Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Method
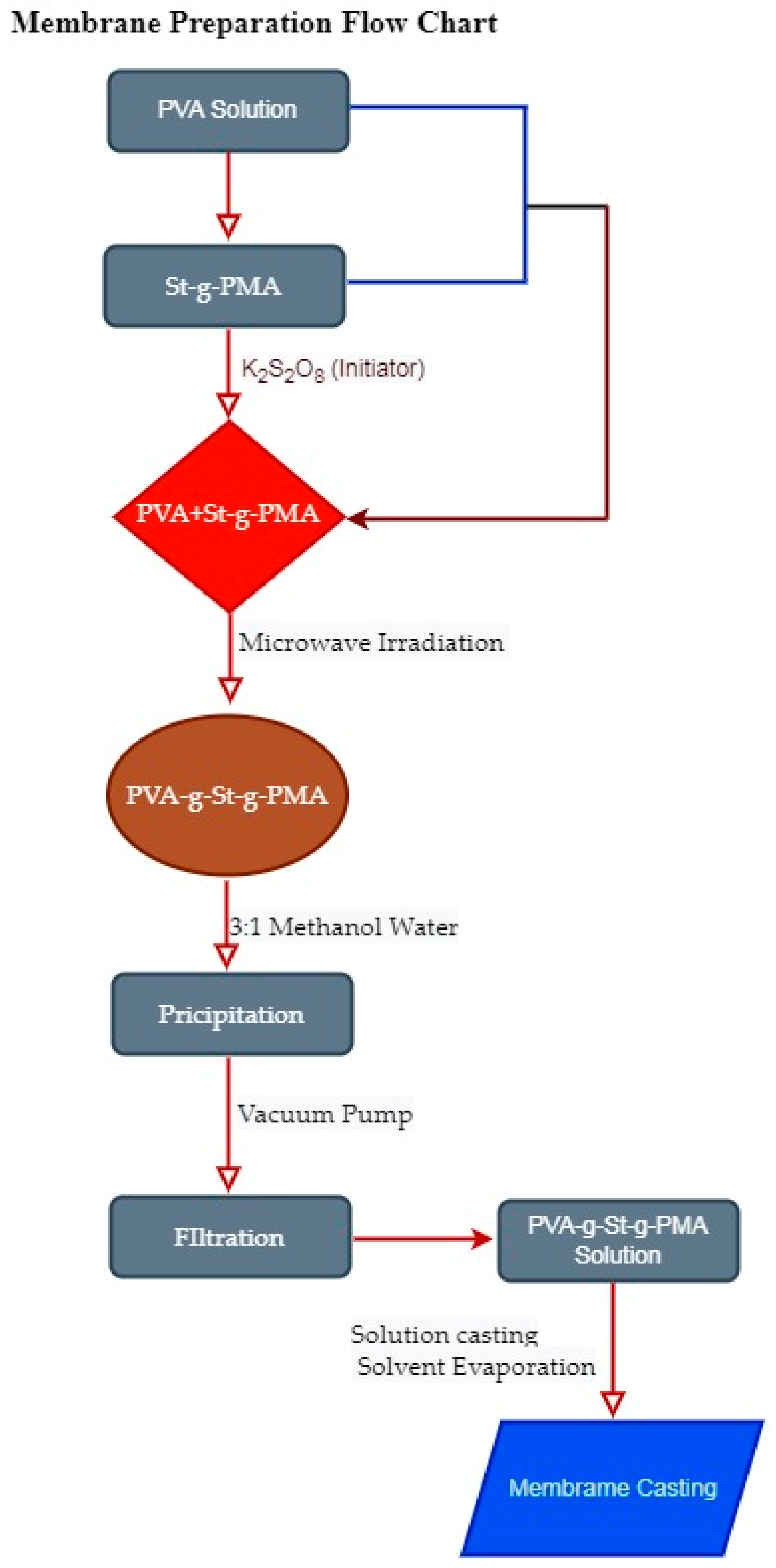
2.2. Synthesis of PVA-g-St-g-PMAcopolymer
2.3. Membrane Fabrication
2.4. Permeability Measurements
- -
- P is the permeability of the gas through the membrane (barrer), (1 Barrer = 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1).
- -
- V is the permeate volume (cm3).
- -
- l is the thickness of the membrane layer (cm).
- -
- A is the effective area of the membrane (cm2).
- -
- Pf is the feed pressure (cmHg).
- -
- P0 is the pressure at standard state (76 cmHg).
- -
- T is the absolute operating temperature (K).
- -
- T0 is the temperature at standard state (273.15 K).
- -
- (dp/dt)ss is the rate of pressure increase in the permeate side at the steady state (cmHg s−1) under the feed pressure.
- -
- (dp/dt)leak is the pressure increase in the permeate side under vacuum (leakage pressure increase).
3. Characterization
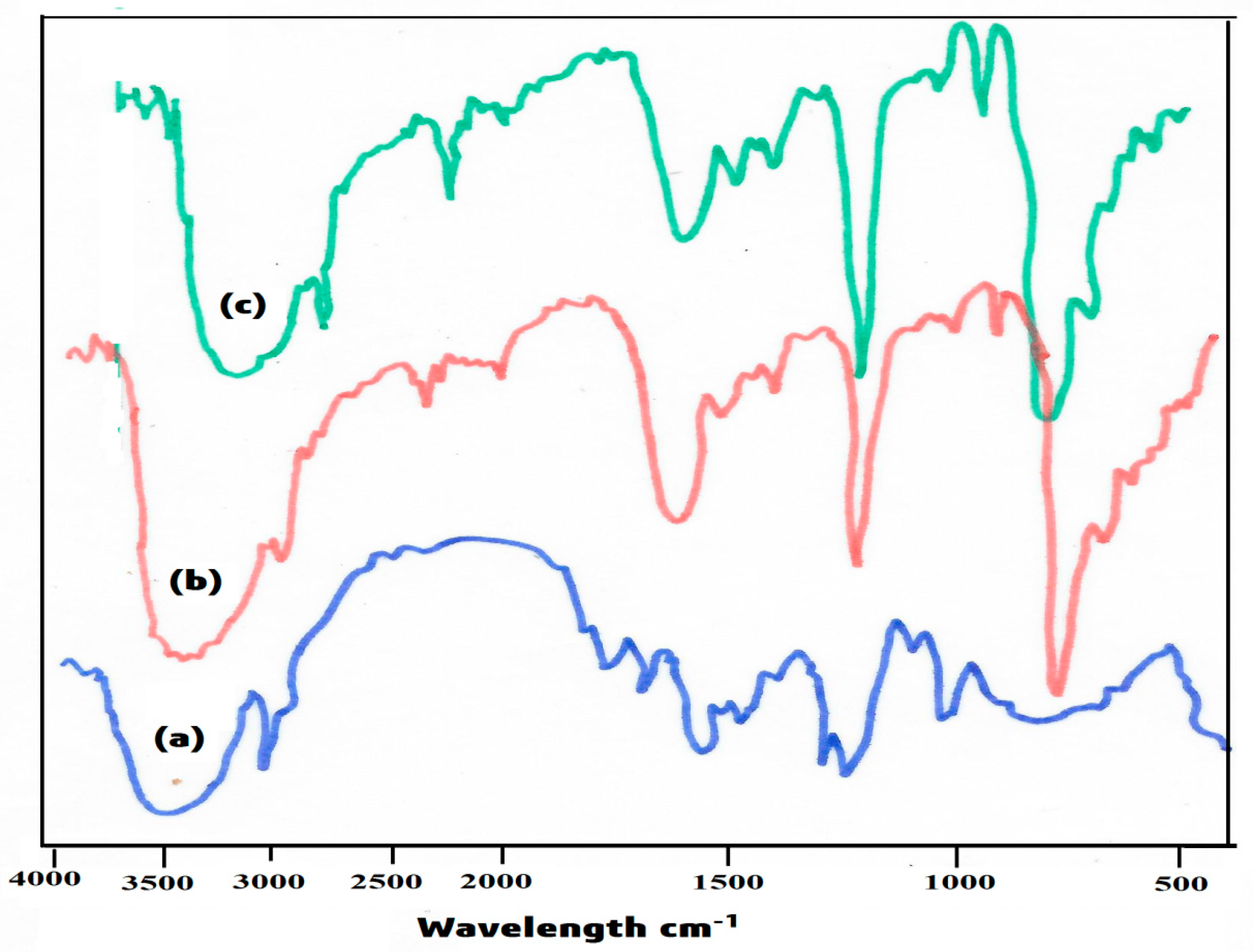
3.1. FTIR
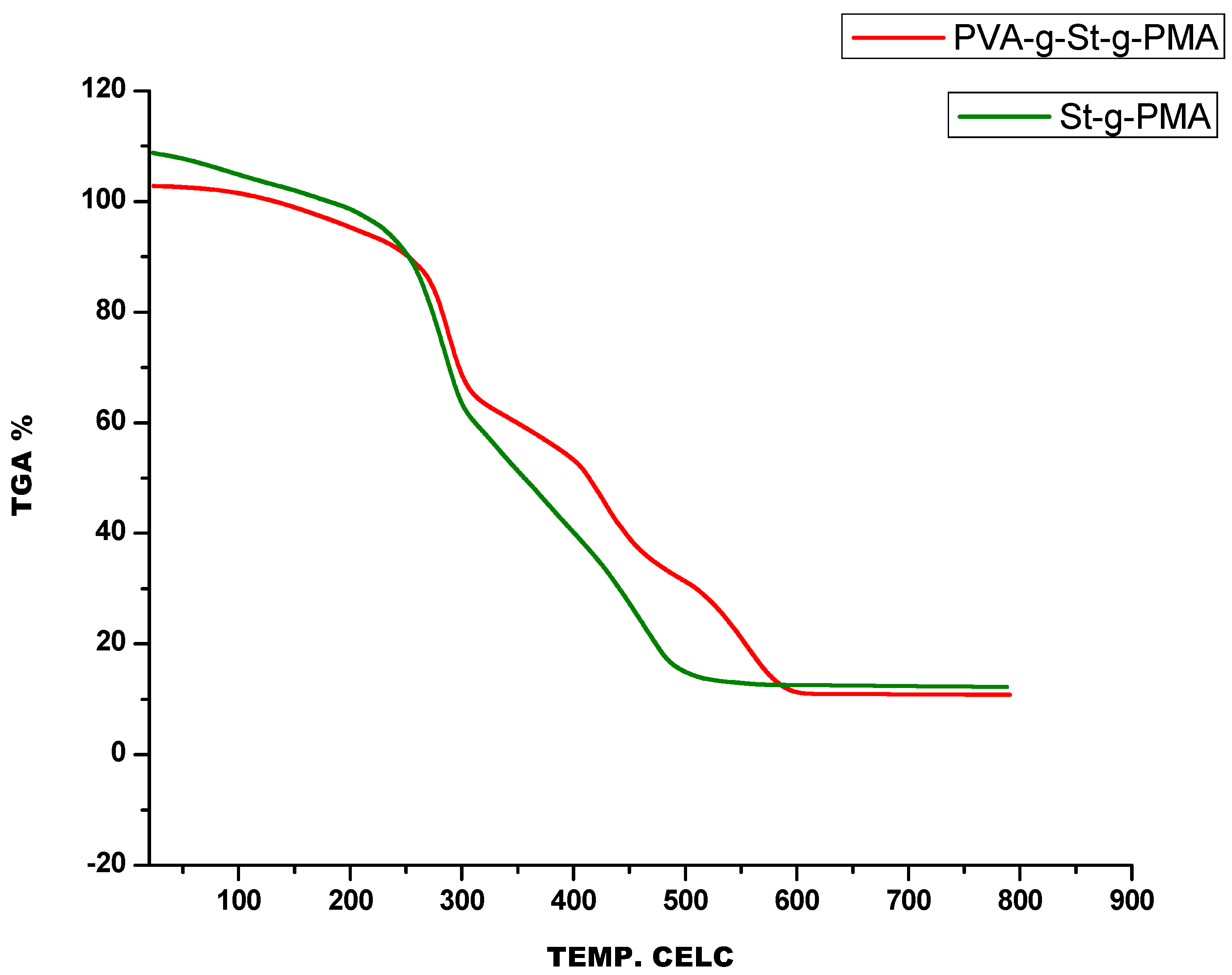
3.2. Thermogravimetric Analysis
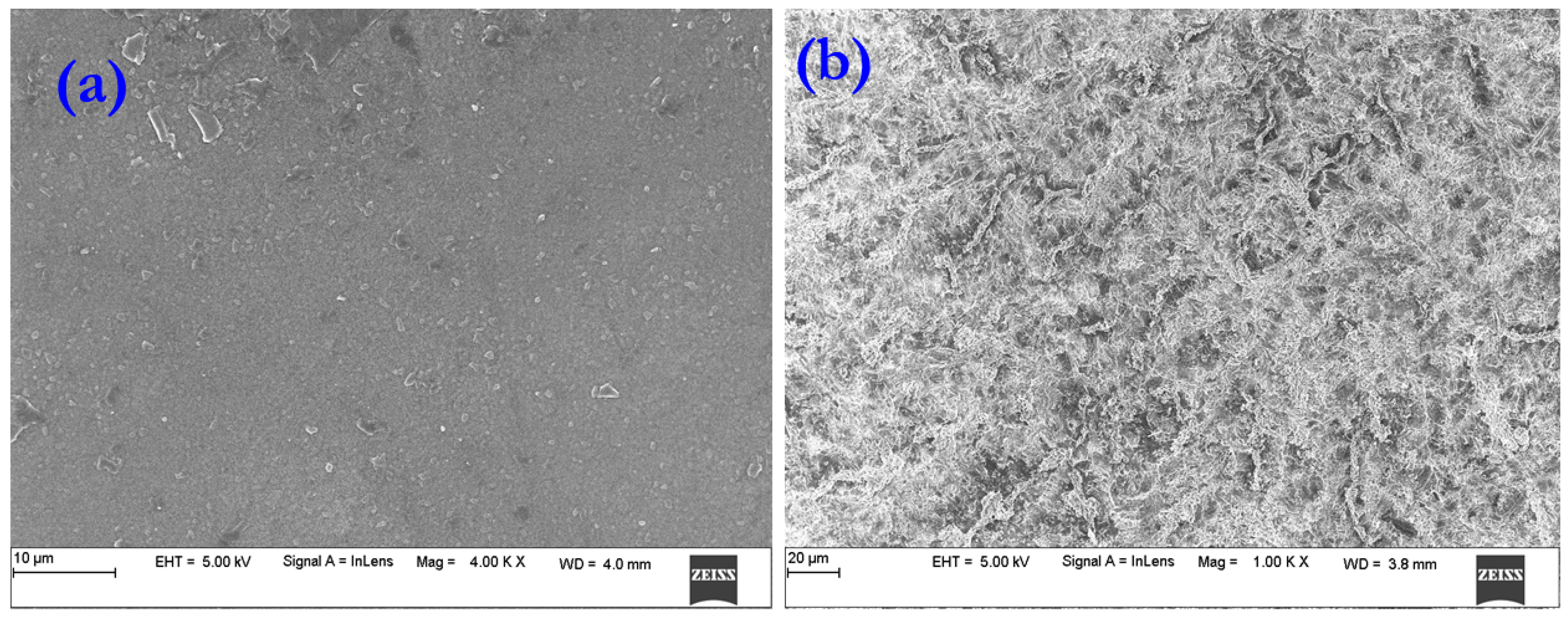
3.3. Scanning Electron Microscopy (SEM) Studies
3.4. Mechanical Properties
4. Result and Discussion
4.1. FTIR Spectroscopy
4.2. Thermogravimetric Analysis
4.3. Scanning Electron Microscopy(SEM) Study
4.4. Mechanical Properties
4.5. Gas Permeability Studies
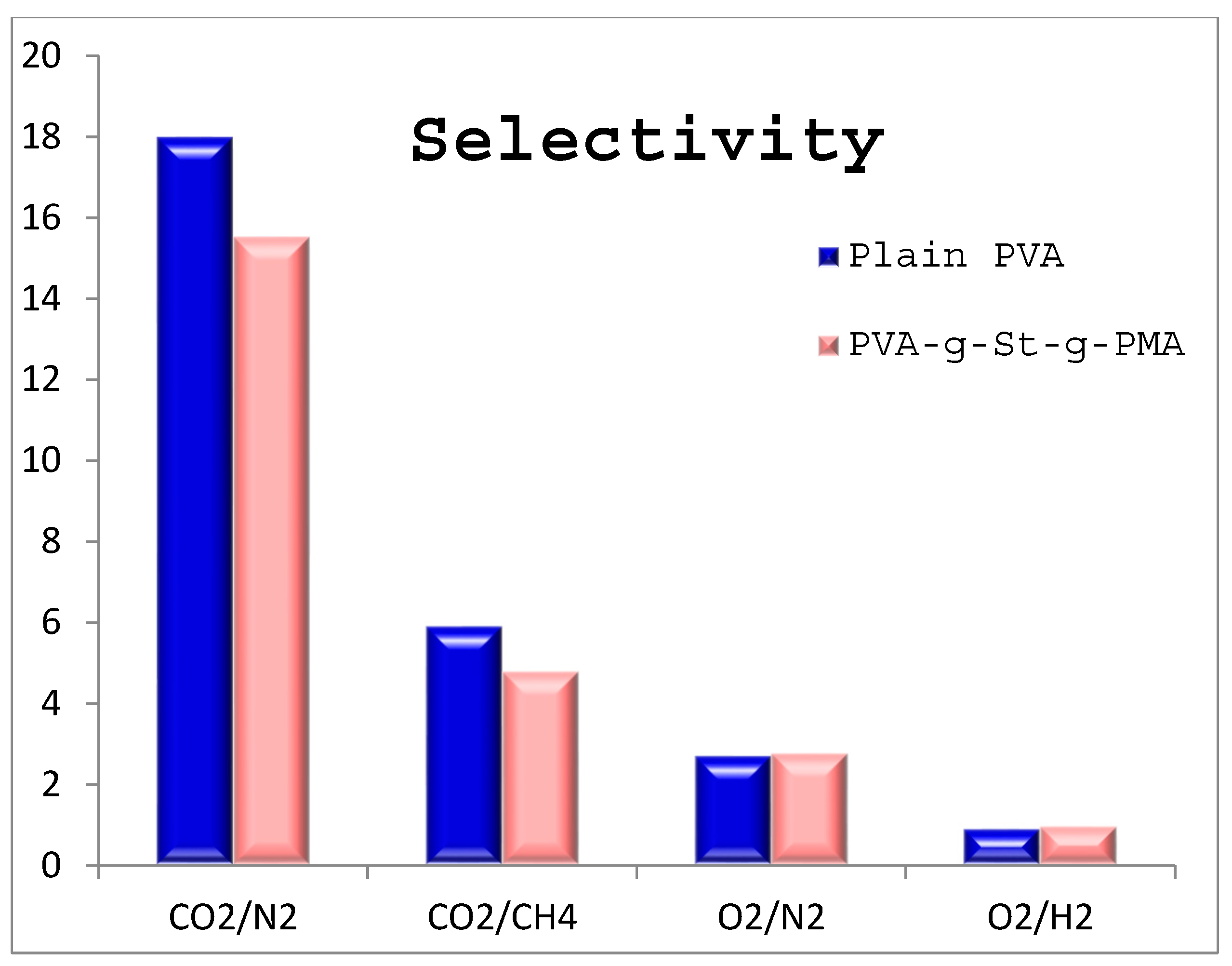
4.6. Selectivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, S.Q.; Manning, S.Q.D.M. Book reviews. S. Afr. Geogr. J. 2009, 91, 103–104. [Google Scholar] [CrossRef]
- McMillan, C.A.; Keoleian, G.A.; Spitzley, D.V. Greenhouse Gases; University of Michigan: Ann Arbor, MI, USA, 2005. [Google Scholar]
- Blasing, T.J. Recent Greenhouse Gas Concentrations, 32nd ed.; US Department of Energy: Washington, DC, USA, 2012.
- Granite, E.J.; O’Brien, T. Fuel Processing Technology; Elsvier: Amsterdam, The Netherlands, 2005; Volume 86, pp. 1421–1706. [Google Scholar]
- Yampolskii, Y.; Freeman, B. Membrane Gas Separation; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 370. [Google Scholar]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Carta, M.; Evans, R.M.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science (Washington, DC, USA) 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and New Perspectives on Integrated Membrane Operations for Sustainable Industrial Growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Patil, M.B.; Amshumali, M.K. Sweetening of Natural Gas Through Hollow Silica Nanoparticles Embedded Hydroxyethyl Cellulose Membrane. Mat. Sci. Res. India 2018, 15, 256–262. [Google Scholar] [CrossRef]
- Murali, R.; Sankarshana, T.; Sridhar, S. Air separation by polymer-based membrane technology. Sep. Purif. Rev. 2013, 42, 130–186. [Google Scholar] [CrossRef]
- Murali, R.S.; Sridhar, S.; Sankarshana, T.; Ravikumar, Y.V.L. Gas permeation behavior of Pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2010, 49, 6530–6538. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Aminabhavi, T.M. Separation of carbon dioxide from natural gas mixtures through polymeric membranes—A review. Sep. Purif. Rev. 2007, 36, 113–174. [Google Scholar] [CrossRef]
- Deng, L.; Kim, T.J.; Sandru, M.; Hägg, M.B. PVA/PVAm blend FSC membrane for natural gas sweetening. In Proceedings of the 1st Annual Gas Processing Symposium; Hassan, E.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 247–255. [Google Scholar]
- Singha, A.S.; Thakur, B.P. Analysis and Characterization of Microwave Irradiation–Induced Graft Copolymerization of Methyl Methacrylate onto Delignified Grewia Optiva Fiber. Int. J. Polym. Anal. Char. 2014, 19, 115–119. [Google Scholar] [CrossRef]
- Mansour, O.Y.; Nagaty, A.J. Graft polymerization of vinyl monomers onto cellulose in presence of soda lime glass. I. J. Polym. Sci. Pol. Chem. 1975, 13, 2785–2793. [Google Scholar] [CrossRef]
- Kaith, B.S.; Kalia, S. Grafting of flax fiber (Linum usitatissimum) with vinyl monomers for enhancement of properties of flax-phenolic composites. Polym. J. 2007, 39, 1319–1327. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; Rao, K.J. On the formation of poly(ethyleneoxide)–poly(vinylalcohol) blends. Eur. Polym. J. 1999, 35, 1883. [Google Scholar] [CrossRef]
- Sato, H.; Ohtani, H.; Tsuge, S.; Aoi, K.; Takasu, K.; Okada, M. Characterization of Chitin-Based Polymer Hybrids by Temperature-Programmed Analytical Pyrolysis Techniques. 2. Chitin-graft-poly(2-methyl-2-oxazoline)/Poly(vinyl alcohol) Blends. Macromolecules 2000, 33, 357–362. [Google Scholar] [CrossRef]
- Watanabe, R.; Hagihara, H.; Sato, H. Structure−property relationships of polypropylene-based nanocomposites obtained by dispersing mesoporous silica into hydroxyl-functionalized polypropylene. Part 2: Matrix−filler interactions and pore filling of mesoporous silica characterized by evolved gas analysis. Polym. J. 2018, 50, 1067–1077. [Google Scholar] [CrossRef]
- Chen, C.H.; Niko, Y.; Konishi, G. Amphiphilic gels of solvatochromic fluorescent poly(2-oxazoline) s containing D−π−A pyrenes. RSC Adv. 2016, 6, 42962–42970. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Mali, S.; Grossmann, M.V.E.; García, M.A. Effects of plasticizers on the properties of oat starch films. Mater. Sci. Eng. C 2009, 29, 532–538. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W. Carbon dioxide separation through polymeric membrane systems for flue gas applications. Rec. Patents. Chem. Eng. 2008, 1, 52–66. [Google Scholar]
- Khudyakov, I.V.; Zopf, D.R.; Turro, N.J. Polyurethane nanocomposites. Des. Monomers Polym. 2009, 12, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Wahab, M.F.A.; Ismail, A.F.; Shilton, S.J. Studies on gas permeation performance of asymmetric polysulfone hollow fiber mixed matrix membranes using nanosized fumed silica as fillers. Sep. Purif. Technol. 2012, 86, 41–48. [Google Scholar] [CrossRef]
- Nor, F.M.; Karim, N.H.A.; Abdullah, I.; Othaman, R.J. Permeability of carbon dioxide and nitrogen gases through SiO2 and MgO incorporated ENR/PVC membranes. Elastomers Plast. 2016, 48, 483–498. [Google Scholar] [CrossRef]
- Jankowski, A.; Grabiec, E.; Noco’n-Szmajda, K.; Marcinkowski, A.; Janeczek, H.; Woli’nska-Grabczyk, A. Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides. Membranes 2021, 11, 274. [Google Scholar] [CrossRef]
- Jaschik, M.; Ta’nczyk, M.; Janusz-Cygan, A.; Wojdyła, A.; Warmuzi’nski, K. The separation of carbon dioxide from CO2/N2/O2 mixtures using polyimide and polysulphone membranes. Chem. Proc. Eng. 2018, 39, 449–456. [Google Scholar]
- Aleksandra, J.C.; Jaschik, J.; Wojdyła, A.; Ta’nczyk, M. The Separative Performance of Modules with Polymeric Membranes for a Hybrid Adsorptive/Membrane Process of CO2 Capture from Flue Gas. Membranes 2020, 10, 309. [Google Scholar] [CrossRef]

| Membrane | Tensile Strength M.Pa ± S.D | % Elongation ± S.D |
|---|---|---|
| Plain PVA Membrane | 84.7 ± 0.5 | 91.4 ± 0.3 |
| PVA-g-St-g-PMA | 71.0 ± 0.4 | 863.6 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, M.; Mathad, S.N.; Patil, A.Y.; Arshad, M.N.; Alorfi, H.S.; Puttegowda, M.; Asiri, A.M.; Khan, A.; Azum, N. Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties. Polymers 2022, 14, 350. https://doi.org/10.3390/polym14020350
Patil M, Mathad SN, Patil AY, Arshad MN, Alorfi HS, Puttegowda M, Asiri AM, Khan A, Azum N. Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties. Polymers. 2022; 14(2):350. https://doi.org/10.3390/polym14020350
Chicago/Turabian StylePatil, Mallikarjunagouda, Shridhar N. Mathad, Arun Y. Patil, Muhammad Nadeem Arshad, Hajar Saeed Alorfi, Madhu Puttegowda, Abdullah M. Asiri, Anish Khan, and Naved Azum. 2022. "Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties" Polymers 14, no. 2: 350. https://doi.org/10.3390/polym14020350
APA StylePatil, M., Mathad, S. N., Patil, A. Y., Arshad, M. N., Alorfi, H. S., Puttegowda, M., Asiri, A. M., Khan, A., & Azum, N. (2022). Synthesis and Characterization of Microwave-Assisted Copolymer Membranes of Poly(vinyl alcohol)-g-starch-methacrylate and Their Evaluation for Gas Transport Properties. Polymers, 14(2), 350. https://doi.org/10.3390/polym14020350










