers, G.F. Polymer Microscopy, 3rd ed.; Chapter 5; Springer: New York, NY, USA, 2008. [Google Scholar]
- Valko, L.; Klein, E.; Kovařík, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1132. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.-M.; Jiang, G.-D. Different photodegradation processes of PVC with different average degrees of polymerization. J. Appl. Polym. Sci. 2008, 107, 528–540. [Google Scholar] [CrossRef]
- Mousa, O.G.; El-Hiti, G.A.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Synthesis of carvedilol-organotin complexes and their effects on reducing photodegradation of poly(vinyl chloride). Polymers 2021, 13, 500. [Google Scholar] [CrossRef]
- Yaseen, A.A.; Yousif, E.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Kariuki, B.M.; Ahmed, D.S.; Bufaroosha, M. FTIR, weight, and surface morphology of poly(vinyl chloride) doped with tin complexes containing aromatic and heterocyclic moieties. Polymers 2021, 13, 3264. [Google Scholar] [CrossRef]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly(vinyl chloride) films containing naproxen diorganotin(IV) complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly(vinyl vhloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [Green Version]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghani, H.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Tin complexes of 4-(benzylideneamino)benzenesulfonamide: Synthesis, structure elucidation and their efficiency as PVC photostabilizers. Polymers 2021, 13, 2434. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [Green Version]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohamed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdul Naby, A.S.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]


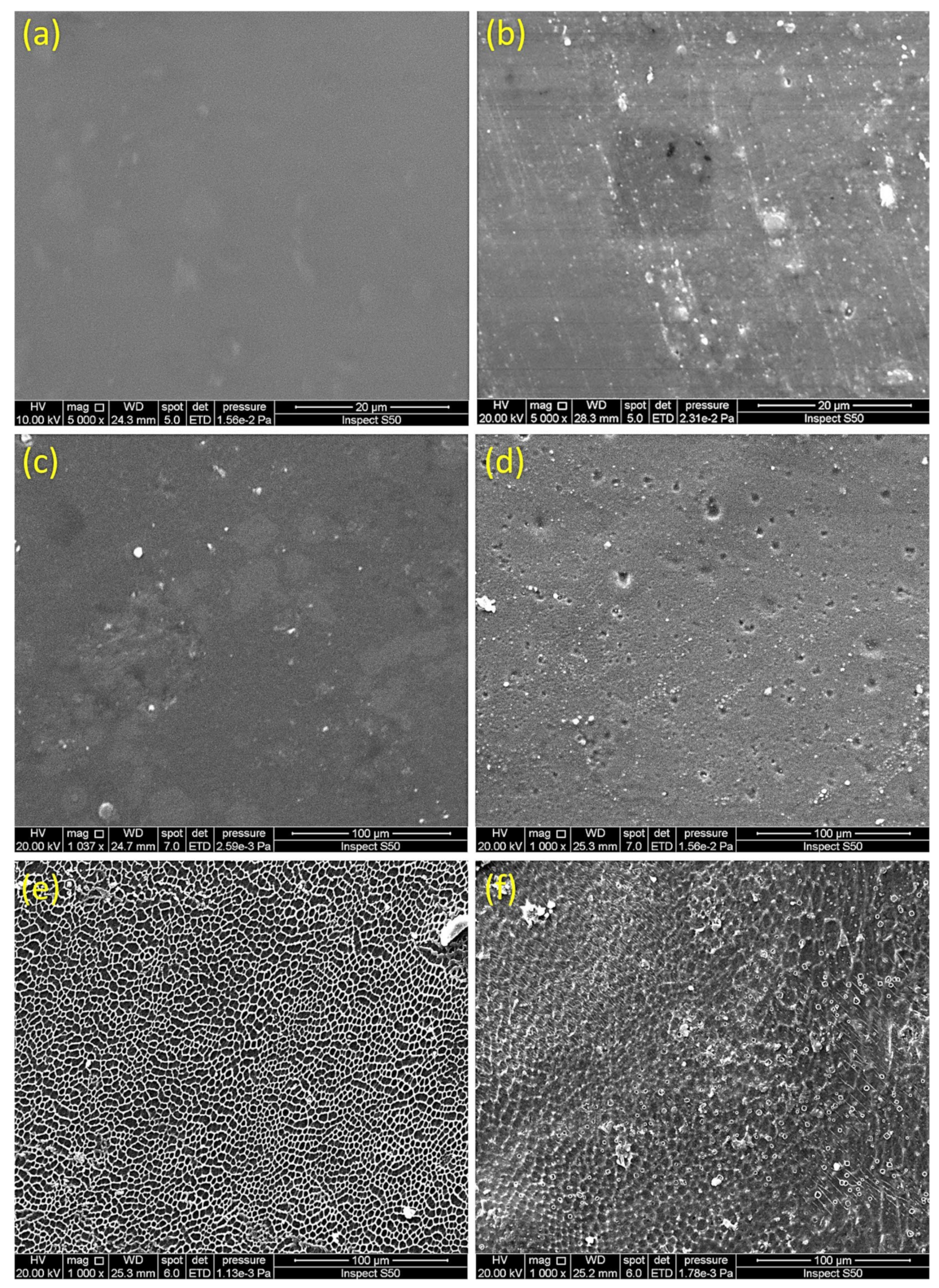
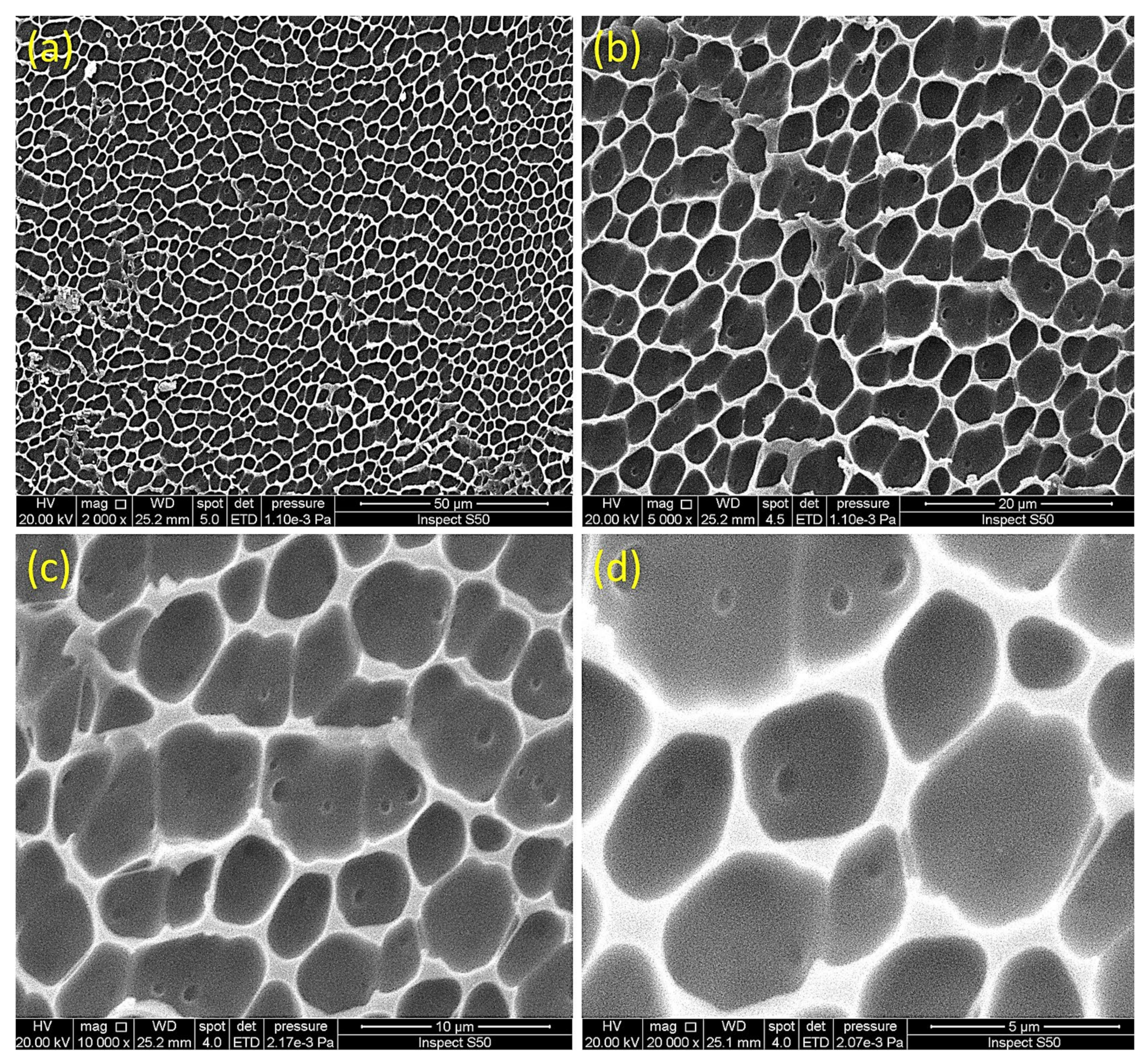

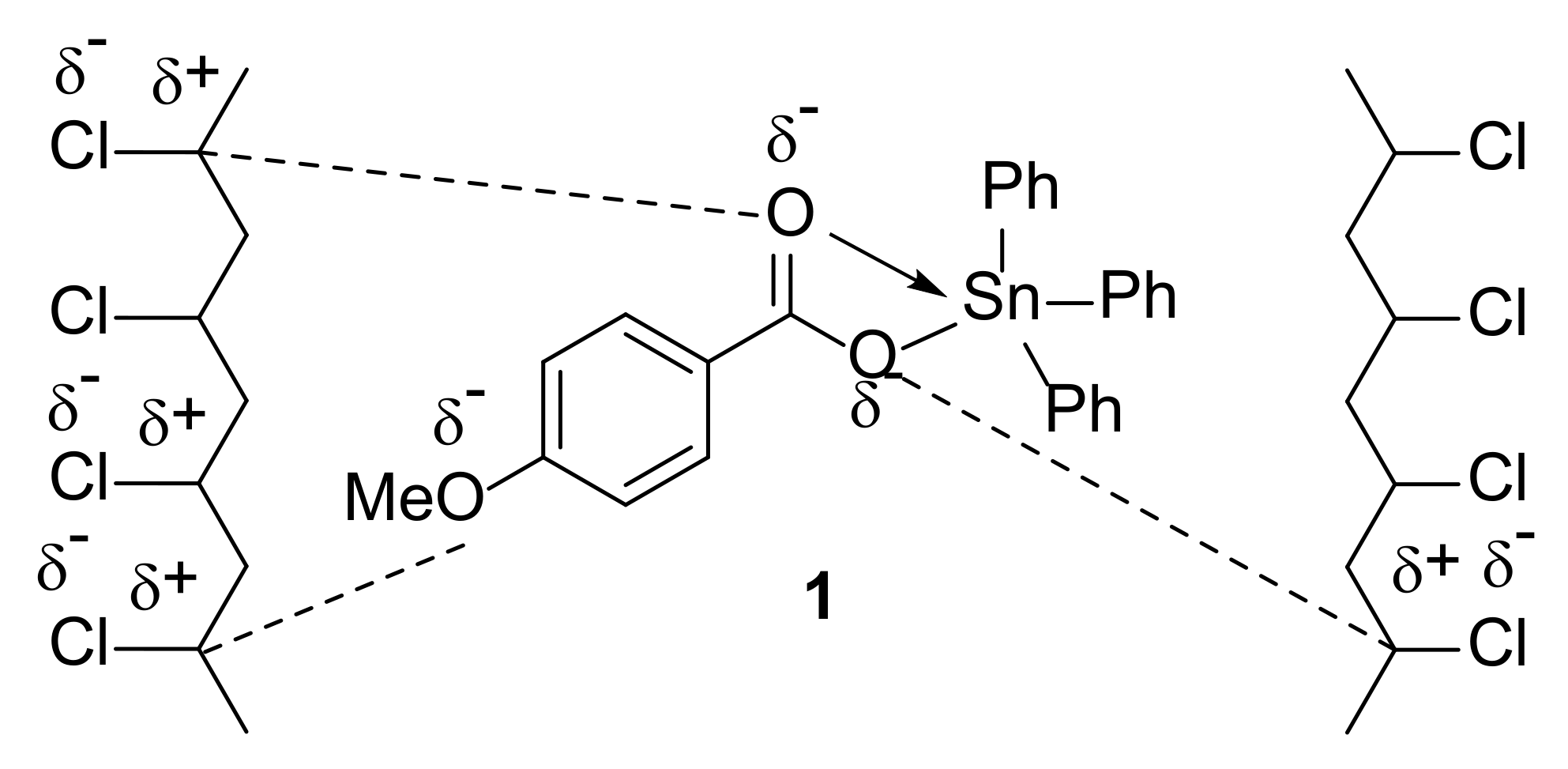
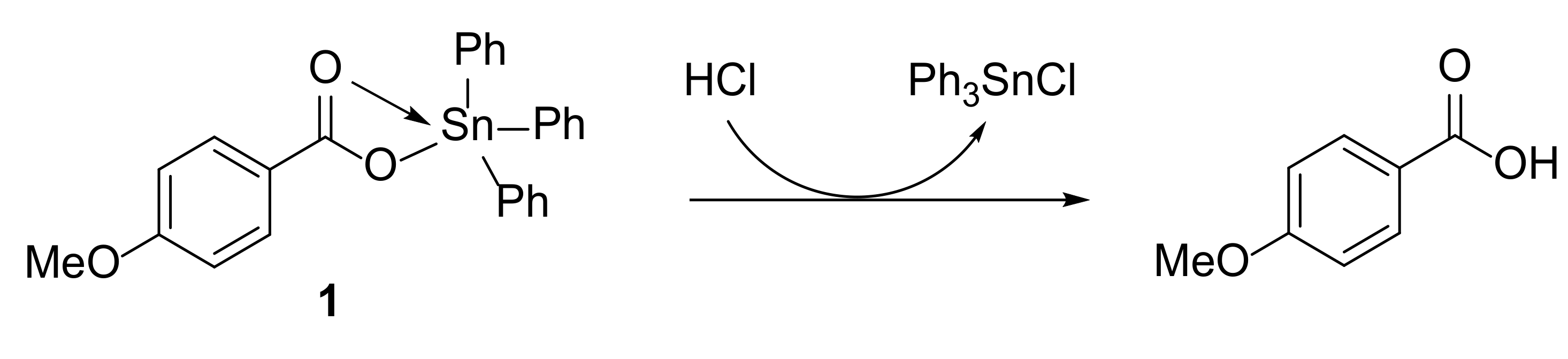
| Sn Complex | R | Color | Yield (%) | M.P. (°C) | Calculated (Found; %) | ||
|---|---|---|---|---|---|---|---|
| C | H | Sn | |||||
| 1 | Ph | Off white | 78 | 153–155 | 62.31 (62.42) | 4.42 (5.48) | 23.69 (23.52) |
| 2 | Bu | White | 71 | 144–146 | 54.45 (54.55) | 7.77 (7.80) | 26.91 (26.75) |
| 3 | Bu | White | 76 | 147–149 | 53.86 (53.94) | 6.03 (6.12) | 22.18 (22.04) |
| 4 | Me | Off white | 75 | 136–138 | 47.93 (47.98) | 4.47 (5.56) | 26.32 (26.24) |
| Sn Complex | FTIR, Frequency (ν, cm−1) | |||||
|---|---|---|---|---|---|---|
| C=O | C=C | Sn–O | Sn–C | |||
| asym | sym | Δν (asym – sym) | ||||
| 1 | 1680 | 1508 | 172 | 1591 | 518 | 468 |
| 2 | 1682 | 1508 | 174 | 1595 | 515 | 459 |
| 3 | 1682 | 1514 | 168 | 1592 | 515 | 468 |
| 4 | 1683 | 1508 | 175 | 1596 | 515 | 472 |
| Complex | 1H NMR | 19Sn NMR |
|---|---|---|
| 1 | 7.94 (d, J = 8.6 Hz, 2H, Ar), 7.66–7.32 (m, 15H, 3 Ph), 6.97 (d, J = 8.6 Hz, 2H, Ar), 3.84 (s, 3H, OMe) | –178 |
| 2 | 7.97 (d, J = 8.5 Hz, 2H, Ar), 6.94 (d, J = 8.5 Hz, 2H, Ar), 3.78 (s, 3H, OMe), 1.71 (t, J = 7.6 Hz, 6H, 3 MeCH2CH2CH2), 1.50 (quintet, J = 7.6 Hz, 6H, 3 MeCH2CH2),1.35 (sextet, J = 7.6 Hz, 6H, 3 MeCH2), 0.93 (t, J = 7.6 Hz, 9H, 3 Me) | –163 |
| 3 | 7.66 (d, J = 8.4 Hz, 4H, 2 Ar), 6.98 (d, J = 8.4 Hz, 4H, 2 Ar), 3.80 (s, 6H, 2 OMe), 1.55 (t, J = 7.6 Hz, 4H, 2 MeCH2CH2CH2), 1.42 (quintet, J = 7.6 Hz, 4H, 2 MeCH2CH2),1.30 (sextet, J = 7.6 Hz, 4H, 2 MeCH2), 0.83 (t, J = 7.6 Hz, 6H, 2 Me) | –279 |
| 4 | 7.64 (d, J = 8.5 Hz, 4H, 2 Ar), 6.97 (d, J = 8.5 Hz, 4H, 2Ar), 3.79 (s, 6H, 2 OMe), 0.75 (s, 6H, 2 Me) | –269 |
| Organic Moiety in Sn Complex | Reduction in Rq (Fold) | Reference |
|---|---|---|
| 4-Methoxybenzoic acid | 21.2 | [current work] |
| Naproxen | 5.2 | [56] |
| Carvedilol | 6.4 | [54] |
| Furosemide | 6.6 | [57] |
| Valsartan | 7.4 | [58] |
| Telmisartan | 9.4 | [59] |
| Trimethoprim | 11.3 | [55] |
| Ciprofloxacin | 16.6 | [60] |
| 4-(Benzylideneamino)benzenesulfonamide | 18.4 | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, A.G.; Baqir, S.J.; Ahmed, D.S.; El-Hiti, G.A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; Yousif, E. Substituted Organotin Complexes of 4-Methoxybenzoic Acid for Reduction of Poly(vinyl Chloride) Photodegradation. Polymers 2021, 13, 3946. https://doi.org/10.3390/polym13223946
Hadi AG, Baqir SJ, Ahmed DS, El-Hiti GA, Hashim H, Ahmed A, Kariuki BM, Yousif E. Substituted Organotin Complexes of 4-Methoxybenzoic Acid for Reduction of Poly(vinyl Chloride) Photodegradation. Polymers. 2021; 13(22):3946. https://doi.org/10.3390/polym13223946
Chicago/Turabian StyleHadi, Angham G., Sadiq J. Baqir, Dina S. Ahmed, Gamal A. El-Hiti, Hassan Hashim, Ahmed Ahmed, Benson M. Kariuki, and Emad Yousif. 2021. "Substituted Organotin Complexes of 4-Methoxybenzoic Acid for Reduction of Poly(vinyl Chloride) Photodegradation" Polymers 13, no. 22: 3946. https://doi.org/10.3390/polym13223946
APA StyleHadi, A. G., Baqir, S. J., Ahmed, D. S., El-Hiti, G. A., Hashim, H., Ahmed, A., Kariuki, B. M., & Yousif, E. (2021). Substituted Organotin Complexes of 4-Methoxybenzoic Acid for Reduction of Poly(vinyl Chloride) Photodegradation. Polymers, 13(22), 3946. https://doi.org/10.3390/polym13223946