Nutritional and Functional Properties of Colostrum in Puppies and Kittens
Abstract
Simple Summary
Abstract
1. Introduction
2. Development of the Gastrointestinal System and Food Inputs
3. Composition and Physiological Properties of Colostrum
3.1. Nutritional Function and Composition
3.2. Immune Function
3.3. Absorption Efficiency
3.4. Other Bioactive Compounds
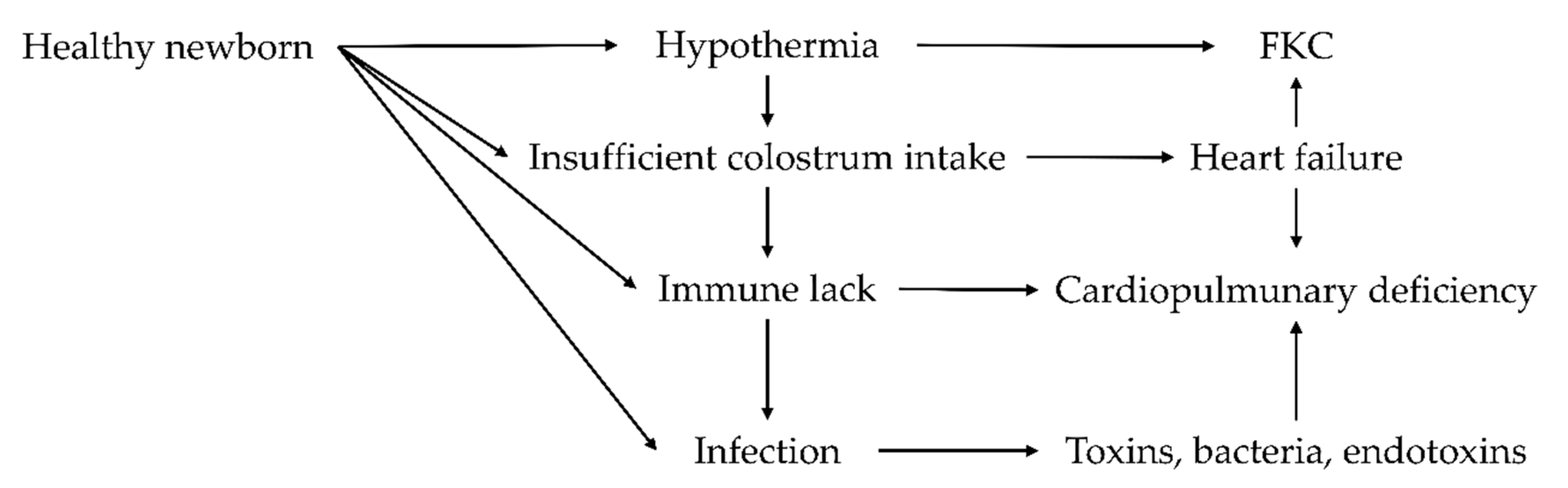
4. Colostrum Deficiencies: Causes and Consequences
4.1. Cardiovascular Troubles
4.2. Hypothermia
4.3. Hypoxia, Anoxia and Acidosis
4.4. Hypoglycemia
5. Supporting Newborns in the Case of Colostrum Deficiencies
6. Nutraceutical Compounds in Newborns
- Cow colostrum:
- Polyunsaturated Fatty Acids (PUFAs).
- Pre- and probiotics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lawler, D.F. Neonatal and pediatric care of the puppy and kitten. Theriogenology 2008, 70, 384–392. [Google Scholar] [CrossRef]
- Cravo, C.D.; Teixeira, C.V.; Passos, M.C.F.; Dutra, S.C.P.; de Moura, E.G.; Ramos, C. Leptin treatment during the neonatal period is associated with higher food intake and adult body weight in rats. Horm. Metab. Res. 2002, 34, 400–405. [Google Scholar] [CrossRef]
- Costăchescu, E.; Hoha, G.; Fotea, L. Research regarding the lactating period of the bitch. Lucr. Ştiinţifice Ser. Zooteh. 2011, 55, 180–183. [Google Scholar]
- Blaxter, K. Lactation and the Growth of the Young. In Milk: The Mammary Gland and Its Secret; Elsevier: Amsterdam, The Netherlands, 1961; Volume 2, pp. 305–361. [Google Scholar]
- Schroeder, G.; Smith, G. Bodyweight and feed intake of German shepherd bitches during pregnancy and lactation. J. Small Anim. Pract. 1995, 36, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Chastant-Maillard, S.; Aggouni, C.; Albaret, A.; Fournier, A.; Mila, H. Canine and feline colostrum. Reprod. Domest. Anim. 2017, 52, 148–152. [Google Scholar] [CrossRef]
- Smith, F.O. Prenatal care of the bitch and queen. Small Anim. Pediatrics 2011, 1–10. [Google Scholar] [CrossRef]
- Fontaine, E. Food intake and nutrition during pregnancy, lactation and weaning in the dam and offspring. Reprod. Domest. Anim. 2012, 47, 326–330. [Google Scholar] [CrossRef]
- Wagner, C.L.; Taylor, S.N.; Johnson, D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin. Rev. Allergy Immunol. 2008, 34, 191–204. [Google Scholar] [CrossRef]
- Elliott, D. Nutritional considerations for optimal puppy growth. Vet. Focus 2012, 22, 2–8. [Google Scholar] [CrossRef]
- Paulsen, D.B.; Buddington, K.K.; Buddington, R.K. Dimensions and histologic characteristics of the small intestine of dogs during postnatal development. Am. J. Vet. Res. 2003, 64, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.; Cappel, T.; Sunvold, G.; Reinhart, G.; Clemens, E. Feline colonic morphology and mucosal tissue energetics as influenced via the source of dietary fiber. Nutr. Res. 2000, 20, 985–993. [Google Scholar] [CrossRef]
- Heird, W.C.; Schwarz, S.M.; Hansen, I.H. Colostrum-induced enteric mucosal growth in beagle puppies. Pediatric Res. 1984, 18, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Chastant, S.; Mila, H. Passive immune transfer in puppies. Anim. Reprod. Sci. 2019, 207, 162–170. [Google Scholar] [CrossRef]
- Van Ierland, Y.; De Beaufort, A. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum. Dev. 2009, 85, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, Ø.; Hove, K.; Sand, O. Physiology of Domestic Animals; Steel, C., Ed.; Scandinavian Veterinary Press: Oslo, Norway, 2003; Volume 211, pp. 632–636. [Google Scholar]
- Mila, H.; Feugier, A.; Grellet, A.; Anne, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S. Inadequate passive immune transfer in puppies: Definition, risk factors and prevention in a large multi-breed kennel. Prev. Vet. Med. 2014, 116, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Godhia, M.L.; Patel, N. Colostrum–its Composition, Benefits as a Nutraceutical–A Review. Curr. Res. Nutr. Food Sci. J. 2013, 1, 37–47. [Google Scholar] [CrossRef]
- Uruakpa, F.; Ismond, M.; Akobundu, E.N. Colostrum and its benefits: A review. Nutr. Res. 2002, 22, 755–767. [Google Scholar] [CrossRef]
- Kienzle, E.; Edtstadtler-Pietsch, G.; Rudnick, R. Retrospective study on the energy requirements of adult colony cats. J. Nutr. 2006, 136, 1973S–1975S. [Google Scholar] [CrossRef]
- Lopate, C. Management of Pregnant and Neonatal Dogs, Cats, and Exotic Pets; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Adkins, Y.; Zicker, S.C.; Lepine, A.; Lonnerdal, B. Changes in nutrient and protein composition of cat milk during lactation. Am. J. Vet. Res. 1997, 58, 370–375. [Google Scholar]
- Adkins, Y.; Lepine, A.J.; Lonnerdal, B. Changes in protein and nutrient composition of milk throughout lactation in dogs. Am. J. Vet. Res. 2001, 62, 1266–1272. [Google Scholar] [CrossRef]
- Debier, C.; Pottier, J.; Goffe, C.; Larondelle, Y. Present knowledge and unexpected behaviours of vitamins A and E in colostrum and milk. Livest. Prod. Sci. 2005, 98, 135–147. [Google Scholar] [CrossRef]
- Hand, M.S.; Lewis, L.D. Small Animal Clinical Nutrition, 4th ed.; Mark Morris Institute: Topeka, KS, USA, 2000; p. 1192. [Google Scholar]
- Coates, P.M.; Blackman, M.; Betz, J.M.; Cragg, G.M.; Levine, M.A.; Moss, J.; White, J.D. Encyclopedia of Dietary Supplements; CRC Press: Boca Raton, FL, USA, 2010; p. 920. [Google Scholar]
- Hurst, E.A.; Homer, N.Z.; Gow, A.G.; Clements, D.N.; Evans, H.; Gaylor, D.; Campbell, S.; Handel, I.; Mellanby, R.J. Vitamin D status is seasonally stable in northern European dogs. Vet. Clin. Pathol. 2020, 49, 279–291. [Google Scholar] [CrossRef]
- Zafalon, R.V.A.; Ruberti, B.; Rentas, M.F.; Amaral, A.R.; Vendramini, T.H.A.; Chacar, F.C.; Kogika, M.M.; Brunetto, M.A. The Role of Vitamin D in Small Animal Bone Metabolism. Metabolites 2020, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Wooding, P.; Burton, G. Endotheliochorial Placentation: Cat, Dog, Bat. In Comparative Placentation: Strucures, Functions and Evolution; Springer: Berlin/Heidelberg, Germany, 2008; pp. 169–183. [Google Scholar]
- Hand, M.S.; Thatcher, C.D.; Remillard, R.L.; Roudebush, P. Body Weights and Feeding Guides for Growing Dogs and Cats. In Small Animal Clinical Nutrition, 4th ed.; Mark Morris Institute: Topeka, KS, USA, 2000; p. 1020. [Google Scholar]
- Zentek, J.; Meyer, H. Normal Handling of Diets Are All Dogs Created Equal. J. Small Anim. Pract. 1995, 36, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, S. Allaitement Artificiel du Chiot Orphelin et Troubles du Comportement. Ph.D. Thesis, University of Nantes, Nantes, France, 2004. [Google Scholar]
- Poffenbarger, E.M.; Olson, P.N.; Chandler, M.L.; Seim, H.B.; Varman, M. Use of Adult Dog Serum as a Substitute for Colostrum in the Neonatal Dog. Am. J. Vet. Res. 1991, 52, 1221–1224. [Google Scholar]
- Chastant-Maillard, S.; Freyburger, L.; Marcheteau, E.; Thoumire, S.; Ravier, J.F.; Reynaud, K. Timing of the Intestinal Barrier Closure in Puppies. Reprod. Domest. Anim. 2012, 47, 190–193. [Google Scholar] [CrossRef]
- Voldoire, E. Physiologie et Pathologie Néonatales du Chiot de Moins de Quinze Jours. Ph.D. Thesis, Université Claude Bernard, Lyon, France, 2002. [Google Scholar]
- Langer, P. Differences in the Composition of Colostrum and Milk in Eutherians Reflect Differences in Immunoglobulin Transfer. J. Mammal. 2009, 90, 332–339. [Google Scholar] [CrossRef]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.-J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Crawford, P.C.; Hanel, R.M.; Levy, J.K. Evaluation of treatment of colostrum-deprived kittens with equine IgG. Am. J. Vet. Res. 2003, 64, 969–975. [Google Scholar] [CrossRef]
- Buddington, R.K. Structure and functions of the dog and cat intestine. In Recent advances in Canine and Feline Nutritional Research: Proceedings of the 1996 Lams International Nutrition Symposium; Orange Frazer Press: Wilmington, OH, USA, 1996; pp. 61–77. [Google Scholar]
- Baintner, K. Transmission of antibodies from mother to young: Evolutionary strategies in a proteolytic environment. Vet. Immunol. Immunopathol. 2007, 117, 153–161. [Google Scholar] [CrossRef]
- Bagwe, S.; Tharappel, L.J.; Kaur, G.; Buttar, H.S. Bovine colostrum: An emerging nutraceutical. J. Complementary Integr. Med. 2015, 12, 175–185. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, T.; Chaturvedi, I. Nutritional importance of colostrum in different farm animals-A Critical. J. Sci. 2016, 2, 16–29. [Google Scholar]
- Case, L.P.; Daristotle, L.; Hayek, M.G.; Raasch, M.F. Chapter 21: Pregnancy and Lactation. In Canine and Feline Nutrition 3; Elsevier: Amsterdam, The Netherlands, 2011; pp. 199–207. [Google Scholar]
- Casal, M.L.; Giger, U. Transfer of colostral antibodies from queens to their kittens. Am. J. Vet. Res. 1996, 57, 1653–1658. [Google Scholar] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Stefaner, I.; Praetor, A.; Hunziker, W. Nonvectorial surface transport, endocytosis via a di-leucine-based motif, and bidirectional transcytosis of chimera encoding the cytosolic tail of rat FcRn expressed in Madin-Darby canine kidney cells. J. Biol. Chem. 1999, 274, 8998–9005. [Google Scholar] [CrossRef]
- West, A.P.; Bjorkman, P.J. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry 2000, 39, 9698–9708. [Google Scholar] [CrossRef]
- Pakkanen, R.; Aalto, J. Growth factors and antimicrobial factors of bovine colostrum. Int. Dairy J. 1997, 7, 285–297. [Google Scholar] [CrossRef]
- Sparks, A.; Kirkpatrick, J.; Chamberlain, C.; Waldner, D.; Spicer, L. Insulin-like growth factor-I and its binding proteins in colostrum compared to measures in serum of Holstein neonates. J. Dairy Sci. 2003, 86, 2022–2029. [Google Scholar] [CrossRef]
- Stojić, V.; Stevanović, J.; Kirovski, D. Effect of colostrum on immunity of newborn domestic mammals during the first days of life. Veterinarski Glasnik 54 (3/4) 2000, 93–106. [Google Scholar]
- Blum, J.W.; Hammon, H. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Livest. Prod. Sci. 2000, 66, 151–159. [Google Scholar] [CrossRef]
- Burrin, D.G.; Davis, T.A.; Ebner, S.; Schoknecht, P.A.; Fiorotto, M.L.; Reeds, P.J. Colostrum enhances the nutritional stimulation of vital organ protein synthesis in neonatal pigs. J. Nutr. 1997, 127, 1284–1289. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef]
- Schwarz, S.M.; Heird, W.C. Effects of Feeding on the Small-Intestinal Mucosa of Beagle Pups During the First 5 d of Life. Am. J. Clin. Nutr. 1994, 60, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.W.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of beta-lactoglobulin and alpha-lactalbumin - Technological implications for processing. Int. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- Xijier; Mori, Y.; Fukuoka, M.; Cairangzhuoma; Inagaki, M.; Iwamoto, S.; Yabe, T.; Kanamaru, Y. Comparison of the Efficacy of Alpha-Lactalbumin from Equine, Bovine, and Human Milk in the Growth of Intestinal IEC-6 Cells. Biosci. Biotechnol. Biochem. 2012, 76, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Adamkin, D.H.; Radmacher, P.G.; Sherman, J.; Niklas, V. Protective Proteins in Mammalian Milks: Lactoferrin Steps Forward. Neoreviwes 2012, 13, e293–e301. [Google Scholar] [CrossRef]
- Sacerdote, P.; Mussano, F.; Franchi, S.; Panerai, A.E.; Bussolati, G.; Carossa, S.; Bartorelli, A.; Bussolati, B. Biological components in a standardized derivative of bovine colostrum. J. Dairy Sci. 2013, 96, 1745–1754. [Google Scholar] [CrossRef]
- Drozdowski, L.; Thomson, A.B.R. Intestinal hormones and growth factors: Effects on the small intestine. World J. Gastroenterol. 2009, 15, 385–406. [Google Scholar] [CrossRef][Green Version]
- Bharti, R.; Khan, A.; Azmi, S. Benefits of feeding bovine colostrum in variety of species. North-East Vet. 2012, 12, 8–9. [Google Scholar]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal. Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef]
- Stewart, C.E.; Pell, J.M. IGF is/is not the major physiological regulator of muscle mass. J. Appl. Physiol. 2010, 108, 1820–1821. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Nykanen, T.; Keinanen, O.; Knuutinen, J.; Lahti, K.; Alen, M.; Rasi, S.; Leppaluoto, J. Protein metabolism and strength performance after bovine colostrum supplementation. Amino Acids 2005, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, Y.; Gauthier, S.F. Milk growth factors as health products: Some technological aspects. Int. Dairy J. 2006, 16, 1415–1420. [Google Scholar] [CrossRef]
- Wynn, P.C. Minor Proteins, Including Growth Factors. In Advanced Dairy Chemistry; Springer: Boston, MA, USA, 2013; pp. 317–335. [Google Scholar]
- Barrington, G.M.; McFadden, T.B.; Huyler, M.T.; Besser, T.E. Regulation of colostrogenesis in cattle. Livest. Prod. Sci. 2001, 70, 95–104. [Google Scholar] [CrossRef]
- Hammon, H.; Blum, J. Feeding different amounts of colostrum or only milk replacer modify receptors of intestinal insulin-like growth factors and insulin in neonatal calves. Domest. Anim. Endocrinol. 2002, 22, 155–168. [Google Scholar] [CrossRef]
- Kelly, D.; King, T.P.; McFadyen, M.; Coutts, A.G.P. Effect of Preclosure Colostrum Intake on the Development of the Intestinal Epithelium of Artificially Reared Piglets. Biol. Neonate 1993, 64, 235–244. [Google Scholar] [CrossRef]
- Christoffersen, R.E. Antibiotics—An investment worth making? Nat. Biotechnol. 2006, 24, 1512–1514. [Google Scholar] [CrossRef]
- Fernandes, P. Antibacterial discovery and development—The failure of success? Nat. Biotechnol. 2006, 24, 1497–1503. [Google Scholar] [CrossRef]
- Sokolowska, A.; Bednarz, R.; Pacewicz, M.; Georgiades, J.A.; Wilusz, T.; Polanowski, A. Colostrum from different mammalian species - A rich source of colostrinin. Int. Dairy J. 2008, 18, 204–209. [Google Scholar] [CrossRef]
- Urashima, T.; Saito, T.; Nakamura, T.; Messer, M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 2001, 18, 357–371. [Google Scholar] [CrossRef]
- Lane, J.A.; Mariño, K.; Naughton, J.; Kavanaugh, D.; Clyne, M.; Carrington, S.D.; Hickey, R.M. Anti-infective bovine colostrum oligosaccharides: Campylobacter jejuni as a case study. Int. J. Food Microbiol. 2012, 157, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Lehto, E.; Salminen, S.; Mikelsaar, M. Inhibition of adhesion of Clostridium difficile to Caco-2 cells. FEMS Immunol. Med. Microbiol. 1996, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Lindbaek, M.; Francis, N.; Cannings-John, R.; Butler, C.C.; Hjortdahl, P. Clinical course of suspected viral sore throat in young adults: Cohort study. Scand. J. Prim. Health Care 2006, 24, 93–97. [Google Scholar] [CrossRef]
- van den Berg, A.; van Elburg, R.M.; Westerbeek, E.A.M.; Twisk, J.W.R.; Fetter, W.P.F. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: A randomized controlled trial. Am. J. Clin. Nutr. 2005, 81, 1397–1404. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.; Suzuki, K.; Okutsu, M.; Pereira, R.; Stevenson, L.; Jenkins, D.G.; Coombes, J.S. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J. Appl. Physiol. 2007, 102, 1113–1122. [Google Scholar] [CrossRef]
- Crooks, C.V.; Wall, C.R.; Cross, M.L.; Rutherfurd-Markwick, K.J. The effect of bovine colostrum supplementation on salivary IgA in distance runners. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 47–64. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeon, W.K.; Yun, J.W.; Park, D.I.; Cho, Y.K.; Sung, I.K.; Sohn, C.I.; Kim, B.I.; Yeom, J.S.; Park, H.S.; et al. Protective effects of bovine colostrum on non-steroidal anti-inflammatory drug induced intestinal damage in rats. Asia Pac. J. Clin. Nutr. 2005, 14, 103–107. [Google Scholar]
- Purup, S.; Vestergaard, M.; Pedersen, L.O.; Sejrsen, K. Biological activity of bovine milk on proliferation of human intestinal cells. J. Dairy Res. 2007, 74, 58–65. [Google Scholar] [CrossRef]
- Du, M.; Xu, W.; Yi, H.; Han, X.; Wang, C.; Zhang, L. Protective effects of bovine colostrum acid proteins on bone loss of ovariectomized rats and the ingredients identification. Mol. Nutr. Food Res. 2011, 55, 220–228. [Google Scholar] [CrossRef]
- Sheridan, L. Kitten nutrition. Vet. Nurs. J. 2012, 27, 232–234. [Google Scholar] [CrossRef]
- Séverin, S.; Wenshui, X. Milk biologically active components as nutraceuticals. Crit. Rev. Food Sci. Nutr. 2005, 45, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M. Colostrum vs vaccination for influenza prevention. Inpharma 2007, 1586, 5. [Google Scholar]
- Struff, W.; Sprotte, G. Bovine colostrum as a biologic in clinical medicine: Review. Int. J. Clin. Pharm. Ther. 2007, 45, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Claus, M.A.; Levy, J.K.; MacDonald, K.; Tucker, S.J.; Crawford, P.C. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J. Feline Med. Surg. 2006, 8, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Crawford, P.C.; Collante, W.R.; Papich, M.G. Use of adult cat serum to correct failure of passive transfer in kittens. J. Am. Vet. Med Assoc. 2001, 219, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.R.; Abonyi-Tóth, Z.; Sprenger, N.; Austin, S.C.; Wichert, B.A.; Liesegang, A.; Oei, C.H.; Balogh, O.; Reichler, I.M. Effect of metoclopramide treatment of bitches during the first week of lactation on serum prolactin concentration, milk composition, and milk yield and on weight gain of their puppies. Am. J. Vet. Res. 2018, 79, 233–241. [Google Scholar] [CrossRef]
- Moura, E.; Pimpão, C.T. Cleft Lip and Palate in the Dog: Medical and Genetic Aspects. In Designing Strategies for Cleft Lip and Palate Care; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Silvestre-Ferreira, A.C.; Pastor, J. Feline neonatal isoerythrolysis and the importance of feline blood types. Vet. Med. Int. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Fisher, E.W. Diagnostic and therapeutic checklists. Neonatal diseases of dogs and cats. Br. Vet. J. 1982, 138, 277–284. [Google Scholar] [CrossRef]
- Giffard, C.J.; Seino, M.M.; Markwell, P.J.; Bektash, R.M. Benefits of bovine colostrum on fecal quality in recently weaned puppies. J. Nutr. 2004, 134, 2126S–2127S. [Google Scholar] [CrossRef]
- Ogbu, K.; Ochai, S.; Danladi, M.; Abdullateef, M.; Agwu, E.; Gyengdeng, J. A review of Neonatal mortality in Dogs. Int. J. Life Sci. 2016, 4, 451–460. [Google Scholar]
- Mortola, J.P. How newborn mammals cope with hypoxia. Respir. Physiol. 1999, 116, 95–103. [Google Scholar] [CrossRef]
- Touzeau, N. Induction de la Parturition de la Chienne par une Molécule Antiprogestérone: L‘Aglépristone. Ph.D. Thesis, The College of Veterinary Medicine, Nantes, France, 2000. [Google Scholar]
- Blunden, T. Fading puppies–Reality or myth? Practice 2012, 34, 314–321. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Casas-Alvarado, A.; José, N.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Nunez, A.; Benavente, I.; Blanco, D.; Boix, H.; Cabanas, F.; Chaffanel, M.; Fernández-Colomer, B.; Fernández-Lorenzo, J.R.; Loureiro, B.; Moral, M.T. Estrés Oxidativo en la Asfixia Perinatal y la Encefalopatía Hipóxico-Isquémica. An. De Pediatría 2018, 88, 221–228. [Google Scholar] [CrossRef]
- Chitty, H.; Wyllie, J. Importance of Maintaining the Newly Born Temperature in the Normal Range from Delivery to Admission. Semin. Fetal Neonatal Med. 2013, 18, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Vanderweyden, G.; Taverne, M.; Vanoord, R. Changes of Jugular Blood-Ph, Blood-Gases and Base Excess and Body-Temperature of Newborn Pups During the 1st Few Hours After Birth. Tijdschr. Voor Diergeneeskd. 1986, 111, S8–S10. [Google Scholar]
- Swann, H.G.; Christian, J.J.; Hamilton, C. The process of anoxic death in newborn pups. Surg. Gynecol. Obstet. 1954, 99, 5–8. [Google Scholar]
- Macintire, D.K. Pediatric fluid therapy. Vet. Clin. North Am. Small Anim. Pract. 2008, 38, 621–627. [Google Scholar] [CrossRef]
- Poffenbarger, E.; Ralston, S.; Chandler, M.; Olson, P. Canine neonatology. Part 1. Physiologic differences between puppies and adults. Compend. Contin. Educ. Pract. Vet. 1990, 12, 1601–1609. [Google Scholar]
- Davidson, A.P. Approaches to Reducing Neonatal Mortality in Dogs. In Recent Advances in Small Animal Reproduction; International Veterinary Information Service: Ithaca, NY, USA, 2003. [Google Scholar]
- England, G.C.W.; Heimendahl, A.V. BSAVA Manual of Canine and Feline Reproduction and Neonatology; British Small Animal Vetiranry Associaion: Gloucester, UK, 2010; p. 230. [Google Scholar]
- Little, S. Feline pediatrics: How to treat the small and the sick. Compend. Contin. Educ. Vet. 2011, 33, 1–6. [Google Scholar]
- McMichael, M. Pediatric emergencies. Vet. Clin. Small Anim. Pract. 2005, 35, 421–434. [Google Scholar] [CrossRef]
- Moon, P.F.; Massat, B.J.; Pascoe, P.J. Neonatal critical care. Vet. Clin. North Am. Small Anim. Pract. 2001, 31, 343–367. [Google Scholar] [CrossRef]
- Hoskins, J.D. Veterinary Pediatrics; Elsevier Sci. Ltd.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- McMichael, M.; Dhupa, N. Pediatric critical care medicine: Specific syndromes. Compend. Contin. Educ. Pract. Vet. 2000, 22, 353. [Google Scholar]
- Casal, M. Clinical Approach to Neonatal Conditions; British Small Animal Vetiranry Associaion: Gloucester, UK, 2010; pp. 147–154. [Google Scholar]
- Köse, A.; Tekelİ, T. The problems faced in puppies and kittens during the neonatal period. Atatürk Üniversitesi Vet. Bilimleri Derg. 2013, 8, 158–165. [Google Scholar]
- Grandjean, D.; Pierson, P.; Rivière, S.; Grellet, A.; Boogaerts, C.; Colliard, L.; Thorel, J.; Overall, K.; Zabel, U. Practical Guide to Dog Breeding, 4th ed.; Royal Canin: Aimargues, France, 2009. [Google Scholar]
- Lawrence, R. Storage of human milk and the influence of procedures on immunological components of human milk. Acta Paediatr. 1999, 88, 14–18. [Google Scholar] [CrossRef]
- Mila, H.; Grellet, A.; Mariani, C.; Feugier, A.; Guard, B.; Suchodolski, J.; Steiner, J.; Chastant-Maillard, S. Natural and artificial hyperimmune solutions: Impact on health in puppies. Reprod. Domest. Anim. 2017, 52, 163–169. [Google Scholar] [CrossRef]
- Van Nguyen, S.; Umeda, K.; Yokoyama, H.; Tohya, Y.; Kodama, Y. Passive protection of dogs against clinical disease due to Canine parvovirus-2 by specific antibody from chicken egg yolk. Can. J. Vet. Res. 2006, 70, 62. [Google Scholar] [PubMed]
- Reynolds, A.; Knorr, R. Effect of feeding hyperimmunized egg powder on indices of gastrointestinal stress in exercising puppies. Compend. Contin. Educ. Pract. Vet. North Am. Ed. 2006, 28, 53. [Google Scholar]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Weiser, M.J. Bovine colostrum: Its constituents and uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Gore, A.M.; Satyaraj, E.; Labuda, J.; Engler, R.; Sun, P.; Kerr, W.; Conboy-Schmidt, L. Supplementation of diets with bovine colostrum influences immune and gut function in kittens. Front. Vet. Sci. 2021, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional roles of Lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.E.; Dunbar, B.L.; Bigley, K.E. Dietary flaxseed in dogs results in differential transport and metabolism of (n-3) polyunsaturated fatty acids. J. Nutr. 1998, 128, 2641S–2644S. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, A.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Uauy, R.; Dangour, A.D. Nutrition in brain development and aging: Role of essential fatty acids. Nutr. Rev. 2006, 64, S24–S33. [Google Scholar] [CrossRef]
- Heinemann, K.M.; Waldron, M.K.; Bigley, K.E.; Leeds, G.E.; Bauer, E.J. Long-Chain (n-3) Polyunsaturated Fatty Acids Are More Efficient than apha- Linolenic Acid in Improving Electroretinogram Responses of Puppies Exposed during Gestation, Lactation, and Weaning. Am. Soc. Nutr. Sci. 2005, 135, 1960–1966. [Google Scholar]
- Corey, E.J.; Shih, C.; Cashman, J.R. Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc. Natl. Acad. Sci. USA 1983, 80, 3581–3584. [Google Scholar] [CrossRef]
- Vonschacky, C.; Weber, P.C. Metabolism and Effects on Platelet-Function of the Purified Eicosapentaenoic and Docosahexaenoic Acids in Humans. J. Clin. Investig. 1985, 76, 2446–2450. [Google Scholar] [CrossRef]
- Czarnecki-Maulden, G. Effect of dietary modulation of intestinal microbiota on reproduction and early growth. Theriogenology 2008, 70, 286–290. [Google Scholar] [CrossRef]
- Alonge, S.; Aiudi, G.G.; Lacalandra, G.M.; Leoci, R.; Melandri, M. Pre-and probiotics to increase the immune power of colostrum in dogs. Front. Vet. Sci. 2020, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Melandri, M.; Aiudi, G.G.; Caira, M.; Alonge, S. A biotic support during pregnancy to strengthen the gastrointestinal performance in puppies. Front. Vet. Sci. 2020, 7, 417. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.C.; Rudinsky, A.J.; Parker, V.J.; Gilor, C. Use of probiotics in small animal veterinary medicine. J. Am. Vet. Med. Assoc. 2017, 250, 519–528. [Google Scholar] [CrossRef] [PubMed]
| Species | Stomach Capacity | Calories Needs | Calories of Colostrum | Feeding Frequency | References |
|---|---|---|---|---|---|
| Kitten | 40–50 mL/kg | 240–275 kcal/kg | 1170 kcal/L | Every 4–6 h | [20,21,22] |
| Puppy | 40–50 mL/kg | 130–220 kcal/kg | 1800 kcal/L | Every 3–6 h | [1,23] |
| Nutrient | Bitch Lactation | Queen Lactation | ||||
|---|---|---|---|---|---|---|
| Composition | Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 |
| True protein (g/L) | 143.00 ± 19.20 | 102.30 ± 13.20 | 81.70 ± 8.80 | 83.00 ± 13.00 | 54.00 ± 3.00 | 63.00 ± 4.00 |
| NPN (g/L) | 1.19 ± 0.05 | 1.07 ± 0.02 | 1.15 ± 0.05 | 0.91 ± 0.12 | 0.78 ± 0.04 | 0.81 ± 0.03 |
| NPN (%/N) | 5.70 ± 0.70 | 6.70 ± 0.50 | 9.10 ± 1.20 | 7.20 ± 1.20 | 8.40 ± 0.80 | 7.50 ± 0.40 |
| Casein (% total protein) | 60.70 ± 4.10 | 75.4.00 ± 2.70 | 68.20 ± 3.70 | 40.40 ± 3.80 | 49.00 ± 0.60 | 50.40 ± 1.60 |
| Whey (% total protein) | 39.30 ± 4.10 | 24.60 ± 2.70 | 31.80 ± 3.70 | 59.60 ± 3.80 | 51.00 ± 0.06 | 49.60 ± 1.60 |
| Lipid (g/L) | 132.20 ± 16.70 | 137.20 ± 11.70 | 132.10 ± 8.30 | 93.00 ± 19.00 | 53.00 ± 9.00 | 76.00 ± 14.00 |
| Lactose (g/L) | 16.60 ± 1.30 | 29.30 ± 2.30 | 35.40 ± 1.00 | 29.90 ± 3.40 | 40.30 ± 1.50 | 39.00 ± 1.60 |
| Citrate (mM) | 4.80 ± 0.40 | 5.30 ± 0.24 | 6.60 ± 0.34 | 6.50 ± 0.30 | 5.80 ± 0.50 | 3.90 ± 0.40 |
| Iron (mg/L) | 3.70 ± 0.29 | 6.90 ± 0.57 | 5.70 ± 0.39 | 1.85 ± 0.300 | 3.90 ± 0.55 | 3.19 ± 0.26 |
| Copper (mg/L) | 1.30 ± 0.66 | 1.40 ± 0.22 | 1.00 ± 0.13 | 0.36 ± 0.07 | 1.34 ± 0.25 | 1.28 ± 0.16 |
| Zinc (mg/L) | 5.00 ± 1.30 | 5.40 ± 1.00 | 5.80 ± 0.40 | 5.78 ± 0.76 | 6.77 ± 1.24 | 6.49 ± 0.58 |
| Magnesium (mg/L) | 128.50 ± 17.80 | 85.80 ± 4.90 | 95.60 ± 5.30 | 111.00 ± 12.00 | 82.00 ± 6.00 | 79.00 ± 7.00 |
| Calcium (mg/L) | 1363.00 ± 108.00 | 1366.00 ± 118.00 | 1773.00 ± 128.00 | 462.00 ± 57.00 | 1162.00 ± 110.00 | 1586.00 ± 122.00 |
| Phosphorus (mg/L) | 935.00 ± 83.00 | 914.00 ± 162.00 | 1166.00 ± 136.00 | 1137.00 ± 45.00 | 1305.00 ± 55.00 | 1529.00 ± 57.00 |
| Ca:P | 1.50 | 1.50 | 1.50 | 0.10 | 0.89 | 1.40 |
| Energy (kcal/L) | 1831.00 ± 506.00 | 1761.00 ± 282.00 | 1657.00 ± 292.00 | 1287.00 ± 141.00 | 853.00 ± 80.00 | 1093.00 ± 137.00 |
| Amino Acids | Bitch (mmol/L) | Queen (mmol/g protein) | ||||
| Composition | Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 |
| Alanine | 0.170 ± 0.012 | 0.052 ± 0.007 | 0.082 ± 0.010 | 0.539 ± 0.006 | 0.571 ± 0.010 | 0.530 ± 0.008 |
| Arginine | 0.104 ± 0.007 | 0.032 ± 0.004 | 0.056 ± 0.008 | 0.352 ± 0.011 | 0.400 ± 0.004 | 0.400 ± 0.003 |
| Asparagine + Aspartic acid | 0.235 ± 0.015 | 0.073 ± 0.009 | 0.117 ± 0.014 | 0.691 ± 0.014 | 0.799 ± 0.011 | 0.769 ± 0.010 |
| Cysteine | 0.058 ± 0.004 | 0.021 ± 0.003 | 0.032 ± 0.005 | 0.130 ± 0.004 | 0.149 ± 0.005 | 0.122 ± 0.004 |
| Glutamine + Glutamic acid | 0.426 ± 0.021 | 0.124 ± 0.018 | 0.242 ± 0.004 | 1.474 ± 0.013 | 1.516 ± 0.015 | 1.581 ± 0.013 |
| Glycine | 0.079 ± 0.008 | 0.020 ± 0.003 | 0.027 ± 0.003 | 0.241 ± 0.016 | 0.222 ± 0.011 | 0.198 ± 0.008 |
| Histidine | 0.076 ± 0.004 | 0.021 ± 0.003 | 0.041 ± 0.007 | 0.193 ± 0.009 | 0.198 ± 0.010 | 0.206 ± 0.008 |
| Isoleucine | 0.128 ± 0.007 | 0.037 ± 0.005 | 0.069 ± 0.011 | 0.290 ± 0.008 | 0.277 ± 0.007 | 0.291 ± 0.006 |
| Leucine | 0.301 ± 0.017 | 0.093 ± 0.014 | 0.169 ± 0.026 | 0.988 ± 0.008 | 0.972 ± 0.004 | 0.997 ± 0.006 |
| Lysine | 0.128 ± 0.008 | 0.037 ± 0.005 | 0.061 ± 0.008 | 0.491 ± 0.019 | 0.526 ± 0.016 | 0.516 ± 0.017 |
| Methionine | 0.073 ± 0.005 | 0.022 ± 0.003 | 0.036 ± 0.005 | 0.213 ± 0.007 | 0.225 ± 0.003 | 0.206 ± 0.016 |
| Phenylalanine | 0.096 ± 0.006 | 0.027 ± 0.004 | 0.048 ± 0.007 | 0.251 ± 0.029 | 0.234 ± 0.003 | 0.224 ± 0.002 |
| Proline | 0.302 ± 0.017 | 0.083 ± 0.013 | 0.168 ± 0.028 | 1.073 ± 0.011 | 0.910 ± 0.020 | 0.929 ± 0.007 |
| Serine | 0.138 ± 0.007 | 0.036 ± 0.005 | 0.065 ± 0.009 | 0.545 ± 0.026 | 0.482 ± 0.006 | 0.467 ± 0.006 |
| Threonine | 0.149 ± 0.009 | 0.045 ± 0.006 | 0.070 ± 0.007 | 0.517 ± 0.011 | 0.479 ± 0.008 | 0.480 ± 0.019 |
| Tryptophan | 0.007 ± 0.002 | n.d. | 0.005 ± n.d. | 0.094 ± 0.004 | 0.095 ± 0.007 | 0.094 ± 0.002 |
| Tyrosine | 0.068 ± 0.005 | 0.021 ± 0.005 | 0.031 ± 0.004 | 0.278 ± 0.003 | 0.274 ± 0.001 | 0.275 ± 0.001 |
| Valine | 0.206 ± 0.013 | 0.060 ± 0.008 | 0.102 ± 0.014 | 0.415 ± 0.014 | 0.365 ± 0.012 | 0.376 ± 0.010 |
| Bioactive Compounds | Function | Reference |
|---|---|---|
| ß-lactalbulin | Potential antiviral, prevention of pathogen adhesion anticarcinogenic and hypocholesterolemic, and hydrophobic components binding ability, including retinol and long-chain fatty acids. | [55] |
| α-lactalbulin | Calcium metalloprotein, in which Ca plays a crucial role in the folding and structure. Effector of lactose synthesis in mammary gland, calcium carrier, immunomodulatory, precursor for bioactive peptides potentially anticarcinogenic. | [56] |
| Lactoferrin | Antimicrobial, antioxidative, anticarcinogenic, anti-inflammatory, iron transport, cell growth regulation, precursor for bioactive peptides, immunomodulatory, stimulation of osteoblast proliferation. | [57] |
| Lactoperoxidase | Antimicrobial, synergistic effects with immunoglobulins, lactoferrin, and lysozyme. | [53] |
| Lysozyme | Antimicrobial, synergistic effects with immunoglobulins, lactoferrin, and lactoperoxidase. | [58] |
| Growth factors | Function | Reference |
| Epidermal growth factor (EGF) | Stimulation of cell growth, intestinal cell protection and repair, regulation of immune system. | [59] |
| Binding proteins (IGFBP) | Marked anabolic characteristics, gastrointestinal maturation, and wound healing contribution. | [60] |
| Transforming growth factor -alpha (TGF-α) and beta (TGF-ß) | Cellular proliferation and tissue growth, maturation, and repair activation. Inductive effect on IgA production in Peyer’s patch lymphocytes and spleen lymphocytes. | [61] |
| Insulin-like growth factor 1 (IGF-1). | Maintenance of adult muscle mass depending on satellite cells activation, proliferation, survival, and differentiation, processes. Lean muscle growth and beta oxidation of fats. | [62] |
| Hepatocyte growth factor (HGF). | Produced by macrophages, important factor for intestinal cells growth in neonates after birth. DNA and proteins synthesis and nutrient uptake enhancement, particularly in muscles and cartilages. | [63] |
| Platelet derived growth factor (PDGF) | Gastrointestinal development and maturation. | [64] |
| Vascular endothelial growth factor vascular (VEGF) | Gastrointestinal growth and perivascular maturation. | [65] |
| Growth hormone (GH) | Maturation of gastrointestinal mucous membrane development and closing of antibody transport at intestinal level. | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, L.; Lumbreras, A.E.V.; Vagni, S.; Dell’Anno, M.; Bontempo, V. Nutritional and Functional Properties of Colostrum in Puppies and Kittens. Animals 2021, 11, 3260. https://doi.org/10.3390/ani11113260
Rossi L, Lumbreras AEV, Vagni S, Dell’Anno M, Bontempo V. Nutritional and Functional Properties of Colostrum in Puppies and Kittens. Animals. 2021; 11(11):3260. https://doi.org/10.3390/ani11113260
Chicago/Turabian StyleRossi, Luciana, Ana Elena Valdez Lumbreras, Simona Vagni, Matteo Dell’Anno, and Valentino Bontempo. 2021. "Nutritional and Functional Properties of Colostrum in Puppies and Kittens" Animals 11, no. 11: 3260. https://doi.org/10.3390/ani11113260
APA StyleRossi, L., Lumbreras, A. E. V., Vagni, S., Dell’Anno, M., & Bontempo, V. (2021). Nutritional and Functional Properties of Colostrum in Puppies and Kittens. Animals, 11(11), 3260. https://doi.org/10.3390/ani11113260








